Abstract
Although olfaction is the main sensory domain in dogs and urinary markings are crucial behavioral patterns involved in canids’ communication, the scientific literature on this topic is quite scarce. In the present work, we investigated the relationship between male dogs marking behavior (scored throughout different facial expression, marking distance from the target odor etc.) and physiological response (urine concentrations of the hormones mainly involved in the physiological responses to social stressors: cortisol, oxytocin, adrenaline, testosterone; and the neurotransmitters most responsible for cognitive flexibility: norepinephrine and dopamine) to different emotional odors. Specifically, the odour stimuli were urine odors collected from (1) an unfamiliar female in estrus, which were expected to increase male dogs’ arousal level due to sexual interest; and of (2) an unfamiliar intact male showing overt agonistic behaviors toward conspecific males, which were expected to elicit the arousal level in the tested subjects representing a potential threat. Although preliminarily, our data would indicate a relationship between differences in the type of marking behavior of male dogs and both hormonal and neurotransmitters levels in their urines, suggesting a modulation in canine cognitive strategy during marking.
Similar content being viewed by others
Introduction
Olfaction plays a pivotal role in canids’ communication1,2. Chemiosignals, better defined as pheromones3,4, are an efficient mean to convey social information about the individuals, their sex, reproductive and emotional state without the need for subjects’ direct contacts2. The indirect transfer of information provides considerable advantages. It allows individuals to assess the others’ features and decide whether and how to approach them5,6, helping to avoid potentially dangerous direct interactions. On the other hand, the chemical communication contributes to the development and maintenance of social relationship; it aids courtship, mate identification and resources defense (e.g. territory or mates)5. In dogs, pheromones are mainly excreted in urine, feces and secretions of several glands (including anal sacs and pedal scent gland) through micturition, defecation and rubbing behaviors7,8.
It has been found that scent-marking behavior, the chemical compositions of scent marks and mark investigation are affected by different factors. They vary according to dogs’ age9,10, size6 and sex11,12,13 and appear to be influenced by hormonal levels14,15. In particular, small-sized dogs display a higher rate of urine-marking behavior than large breeds, demonstrating their potential preference for an indirect communication, which prevents any costly direct encounters6. On the other hand, the frequency of urine-marking behavior differs between male and female dogs and specifically it is higher in males9,11. Sexual dimorphism has also been reported for the composition of anal gland secretions but has no effect on defecation and ground scratching behavior16. The role of sexual hormones appears to be crucial for the regulation of canids’ scent marking and the chemical composition of scent marks10,14. In females, during proestrus and estrus both the amount of chemical compounds in urines, including carbonyl aromatic compounds and methyl ketones14 and the frequency of urine marking behavior increase10. The latter facilitates the pheromones diffusion in the environment enhancing the females’ chance to mate10. On the other hand, the transition to diestrus, i.e. a phase of the ovarian cycle where bitches are not fertile, is accompanied by the sharp increase of sulfide compounds, which have a repelling effect on males14. The gonadal hormones have a key role also in males’ chemical communication. It has been found indeed that gonadectomy, which cause a significant decrease in androgens, causes a significant decrease in the rate of urination and investigative behavior of conspecific urine marks15. In addition, androgens affect the chemical composition of urine marks, as the exogenous testosterone administration influence the volatile urine components in grey wolves, which share many characteristics of social behavior with dogs17. The gonadal hormones support also the expression of countermarking behavior in male dogs, i.e. the deposition of urine marks in response to previous scent marks5. Overmarking (i.e. scent marking behavior on previous scent marks) was observed only in males, and only intact males (not gonadectomized ones) overmarked on intact female urines, suggesting a role of androgens in mate guarding behavior5. Nevertheless, beside sex, overmarking behavior is also affected by different factors like dog social status5 or emotional state2. Lisberg and colleagues5 observed that dogs’ tail base position varied according to the expression of overmarking behavior. In particular, overmarking males displayed higher tail than no countermarking males during urination, suggesting a role of chemical signals for the transmission of social information to conspecifics. However, considering that the tail wagging conveys information about the individuals’ emotional state2,18,19, the relationship between countermarking behavior and tail base position suggests that emotional states may affect dogs’ scent communication. Indeed, previous studies report that dogs are able to recognize the odors of conspecifics collected during stressful events and increased their arousal level accordingly20. This suggests that both hormones and neurotransmitters related to stress intervene in olfactory-based communication and empathy21 but the underlying mechanisms remain to be defined. In humans, hormones regulating stress response, i.e. catecholamine, induce the spreading of olfactory signals by increasing sweating and micturition and alter the properties of the microbial communities living in the body (e.g. skin and digestive tract) which cause a variation of odorant metabolites21. Similarly, it could be possible that the changes in emotional states could affect dog scent marking behavior and the chemical composition of scent marks, which in turn could be detected by conspecifics causing their physiological and behavioral responses.
The aim of the present study was to investigate whether male dogs show different marking behaviors (i.e. facial expression during sniffing the target odor, marking distance from the target odor etc.) and physiological responses (urine concentrations of epinephrine, norepinephrine, dopamine, cortisol, testosterone and oxytocin before and during marking) to conspecific urine odors collected from an unfamiliar female in estrus (which were expected to increase male dogs’ arousal level due to sexual interest) and of an unfamiliar intact male showing overt agonistic behaviors toward conspecific males (which were expected to elicit the arousal level in the tested subjects representing a potential threat). In particular, regarding the physiological responses, we decided to measure the urinary levels of the hormones mainly involved in responding to social stressors (cortisol, oxytocin, epinephrine, testosterone12,22,23,24 and the neurotransmitters most responsible for cognitive flexibility (norepinephrine and dopamine)25,26,27,28, whose possible variations would justify a different marking behavior with direct implications on canine intraspecific communication.
Results
The analysis revealed an effect of the “remote marks” on the cortisol levels in dogs’ urine; specifically, overall cortisol levels were higher when urine were deposited at a distance longer than 2 m from the swab with respect to other type of marks (F(1,26) = 8.772, P < 0.01); GLMM analysis; see Fig. 1A); in addition, an interaction between “remote marks” and “order of marks” indicated that this differences occurred during the second presentation of the odor stimuli ((F(1, 26) = 5.154, P < 0.05; GLMM analysis; see Fig. 1B). No other significant differences with respect to urine cortisol levels were observed (order (F(1,26) = 0.736, P = 0.399); stimulus (F(1,26) = 0.522; P = 0.476); condition (F(1,26) = 1.526, P = 0.228); close marks (F(1,26) = 0.948, P = 0.339); stimulus x order (F(1,26) = 2.637, P = 0.116); condition x order (F(1,26) = 0.004, P = 0.952); order x close marks (F(1,26) = 0.031, P = 0.861); condition x stimulus (F(1,26) = 0.112, P = 0.741); condition x close marks (F(1,26) = 1.221, P = 0.279); condition x remote marks (F(1,26) = 0.274, P = 0.605); stimulus x close marks (F(1,26) = 0.200, P = 0.658); stimulus x remote marks (F(1,26) = 1.088, P = 0.307).
Urinary cortisol levels. A) Overall cortisol levels in the presence of close-medium distant and remote marks (N = 41; group means with SEM are shown; **P < 0.01; Fisher’s LSD test). B) Overall cortisol levels in the presence of close-medium distant and remote marks during the first and the second presentation of the odour stimuli (N = 41; group means with SEM are shown; *P < 0.05; Fisher’s LSD test).
Regarding oxytocin, overall levels in urine were higher during the second presentation of the odor stimuli with respect to the first (order: F(1,26) = 4.251, P < 0.05); GLMM analysis; see Fig. 2A); furthermore, this evidence was more manifested comparing remote with respect to close-medium distance marks (order x remote marks interaction (F(1,26) = 31.370, P < 0.001; GLMM analysis; Fig. 2B). No other significant differences with respect to urine oxytocin levels were observed (stimulus (F(1,26) = 0.679; P = 0.417); condition (F(1,26) = 2.486, P = 0.127); close marks (F(1,26) = 0.417, P = 0.524); stimulus x order (F(1,26) = 1.712, P = 0.202); condition x order (F(1,26) = 1.196, P = 0.170); order x close marks (F(1,26) = 0.077, P = 0.784); condition x stimulus (F(1,26) = 1.723, P = 0.201); condition x close marks (F(1,26) = 0.023, P = 0.880); condition x remote marks (F(1,26) = 0.702, P = 0.410); stimulus x close marks (F(1,26) = 0.355, P = 0.557); stimulus x remote marks (F(1,26) = 2.080, P = 0.161).
Urinary oxytocin levels. A) Overall oxytocin levels in urine (i.e. baseline and marks) during the first and the second presentations of the odour stimuli (N = 41; group means with SEM are shown; *P < 0.05; Fisher’s LSD test). B) Overall urine oxytocin levels in the presence of remote marks and close-medium distant marks during the first and the second presentation of the odour stimuli (N = 41; ***P < 0.001, estimated by GLMM analysis; means with S.E.M. are shown).
As for testosterone, the analysis revealed an interaction between type of stimulus and order (F(1,29) = 12.674, P < 0.001; GLMM analysis; see Fig. 3A) indicating that overall testosterone urine levels during the sessions in which the male urine odor was presented as the first were higher while the opposite pattern was observed during presentation of the female urine odor; in addition, the analysis revealed that, during the second experimental sessions, overall testosterone levels in the presence of remote marks were higher with respect to dogs which showed the other type of marks; on the other hand, a reversed pattern was observed during the first experimental sessions (order x remote marks: (F(1,29) = 9.649, P < 0.001; GLMM analysis; see Fig. 3B); finally, an interaction between type of stimulus and remote marks revealed that during the sessions in which the female odor was presented, overall testosterone levels were higher in subjects displaying remote marks with respect to dogs which displayed the other type of marking behaviors (F(1,29) = 6.671, P < 0.05; GLMM analysis; see Fig. 3C). No other significant differences with respect to urine testosterone levels were observed (order (F(1,29) = 0.001; P = 0.974); stimulus (F(1,29) = 0.154; P = 0.698); condition (F(1,29) = 0.679, P = 0.417); close marks (F(1,29) = 0.128, P = 0.723); remote marks (F(1,29) = 0.280; P = 0.601); condition x order (F(1,29) = 0.103, P = 0.750); order x close marks (F(1,29) = 0.516, P = 0.478); condition x stimulus (F(1,29) = 0.035, P = 0.852); stimulus x close marks (F(1,29) = 0.006, P = 0.55); condition x close marks (F(1,29) = 0.270, P = 0.938); condition x remote marks (F(1,29) = 0.965, P = 0.334).
Urinary testosterone levels. A) Overall testosterone levels during the experimental sessions in which the male and female urine odors were presented as the first and the second (N = 44; group means with SEM are shown; **P < 0.01; Fisher’s LSD test). B) Overall testosterone levels during the first and the second experimental sessions in remote vs. close-medium distant marks (N = 44; group means with SEM are shown; **P < 0.01; Fisher’s LSD test). C) Overall testosterone levels in the presence of close-medium distant and remote marks during the experimental sessions in which the male and female urine odors were presented (N = 44; group means with SEM are shown; *P < 0.05; Fisher’s LSD test).
With regard to urine catecholamine levels, GLMM analysis revealed an effect of order on overall norepinephrine urine levels (order: F(1,27) = 5.796, P < 0.05 Fig. 4A) indicating that dogs that had higher levels of this catecholamine during the second experimental session with respect to the first; in addition an effect of condition was revealed indicating higher levels of norepinephrine in marks with respect to the baselines (condition: (F(1,27) = 7.338, P < 0.05 Fig. 4B); furthermore, a condition x order interaction showed that this finding was manifested during the second experimental sessions and not during the first (condition x order: (F(1,27) = 7.265, P < 0.05 Fig. 4C); in addition, the analysis revealed that overall norepinephrine levels in urine were higher in the presence of remote marks with respect to the other type of marks; (F(1,27) = 5.280, P < 0.05); GLMM analysis; see Fig. 4D); finally, an interaction between condition and remote mark revealed an increase in norepinephrine urinary levels of remote marks compared to baseline values (F(1,27) = 5.972, P < 0.05); GLMM analysis; (Fig. 4E).
Urinary norepinephrine levels. A) Overall norepinephrine levels in urine during the first and the second presentations of the odour stimuli (N = 44; group means with SEM are shown; *P < 0.05; Fisher’s LSD test). B) Norepinephrine levels in marks and basal levels (N = 44; group means with SEM are shown; *P < 0.05; Fisher’s LSD test). C) Norepinephrine levels in baselines and marks during the first and the second presentations of the odour stimuli (N = 44; group means with SEM are shown; *P < 0.05; Fisher’s LSD test). D) Overall norepinephrine levels in the presence of close-medium distant and remote marks (N = 44; group means with SEM are shown; *P < 0.05; Fisher’s LSD test). E) Norepinephrine levels in marks and baselines in dogs which displayed remote and close-medium distant marks (N = 44; group means with SEM are shown; *P < 0.05; Fisher’s LSD test).
Regarding dopamine, the GLM analysis revealed a “remote” marking effect, indicating that overall urinary levels of dopamine were higher in the presence of remote marks (remote mark (F(1,26) = 14.550, P < 0.001; Fig. 5A). The analysis also highlighted a stimulus effect, indicating that dogs had higher overall levels of dopamine in their urine during sessions in which the odor of the male agonistic dog was presented (stimulus (F(1,26) = 9.084, P = 0.005; Fig. 5B), an effect, the latter, that was significantly evident in the presence of remote marks (stimulus x remote mark (F(1,26) = 13.684, P < 0.001; Fig. 5C).
No other significant differences with respect to catecholamine were observed: norepinephrine (stimulus (F(1,27) = 2.777; P = 0.107); close mark (F(1,27) = 0.011, P = 0.917); stimulus x order (F(1,27) = 0 0.06, P = 0.938); order x close mark (F(1,27) = 0.650, P = 0.427); order x remote mark (F(1,27) = 2.580, P = 0.120); condition x stimulus (F(1,27) = 2.783, P = 0.107); stimulus x close marks (F(1,27) = 2.198, P = 0.150); stimulus x remote mark (F(1,27) = 2.219, P = 0.148); condition x close mark (F(1,27) = 3.094, P = 0.090)); epinephrine (order (F(1,28) = 2.579; P = 0.120); stimulus (F(1,28) = 3.515; P = 0.071); condition (F(1,28) = 0.000, P = 0.984); close marks (F(1,28) = 0.811, P = 0.375); remote marks (F(1,28) = 1.939; P = 0.175); condition x order (F(1,28) = 0.099, P = 0.755); order x close marks (F(1,28) = 1.570, P = 0.221); condition x stimulus (F(1,28) = 0.057, P = 0.813); condition x close marks (F(1,28) = 0.000, P = 0.984); condition x remote marks (F(1,28) = 0.944, P = 0.340)) stimulus x close marks (F(1,28) = 0.443, P = 0.511);); stimulus x remote mark (F(1,28) = 1.479, P = 0.234)); dopamine (order (F(1,26) = 4.090; P = 0.054); condition (F(1,26) = 1.888, P = 0.181); close marks (F(1,26) = 0.089, P = 0.767); stimulus x order (F(1,26) = 3.938, P = 0.058); condition x order (F(1,26) = 0.273, P = 0.606); order x remote mark (F(1,26) = 2.478, P = 0.128); condition x close marks (F(1,26) = 2.478, P = 0.128); condition x remote marks (F(1,26) = 0.202, P = 0.657).
A) Dopamine levels in close-medium distant marks and remote marks (N = 40; group means with SEM are shown; **P < 0.01; Fisher’s LSD test). B) Overall dopamine levels during sniffing at male and female urine odors (N = 40; group means with SEM are shown; **P < 0.01; Fisher’s LSD test). C) Dopamine levels in close-medium distant marks and remote marks during sniffing at male and female urine odors (N = 40; group means with SEM are shown; **P < 0.01; Fisher’s LSD test).
Correlations between physiological parameters and behavioral measures
The low scores observed for the “Neutral” and “Anxious” did not allow the inclusion of these behavioural categories in the final analysis.
Negative correlation was found between basal levels of oxytocin in urine and “attentive” facial expression during sniffing at the swab impregnated of female odor (r10 = −0.642, P = 0.045; Spearman) indicating that the higher the baseline of oxytocin levels in the subjects tested, the least was the attention towards the odor stimulus. Furthermore, the analysis reported a negative correlation between the urinary epinephrine basal levels and “attentive” face scores during sniffing the male odor target (r11=−0.619, P = 0.042). As regards the correlations between the urinary levels of hormones in the marks and the behavior during sniffing, the analysis reported a positive correlation between both cortisol and oxytocin urine levels and “fearful” facial expression during sniffing the male odor (cortisol: r11 = 0.684, P = 0.020; oxytocin: r11 = 0.790, P = 0.004) indicating that higher levels of these hormones in the urinary marks correspond to higher levels of “fearful” facial expression during the sniffing of the urine of the male dog with an agonistic behavior. As for the “attentive” facial expression, the results indicated a negative correlation with oxytocin (r11=−0.752, P = 0.008) and cortisol (r = 11 −0.771, P = 0.014) levels in the marks during sniffing at male urine odor. Finally, testosterone urinary levels in marks were positively correlated with “agonistic” facial expression during sniffing at female odor, indicating that higher testosterone levels are accompanied by a greater degree of aggressiveness in the facial expression of dogs during the sniffing of this odor (r11 = 0.621; P = 0.041).
Discussion
Overall, the results showed an evident increase in the urinary levels of cortisol in the presence of “remote marks” and this phenomenon was more manifested during the second experimental sessions. In dogs, urinary cortisol levels have been validated as a non-invasive measure of stress responses7. Cortisol, in fact, is a well-known stress marker in dogs and reflects the activity of the hypothalamic-pituitary-adrenal (HPA) axis in different animal models29,30. An increase in the levels of this hormone have been related to both acute and long-term stress, although the latter could be also associated with low cortisol levels for the well -known negative feedback effect24. Our result could therefore indicate a relationship between “remote marks” and higher stress responsiveness of the dogs, the latter independent of the odor stimulus. However, a more subtle and deep analysis of the fact that higher urinary levels of cortisol in the presence of “remote marks” was more manifested during the second experimental sessions (order effect) could find a reasonable explanation in the difference between the odour stimuli. More specifically, given that, the presentation order of the stimuli was unbalanced, most of the dogs have sniffed the agonistic dog odor during the first experimental session (seven presentations out of a total of twelve) which, reasonably has to be considered as an arousing stimulus. The latter was confirmed by the analysis of the behavioral score which highlighted a positive correlation between cortisol urine levels in the marks and “fearful” facial expression during sniffing this odor. Although there is no literature specifically referring to dogs, data on bonobos indicate that, after psychological stressor, urinary cortisol levels are present within an average time-lag of 160 min, which could explain the absence of an increase in urinary cortisol levels in the short time between smelling the stimulus and marking in our experiment31. Consequently, our hypothesis is that during the second experimental session, the dogs exhibited overall higher cortisol levels (irrespective of baseline or mark values), as they anticipated—based on their prior experience with the identical experimental setup in the first session—either an encounter or at minimum, the opportunity to detect the male odor. This hypothesis could be further supported by our very similar findings on the oxytocin levels in urines (please see Fig. 2).
There is now scientific evidence that oxytocin is released in response to psychological and physical stressors as well as in reaction to a variety of social stimuli8,32,33,34,35. Oxytocin regulates stress responses at different levels, including the neuroendocrine and autonomic nervous system activities as well as behavioral responses36. However, the current literature reports conflicting results: although some studies have shown a positive relationship between urinary oxytocin levels and positive emotional states in dogs37, including positive social interactions during the dog-human relationship38, more recent studies were unable to show that a socio-positive interaction with either a bonded or familiar partner significantly increased oxytocin levels in dogs39. Oxytocin has been found to promote prosocial or socially positive behaviors, but also elicit anti-social or socially negative behaviors, including aggression, depending on the situation40. Furthermore, studies in both rats and mice22,41,42 have shown that various physiological and psychological stressful stimuli, such as noxious stimuli22 and social defeat stress41 facilitate oxytocin release into the plasma and in the brain, which potentially facilitate social fear memory41. In humans, oxytocin release after stressful physical43, and psychological stimuli44 has also been reported. In addition, a positive correlation between plasma oxytocin levels and relational distress or anxiety has been shown in healthy humans45 and in patients affected by social anxiety disorders46. In many studies, oxytocin has been shown to attenuate the autonomic stress responses33,36,37,38,39,40,41,42,43,44,45,46 and reduce anxiety-related behaviors47,48. Overall, findings using both animal and human subjects suggest that the oxytocin system works in parallel with cortisol release to alleviate the autonomic stress response in distressed individuals by reducing the activity of the hypothalamic-pituitary-adrenal (HPA) axis23,49,50. In addition, animal studies have shown that oxytocin has persistent effects facilitating both resilience and allostasis in a variety of stress models facilitating adaptation to stress36. Hence, stressful stimuli activate oxytocin neurons and promote oxytocin release modulating behavioral and physiological responses that facilitate the individual adaptation to stress33,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51. Therefore, a possible explanation could be that subjects who have a greater responsiveness to stress which increase even more after having smelled the odor of the male dog with agonistic behavior, as corroborate by the positive correlation between the levels of both cortisol and oxytocin in the marks and the dog’s “fearful” facial expression displayed during sniffing this odor, have overall high urinary levels of cortisol, which represents the adaptation of the hypothalamic pituitary adrenal axis to a stressful situation, and at the same time have higher levels of oxytocin which would have the function on the one hand of promoting the memory of the social alarm stimulus and on the other hand of stimulating the brain to strengthening the allostatic and resilience functions of stressed subjects. It is interesting to note that higher urinary levels of both cortisol and oxytocin levels are associated with “remote marks” indicating that this type of marking would communicate a stress behavioral response toward a particular odor. As for the cortisol, the absence of an increase in the levels of this hormone in markings could be due to the short interval of time that elapsed between the latter and the sniffing of the odors since in beagle dogs has been reported that the peak urinary oxytocin concentration occurred between 45 and 60 min after nasal administration52.
The overall higher levels of testosterone in the urine when the female was presented during the second experimental session compared to the male would align with our hypothesis that the order effect is precisely due to the type of stimulus that the dogs smelled during the first session: the smell of the male dog with agonistic behavior, which was smelled most of the time during the first session, could in fact be the cause of the increase in testosterone levels during the second experimental session. In male hamsters, for example, aggressive encounters can alter responses to future stressful events, increasing testosterone plasma levels after just 15 min53. Furthermore, testosterone urine levels in the markings were also positively correlated with the “agonistic” facial expression during sniffing at female odor. A possible explanation for this result could be found in possessiveness related aggressive behaviors54, in fact, the presence of a female dog in heat could represent an important resource to guard against other competing males. This would lead to a conflictual response on marking as the dog could prepare to protect the female as a resource and at the same time turn away the other male as a competitor. In our experience with dogs that live on the territory (i.e. free-ranging dogs in the “Alta Murgia” countryside in Puglia, Italy; unpublished data), it has often happened to see male dogs displaying aggressive behaviors towards the female in heat to prevent her from approaching the other males.
Regarding catecholamines, in line with previous findings, during the second experimental session, overall higher levels of norepinephrine were recorded in urine, confirming the presence of an effect of the order of presentation of the stimuli on this neurotransmitter as well. However, during the second experimental session, higher norepinephrine levels were recorded in remote marks compared to baseline values, demonstrating changes of this neurotransmitter levels in urine in response to olfactory stimuli; finally, marks at a distance longer than 2 m from the swab of male urine were associated with higher levels of dopamine urine levels with respect to female odor. Urinary catecholamine levels in dogs have been studied as non-invasive technique for measuring stress responses7 and, more specifically, short-term stressor (i.e. boarding kennel) affected urinary levels of both NE and dopamine55. Like dopamine, norepinephrine typically cannot cross the blood-brain barrier56, therefore, norepinephrine urine levels are most likely of peripheral origin. Plasma and urinary catecholamine levels are known to be strongly correlated57. Recently, it has been hypothesized that peripheral catecholamines may influence cognitive function—either as possibly direct peripheral-to-central afferent pathways, partly via their action on vagal sensory nerves58,59 or parallel peripheral markers of central catecholaminergic activity60. The latter is a very interesting hypothesis given that there is now evidence that these neurotransmitters play a crucial role in various aspects of cognition including attention, working memory and behavioral flexibility25,26,27,28. Interestingly, NE/dopamine reuptake inhibitors simultaneously improve cognitive functions such as attention and working memory as well as prefrontal neuronal responsiveness61. NE at particular levels might increase animals’ alertness to salient sensory stimuli in their environments higher26. However, NE not only modulates cognitive process related to attention in PFC (prefrontal cortex) circuits, but there is now evidence that the increase of prefrontal NE concentration increase, as might occur during stress, inhibited prefrontal cortical function and working memory26,27,28,61,62,63. The inhibition of PFC and brain functions such as working memory and sustained attention toward an alarming stimulus might be a valuable cognitive strategy in the animal kingdom for promoting behavioral flexibility under particular circumstances, such as during stress63. In this way, attention can be distracted from specific stimuli and redirected to other elements in the environment, facilitating withdrawal from the stressor and the identification of new behavioral strategies. Interestingly, norepinephrine is the only molecule among those studied whose urinary levels significantly changed in the short time interval between sniffing the olfactory stimuli and urinary marking, thus potentially representing the key neurotransmitter in modulating the short-term behavioral response and the different type of marking in response to the odor. The role played by dopamine in the process of cognitive flexibility could also be crucial: once changes in the environment have been detected, the action of dopamine would be to destabilize the functioning of the neural networks of the prefrontal cortex (activity on type 2 dopamine receptors), giving the brain the possibility of finding a new cognitive strategy that, in the case is more effective, it would subsequently be stabilized (activity on type 1 dopamine receptors)28. Furthermore, dopamine can boost both ongoing psychomotor64 and cognitive activities65 to support motivational engagement, as well as reinforce behaviors that produce desirable results27,65,66. Dopamine neurons become engaged also in response to negative, aversive and stressful stimuli66,67,68 and pharmacological dopamine depletions and antagonists applied within the brain reward pathway impair active withdrawal responses blocking the development of place aversion69. Furthermore, distinct populations of dopamine neurons encode motivational salience, as show weak responses to neutral events but are excited by salient events (i.e. regardless of whether the event was appetitive or aversive in nature)70. Taken together these results would indicate that dogs with higher dopamine and norepinephrine levels mark a distance from a potentially alarm stimulus, implementing a more flexible behavioral strategy that could also have a clear communicative function. An interesting hypothesis would be that a dog that remotely marked from the smell of the urine of a conspecific that could represent a potential threat could represent for the latter a sign of avoidance; in a natural context, the dog that marks at a distance from the urine of another conspecific could want to communicate his desire not to want to interact with the latter by requesting social distance. It would certainly be interesting in future studies to verify the hypothesis that the different marking behavior (e.g. urines deposited on the urine of another dog or deposited at a certain distance) may be predictive of the encounter between the two subjects.
In conclusion, the data would indicate a relationship between the different hormonal and neurotransmitters levels in urine and the type of marks of dogs in response to the urine of intraspecifics indicating a different cognitive flexibility in the behavioral response.
Materials and methods
Participants
Seventeen domestic male dogs of various breeds were recruited for this study. We excluded 6 dogs: two subjects because they showed distress behaviors soon after entry into the testing area (during the first minute of the session); three subjects did not mark after sniffing the swabs; one subject was influenced by the owner during the test. Hence the final sample consisted of 11 male dogs whose ages ranged from 3 to 11 years (6.91±3.14; M±S.D.). They were six mixed-breed dogs, one Border Collie, one Labrador Retriever, one Cocker Spaniel, one German Shepherd and one Czechoslovakian wolf. Since previous studies showed that the female ovarian cycle deeply influences scent marking behavior and the composition of scent marks10,14, we included only intact males. All dogs were pets living in households selected from a general population of volunteers taking part in behavioral studies at the Animal Physiology and Behavior Unit of the University of Bari. Before the beginning of the experiment, they underwent clinical and behavioral evaluation at the Department of Veterinary Medicine, University of Bari, to certify the absence of any organic and behavioral disorders.
Stimuli
Two dogs, an intact male and female, aged 6 and 3 years respectively, participated as donors. Their urines were collected to obtain two different samples aiming at eliciting different emotional responses: the urines of an unfamiliar female in estrus, which were expected to increase males’ arousal level due to sexual interest, and the urines of unfamiliar intact male showing overt agonistic behaviors toward conspecific males, which pose a potential threat to the tested subjects. The donors were selected by veterinary clinician and behaviorists of the Department of Veterinary Medicine, University of Bari, among dogs carried out at the Department for specialist consulting but showing no behavioral disorders. Specifically, the male donor was selected evaluating the agonistic behavior towards other males during social interactions. The exact stage of the estrous cycle of the female donor was determined on the basis of owners’ reports, clinical examination, laboratory tests (vaginal cytology and measurement of progesterone concentrations) and evaluation of adult male behavior towards the female14. Urine samples were collected during natural and spontaneous urination during a walk with the owners at the Department of Veterinary Medicine, in a familiar context. Specifically, a stainless-steel kidney dish was used to collect the samples by an experimenter wearing gloves and already familiar to the donor dogs. The male and female urine samples (15 mL each) were divided into ten 1.5-mL aliquots in Eppendorf tubes. All aliquots were then stored at − 80 °C71. For each testing session, a single 1.5-mL aliquot was thawed at ambient temperature (approximately 15 min) and used to conduct two tests. In each session, two dogs were tested with samples of the same sex; therefore, the same aliquot provided the material needed for both tests. Olfactory stimuli were presented via cotton swabs impregnated with urine. Each swab was dipped into the Eppendorf tube for 2 min and absorbed approximately 0.3 mL of urine, resulting in two swabs (one per dog) prepared from each aliquot. The swabs were positioned on the fence immediately before the start of each test. Around 0.3 mL of urine was used to prepare each olfactory stimulus. During pilot tests, a “blank” swab dipped in water was included as a “neutral” control and presented to the dogs. However, since no behavioral reactions were observed—specifically, no interest or sniffing directed toward the control stimulus—we decided to exclude it from the final experimental design.
Procedure
Each dog was tested twice, with a week interval. In each session, one odor sample type (male or female) was presented. The odors were presented only once to each dog in a random order between subjects. Over 18 weeks, the experimental group completed all the trials.
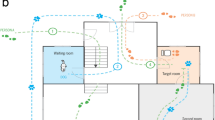
The experiment took place in a fenced area (5 × 13,5 m; see Fig. 6). Sterile cotton swabs impregnated with the donors’ urine were positioned at the dogs’ head height along the perimeter of the area. Moreover, a removable plastic coating with a folded base was placed around the fence to collect the participants’ marks (Fig. 7A). This support has proven to be efficient for urine collection in previous pilot tests. The owners with their dogs on the leash entered in the testing area and reach the swabs position. Dogs were then let free to spontaneously investigate the swab (Fig. 7B) and mark at a variable distance from the donors’ urine (Fig. 7C). Owners were asked not to influence their dogs’ behavior (e.g. either to indicate the swab or to force sniffing behavior). Each subject had a maximum of 5 min to spontaneously approach the swab. Immediately after the deposit of each mark, urine samples (approximately 10 mL) were collected using sterile 2.5-mL syringes (Fig. 7D), with multiple draws to achieve the target volume, and transferred into a plain silicone-coated tube, protected from light and stored at − 80 °C until analysis. If a dog smelled a stimulus other than the swab before the deposition of a urine mark, the animal was excluded from the analysis.
On each testing day, two dogs were tested with an intersession interval of 15 min. Each test was performed at opposite corners of the fence to avoid odor contamination originating from the presented stimulus or from any urine the dog may have deposited in response to it. Moreover, all urine marks were deposited on a plastic coating that was removed at the end of each test, effectively preventing potential odor contamination. The test was video recorded by two high-resolution cameras (Sony 4 K FDR-AX43®) placed on a tripod in front of the swab and on one side at a distance of 3 m (see Fig. 6). Before the beginning of each session further urine samples were collected to obtain a baseline of physiological parameters levels for each dog. In particular, urines were collected during spontaneous urination while dogs walked with the owner in a familiar area of the Department of Veterinary Medicine. An experimenter familiar to the dogs collected the urine samples using a stainless-steel kidney dish while wearing gloves. 10 mL of urine were placed in a plain silicone-coated tube, urine samples were light-protected and stored at − 80 °C until analysis. Biochemical analyses were then performed in a single batch.
Data analysis
Behavioral analysis
Behavioral analysis was performed using The Observer XT (Noldus®) by two trained observers, who were blind to the odor stimuli presented to the dogs. The dogs’ behaviors (i.e. the distance of urine marks from the swab, dogs’ facial expressions during sniffing the swab) were analyzed during the sessions. Specifically, urine marks were classified according to the distance from the swab (i.e. close marks, if ’s urine was deposited on top or within a distance of 1 m from the swab; medium distant-marks, for urine deposited at a distance between 1 and 2 m from the swab, and remote-marks, for marks deposited at a distance of more than 2 m from the swab, see Fig. 6). The total duration of each behavioral category during sniffing (i.e. “neutral”, “attentive”, “stress-induced expressions” and “anxiety”, see Table 1) was coded. Finally, the total time spent sniffing the different odors was computed. The inter-rater reliability was assessed by means of independent parallel coding of dogs’ behaviour during the test and was calculated as the percentage agreement. It was always higher than 95% for each tested variable.
Urine sample analysis
Oxytocin
Urinary oxytocin concentrations were measured by competitive ELISA kits. Samples were extracted as it is prescribed in assay procedure (Oxytocin ELISA DE-3117, Demeditec Diagnostics GmbH, Germany) detection range: 15.6–1,000 pg/ml. The oxytocin ELISA kit is a competitive immunoassay for the quantitative determination of oxytocin in samples. The kit uses a polyclonal antibody to oxytocin to bind, in a competitive manner, the oxytocin in the standard or sample or an alkaline phosphatase molecule which has oxytocin covalently attached to it. After a simultaneous incubation at 4 °C the excess reagents are washed away and substrate is added. After a short incubation time the enzyme reaction is stopped, and the yellow color generated read on a microplate reader at 405 nm. The intensity of the bound yellow color is inversely proportional to the concentration of oxytocin in either standards or samples. The measured optical density of the standards is used to calculate the concentration of oxytocin in the sample.
Testosterone
This ELISA kit is based on the competitive binding enzyme immunoassay technique. The microtiter plate provided in this kit has been pre-coated with the analyte. During the reaction, the analyte in the sample or standard competes with a fixed amount of biotin-labeled analyte for sites on an antibody specific to the analyte. Excess conjugate and unbound sample or standard are washed from the plate. Next, Avidin conjugated to Horseradish Peroxidase (HRP) is added to each microplate well and incubated. Then a TMB substrate solution is added to each well. The enzyme-substrate reaction is terminated by the addition of a sulphuric acid solution and the color change is measured spectrophotometrically at a wavelength of 450 nm ± 2 nm. The concentration of the analyte in the samples is then determined by comparing the O.D. of the samples to the standard curve. Detection Range: 3.9–250 pg/mL.
Cortisol
This ELISA kit is based on the competitive binding enzyme immunoassay technique. The microtiter plate provided in this kit has been pre-coated with the analyte. During the reaction, the analyte in the sample or standard competes with a fixed amount of biotin-labeled analyte for sites on an antibody specific to the analyte. Excess conjugate and unbound sample or standard are washed from the plate. Next, Avidin conjugated to Horseradish Peroxidase (HRP) is added to each microplate well and incubated. Then a TMB substrate solution is added to each well. The enzyme-substrate reaction is terminated by the addition of a sulphuric acid solution and the color change is measured spectrophotometrically at a wavelength of 450 nm ± 2 nm. The concentration of the analyte in the samples is then determined by comparing the O.D. of the samples to the standard curve. Detection range: 0.78–50.0.78.0 ng/mL.
The minimum urine volume required to perform ELISA analyses for oxytocin, testosterone and cortisol, was 100 µl for each assay, including the calibration curve.
Dopamine, epinephrine and norepinephrine
Urinary free catecholamines were separated and concentrations were determined quantitatively by high pressure liquid chromatography with electrochemical detection with commercial reagents from Bio-Rad. For all biogenic amines, the minimum urine volume required to perform HPLC determination was 3 mL.
Ethical statement
The experiment was conducted according to the protocols approved by the Italian Minister for Scientific Research in accordance with EC regulations and were approved by the Department of Veterinary Medicine (University of Bari) Ethics Committee EC (Approval Number: 30/2024). Written informed consent was obtained from the owners before the beginning of the test. Furthermore, informed consent has been obtained from the study participant included in Fig. 7 to publish the image in an online open-access publication. In addition, the study is reported in accordance with ARRIVE guidelines (https://arriveguidelines.org).
Statistical analysis
GLMM analysis was carried out to assess the influence of “distance of urine marks” (given that our primary interest was to identify differences in extreme marking behaviors, we included only “close-marks” and “remote-marks” in the final model comparing “close marks” vs. “medium-distant and remote marks” and “remote marks” vs. “close and medium-distant marks”), “conditions” (i.e. “baseline” and “marks”), “stimuli” (i.e. swabs impregnated with urines of “male” and “female”) on the test variables urine mark metabolites (i.e. “cortisol”, “oxytocin”, “testosterone”, “norepinephrine”, “epinephrine” and “dopamine”), with “subjects” as a random variable and condition and stimulus as repeated measures. Gaussian distribution was verified with the Wilcoxon test, and the variables were found to have a nonparametric data distribution. Consequently, “Gamma” and “Inverse Gaussian” distributions and identity-link function were used to specify the target of the model. Bayesian information criterion (BIC) was used for selecting and comparing models based on the − 2 log likelihood. To detect differences between different groups, Fisher’s Least Significant Difference (LSD) pairwise comparisons were performed.
As for facial expressions, according to data distribution, Spearman and Pearson correlations were used to measure their association with urine-marks metabolites.
Data availability
Datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Siniscalchi, M. Olfaction and the Canine Brain in Canine Olfaction Science and Law. (eds. Jezierski, T., Ensminger, J., & Papet, L. E) 31–37CRC Press, https://doi.org/10.1201/b20027 (2016).
Siniscalchi, M., D’Ingeo, S., Minunno, M. & Quaranta, A. Communication Dogs Animals 8, 131 (2018).
Karlson, P. & Lüscher, M. Pheromones’: A new term for a class of biologically active substances. Nature 183, 55–56 (1959).
Baum, M. J. & Bakker, J. Roles of sex and gonadal steroids in mammalian pheromonal communication. Front. Neuroendocrinol. 34, 268–284 (2013).
Lisberg, A. E. & Snowdon, C. T. Effects of sex, social status and gonadectomy on countermarking by domestic dogs, canis familiaris. Anim. Behav. 81, 757–764 (2011).
McGuire, B. & Bemis, K. E. Scent marking in shelter dogs: effects of body size. Appl. Anim. Behav. Sci. 186, 49–55 (2017).
Beerda, B., Schilder, M. B. H., Janssen, N. S. C. R. M. & Mol, J. A. The use of saliva Cortisol, urinary Cortisol, and catecholamine measurements for a noninvasive assessment of stress responses in dogs. Horm. Behav. 30, 272–279 (1996).
Lang, R. E. et al. Oxytocin unlike vasopressin is a stress hormone in the rat. Neuroendocrinology 37, 314–316 (1983).
McGuire, B. Scent marking in shelter dogs: effects of sex and age. Appl. Anim. Behav. Sci. 182, 15–22 (2016).
Wirant, S. C., Halvorsen, K. T. & McGuire, B. Preliminary observations on the urinary behaviour of female Jack Russell terriers in relation to stage of the oestrous cycle, location, and age. Appl. Anim. Behav. Sci. 106, 161–166 (2007).
McClanahan, K. & Rosell, F. Conspecific recognition of pedal scent in domestic dogs. Sci. Rep. 10, 17837 (2020).
Lisberg, A. E. & Snowdon, C. T. The effects of sex, gonadectomy and status on investigation patterns of unfamiliar conspecific urine in domestic dogs, canis familiaris. Anim. Behav. 77, 1147–1154 (2009).
Holcova, K., Koru, E., Havlicek, Z. & Rezac, P. Factors associated with sniffing behaviors between walking dogs in public places. Appl. Anim. Behav. Sci. 244, 105464 (2021).
Dzięcioł, M. et al. Identification of putative volatile sex pheromones in female domestic dogs (Canis familiaris). Anim. Reprod. Sci. 197, 87–92 (2018).
McGuire, B. Effects of gonadectomy on scent-marking behavior of shelter dogs. J. Veterinary Behav. 30, 16–24 (2019).
Natynczuk, S., Bradshaw, J. W. S. & Mcdonald, D. W. Chemical constituents of the anal sacs of domestic dogs. Biochem. Syst. Ecol. 17, 83–87 (1989).
Udell, M. A. R., Dorey, N. R. & Wynne, C. D. L. What did domestication do to dogs? A new account of dogs’ sensitivity to human actions. Biol. Rev. 85, 327–345 (2010).
Siniscalchi, M., Lusito, R., Vallortigara, G. & Quaranta, A. Seeing Left- or Right-Asymmetric tail wagging produces different emotional responses in dogs. Curr. Biol. 23, 2279–2282 (2013).
Quaranta, A., Siniscalchi, M. & Vallortigara, G. Asymmetric tail-wagging responses by dogs to different emotive stimuli. Curr. Biol. 17, R199–R201 (2007).
Siniscalchi, M., d’Ingeo, S. & Quaranta, A. The dog nose KNOWS fear: asymmetric nostril use during sniffing at canine and human emotional stimuli. Behav. Brain. Res. 304, 34–41 (2016).
Bombail, V. Perception and emotions: on the relationships between stress and olfaction. Appl. Anim. Behav. Sci. 212, 98–108 (2019).
Onaka, T., Okabe, S., Takayanagi, Y. & Yoshida, M. Noxious or Non-noxious inputs to Oxytocin neurons: possible roles in the control of behaviors. Interdiscip Inf. Sci. 21, 189–195 (2015).
Ditzen, B. et al. Intranasal Oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biol. Psychiatry. 65, 728–731 (2009).
Siniscalchi, M., McFarlane, J. R., Kauter, K. G., Quaranta, A. & Rogers, L. J. Cortisol levels in hair reflect behavioural reactivity of dogs to acoustic stimuli. Res. Vet. Sci. 94, 49–54 (2013).
Sara, S. J. The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci. 10, 211–223 (2009).
Borodovitsyna, O., Flamini, M. & Chandler, D. Noradrenergic modulation of cognition in health and disease. Neural Plast. 2017, 1–14 (2017).
Hamid, A. A. Dopaminergic specializations for flexible behavioral control: linking levels of analysis and functional architectures. Curr. Opin. Behav. Sci. 41, 175–184 (2021).
Floresco, S. B. Prefrontal dopamine and behavioral flexibility: shifting from an inverted-U toward a family of functions. Front Neurosci 7, 62 (2013).
Lensen, R. C. M. M., Moons, C. P. H. & Diederich, C. Physiological stress reactivity and recovery related to behavioral traits in dogs (Canis familiaris). PLoS One. 14, e0222581 (2019).
Hekman, J., Karas, A. & Sharp, C. Psychogenic stress in hospitalized dogs: cross species Comparisons, implications for health Care, and the challenges of evaluation. Animals 4, 331–347 (2014).
Verspeek, J. et al. Time-lag of urinary and salivary cortisol response after a psychological stressor in bonobos (Pan paniscus). Sci. Rep. 11, 1–12 (2021).
Jezova, D., Skultetyova, I., Tokarev, D. I., BAKOS, P. & VIGAS, M. Vasopressin and Oxytocin in stressa. Ann. N Y Acad. Sci. 771, 192–203 (1995).
Onaka, T. Neural pathways controlling central and peripheral Oxytocin release during stress. J. Neuroendocrinol. 16, 308–312 (2004).
Robinson, D. A. et al. Oxytocin mediates stress-induced analgesia in adult mice. J. Physiol. 540, 593–606 (2002).
Wigger, A. & Neumann, I. D. Endogenous opioid regulation of stress-induced Oxytocin release within the hypothalamic paraventricular nucleus is reversed in late pregnancy: a Microdialysis study. Neuroscience 112, 121–129 (2002).
Takayanagi, Y. & Onaka, T. Roles of Oxytocin in stress Responses, allostasis and resilience. Int. J. Mol. Sci. 23, 150 (2021).
Mitsui, S. et al. Urinary Oxytocin as a noninvasive biomarker of positive emotion in dogs. Horm. Behav. 60, 239–243 (2011).
Nagasawa, M. et al. Oxytocin-gaze positive loop and the Coevolution of human-dog bonds. Sci. (1979). 348, 333–336 (2015).
Marshall-Pescini, S. et al. The role of Oxytocin in the Dog–Owner relationship. Animals 9, 792 (2019).
Ferris, C. F. et al. Oxytocin in the amygdala facilitates maternal aggression. Ann. N Y Acad. Sci. 652, 456–457 (1992).
Nasanbuyan, N. et al. Oxytocin–Oxytocin receptor systems facilitate social defeat posture in male mice. Endocrinology 159, 763–775 (2018).
Engelmann, E. & Landgraf, H. Wotjak. Emotional stress triggers intrahypothalamic but not peripheral release of Oxytocin in male rats. J. Neuroendocrinol. 11, 867–872 (1999).
Hew-Butler, T., Noakes, T. D., Soldin, S. J. & Verbalis, J. G. Acute changes in endocrine and fluid balance markers during high-intensity, steady-state, and prolonged endurance running: unexpected increases in Oxytocin and brain natriuretic peptide during exercise. Eur. J. Endocrinol. 159, 729–737 (2008).
Pierrehumbert, B. et al. Oxytocin response to an experimental psychosocial challenge in adults exposed to traumatic experiences during childhood or adolescence. Neuroscience 166, 168–177 (2010).
Taylor, S. E., Saphire-Bernstein, S. & Seeman, T. E. Are plasma Oxytocin in women and plasma vasopressin in men biomarkers of distressed Pair-Bond relationships? Psychol. Sci. 21, 3–7 (2010).
Hoge, E. A., Pollack, M. H., Kaufman, R. E., Zak, P. J. & Simon, N. M. Oxytocin levels in social anxiety disorder. CNS Neurosci. Ther. 14, 165–170 (2008).
Neumann, I. D. & Slattery, D. A. Oxytocin in general anxiety and social fear: A translational approach. Biol. Psychiatry. 79, 213–221 (2016).
Janeček, M. & Dabrowska, J. Oxytocin facilitates adaptive fear and attenuates anxiety responses in animal models and human studies—potential interaction with the corticotropin-releasing factor (CRF) system in the bed nucleus of the stria terminalis (BNST). Cell. Tissue Res. 375, 143–172 (2019).
Neumann, W., Torner, H. & Landgraf Brain Oxytocin inhibits basal and Stress-Induced activity of the Hypothalamo‐Pituitary‐Adrenal axis in male and female rats: partial action within the paraventricular nucleus. J. Neuroendocrinol. 12, 235–243 (2000).
Cavanaugh, J., Carp, S. B., Rock, C. M. & French, J. A. Oxytocin modulates behavioral and physiological responses to a stressor in marmoset monkeys. Psychoneuroendocrinology 66, 22–30 (2016).
Quintana, D. S., Kemp, A. H., Alvares, G. A. & Guastella, A. J. A role for autonomic cardiac control in the effects of Oxytocin on social behavior and psychiatric illness. Front Neurosci 7, 48 (2013).
Temesi, A., Thuróczy, J., Balogh, L. & Miklósi, Á. Increased serum and urinary Oxytocin concentrations after nasal administration in beagle dogs. Front. Vet. Sci. 4, 268535 (2017).
Clinard, C. T., Barnes, A. K., Adler, S. G. & Cooper, M. A. Winning agonistic encounters increases testosterone and androgen receptor expression in Syrian hamsters. Horm. Behav. 86, 27–35 (2016).
Kleszcz, A. et al. Review on selected aggression causes and the role of neurocognitive science in the diagnosis. Animals 12, 281 (2022).
Albright, J. D. & Ng, Z. Y. Measurement of neurotransmitters excreted in the urine of behaviorally healthy dogs in home and boarding kennel conditions. J. Veterinary Behav. 48, 74–77 (2022).
Moleman, P., Tulen, J. H. M., Blankestijn, P. J., Man’Veld, A. J. & Boomsma, F. Urinary excretion of catecholamines and their metabolites in relation to Circulating catecholamines: Six-Hour infusion of epinephrine and norepinephrine in healthy volunteers. Arch. Gen. Psychiatry. 49, 568–572 (1992).
Barbeito, L., Lista, A., Silveira, R. & Dajas, F. Resting urinary catecholamine excretion in schizophrenics: methodology and interpretation of results. Biol. Psychiatry. 19, 1419–1425 (1984).
Tank, A. W. & Wong, D. L. Peripheral and central effects of Circulating catecholamines. Compr. Physiol. 5, 1–15 (2015).
Mravec, B. Role of catecholamine-induced activation of vagal afferent pathways in regulation of sympathoadrenal system activity: negative feedback loop of stress response. Endocr. Regul. 45, 37–41 (2011).
Savransky, A. et al. Association of working memory and elevated overnight urinary norepinephrine in patients with schizophrenia. J. Psychiatr Res. 137, 89–95 (2021).
Devilbiss, D. M. & Berridge, C. W. Cognition-Enhancing doses of methylphenidate preferentially increase prefrontal cortex neuronal responsiveness. Biol. Psychiatry. 64, 626–635 (2008).
Wang, M. et al. α2A-Adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell 129, 397–410 (2007).
Nicholls, R. E. et al. Transgenic mice lacking NMDAR-Dependent LTD exhibit deficits in behavioral flexibility. Neuron 58, 104–117 (2008).
Salamone, J. D. & Correa, M. The mysterious motivational functions of mesolimbic dopamine. Neuron 76, 470–485 (2012).
Westbrook, A. et al. Dopamine promotes cognitive effort by biasing the benefits versus costs of cognitive work. Sci. (1979). 367, 1362–1366 (2020).
Abercrombie, E. D., Keefe, K. A., DiFrischia, D. S. & Zigmond, M. J. Differential effect of stress on in vivo dopamine release in Striatum, nucleus Accumbens, and medial frontal cortex. J. Neurochem. 52, 1655–1658 (1989).
Scott, D. J., Heitzeg, M. M., Koeppe, R. A., Stohler, C. S. & Zubieta, J. K. Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. J. Neurosci. 26, 10789–10795 (2006).
Love, T. M. Oxytocin, motivation and the role of dopamine. Pharmacol. Biochem. Behav. 119, 49–60 (2014).
Salamone, J. D. The involvement of nucleus accumbens dopamine in appetitive and aversive motivation. Behav. Brain. Res. 61, 117–133 (1994).
Bromberg-Martin, E. S., Matsumoto, M. & Hikosaka, O. Dopamine in motivational control: Rewarding, Aversive, and alerting. Neuron 68, 815–834 (2010).
Siniscalchi, M. et al. Sniffing with the right nostril: lateralization of response to odour stimuli bydogs. Anim. Behav. 8, 399–404 (2011).
Acknowledgements
M.S. discloses support for the research of this work from the Italian Ministry of University and Research (MUR) and the European Commission, Next Generation EU: PRIN 2022 (DD 104 DEL 02/02/22) – PNRR – M4 – C2 – INV 1.1. [Project N°: 202222EJZE – CUP: H53D23003970006].
Funding
PRIN “Research Projects of Significant National Interest” funding program, administered by the Italian Ministry of University and Research (MUR). [Project N°: 202222EJZE – CUP: H53D23003970006].
Author information
Authors and Affiliations
Contributions
A.Q., S.d., M.M., G.V., E.C., M.S. designed the research; A.Q., S.d., M.M., V.S., M.N., G.V., E.C., M.S. analysed the data; A.Q., S.d., M.M., G.V., E.C., M.S. wrote the paper. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Quaranta, A., d’Ingeo, S., Minunno, M. et al. Decoding dog communication through the physiology and behavior of urine marking. Sci Rep 16, 1711 (2026). https://doi.org/10.1038/s41598-025-31373-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-31373-8