Abstract
Diverse fungi have been historically vital reservoirs of drug discovery, providing life-saving pharmaceuticals. Many species of fungi, yeasts in particular, are highly resistant to radiation, with their cellular contents potentially conferring dietary radioresistance. We developed a Drosophila model to test whether feeding two highly radioresistant fungi, Aureobasidium pullulans and Rhodotorula taiwanensis, could improve fly lifespan and gut morphology after acute irradiation. We constructed a dosimetry curve for the lifespan response of males and females to irradiation and found dose-dependent and sex-specific effects on lifespan. We also determined that the sex-specific response to irradiation correlated with nuclear morphology defects in the gut, with the more radiosensitive males displaying increased midgut cellular holes and aberrant nuclear morphology. To determine if feeding Aureobasidium pullulans and Rhodotorula taiwanensis before irradiation could improve survival and gut morphology, we first exclusively fed males and females each fungus and observed that they tolerated the diet well. Using these methods, we found that only two days of pre-feeding Aureobasidium pullulans increased male lifespan, but not female, after irradiation, and improved nuclear morphology in the gut. However, dietary Rhodotorula taiwanensis was not protective. Overall, this study identified a highly radioresistant dietary fungus, Aureobasidium pullulans, as effective for extending male Drosophila lifespan and improving gut morphology following irradiation. Since the gut is particularly sensitive to the effects of irradiation, this fungus indicates a potential therapeutic for patients undergoing radiotherapy. Furthermore, this method could identify additional radioresistant fungi that protect the gut from radiation injury.
Similar content being viewed by others
Introduction
Fungi synthesize a large number of naturally occurring bioactive molecules from which researchers have identified a wide array of vital pharmaceuticals such as antibiotics, immunosuppressants, and cholesterol-lowering drugs1,2. Within this large and diverse kingdom are species that are highly radiation-resistant, with fungal species growing in the reactor of the damaged Chernobyl Nuclear Power Plant among the first ones identified3. Since then, many species of fungi have been shown to survive acute and chronic exposures to astoundingly high levels of ionizing radiation (IR), far beyond any that are naturally occurring4,5. Several mechanisms contribute to the astonishing ability of these microbes to grow and reproduce in highly irradiated environments. Some radiation-resistant organisms accumulate antioxidant compounds such as manganous peptide phosphate complexes to survive the oxidative stress of IR6,7. Melanin often accumulates in response to stress in various fungi, and its chemical properties include broad optical absorption and the ability to interact with ionizing radiation, lending it antioxidant activity3,8,9. Given the diversity of the kingdom and the fact that so many species demonstrate radioresistance to levels of IR not found in nature, this suggests that additional metabolites and molecules conferring radioresistance have yet to be identified.
Aureobasidium pullulans, a dimorphic ascomycetous yeast, grows on the inner parts of the damaged Chernobyl Nuclear Power Plant and the International Space Station, and is considered a polyextremotolerant fungus3,10,11. Aureobasidium pullulans (A. pullulans) is usually light in color but becomes darker under stress due to melanin production, which is thought to confer enhanced survival10,12. Rhodotorula taiwanensis (R. taiwanensis), a basidiomycetous carotenogenic yeast, was characterized for its ability to tolerate high levels of chronic radiation and low pH as part of an initiative to identify fungi that could be harnessed for the bioremediation of radioactive waste sites5. R. taiwanensis can grow under chronic 66 Gy/h gamma radiation at pH 2.3, giving this fungus remarkable resiliency5. Since R. taiwanensis and A. pullulans survive IR in the absence of melanin, there are likely unidentified metabolites and small molecules that function to protect them against the damaging effects of IR.
IR damages DNA by causing double-strand breaks (DSBs), lipids, such as those in membranes, through peroxidation and proteins through various modifications13. It is generally accepted that DNA is most sensitive to IR since the DSBs are caused directly by the radiation exposure. For example, the effects of cytoplasmic irradiation can cause nuclear mutations and DSBs while being minimally cytotoxic14,15. In addition, studies have indicated that increased amounts of DNA in the nucleus make cells more susceptible to DSBs16. Thus, human mitotic cells, such as in the bone marrow, gut and reproductive organs, are highly sensitive to the effects of IR exposure17. In people, acute radiation syndrome (ARS) results from exposures as low as 1 Gy, with the hematopoietic system, the gastrointestinal tract, and cerebrovascular system becoming damaged with increasing amounts of IR18.
Using Drosophila, we investigated whether ingestion of A. pullulans and R. taiwanensis could provide radioprotection to the gut and extend lifespan. Drosophila have a rapid lifecycle, short lifespan, and large genetic toolkit, making them an ideal animal in which to test for dietary protection against IR. Furthermore, Drosophila have organ systems that are highly homologous to human physiology, sufficient for modeling numerous human diseases19,20. For example, the adult gut exhibits clear regional differences in morphology, physiology, and gene expression along its anterior–posterior axis and has emerged as an important model to study epithelial damage and intestinal stem cell biology21,22,23,24,25,26. DNA damage and DSBs also occur in the fly gut after exposure to IR27,28. Drosophila are natural fungivores and readily consume diverse yeast species with notable effects on physiology and behavior29,30. Thus, natural feeding would allow direct contact between the radioresistant fungi and highly radiosensitive gut cells, optimizing any potential radioprotective benefits.
To test the protective effects of dietary A. pullulans and R. taiwanensis after acute exposure to gamma radiation, we first constructed radiation survival curves for male and female lifespans to identify doses that were not acutely detrimental to fly health, yet high enough to make lifespan measurements manageable. Males were more sensitive to the effects of IR compared to females, as expected31,32,33. In addition, we observed that male gut morphology was significantly more disrupted compared to females receiving the same IR dose. Next, we fed males and females exclusive diets of both fungi and determined that, although they shortened lifespan, neither was acutely toxic, nor did they have detrimental effects on development. To test for dietary protection, we fed flies either fungus for two days before IR exposure and determined that A. pullulans, but not R. taiwanensis, enhanced radiation survival of males, but not females, for the next fifteen to twenty days. Males fed A. pullulans and subsequently exposed to IR showed improved nuclear morphology in the gut compared to non-fed control males, suggesting this could be a contributing factor to the increased survival. Overall, this study demonstrates that Drosophila is an effective model to successfully identify highly radioresistant dietary fungi that confer radioresistance to the host. Notably, we identified A. pullulans as a promising radioprotectant, which could be further tested in higher eukaryotes.
Materials and methods
Drosophila and fungal strains
Fly strains used in this study: w1118 and catalasen1/TM3, Sb1, Ser (Kyoto Drosophila Stock Center, #107554). Fly stocks were maintained in standard cornmeal fly food vials at room temperature and a 12-h light cycle. The strain of Aureobasidium pullulans var. namibiae (A. pullulans) (EXF-1147) used in this study was obtained from Microbial Culture Collection EX curated by the Biotechnical Faculty of the University of Ljubljana in Ljubljana, Slovenia. The strain of Rhodotorula taiwanensis (R. taiwanensis) (MD-1149) was isolated from an acid mine drainage facility in Allegany County, Maryland, USA, and curated in the collection of Dr. Michael Daly of the Department of Pathology of the Uniformed Services University of the Health Sciences in Bethesda, Maryland, USA. Dietary yeast cultures were grown at room temperature on standard YPD agar plates [1% Bacto Yeast Extract (Cat# 212750, Thermo Fisher Scientific, Waltham, MA, USA), 2% Peptone (Cat# 211677, Thermo Fisher Scientific, Waltham, MA, USA), 2% Dextrose (Cat# PHR1000, Millipore Sigma, St. Louis, MO, USA), 2% Bacto Agar (Cat# DF-0140-01-0, Thermo Fisher Scientific, Waltham, MA, USA)] covered with gamma-sterilized dialysis membrane (Frey Scientific Dialysis Tubing, Cat# 1591654, Dialysis Tubing, Frey Scientific®, Greenville, WI, USA) to ensure only yeast was removed during collection. For pre-feeding IR exposured cultures, A. pullulans and R. taiwanensis were grown for 4 days and 1 day, respectively, under chronic irradiation at 35 Gy/h in a 137Cs-sourced gamma irradiator (Shepard and Associates 68A Mark1, San Fernando, CA, USA). Unirradiated fungi cultures were grown in the same location outside the irradiator to ensure both cultures grew under otherwise identical conditions. Acute radiation tolerances (D10) for both strains were determined by incremental irradiation of liquid YPD culture (OD600 = 0.8) in a 60Co-sourced gamma irradiator (Shepard and Associates, San Fernando, CA, USA) followed by colony forming unit (CFU) assay on solid YPD medium as previously described34. To achieve a bisecting exposure of A. pullulans culture, we cast a cylindrical radiation shield of pure lead (Rotometals, Inc., San Leandro, CA, USA) with a central cavity of approximately 65 mm × 30 mm × 12 mm to snugly fit a standard 60 mm petri dish. A. pullulans was streaked on solid YPD agar plate, placed in the radiation shield leaving half the plate exposed, and irradiated at ~ 35 Gy/h for 24 h in a 137Cs-sourced irradiator. The partially irradiated culture was then allowed to recover for 3 days. To observe phenotypic switching of melanin expression in response to cyclical irradiation, 20 µL of A. pullulans in liquid YPD (OD600 0.8) was aliquoted to the center of a YPD agar plate. After one day of growth, the culture was exposed to alternating cycles of chronic radiation at 35 Gy/hr for 2 days followed by 2 days without exposure. To visualize accumulation of melanin in the cell walls of A. pullulans hyphae, cultures were imaged using a Motic BA210E (Motic, Universal City, TX, USA) at 400x (40 × objective x 10 × eyepiece).
Fungal feeding
For administration of fungal diets, flies were housed in feeding chambers made from agar feeding plates encapsulated by perforated plastic beakers. Aliquots of fungi were carefully harvested with a silicone spatula from 2–3 day-old cultures grown on YPD plates and added to agar plates. Newly eclosed flies age 0–2 h old were collected and allowed to feed for two days, irradiated, then transferred to standard food vials supplemented with a small amount of live dry yeast (Saccharomyces cerevisiae (S. cerevisiae) (Red Star Active Dry Yeast, Red Star Yeast Company LLC, Milwaukee, WI, United States)). For determining the toxicity of, and survivorship on, A. pullulans and R. taiwanensis diets, twenty males or females, in triplicate, were continuously fed fungal paste for the entire lifespan, changing the plate and fungal paste every 1–2 days. A paste of water and S. cerevisiae was used as a control. To determine pupation and eclosion rates, twenty first instar larvae collected one day after egg laying were transferred into agar plates supplemented with A. pullulans, R. taiwanensis or a paste of control S. cerevisiae and grown at room temperature. The number of pupae was counted every 24 h after the onset of pupation. The number of eclosed adults was counted each day after the onset of eclosion. Each experiment was performed in triplicate. To visualize consumption of the fungi, representative flies and larvae were imaged using an Accu-scope 3076 digital microscope 0.67x-4.5x (Accu-Scope, Commack, NY, USA).
Irradiation, dosimetry, and lifespan analysis
Flies were exposed to gamma radiation in standard polystyrene Drosophila vials (Genesee Scientific, Cat# 32-109, Morrisville, NC, USA) capped with cellulose-acetate stoppers (Genesee Scientific, Cat# 49-102, Morrisville, NC, USA) containing standard cornmeal fly media (8.25% corn syrup (Karo Light Corn Syrup, ACH Food Companies, Oakbrook Terrace, IL, United States), 5% corn meal (Cat# 62-100, Genesee Scientific, El Cajon, CA, United States), 1.25% dry inactivated yeast (Cat# 62-103, Genesee Scientific, El Cajon, CA, United States), 0.75% soy flour (Cat# 62-115, Genesee Scientific, El Cajon, CA, United States), 0.5% propionic acid (Cat# P1386, Millipore Sigma, St. Louis, MO, United States), 0.1% Tegosept (Ca# 20-258, Genesee Scientific, El Cajon, CA, United States) with 0.04 g live dry yeast (Red Star Active Dry Yeast, Red Star Yeast Company LLC, Milwaukee, WI, United States)). Six vials, two sets of matching triplicates, were irradiated per radiation treatment using a vendor-calibrated, 137Cs-sourced gamma irradiator. Vials were placed on a rotating platform to ensure uniform exposure of all samples to the source emission. Consistent and precise dosimetry for all sample irradiations was achieved by calculating the length of each exposure, accounting for daily source decay using the standard decay equation. Exposures were delivered at a dose rate of ~ 12.62 Gy/min. For lifespan analysis, twenty male and female adults were monitored daily after irradiation for their full lifespan, which was performed minimally in triplicate as described in Fungal feeding above. Since irradiation significantly weakens the animals, the determination of death for each fly was made by observing whether it failed to respond repeatedly to gentle probing, as indicated by head twitching and/or leg movements. To perform statistical analysis, the average daily survivorship data from the triplicates were compiled and entered into the Online Application for Survival Analysis 2 (OASIS) (https://sbi.postech.ac.kr/oasis2/)35. Statistical significance between control and experimental conditions was determined in OASIS using the Wilcoxon-Breslow-Gehan test (Supplementary Table 1).
Immunostaining and gut injury analysis
Post-irradiated 4-day-old adult flies were dissected and immunostained as previously described, with slight modifications36. Briefly, 2-day-old adult flies were irradiated and subsequently maintained in standard food vials for an additional two days. The guts were dissected in 1 × phosphate-buffered saline (PBS) and fixed with 4% formaldehyde in 1 × PBS for thirty minutes at room temperature. After washing with Antibody wash solution (AWS, 0.1% TritonX-100, and 1% BSA in PBS) three times for twenty minutes, the tissues were stained with Alexa Fluor™ 488 Phalloidin (Cat# A12379, Invitrogen, Waltham, MA, USA) overnight at 4 °C. Following washes with AWS twice for 20 min, tissues were stained with 4′,6-Diamidino-2-phenylindole (DAPI) for 10 min at room temperature and mounted in Vectashield Antifade Mounting Medium (Cat# H-1000, Vector Laboratories, Newark, CA, USA). Images were obtained using a Zeiss LSM 980 confocal laser scanning microscope with a 63 × objective lens (Carl Zeiss Microscopy LLC, White Plains, NY, USA). The R4 region of the midgut was chosen, and a z-stacked image was obtained for the whole layer of enterocytes of randomly selected regions in R4, from just beneath the muscle cells to the lumen. The total number of guts analyzed is indicated in each figure legend. Quantification of abnormal nuclear shape, disrupted cellular barriers, and holes in the actin filament layer of enterocytes was determined by subjective measurement. Abnormal nuclear shape was determined if a nucleus had a smaller size relative to control and decreased DAPI staining in the nucleus. Disrupted cellular barriers between enterocytes were evaluated by loss of actin and abnormal cellular shapes, as demonstrated by the actin staining. We did not include cells which had lost actin labeling and had fainter DAPI staining in the category of disrupted cellular barriers since this phenotype was found in control non-irradiated animals. Holes in an actin filament layer were counted if the following conditions were satisfied: at least five clear round-shaped holes with a diameter of more than 1.5 µm in an actin layer within a single cell, or two holes with a diameter of more than 2.5 µm. For nuclei, the percentage of abnormal nuclei relative to total nuclei within each image of the guts was calculated and plotted in a graph generated using GraphPad Prism (GraphPad Software, version 10.4.2, Boston, Massachusetts USA, www.graphpad.com). For disruption to cellular barriers and holes in the actin filament later, the percentage of animals with gut injury in each replicate was calculated, averaged, and plotted in a graph generated using GraphPad Prism (GraphPad Software, version 10.4.2, Boston, Massachusetts USA, www.graphpad.com). A summary table of the image analyses is in Supplemental Spreadsheet 1. Significant differences between groups were tested as described in Statistical analyses below.
Statistical analyses
To quantify the relationship between radiation dose and mortality, linear regression was conducted in Microsoft Excel (version 16.94.1), and the coefficient of determination (R2) was reported as a measure of goodness-of-fit. Lifespan data were analyzed using the OASIS 2 online platform for survival analysis (35; https://sbi.postech.ac.kr/oasis2/) using compiled average of three triplicates. Mean lifespan statistical significance between groups was determined using log-rank and weighted log-rank (Wilcoxon–Breslow-Gehan) test37. The analysis of gut injury was performed using GraphPad Prism (GraphPad Software, version 10.4.2, Boston, Massachusetts USA, www.graphpad.com). Two-way analysis of variance (ANOVA) with multiple comparisons followed by Tukey’s post hoc test was performed to evaluate the effect of sex (male, female) and irradiation (− IR, + IR) on abnormal physiological events. Mean lifespans, standard errors, and Bonferroni p-values were reported to assess the impact of dietary intervention on survival following irradiation and are presented in Supplementary Table 1.
Biological, chemical, and radiological safety
All experimental work was performed in strict adherence to the biological, chemical, and radiological safety protocols and regulations established by the Uniformed Services University of the Health Sciences and its Environmental Health and Radiation Safety Divisions.
Results
Drosophila exhibit dose-dependent lifespan sensitivity to exposure to acute irradiation
In order to use Drosophila as a model to test dietary radioprophylactic properties of highly radioresistant fungi, we designed a protocol for irradiating adult flies and determined lifespan dose curves for a range of radiation exposures. For consistent irradiation, six standard fly food vials were elevated to the appropriate height on a rotating platform in a 137Cs-source irradiator (Fig. 1A). A blank vial was placed in the middle of the vial cluster. This configuration ensured that all flies were exposed to the correct dose of irradiation. This also enabled us to simultaneously test experimental and control groups in triplicate. We tested the effect of 0–1500 Gy gamma radiation on the lifespan of w1118 male and female adult flies (Fig. 1B). The normal (w1118) Drosophila lifespan is approximately 100 days (Fig. 1B, black lines). Twenty newly eclosed males and females, in triplicate, were simultaneously exposed to increasing doses of acute gamma radiation (Fig. 1B). Exposed males (Fig. 1B, dashed lines) were more sensitive to the effect of IR compared to females (Fig. 1B, solid lines) for every dose. In addition, the exponential decline in the number of days to reach LD50 of males and females was observed with increased IR dose, with strong coefficient of determination (R2) values of 0.9728 and 0.9095 for males and females, respectively (Fig. 1C). Finally, as a positive control, we tested whether Drosophila mutants lacking the free-radical scavenging enzyme catalase (cat) are more sensitive to IR exposure using our method. cat null flies normally exhibit wild type lifespans but are sensitive to oxidative stress38,39. Two-day-old adult catn1 null mutants exposed to 1000 Gy had shorter lifespans compared to w1118, with males more sensitive than females (Fig. 1D, p = 0.0002 (***) (female) and p = 0.000049 (****) (male)). Together, these data demonstrate that our method exposing male and female Drosophila to IR consistently shortened lifespan in a dose-sensitive and sex-specific manner.
Dosimetry and radiation sensitivity of Drosophila. (A) Schematic of Drosophila irradiation sequence. (B) Lifespan of male and female flies acutely exposed to 0–1500 Gy gamma radiation. Radiation survival negatively correlates with dose. Males (dashed lines) were more sensitive than females (solid line) for all doses. (C) Graph showing a negative exponential relationship between the days to LD50 for males and females and increasing IR dose, indicated by R2 close to 1.0 for both sexes. (D) Lifespan of control (w1118) and catalasen1 (catn1) mutants after 1000 Gy acute irradiation. catn1 null flies are more sensitive to irradiation than control, and males are more sensitive than females for both genotypes. IR = ionizing radiation. Graphs were plotted using Microsoft Excel. Each point on the lifespan represents the average of triplicates of twenty flies that were irradiated simultaneously. Significance: (D) p = 0.0002 (***) for females and 0.000049 (****) for males.
Drosophila male guts are more sensitive to the effects of irradiation compared to females
As a single-layered epithelium, the adult fly midgut responds quickly to environmental changes, including nutrient fluctuations and tissue damage, in order to maintain homeostasis. Given this rapid physiological response, we conducted immunostaining on dissected midguts from flies exposed to 1000 Gy to identify physiological differences between males and females (Fig. 2, Fig. S1). The Drosophila adult gut is comprised of the foregut, midgut, and hindgut (Fig. 2A)24,26. The midgut is divided into five regions (R1-5) primarily by cellular function40. R4 is commonly studied due to its high incident of intestinal stem cells (ISCs), high cellular turnover, and high expression of stress-responsive genes, thus making it a good region to examine potential tissue damage from irradiation22,23,25. Enterocytes comprise ~ 80% of gut cells and are large, polyploid and in a single epithelial later (Fig. 2B,C)23,41. Other cell types, including ISCs, enteroblasts and enteroendocrine cells are less frequent, smaller and diploid (Fig. 2B). To examine potential irradiation-induced damage to the gut epithelium, we labeled the midgut enterocytes to visualize actin filaments and nuclei, then imaged the R4 region of the midguts using confocal microscopy. Irradiated males had abnormal nuclear shape (Fig. 2E,E”, H, Fig. S1D,D″,E,E″,F,F″, arrowheads), disrupted cellular barriers (Fig. 2E,E’,I, Fig. S1D,D′,E,E′), or visible holes within actin alignments (Fig. 2J, Fig. S1D,D′,E,E′, asterisks) compared to non-irradiated males that had normal nuclear shape (Fig. 2D,D″,H, Fig. S1A,A″,B,B″,C,C″) and well-organized actin filament distribution (Fig. 2D,D’,J, Fig. S1A,A′,B,B′,C,C′). In contrast, irradiated females had normal nuclear shape (Fig. 2G,G″,H, Fig. S1J,J″,K,K″,L,L″) and fewer holes within the actin cytoskeleton (Fig. 2G,G′,J) compared to irradiated males. However, irradiated females had significant numbers of enterocytes with indistinct cellular barriers compared to non-irradiated females (Fig. 2G,G′,I, Fig. S1J,J′,K,K′,L,L′). This suggests that the enterocytes of male guts are more vulnerable to the effects of IR compared to female guts, which could contribute to the difference between male and female survival after irradiation.
Drosophila male guts are more sensitive to the effects of irradiation compared to females. (A,B) Schematics of adult Drosophila gut structure. (A) Schematic of anatomical structure of the adult gut indicating the foregut, midgut and hindgut. R = Region. (B) Schematic of the cell types and general composition of the midgut. EE enteroendocrine cells, ISC intestinal stem cell, EB enteroblast, BM basement membrane. Arrowhead indicates the focal plane for D–G″. (C) General schematic of B rotated 90° to represent the en face view for D–G″. (D–G″) Dissected midguts from 4-day-old adults exposed to IR (+ IR) at day 2 and unexposed controls (− IR) labeled with phalloidin to label actin filaments and DAPI to label nuclei. The images of the R4 regions of midguts from each experimental condition were obtained by confocal microscopy using z-stacked images to show the layer of enterocytes. (D–D″) The enterocytes of a representative non-irradiated male control showing clear cellular barriers (D,D′) and good shape of nuclei (D,D″). Arrows in D,D′ indicate normal cellular gaps as visualized by phalloidin potentially due to erebosis that we did not include in our analysis58. (E–E″) The enterocytes of a representative male two days after irradiation showing abnormal nuclear shape (E,E″, arrowheads) and ambiguous cellular morphology due to disrupted cellular barriers (E,E′). (F–F″) The enterocytes of a representative female control and (G–G″) a representative female two days after irradiation showing normal nuclear morphology (F,F″,G,G″) compared to irradiated males (H). Irradiated females had disrupted cellular barriers between enterocytes (G,G′,I) similar to irradiated males (E,E′,I). (H) Graph indicates the percentage of abnormal nuclei relative to total nuclei within each image of the guts from irradiated or non-irradiated males and females in (A–D″). The triangles represent the arithmetic mean of each replicate. The dots represent the individual images analyzed in each replicate. The colors are: Replicate 1: dark blue triangle, light blue dots; Replicate 2: dark purple triangles, light purple dots: Replicate 3; dark green triangle, light green dots. The following numbers of the midguts were assayed per experiment: male (− IR) (6, 7, 7); male (+ IR) (5, 7, 4); female (− IR) (4, 8, 9); female (+ IR) (5, 8, 9). n: total image numbers analyzed. (I,J) Each graph indicates the percentage of animals with guts showing disrupted cellular barriers (I) and holes in the actin filament layer in enterocytes (J). Each point on the graph represents the percentage of each replicate, and each bar represents the arithmetic mean of triplicates with a standard error of the mean (SEM). The quantification method is further described in the Materials and Methods section. Data were analyzed statistically using a two-way ANOVA with multiple comparisons, followed by Tukey’s post hoc analysis. Significance of multiple comparisons: (E) p = 0.0077 (**) for males and 0.0384 (*) for male (+ IR) vs female (+ IR); (F) p = 0.0016 (**) for males and 0.0030 (**) for females; (G) p = 0.0379 (*) for males. ns not significant. (D,E,F,G) Green = phalloidin, blue = DAPI. (D′,E′,F′,G′) White = phalloidin. (D″,E″,F″,G″) White = DAPI. (D–J) − IR: non-irradiated, + IR: 1000 Gy irradiation. Scale bar: 10 μm in D for (D–G″).
Rhodotorula taiwanensis and Aureobasidium pullulans are highly radioresistant fungi
R. taiwanensis and A. pullulans (var. namibiae) are highly radioresistant fungi and potential candidates for a dietary prevention strategy to injury from acute IR (Fig. 3A)5,42. R. taiwanensis, a basidiomycetous carotenogenic fungus, is radioresistant to acute exposures of 2500 Gy and chronic exposures of 60 Gy/h (Fig. 3A,B5). A. pullulans, a dimorphic ascomycetous fungus, normally grows as a white colony (Fig. 3C). We assessed that A. pullulans is radioresistant to acute exposures as high as 15,000 Gy and chronic exposures of 35 Gy/h (Fig. 3A,C). In response to stress such as IR, A. pullulans turns black due to the production of melanin43. Exposing a partially lead-shielded A. pullulans plated culture to IR caused the exposed half to produce melanin and turn black (Fig. 3D, E). IR-exposed hyphae accumulated melanin in the hyphal tips (Fig. 3F vs G, arrow). The black, melanized culture regenerated over time, with newly formed hyphae from fully exposed or shielded colonies growing white, indicating the non-melanized form replenished growth (Fig. 3H,I, arrowheads).
Rhodotorula taiwanensis and Aureobasidium pullulans are highly radioresistant fungi. (A) Characteristics of R. taiwanensis and A. pullulans. Both are highly radioresistant. (B) Agar plate showing streaked red R. taiwanensis. (C) Agar plate with a colony of white A. pullulans. (D) Lead shield used for irradiation experiments on an agar plate. Only the top half of the plate is exposed to irradiation. (E) Culture streak of A. pullulans protected (left) and unprotected (right) from exposure to irradiation. The right half of the culture is black due to melanin production following irradiation. (F,G) Micrographs of A. pullulans hyphae without (F, − IR) and with (G, + IR) irradiation. Melanin deposits (arrow) are visible in the hyphae. (H,I) Agar plate (H) and colony streak (I) of radiation-induced melanized A. pullulans allowed to grow without radiation. New fungal growth after irradiation is non-melanized and white (arrow heads). The colony shown in I is the same as the colony in E. Scale bar: 50 μm in (G) for (F,G).
Drosophila tolerate a diet of highly radioresistant fungi
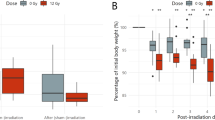
The preferred diet of Drosophila species is yeast growing on various organic matter, including fruit44,45. In a lab setting, Drosophila melanogaster prefers Saccharomyces cerevisiae (S. cerevisiae). To determine whether radiation-resistant fungi are a preventative dietary approach against IR, we first tested whether Drosophila tolerated a diet of exclusive R. taiwanensis or A. pullulans (Fig. 4). To feed flies both fungal strains, we harvested the fungi and placed a smear on a small agar plate. Flies fed ad libitum on the fungi while placed in a standard egg-laying cup with the plate on the bottom (Fig. 4A). Both larvae and adult flies ingested R. taiwanensis as indicated by their red guts (Fig. 4B,C, arrows). To better visualize ingested A. pullulans, we fed the flies irradiated, melanized fungi. The black fungi could also be seen in the larval and adult fly guts (Fig. 4D,E, arrows). This indicated that the flies readily consumed both fungal strains. We also examined the sensitivity of flies at developmental stages to determine whether fungal feeding affects pupation and eclosion rates (Fig. 4F). All larvae developed normally into pupae and eclosed at the same rate compared to control (S. cerevisiae) regardless of the kind of fungus (Fig. 4F). In addition, the flies survived for weeks exclusively eating either fungus (Fig. 4G). Flies fed on both strains had a reduced lifespan compared to those fed exclusive S. cerevisiae (Fig. 4G, blue and green vs black lines), with A. pullulans having a greater effect (Fig. 4G). However, neither was acutely toxic, with flies able to survive approximately 30 or 50 days, respectively, for A. pullulans or R. taiwanensis.
Drosophila tolerate dietary Aureobasidium pullulans and Rhodotorula taiwanensis. (A) Schematic of egg laying cups. Harvested R. taiwanensis and A. pullulans were placed on an agar plate for fly consumption (bottom). (B,C) Larva (B) and adult female fly (C) after feeding on R. taiwanensis. Red guts (arrows) indicate the animals ingested the fungi. (D,E) Larva (D) and adult female fly (E) after feeding on irradiated A. pullulans. Guts show the animals ingested the radiation-induced melanized fungi (arrows). (F) The percentage of larvae that pupated (solid lines) and eclosed (dashed lines) after being fed exclusively on alternative fungi. Each point on the graph represents the average of triplicates. Error bars represent standard deviation. (G) Lifespan analysis of males and females exclusively fed A. pullulans or R. taiwanensis. Agar only plates had no corn syrup (pink lines). Graphs were plotted using Microsoft Excel. Each point on the lifespan represents the average of triplicates of twenty flies that were fed fungi in parallel.
Feeding A. pullulans, but not R. taiwanensis, improves male survivorship after acute irradiation
Previous research suggested that radiation-induced metabolic changes or antioxidant production may endow fungi with radioresistance12,46,47. To test whether pre-feeding Drosophila radioresistant fungus could protect fly lifespan after acute IR exposure, we fed newly eclosed males or female for two days before exposure to acute IR, then monitored the flies for their entire lifespan (Fig. 5A). We found that flies fed A. pullulans showed a sex-specific improvement to lifespan, with males having increased lifespan, but females not (Fig. 5B, blue, C, green). In contrast, neither males nor females had increased lifespan after irradiation with R. taiwanensis pre-feeding (Fig. 5D, blue, E, green). Consistent with the exclusive diet of R. taiwanensis (Fig. 4G), females exhibited decreased lifespan (Fig. 5E, green). Since fungal exposure to IR upregulates protective cellular components6,7,8,9, we next tested whether IR-exposed, melanized fungi conferred greater dietary protection against acute IR. For A. pullulans, we found that males still exhibited lifespan improvement (Fig. 5B, p = 0.0331 (+ γ, magenta) vs p = 0.0297 (− γ, blue)), but this effect was not enhanced with the melanized fungus. Females again did not show any improvement in lifespan by feeding melanized A. pullulans (Fig. 5C). Feeding irradiated R. taiwanensis decreased lifespan in males and females and thus appeared to make the fungus more toxic (Fig. 5D (+ γ, magenta),E (+ γ, orange)).
Feeding Aureobasidium pullulans improves lifespan in males. (A) Schematic showing the experimental timeline. Newly eclosed males and females are fed control yeast or experimental fungal paste for two days. The flies were subsequently placed in standard food vials and irradiated. Flies were transferred to fresh vials every 1–2 days until all died. (B) Lifespan of males fed non-irradiated A. pullulans (A.p.) (blue, − ɣ) and chronically irradiated A. pullulans (magenta, + ɣ) before exposure to 1000 Gy acute irradiation. (C) Lifespan of females fed non-irradiated A. pullulans (green, − ɣ) and chronically irradiated A. pullulans (orange, + ɣ) before exposure to 1000 Gy acute irradiation. Prophylactic dietary A. pullulans significantly extended the lifespan of males, but not females. (D) Lifespan of males fed non-irradiated R. taiwanensis (R.t.) (blue, − ɣ) and chronically irradiated R. taiwanensis (magenta, + ɣ) before exposure to 700 Gy acute irradiation. Feeding irradiated R. taiwanensis was detrimental to male lifespan. (E) Lifespan of females fed non-irradiated R.t. (green, − ɣ) and chronically irradiated R. taiwanensis (orange, + ɣ) before exposure to 700 Gy acute irradiation. Feeding non-irradiated and irradiated R. taiwanensis to females significantly decreased their lifespan. Graphs were plotted using Microsoft Excel. Each point on the lifespan represents the average of triplicates of twenty flies that were irradiated simultaneously. Error bars represent standard deviation. Statistical analysis was calculated using Online Application for Survival Analysis 2 (OASIS) and statistical significance was calculated using the Wilcoxon-Breslow-Gehan test. P values vs control are as follows: (B) p = 0.0331 (*) for A.p.(+ ɣ), 0.0297 (*) for A.p.(− ɣ) ; (C) p = 0.461 for A.p.(+ ɣ), 1.0 for A.p.(− ɣ) (D) p = 0.0041 (*) for R.t.(+ ɣ), 1.0 for R.t.(− ɣ) (E) p = 0.0002 (***) for R.t.(+ ɣ), 0.0116 (*) for R.t.(− ɣ).
Feeding A. pullulans is associated with reduced IR-induced nuclear morphology defects in male guts
Since male guts were more susceptible to IR exposure (Fig. 2) and pre-feeding with A. pullulans improved male lifespan (Fig. 5B), we examined whether A. pullulans pre-feeding could ameliorate any negative effects of IR on gut morphology (Fig. 6). First, we assessed the effect of feeding A. pullulans on gut morphology without IR exposure. Feeding male fruit flies A. pullulans appeared to have a dual effect on their gut enterocytes. While it reduced the number of enterocytes with abnormal nuclei (Fig. 6C,C″,E, Fig. S2G,G″,H,H″,I,I″), A. pullulans feeding without radiation disrupted the cells’ actin cytoskeleton, leading to compromised cellular barriers and altered cell shape (Fig. 6C,C′,F, Fig. S2G,G′H,H′,I,I′). This disruption might explain the observed shortened lifespan (Fig. 4G). However, when the A. pullulans-fed flies were exposed to radiation, they exhibited fewer abnormal nuclei in their enterocytes compared to flies that were irradiated without dietary A. pullulans (Fig. 6B, B″ vs D,D″,E). Even though radiation still increased the disruption of cell barriers in the A. pullulans-fed group (Fig. 6D,D′,F, SFig. 2J,J′,K,K′,L,L′), these findings collectively support that feeding male Drosophila the radioresistant A. pullulans helped lessen or postpone gut injury which could contribute to their improved survival following radiation exposure.
Feeding Aureobasidium pullulans attenuates IR-induced cellular damage in male guts. (A–D″) Dissected midguts from 4-day-old adult males exposed to IR at day 2 and control males labeled with phalloidin to label actin filaments and DAPI to label nuclei. The images of the R4 regions of midguts from each experimental condition were obtained by confocal microscopy using z-stacked images to show a layer of enterocytes. (A–A″) Representative gut dissected from a male control, showing clear cell barriers (A,A′) and normal nuclear shapes in enterocytes (A,A″). Arrows in A,A′ indicate normal cellular gaps as visualized by phalloidin potentially due to erebosis that we did not include in our analysis58 (B–B″) Representative gut dissected from a male two days after irradiation. Enterocytes had aberrant nuclear shape (B,B″, arrowheads, E) and altered cellular morphology (B,B′,F). Holes were formed within a layer of actin filament (B,B′, asterisk, G) by irradiation. (C–C″) Representative gut from a male fed A. pullulans (Ap) had normal nuclear morphology (C,C″,E) in enterocytes while having a loss of cell barriers with some degree (C,C′,F). (D–D″) Representative gut from a male fed A. pullulans followed by irradiation had normal nuclear shape (D,D″,E) as well as loss of cellular barriers and irregular cell shape (D,D′,F). Many small holes appeared within actin layers (D,D′, number sign) but were not counted because they did not satisfy the criterion as described in Methods. (E) Graph indicates the percentage of abnormal nuclei relative to total nuclei within each image of the guts from irradiated or non-irradiated males with or without A.p. pre-feeding. The triangles represent the arithmetic mean of each replicate. The dots represent the individual images analyzed in each replicate. The colors are: Replicate 1: dark blue triangle, light blue dots; Replicate 2: dark purple triangles, light purple dots: Replicate 3; dark green triangle, light green dots. The following numbers of the midguts were assayed per experiment: male control (11, 6, 7); male + IR (8, 4, 10); male fed A.p. (8, 6, 5); male fed A.p.-IR (7, 8, 7). n: total image numbers analyzed. (F,G) Each graph indicates the percentage of animals having guts with disrupted cellular barriers (F), or holes in the actin filaments layer in enterocytes (G). Each point on the graph represents the percentage of each replicate, and each bar presents the arithmetic mean of triplicates with a standard error of the mean (SEM). Quantification method is described in the Materials and Methods section. Data were analyzed statistically using a two-way ANOVA with multiple comparisons, followed by Tukey’s post hoc analysis. P values of multiple comparisons are as follows: (E) p = 0.0039 (**) for (−/+IR) without A.p. and 0.0235 (*) for (−/+A.p.) with IR; (F) p < 0.0001 (****) for (−/+IR) without A.p., 0.0007 (***) for (−/+IR) with A.p., and 0.0014 (**) for (−/+A.p.) without IR; (G) p = 0.0225 (*) for (−/+IR) without A.p. ns not significant. (A–D) Green = phalloidin, blue = DAPI. (A′,B′,C′,D′) White = phalloidin. (A″,B″,C″,D″) White = DAPI. (A–G) − IR: non-irradiated, + IR: 1000 Gy irradiation. (E–G) − A.p.: non-feeding A. pullulans, + Ap: feeding A. pullulans. Scale bar: 10 μm in A for A–D″.
Discussion
Drosophila as a model to study dietary protection from IR
Drosophila has many advantages for evaluating preventative measures against radiation damage compared to mammalian models, such as experimental numbers in the hundreds, longitudinal analyses to capture survival, developmental, reproductive, and aging metrics, as well as observations of delayed effects of both radiation injury and treatment. Since irradiators can vary sample exposures due to differences in source and geometry, we created the survival curves for male and female lifespan using our 137Cs source, ensuring the flies were at a consistent and correct position in the irradiator and that six vials of adults, three experimental and three control, could be simultaneously exposed. As demonstrated in many species, we found sex-specific IR sensitivity in Drosophila, with males being more sensitive than females and thus having shorter lifespans31,32,33,48. This sex-specific difference occurred at all doses we tested. In general, female flies are more stress-tolerant than males49,50,51.
The survival radiosensitivity we observed with males compared to females was reflected in their gut morphologies, with males experiencing more damage. The GI tract is highly conserved in function and structure between Drosophila and vertebrates52,53,54. The fly intestinal epithelium has become a model to study tissue repair and age-related decline of tissue regeneration, including response to IR exposure24,28,55. The gut epithelium maintains homeostasis via normally quiescent Intestinal Stem Cells (ISCs) that rapidly proliferate in response to oxidative stress55. Previous studies have established that oxidative stressors, including aging, radiation, and ROS-generating compounds, as well as mitochondrial disruption, induce epithelial dysplasia and barrier dysfunction in Drosophila28,56,57. Notably, IR doses lower than those employed in the present study (2 Gy27; 100 Gy28) are sufficient to cause DSBs in the Drosophila midgut. Given that polyploid enterocytes have increased nuclear DNA content, which has been associated with heightened susceptibility to DSBs16, we hypothesize that enterocyte IR damage to the nucleus is exacerbated. The lifespan rescue observed with A. pullulans pre-feeding coincides with reduced IR-induced nuclear morphology deficits, suggesting a potential link between these two phenotypes. This could result in preserved gut function contributing to extended longevity. Although previous work demonstrated that 100 Gy exposure increases both DSBs and gut barrier dysfunction28, our findings did not show an improvement in cellular barrier integrity in irradiated males following A. pullulans feeding. Furthermore, cellular barrier integrity seemed to be the most sensitive of the phenotypes we scored since this was the only disruption that we observed with irradiated females. In addition, we observed increased barrier disruption with A. pullulans pre-feeding alone in males (Fig. 6F), suggesting that this specific diet may induce a mild stress response when given in isolation and supporting that disruption to the cellular barrier integrity may be particularly sensitive to perturbation. For assessing cellular barrier defects, we intentionally excluded cells exhibiting a loss of actin labeling and faint DAPI staining from the disrupted category. This exclusion was based on the observation of this phenomenon in non-irradiated control flies, suggesting it could be due to erebosis—a natural, non-pathological cell death process in the healthy gut58—rather than IR-induced pathology; further marker analysis was not performed to confirm this. Relatedly, pre-feeding R. taiwanensis shortened lifespan. Consistent with these complex microbial interactions, pre-feeding with unirradiated (for females) and irradiated (for males and females) R. taiwanensis shortened the lifespan of flies. We speculate that this detrimental effect may stem from IR exposure increasing the production of toxic compounds by R. taiwanensis or leading to excessive gut overpopulation in IR-compromised hosts. Interestingly, while both R. taiwanensis and A. pullulans reduced lifespan compared to the preferred control fungus, S. cerevisiae, the exclusive R. taiwanensis diet resulted in longer lifespans than the exclusive A. pullulans diet. Despite the lack of observed barrier improvement, the finding that A. pullulans pre-feeding improved male gut nuclei morphology after IR exposure we believe is significant. Given the high degree of conservation in gastrointestinal (GI) architecture and signaling pathways between Drosophila and mammals, dietary A. pullulans represents a promising nutritional preventative measure that could directly protect the GI tract against IR-induced injury.
Mechanisms and molecules for IR protection
Ionizing radiation (IR) causes cellular injury through both direct and indirect mechanisms. Direct damage involves IR-induced modifications to DNA, proteins, and lipids, including critical DNA repair enzymes. The primary indirect mechanism is water radiolysis, which increases the concentration of reactive free radicals that subsequently disrupt cellular integrity. The differential IR tolerance observed across Metazoan species is hypothesized to stem from a combination of superior DNA protection and an enhanced capacity to manage reactive oxygen species (ROS)59. Similarly, the highly radioresistant fungal pathogen, Cryptococcus neoformans, employs dual radioprotection strategies: a robust enzymatic antioxidant system alongside upregulated DNA damage repair genes47,60. If A. pullulans utilizes analogous defensive strategies, it is difficult to postulate a clear pathway for the transport of these fungal enzymes into host enterocytes to confer radioprotection. Nevertheless, compelling evidence suggests that the natural human microbiome mitigates ROS in enterocytes by secreting synthesized metabolites and upregulating host antioxidant enzymes within gut cells61. How might A. pullulans exert its protective effect on the gut? One possibility is that ingested antioxidant metabolites or other small molecules from A. pullulans could be taken up by the enterocytes, thus increasing protection against free radicals. For example, feeding mice melanin-containing Jelly Ear mushrooms compared to porcini mushrooms greatly improved survival post 9 Gy IR, with no signs of GI radiation damage 45 days post-irradiation62. Survival improvement post-irradiation has also been demonstrated with the oral administration of antioxidants63,64. N-acetyl-l-cysteine as a dietary supplement protected the mouse intestinal epithelial barrier after irradiation65. Mice ingesting Aloe vera extract had delayed radiation sickness symptoms and lower acid phosphatase and alkaline phosphatase levels in the liver after 6 Gy irradiation compared to a control66. A mixture of ingested traditional Indian medicinal plants increased survival and improved DNA damage compared to a control after 7.5 Gy exposure67. In Drosophila, feeding curcumin, a plant phenolic compound, increased lifespan and decreased protein carbonylation68. Feeding flies a tea polyphenol and beta-carotene decreased mutation frequency and increased antioxidant levels post 10 Gy exposure compared to controls69. Finally, flies fed ibuprofen or two flavonoids (quercetin and epicatechin) showed increased lifespan compared to a control after 1000 Gy exposure70.
Pre-feeding MnCl2 before IR exposure increased radiation survival in a similar manner to A. pullulans feeding71. The protective effect of dietary manganese was attributed to an increase in small molecule manganous peptide antioxidant content, not an increase in the free radical scavenger Mn Superoxide Dismutase. It is possible that A. pullulans contains high levels of manganous peptides, and this could explain its protective effect. Melanin is recognized as a radioprotectant present in extremophile microorganisms and has been shown to have radioprotective effects in vertebrates as well62,72,73. Mice fed melanin-containing mushrooms had greater gastrointestinal protection compared to mice fed non-melanized mushrooms, supporting that the presence of melanin in the gut could protect the gut lining from the effects of acute irradiation62. A. pullulans melanizes in response to IR10. Since this fungus extended lifespan in irradiated males, we also tested the melanized phenotype to determine whether melanin could increase its effectiveness. However, melanized A. pullulans did not increase the protective effect, suggesting that the concentration of melanin was not sufficient to produce an enhanced radioprotective effect or that other metabolites or molecules are responsible for the protection. Finally, we cannot rule out that the flies pre-fed A. pullulans before transfer to a standard diet supplemented with dry yeast eat less A. pullulans since it is not their preferred food source. Dietary restriction (DR) extends lifespan in many different organisms with female fruit flies responding more robustly compared to male flies74,75. Mair et al. demonstrated that for female fruit flies, this was independent of calories and that restricting yeast (protein) only while maintaining total calories with sugar mimics the lifespan extension observed with DR76. Examining mortality rates with DR, Mair et al. found that switching males from fully fed to DR reduced mortality, but switching them from DR to fully fed increased mortality rates77. Likewise, switching males from a low calorie to a high calorie diet decreased lifespan78. We would posit that males fed A. pullulans for two days before standard food would most likely mimic DR before switching to fully fed conditions. For this reason, it does not seem likely that two days of A. pullulans pre-feeding would be sufficient to increase lifespan in males. In addition, we did not observe any change in lifespan for females which are more responsive to DR.
Wider implications of identifying dietary strategies for reducing radiation-induced cellular damage
Radiotherapy patients are the most recognizable cohort that would benefit from identifying new strategies for reducing the effects of radiation injury. Radiotherapy patients, particularly those exposed to abdominal and pelvic radiation therapy, frequently have the side effect of GI tract damage and enteropathy79,80. An important consideration is that symptoms may be delayed and impact long-term quality of life. In addition to tissue damage, one notable change in this cohort is the alteration in the microbiome81. Changes to metabolites, tryptophan pathway intermediates in particular, and the microbiome occur in mice that exhibit improved survivorship to irradiation as well as in patients with leukemia who experienced fewer adverse effects of whole-body irradiation82. Identifying protective measures would greatly aid these patients.
An additional population at serious health risk from IR are those who are potentially exposed to radiation disasters—either as cleanup workers, military personnel, or the general public. For these groups of people, the diverse and wide-ranging effects of IR exposures are organized into subsyndromes of acute radiation syndrome (ARS). While medical countermeasures to the hematopoietic subsyndrome of acute radiation syndrome (H-ARS) have been developed, no medical countermeasures to the gastrointestinal subsyndrome (GI-ARS) exist. There are only four countermeasures approved as mitigators by the FDA for H-ARS, but none are effective as prophylactic intervention or for GI damage83. Gamma tocotrienol, a naturally occurring vitamin E isoform, reportedly confers some radioprotective survival in mice64,84,85. Effective prophylactic countermeasures targeting the vulnerable gastrointestinal tract need to be identified85,86.
Future directions and limitations
This study identified A. pullulans as a dietary prevention strategy for protection against acute irradiation. However, this observation has certain limitations and opens several avenues for future research. A key limitation is that we have only observed protection when feeding the entire fungus; therefore, a future direction is to identify the precise compound(s) responsible for this effect. While some contributors to the fungus’s radioresistance have been identified, such as manganous peptides, it is likely that other, as-yet-unidentified mechanisms and metabolites are also involved. In addition, we only tested two days of pre-feeding with A. pullulans for radioprotection. Future studies could extend this analysis to feeding for longer or to supplement standard food in various combination with live or heat inactivated or irradiated and non-irradiated A. pullulans to improve the effect. Furthermore, although we demonstrated improved lifespan and gut morphology in male flies, the underlying mechanism remains to be determined. In the future, many other radioresistant fungi could be tested in a similar manner. If subsequent vertebrate studies indicate that dietary A. pullulans can protect against radiation injury, it could prove to be a significant benefit for humans. Employing whole fungi that have adapted to survive high doses of irradiation may be the optimal strategy to protect people from the deleterious effects of IR.
Data availability
The images analyzed during the current study will be made available from the corresponding author on reasonable request. All other data generated are included in this published article and the supplementary information files.
References
Prescott, T.A.K. et al. Fungal drug discovery for chronic disease: history, new discoveries and new approaches. Biomolecules. 13(6) (2023).
Aly, A. H., Debbab, A. & Proksch, P. Fifty years of drug discovery from fungi. Fungal Divers. 50, 3–19 (2011).
Zhdanova, N. N. et al. Changes in micromycete communities in soil in response to pollution by long-lived radionuclides emitted in the Chernobyl accident. Mycol. Res. 98(7), 789–795 (1994).
Shuryak, I. et al. Chronic gamma radiation resistance in fungi correlates with resistance to chromium and elevated temperatures, but not with resistance to acute irradiation. Sci. Rep. 9(1), 11361 (2019).
Tkavc, R. et al. Prospects for fungal bioremediation of acidic radioactive waste sites: characterization and genome sequence of Rhodotorula taiwanensis MD1149. Front. Microbiol. 8, 2528 (2017).
Gaidamakova, E. K. et al. Small-molecule Mn antioxidants in Caenorhabditis elegans and Deinococcus radiodurans supplant MnSOD enzymes during aging and irradiation. MBio 13(1), e0339421 (2022).
Sharma, A. et al. Across the tree of life, radiation resistance is governed by antioxidant Mn(2+), gauged by paramagnetic resonance. Proc. Natl. Acad. Sci. U. S. A. 114(44), E9253–E9260 (2017).
Cordero, R. J. B., Vij, R. & Casadevall, A. Microbial melanins for radioprotection and bioremediation. Microb. Biotechnol. 10(5), 1186–1190 (2017).
Dadachova, E. & Casadevall, A. Ionizing radiation: how fungi cope, adapt, and exploit with the help of melanin. Curr. Opin. Microbiol. 11(6), 525–531 (2008).
Campana, R., Fanelli, F. & Sisti, M. Role of melanin in the black yeast fungi Aureobasidium pullulans and Zalaria obscura in promoting tolerance to environmental stresses and to antimicrobial compounds. Fungal Biol. 126(11–12), 817–825 (2022).
ChecinskaSielaff, A. et al. Characterization of the total and viable bacterial and fungal communities associated with the International Space Station surfaces. Microbiome 7(1), 50 (2019).
Dadachova, E. et al. Ionizing radiation changes the electronic properties of melanin and enhances the growth of melanized fungi. PLoS ONE 2(5), e457 (2007).
Reisz, J. A. et al. Effects of ionizing radiation on biological molecules–mechanisms of damage and emerging methods of detection. Antioxid Redox Signal 21(2), 260–292 (2014).
Munro, T. R. The site of the target region for radiation-induced mitotic delay in cultured mammalian cells. Radiat. Res. 44(3), 748–757 (1970).
Wu, L.-J. et al. Targeted cytoplasmic irradiation with alpha particles induces mutations in mammalian cells. Proc. Natl. Acad. Sci. 96(9), 4959–4964 (1999).
Tang, N. et al. Simulation of early radiation-induced DNA damage on different types of cell nuclei. Radiat. Prot. Dosimetry 183(1–2), 26–31 (2019).
Grammaticos, P., Giannoula, E. & Fountos, G. P. Acute radiation syndrome and chronic radiation syndrome. Hell. J. Nucl. Med. 16(1), 56–59 (2013).
Dainiak, N. & Albanese, J. Medical management of acute radiation syndrome. J. Radiol. Prot. 42(3) (2022).
Bier, E. Drosophila, the golden bug, emerges as a tool for human genetics. Nat. Rev. Genet. 6(1), 9–23 (2005).
Verheyen, E. M., The power of Drosophila in modeling human disease mechanisms. Dis. Models Mech. 15(3) (2022).
Amcheslavsky, A., Jiang, J. & Ip, Y. T. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell 4(1), 49–61 (2009).
Micchelli, C. A. & Perrimon, N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439(7075), 475–479 (2006).
Ohlstein, B. & Spradling, A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439(7075), 470–474 (2006).
Guo, Z. et al. Maintenance of the adult Drosophila intestine: all roads lead to homeostasis. Curr. Opin. Genet. Dev. 40, 81–86 (2016).
Marianes, A. & Spradling, A. C. Physiological and stem cell compartmentalization within the Drosophila midgut. Elife 2, e00886 (2013).
Miguel-Aliaga, I., Jasper, H. & Lemaitre, B. Anatomy and physiology of the digestive tract of Drosophila melanogaster. Genetics 210(2), 357–396 (2018).
Pyo, J.-H. et al. Functional modification of Drosophila intestinal stem cells by ionizing radiation. Radiat. Res. 181(4), 376–386 (2014).
Sharma, A. et al. Musashi expression in intestinal stem cells attenuates radiation-induced decline in intestinal permeability and survival in Drosophila. Sci. Rep. 10(1), 19080 (2020).
Cooper, D. M. Food preferences of larval and adult Drosophila. Evolution 14(1), 41–55 (1960).
Anagnostou, C., Dorsch, M. & Rohlfs, M. Influence of dietary yeasts on Drosophila melanogaster life-history traits. Entomol. Exp. Appl. 136(1), 1–11 (2010).
Parsons, P. A., Macbean, I. T. & Lee, B. T. Polymorphism in natural populations for genes controlling radioresistancein Drosophila. Genetics 61(1), 211–218 (1969).
Westerman, J. M. & Parsons, P. Variations in genetic architecture at different doses of γ-radiation as measured by longevity in Drosophila melanogaster. Can. J. Genet. Cytol. 15(2), 289–298 (1973).
Ogaki, M. & Nakashima-Tanaka, E. Inheritance of radioresistance in Drosophila. I. Mutat. Res. 3(5), 438–443 (1966).
Daly, M. J. et al. Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science 306(5698), 1025–1028 (2004).
Han, S. K. et al. OASIS 2: online application for survival analysis 2 with features for the analysis of maximal lifespan and healthspan in aging research. Oncotarget 7(35), 56147–56152 (2016).
Chen, J. & Johnston, D. S. Dissection, fixation, and immunostaining of the Drosophila midgut. Methods Mol. Biol. 2438, 309–321 (2022).
Landes, R. D. et al. Statistical analysis of survival data from radiation countermeasure experiments. Radiat. Res. 177(5), 546–554 (2012).
Mackay, W. J. & Bewley, G. C. The genetics of catalase in Drosophila melanogaster: isolation and characterization of acatalasemic mutants. Genetics 122(3), 643–652 (1989).
Orr, W. C., Arnold, L. A. & Sohal, R. S. Relationship between catalase activity, life span and some parameters associated with antioxidant defenses in Drosophila melanogaster. Mech. Ageing Dev. 63(3), 287–296 (1992).
Buchon, N. et al. Morphological and molecular characterization of adult midgut compartmentalization in Drosophila. Cell Rep. 3(5), 1725–1738 (2013).
Hung, R. J. et al. A cell atlas of the adult Drosophila midgut. Proc. Natl. Acad. Sci. U. S. A. 117(3), 1514–1523 (2020).
Liu, T. et al. Protective role of trehalose during radiation and heavy metal stress in Aureobasidium subglaciale F134. Sci. Rep. 7(1), 17586 (2017).
Gostinčar, C. et al. Genome sequencing of four Aureobasidium pullulans varieties: biotechnological potential, stress tolerance, and description of new species. BMC Genom. 15(1), 549 (2014).
Hoang, D., Kopp, A. & Chandler, J. A. Interactions between Drosophila and its natural yeast symbionts-Is Saccharomyces cerevisiae a good model for studying the fly-yeast relationship?. PeerJ 3, e1116 (2015).
Meshrif, W. S., Rohlfs, M. & Roeder, T. The effect of nutritive yeasts on the fitness of the fruit fly Drosophila melanogaster (Diptera: Drosophilidae). African Entomology 24(1), 90–99 (2016).
Kelley, M. et al. Ionizing radiation and chemical oxidant exposure impacts on Cryptococcus neoformans transfer RNAs. PLoS ONE 17(3), e0266239 (2022).
Jung, K.-W., et al., Unraveling fungal radiation resistance regulatory networks through the genome-wide transcriptome and genetic analyses of Cryptococcus neoformans. mBio. 7(6). https://doi.org/10.1128/mbio.01483-16 (2016).
Gartner, L. P. Radiation-induced life span shortening in Drosophila. Gerontology 19(5–6), 295–302 (1973).
Kristensen, T. N. et al. Sex and age specific reduction in stress resistance and mitochondrial DNA copy number in Drosophila melanogaster. Sci. Rep. 9(1), 12305 (2019).
Lin, Y. C. et al. Comparisons of lifespan and stress resistance between sexes in Drosophila melanogaster. Heliyon 9(8), e18178 (2023).
Pomatto, L. C. D., Tower, J. & Davies, K. J. A. Sexual dimorphism and aging differentially regulate adaptive homeostasis. J. Gerontol. A Biol. Sci. Med. Sci. 73(2), 141–149 (2018).
Capo, F., Wilson, A., & Di Cara, F. The intestine of Drosophila melanogaster: An emerging versatile model system to study intestinal epithelial homeostasis and host-microbial interactions in humans. Microorganisms. 7(9) (2019).
Douglas, A. E. Which experimental systems should we use for human microbiome science?. PLoS Biol. 16(3), e2005245–e2005245 (2018).
Ludington, W. B. & Ja, W. W. Drosophila as a model for the gut microbiome. PLoS Pathog. 16(4), e1008398–e1008398 (2020).
Biteau, B., Hochmuth, C. E. & Jasper, H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell 3(4), 442–455 (2008).
Rera, M. et al. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell Metab. 14(5), 623–634 (2011).
Rera, M., Clark, R. I. & Walker, D. W. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 109(52), 21528–21533 (2012).
Ciesielski, H. M. et al. Erebosis, a new cell death mechanism during homeostatic turnover of gut enterocytes. PLoS Biol. 20(4), e3001586 (2022).
Kelleher, E. S., Hajiarbabi, S., & Green, L. Extraordinary variation in radiation tolerance: mechanisms and evolution. J. Heredity. esaf015 (2025).
Giles Steven, S. et al. The Cryptococcus neoformans catalase gene family and its role in antioxidant defense. Eukaryot. Cell 5(9), 1447–1459 (2006).
Sun, Y., et al. The role of gut microbiota in intestinal disease: from an oxidative stress perspective. Front. Microbiol. 15-2024 (2024).
Revskaya, E. et al. Compton scattering by internal shields based on melanin-containing mushrooms provides protection of gastrointestinal tract from ionizing radiation. Cancer Biother. Radiopharm. 27(9), 570–576 (2012).
Brown, S. L. et al. Antioxidant diet supplementation starting 24 hours after exposure reduces radiation lethality. Radiat. Res. 173(4), 462–468 (2010).
Ghosh, S. P. et al. Gamma-tocotrienol, a tocol antioxidant as a potent radioprotector. Int. J. Radiat. Biol. 85(7), 598–606 (2009).
Shukla, P.K., et al., Rapid disruption of intestinal epithelial tight junction and barrier dysfunction by ionizing radiation in mouse colon in vivo: protection by N-acetyl-L-cysteine. Am. J. Physiol.-Gastrointest. Liver Physiol. 310(9), G705–G715 (2016).
Gehlot, P., Soyal, D. & Goyal, P. Prevention of radiation-induced hepatic damage in Swiss albino mice by Aloe Vera leaf extract. Nucl. Technol. Radiat. Prot. 25(3), 186–191 (2010).
Sandhya, T. et al. Protection against radiation oxidative damage in mice by Triphala. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 609(1), 17–25 (2006).
Seong, K. M. et al. Curcumin mitigates accelerated aging after irradiation in drosophila by reducing oxidative stress. Biomed. Res. Int. 2015, 1–8 (2015).
Nagpal, I. & Abraham, S. K. Protective effects of tea polyphenols and β-carotene against γ-radiation induced mutation and oxidative stress in Drosophila melanogaster. Genes Environ. 39(1), 24–24 (2017).
Proshkina, E., et al. Geroprotective and radioprotective activity of quercetin, (−)-epicatechin, and ibuprofen in Drosophila melanogaster. Front. Pharmacol. 7 (2016).
Volpe, R. P., et al. Prophylactically feeding manganese to drosophila confers sex-specific protection from acute ionizing radiation independent of MnSOD2 levels. Antioxidants (Basel). 14(2) (2025).
Kunwar, A. et al. Melanin, a promising radioprotector: mechanisms of actions in a mice model. Toxicol. Appl. Pharmacol. 264(2), 202–211 (2012).
Le Na, N.T., et al. Nanomelanin potentially protects the spleen from radiotherapy-associated damage and enhances immunoactivity in tumor-bearing mice. Materials. 12, https://doi.org/10.3390/ma12101725 (2019).
Sinclair, D. A. Toward a unified theory of caloric restriction and longevity regulation. Mech. Ageing Dev. 126(9), 987–1002 (2005).
Bross, T. G., Rogina, B. & Helfand, S. L. Behavioral, physical, and demographic changes in Drosophila populations through dietary restriction. Aging Cell 4(6), 309–317 (2005).
Mair, W., Piper, M. D. W. & Partridge, L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 3(7), e223 (2005).
Mair, W. et al. Demography of dietary restriction and death in Drosophila. Science 301(5640), 1731–1733 (2003).
Li, M. et al. Late-life shift in caloric intake affects fly metabolism and longevity. Proc. Natl. Acad. Sci. 120(50), e2311019120 (2023).
Hauer-Jensen, M., Denham, J. W. & Andreyev, H. J. N. Radiation enteropathy—pathogenesis, treatment and prevention. Nat. Rev. Gastroenterol. Hepatol. 11(8), 470–479 (2014).
Fernandes, D. C. R. & Andreyev, H. J. N. Gastrointestinal toxicity of pelvic radiotherapy: Are we letting women down?. Clin. Oncol. 33(9), 591–601 (2021).
Oh, B., et al. The gut microbiome and gastrointestinal toxicities in pelvic radiation therapy: A clinical review. Cancers. 13, https://doi.org/10.3390/cancers13102353 (2021).
Guo, H., et al., Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science. 370(6516), eaay9097 (2020).
Bene, B. J. et al. Celebrating 60 years of accomplishments of the armed forces radiobiology research institute1. Radiat. Res. 196(2), 129–146 (2021).
Kumar, V. P. et al. Gamma tocotrienol protects mice from targeted thoracic radiation injury. Front. Pharmacol. 11, 587970 (2020).
Singh, V. K. & Seed, T. M. Pharmacological management of ionizing radiation injuries: current and prospective agents and targeted organ systems. Expert. Opin. Pharmacother. 21(3), 317–337 (2020).
Singh, V. K. & Seed, T. M. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: part I. Radiation sub-syndromes, animal models and FDA-approved countermeasures. Int. J. Radiat. Biol. 93(9), 851–869 (2017).
Acknowledgements
Cartoons in Figs. 2A–C, 4A and 5A were created in BioRender. Cox, R. (2025) https://BioRender.com/joqvpie and Cox, R. (2025) https://BioRender.com/fz2l91l.
Funding
This work was supported by the National Institutes of Health grant numbers R21OD034471 and R01GM127938 to R. T. C. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. The content is solely the responsibility of the authors and does not necessarily represent the official views of Uniformed Services University, the Department of Defense, the National Institutes of Health or the Henry M. Jackson Foundation for the Advancement of Military Medicine, LLC.
Author information
Authors and Affiliations
Contributions
Conceptualization, R.P.V. and R.T.C.; methodology, R.P.V., H.J.H., and R.T.C.; validation, R.P.V. and H.J.H.; formal analysis, R.P.V and H.J.H.; writing—original draft preparation, R.P.V. and R.T.C..; writing—review and editing, R.P.V., H.J.H. and R.T.C.; supervision, R.T.C.; funding acquisition, R.T.C. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Volpe, R.P., Hwang, H.J. & Cox, R.T. Feeding Drosophila highly radioresistant fungi improves survival and gut morphology following acute gamma radiation exposure. Sci Rep 16, 1855 (2026). https://doi.org/10.1038/s41598-025-31545-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-31545-6