Abstract
Chronic inflammatory diseases are frequently comorbid with depression and anxiety, often persisting during periods of inflammatory remission. This suggests functional changes to neural circuits involved in the contextual regulation of motivation and threat processing. Here, we test how chronic gut inflammation evoked by dextran sodium sulfate (DSS) affects gene expression in several limbic brain structures associated with these functions. We assessed post-mortem expression of mRNA transcripts in the anterior cingulate cortex (ACC), CA1 hippocampus, nucleus accumbens (NAc), and primary motor cortex (M1) as a non-limbic control. The levels of mRNA associated with mitochondrial function, inflammation, and synaptic connectivity were altered in DSS-treated animals, but the specific pattern of changes was heterogeneous among brain structures. Chronic gut inflammation affected transcript expression in the CA1 and NAc more so than in the ACC and M1. These differences involved genes related to antioxidant systems and mitochondrial function. For example, expression of the cytochrome oxidase 1 gene mt-co1, which is necessary for oxidative phosphorylation, was reduced in ACC and NAc of DSS animals, suggesting reduced capacity for ATP production in these regions. Markers of gut inflammation correlated with expression of several transcripts in the ACC, including markers of synapses and GABA synthesis. The NAc showed strong correlations of mitochondrial function and measures of mitochondrial fission, inflammation, synaptic connectivity, and GABA synthesis. In sum, the effects of chronic relapsing gut inflammation on mitochondrial and antioxidant transcriptional programs were heterogeneous across key limbic brain structures.
Similar content being viewed by others
Introduction
Inflammation appears to promote anxiety and depression, likely by altering neurophysiological processes. This may contribute to the high comorbidity of anxiety and depression in chronic inflammatory diseases such as diabetes1, rheumatoid arthritis2, and cardiovascular diseases3. Such comorbidity is particularly high in people with inflammatory bowel disease (IBD). Approximately 57% of people with active IBD experience anxiety, and 39% have symptoms of depression4. Importantly, these mood disorders persist even during periods of disease remission4. This suggests that chronic somatic inflammation can produce changes in the brain that persist after peripheral inflammation subsides. Neuroinflammation is a likely intermediary. Even transient inflammation in the gut produces a neuroinflammatory response in the brain, leading to changes in behaviour5,6,7 and an array of changes in neural physiology8,9,10. Several of these physiological changes are associated with anxiety and depression, including oxidative stress, mitochondrial dysfunction, changes in neural structure, altered neural activity, and neurotransmitter levels11,12,13,14,15. The diversity and complexity of neuroinflammatory-evoked changes in neural physiology complicates a straightforward theory for the cellular etiology of anxiety and depression. We and others have proposed that bioenergetics and its regulation by reactive oxygen species in a key brain circuit called the limbic system are a core feature that may account for many phenomena16,17.
The brain’s high energy demand is predominantly met by mitochondria, the largest cellular source of ATP. Mitochondria activity influence a variety of processes related to neural structure, function, and circuity, including dendritic and axonal branching18,19 and neurotransmitter synthesis20,21. The metabolic needs of the brain are both spatially and temporally variable, as evidenced by functional and molecular evidence22. For example, the energetic costs of stress and inflammation can deplete metabolic resources in the brain if they reach sufficient magnitude and duration23,24. It has been proposed that prolonged downregulation of energy production in the brain can promote alterations in neuronal architecture and plasticity that promote anxiety and depression25,26.
Chronic gut inflammation elevates circulating cytokines like tumor necrosis factor-α(TNF-α) and monocyte chemoattractant protein-1 (MCP-1), which disrupt the gut-blood barrier and activate microglia27. Active microglia are a key component of inflammatory mechanisms in the brain, which affect many processes. For instance, activated microglia produce nitric oxide, which impairs mitochondrial function by inhibiting cytochrome c oxidase28,29,30. This inhibition leads to mitochondrial depolarization, ATP depletion, and glutamate release31. These dysfunctional mitochondria then release damage-associated molecular patterns (mt-DAMPs)—such as mitochondrial DNA (mtDNA), ATP, and reactive oxygen species (ROS)—that further propagate microglial activation and sustain a cycle of neuroinflammation32.
Specific regions of the brain appear to be more liable to inflammation-driven changes in bioenergetics, and evoke changes in function. For example, clinical and preclinical studies indicate that certain structures within the limbic system, such as the hippocampus33,34, anterior cingulate cortex16,35, and nucleus accumbens19,36 are particularly prone to changes in brain metabolism, which appear to promote anxiety and depressive behaviours37. Moreover, the phenotype of mitochondria varies among brain structures, as does the mitochondrial sensitivity to stressors and endocrines; notably, stress induced changes to identified ‘mitochondrial networks’, which correlated with anxiety-like behaviours in mice38. Whereas structure-specific effects of chronic stress on mitochondrial function have been reported, the consequences of gut inflammation on brain mitochondria have received less attention. We and others hypothesize that chronic gut inflammation may have a similar effect on mitochondrial function as stress39,40.
Much of the previous work on gut inflammation-induced neuroinflammation has employed relatively brief inflammatory treatments. Our previous research has demonstrated that the duration of gut inflammation influences its behavioural effects. Short-term gut inflammation (1 week) produced deficits in learning and memory, and increased motoric response in a forced swim task. These behavioural changes, however, were not observed in mice with chronic gut inflammation (5 weeks)7. In other words, it appears that the mnemonic and motoric alterations were transient effects triggered by the onset of inflammation. Conversely, increased contextual fear only emerged in chronic gut inflammation41. These data suggest that the neural circuits that encode some behaviours (e.g. contextual fear memory) are only affected by prolonged inflammation, possibly by the accumulated effects of neuroinflammation over many weeks. Here, we test the hypothesis that the accumulated effects of gut inflammation involve decreased mitochondrial function in limbic brain structures more so than in non-limbic structures, and that reduced metabolic function will be associated with changes in cellular physiology and metabolics, such as the management of oxidative stress.
Materials and methods
Animals
The study used 16 male C57Bl/6 mice (8 weeks old, Jackson Laboratories, Bar Harbor, ME, USA). Mice were housed in groups of four in standard clear plastic cages in a vivarium with a 12-h light/dark cycle, with free access to standard mouse chow and drinking water. Animals were acclimatized to the facility for 2 weeks prior to the start of experimentation. All experiments were carried out in accordance with the guidelines of the Canadian Council of Animal Care, and were approved by the University of Lethbridge Animal Care Committee (Protocol #2018). The experimental design and reporting of this study comply with ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments).
Colitis model
Dextran Sodium Sulfate (DSS) is widely used to model gut inflammation in rodents, with excellent face validity42. DSS induces lesions resembling ulcerative colitis by eroding the colonic epithelium and compromising the mucosal barrier, permitting bacterial translocation and triggering persistent inflammation42,43. This leads to a systemic increase in pro-inflammatory cytokines that can alter neurotrophic factor expression and central nervous system function44—a pathophysiology our group has previously characterized in an acute and chronic DSS models8,41. For the induction of chronic DSS colitis, we used a protocol similar to our previous published work on chronic colitis7. Briefly, mice were exposed to three cycles (3% wt/v) of DSS (MW 40kD; Fischer Scientific, CAT 9011-18-1) each lasting five days, with 9 days of recovery (access to regular drinking water) between cycles. Control mice received regular drinking water for the duration of the experiment (Fig. 1).
Forced swim task
Our previous research in an acute colitis model demonstrated that disease severity in acute colitis reduced immobility time in the forced swim test (FST)8. However, this relationship was not observed in a model of chronic colitis7. In acute colitis, we demonstrated a positive correlation between immobility time on FST and increased markers of hippocampal activity8. We utilized the same experimental paradigm here, wherein mice were exposed to the FST just prior to euthanasia.
The forced swim task was conducted on day 42. Mice were placed in a clear glass cylinder (30 cm tall, 15 cm diameter) filled 14 cm deep with water (28 C) and video recorded for 6 min. Mice were monitored continuously. Any animal unable to keep its head above water was immediately removed from the cylinder. After completing the task, mice were removed, dried with a paper towel, and placed in a cage with a heating pad before being returned to the home cage. Immobility time, defined by minimal movements to keep its head above water, was recorded in the last 4 min by an observer blind to the experimental treatment. Each mouse was euthanized 30 min after its completion of the FST, and the brain was quickly snap frozen. The timing of testing, euthanasia, and brain freezing was kept as constant as possible among animals in order to normalize any effects of stress and/or motoring output (swimming) on gene expression.
Colitis assessment
Disease activity was assessed on the third cycle of DSS and scored as previously described7. Briefly, the total score was comprised of: the percentage of body weight lost from the start of the cycle on day 28 scored on a 1–4 range based on the percentage of body weight lost (0 = 0%, 1 = < 1 to ≤ 5%, 2 = > 5 to ≤ 10%, 3 = > 10 to ≤ 15%, 4 = > 15%); stool consistency (solid fecal pellets = 0, soft, sticky stools = 2, loose, water stools = 4); and the presence of fecal blood (no blood = 0, fecal blood = 4). The total disease score was computed as a weighted average of each of these three measures. After euthanasia, the length of the colon was measured from anus to ileocecal junction.
Tissue collection
Mice were euthanized 30 min post forced swim task on day 42 by induction into an isoflurane chamber and subsequent injection with ~ 1.6mL/kg euthanyl (Pentobarbital Sodium 240 mg/ml Bimeda MTC 00141704). Brains were immediately removed and snap-frozen via immersion into pre-chilled isopentane for 60–120 s and subsequently stored at −80 °C until downstream processing. Tissue punches from different brain regions (Fig. 3A) were obtained following protocols outlined by Wager-Miller et al.45. Briefly, frozen whole mouse brains were sectioned into 1 mm thick slices using a pre-chilled brain matrix and chilled razor blades. A tissue punch (1 mm) was used to take samples in locations corresponding to the regions of interest determined by visual alignment with a mouse brain atlas46. This process ensures preservation of nucleic acids and proteins for an extended period of time and additionally enabled us to perform our molecular analysis of the brain regions using an already established protocol45. All tools and surfaces were cleaned using RNaseZap™ RNase Decontamination Solution and UltraPure™ DNase/RNase-Free Distilled Water (Thermofisher scientific).
ELISA
Fecal lipocalin-2 (lcn-2) is a biomarker for intestinal inflammation in both humans and rodents. Samples were prepared following previously published protocols41. Briefly, frozen fecal samples were reconstituted in PBS with 1% Tween 20 (10 mg/100uL), and vortexed for 20 min. Samples were centrifuged for 10 min at 12,000 rpm, and supernatants were collected and stored at −20◦C until analysis. At baseline, all processed fecal samples were diluted at 1:1 in reagent diluent. Moving forward, all fecal samples from mice exposed to DSS were diluted at 1:1250 in reagent diluent to ensure optical density was within limits of detection of the standard curve. Lcn-2 levels were quantified in the supernatants using the DuoSet murine Lcn-2 ELISA Kit (R&D Systems, Minneapolis, MN), following the manufacturer’s instructions.
RNA isolation and RT-PCR
RNA was extracted using the standard Trizol reagent protocol (Cat # 1596026, Invitrogen) and reverse transcribed using Superscript IV (CAT 18090010, Invitrogen) by the following method: 100ng of total RNA was mixed with 100ng Random Primer Mix (S1330S, NEB), and 1 uL of 10mM dNTP mix (CAT N0447S, NEB). The mixture was incubated for 5 min at 65 °C and placed immediately on ice for a minimum of 1 min. The mixture was then incubated with 4uL of 5X Superscript IV buffer, 1uL of 0.1 M DTT, and 1uL of Superscript IV (200U/uL) for 10 min at 25 °C, 10 min at 55 °C and 10 min at 80 °C. The produced cDNA was analyzed by qPCR using 2uL of 1:10 diluted cDNA, 0.5uL of each gene-specific primer diluted to 10 μm, 5uL of Luna Universal qPCR master Mix (M3003E, NEB), and 2uL of H2O. qPCR was performed using the Bio-Rad CFX384 Real-time detection system, with the following thermocycler conditions: 3 min at 95 °C (15 s at 95 °C, 30 s at 54 °C, 30 s at 66 °C) × 40 cycles. Fluorometer readings were taken during the extension phase, while standard curves were prepared for relative expression. The analysis of PCR efficiency was achieved by pooling 2uL of each cDNA sample and the subsequent preparation of standard dilutions SD1: 1:2; SD2: 1:4; SD3: 1:8; SD4: 1:16; SD5: 1:32. All samples were run in triplicate. Quantitative measurements of target gene expression relative to controls followed the 2-ΔΔCt method. Group differences were expressed as fold changes, normalized to the housekeeping gene hypoxanthine phosphoribosyltransferase (Hprt). Gene-specific primers were ordered from IDT (USA, CO) as custom DNA oligos. The sequences of primers used are shown in Table 1.
Statistical analysis
Data are presented as mean ± SEM. The threshold for statistical significance was set to p < 0.05. Body weight was analyzed by a two-way repeated measures ANOVA with Sidak post-test, and Lcn-2 expression analyzed by mixed effects analysis with a Tukey’s comparison post-test. Disease activity index was analyzed by One Sample Wilcoxon Signed ranked Test. Comparisons of the mean between groups was analyzed via Student’s t-test or One-Way ANOVA with Tukey’s post-test. Principal component analyses (PCA) were used to generate plots to visualize clustering between treatment groups. Primary and secondary principal components were compared via Mann-Whitney between DSS and control groups. Significant differences between the first or second principal component are reported. Statistical analyses were conducted with GraphPad Prism 10.0.0 (GraphPad Software, La Jolla, CA). Figures created with BioRender.com and Adobe Photoshop.
Results
Exposure to DSS produces signs of gut inflammation and disease
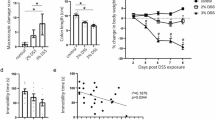
In our study, exposure to DSS in the drinking water led to expected signs of disease. Mice exposed to DSS for 3 cycles exhibited significant weight loss relative to controls (Two-way Rm ANOVA F1,14 = 41.48, p < 0.0001; Fig. 2A). Further, expression of the pro-inflammatory mediator Lcn-2 was significantly elevated in the feces among DSS-treated mice throughout the study (Mixed-effects model F1,14 = 19.70, p = 0.0006; Fig. 2B). The disease activity index was increased after final cycle of DSS in DSS-treated animals compared to controls (One way Wilcoxon Signed Rank Test, W = 36, p = 0.0078; Fig. 2C). Colon length was reduced, but not significantly, among mice exposed to DSS (Students t-test, t = 1.064; Fig. 2D). Overall, mice chronically exposed to DSS exhibited increased weight loss, disease activity, and increased fecal lcn-2 expression relative to controls, indicating that DSS treatment evoked the expected inflammation in the gastrointestinal tract.
(A) Change in weight throughout the duration of the experiment. (B) Disease activity score at peak disease (day 34) on third cycle of DSS exposure. (C) Fecal Lcn-2 expression throughout the study. (D) Colon length at time of necropsy (day 42). (E) Time spent swimming in a forced swim task. Data are mean ± SEM; n = 8 male mice per treatment group. P < 0.05.
Transcript selection
The objective of the study was to determine if DSS-colitis more strongly affects neurons in brain regions associated with anxiety and depression (e.g. limbic structures) as compared to other structures. The limbic structures were the hippocampal CA1, ACC, and NAc. The primary motor cortex (M1) was selected as the non-limbic comparator because of its proximity and general physiological similarity to ACC, and because it is expected to be activated by swimming in the FST. The FST revealed no difference in mobility between DSS and control mice (t = 0.43; Fig. 2E), consistent with a previous study7. We used FST to determine effects of DSS on threat coping, and to place animals in a situation that would evoke activity in the brain for a fixed time in order to evaluate differences in a molecular marker of activation (cFos). To distinguish brain-wide activation from activity specifically related to motor output, we measured cFos in the primary motor cortex (M1). Following the FST, the animals were euthanized, and brain regions were evaluated for a panel of 9 different primers to assess select transcriptional programs (Table 1). The transcripts represented: mitochondrial function (Mfn2, Drp1, Mt-Co1), oxidative stress response (Nrf2, Gpx1), synapse (Snap25 and PSD95), neuronal activation (cFos), and GABAergic signaling (Gad1)47,48,49,50,51,52,53,54,55.
Regional differences in basal transcript expression
We first examined how expression of the selected transcripts varied among brain structures in control animals (Supplementary Fig. 1; Supplementary Table 1). The NAc exhibited an expression profile that most differed from the other structures. The expression of Gad1 (F3,20 = 29.56, p < 0.001) was significantly highest in the NAc relative to all other structures (Tukey’s test for ACC and Ca1, NAc, and M1 are all p < 0.001). Interestingly, the NAc also possessed highest expression of Gpx1 (F3,20 = 76.18, p < 0.001) relative to all other structures (p < 0.0001). The NAc expressed significantly higher expression of Mt-Co1 (F3,20 = 7.554, p = 0.0014) and Psd95 (F3,20 = 8.062, p = 0.0010) compared to the ACC (Mt-co1, p = 0.0060; Psd95, p = 0.0013) and M1 (Mt-co1, p = x; Psd95, p = 0.0033, and significantly lower expression of Snap25 relative to CA1 and M1. The CA1 and NAc displayed lowest expression of cFos relative to the ACC and MA (F3,20 = 12.21, p < 0.0001; CA1 vs. ACC p = 0.0004; CA1 vs. M1, p = 0.0026; NAc vs. ACC p = 0.002, NAc vs. M1 p = 0.014), and highest expression of Nrf2, relative to the ACC and M1 (F3,20 = 20.30, p < 0.0001; CA1 vs. ACC p < 0.0001; CA1 vs. M1 p < 0.0001; NAc vs. ACC p = 0.002; NAc vs. M1 p = 0.0053). No differences in the expression of Mfn2 (F3,20 = 0.6606, p = 0.59) and Drp1 (F3,20 = 1.070, p = 0.38) were observed among neural structures in control animals.
Gut inflammation alters expression of selected gene transcripts in regional manner
We next compared the structure-specific (Fig. 3A) expression of transcripts between treatment groups. Exposure to DSS altered the expression of several transcripts in a region-specific manner (Table 2). The CA1 of the hippocampus was most affected by exposure to DSS; expression of 6 of the 9 transcripts were different (Fig. 3B, C). Specifically, cFos (t = 2.94, p = 0.015), Drp1 (t = 2.64, p = 0.025), Gad1 (t = 2.27, p = 0.046) and Snap25 (t = 2.29, p = 0.045) expression was significantly elevated in the CA1 among DSS-treated mice relative to controls. Conversely, Nrf2 (t = 3.91, p = 0.0029) and Gpx1 (t = 6.707 p = 0.000053) were significantly reduced in the CA1 of DSS-treated mice. Mt-Co1 approached significance (t = 2.092, p = 0.063). The expression of Mt-Co1 was significantly reduced in the NAc (t = 5.19, p = 0.00041) and ACC (t = 2.722, p = 0.021). Gpx (t = 1 3.29, p = 0.0082) was significantly reduced in the NAc of mice with DSS relative to controls (Fig. 3B, C). Treatment did not significantly affect the expression of any selected transcripts in the M1. These data indicate that chronic gut inflammation promotes heterogenous responses of different transcripts that vary among brain regions.
Expression of mRNA transcripts in four brain structures in control and mice with chronic DSS colitis (A) The approximate location of tissue punches in coronal mouse brain sections used for downstream PCR analysis. (B) Mean expression of mRNA transcripts, using Hprt as a reference gene, in the ACC, CA1, NAc, and M1. (C) Average change in mean gene expression relative to controls in mice with chronic DSS-induced colitis. Data represent mean ± SEM. *p < 0.05, n = 6 per treatment group. Treatment comparisons analyzed by t-test. T stat and p values can be found in Table 2.
Gut inflammation drives regional changes in transcriptional programs in the brain
To better understand how transcriptional programs were differentially affected by gut inflammation, we conducted principal component analysis (PCA). When all gene transcripts were loaded (Fig. 4A), and transcript expression from all brain regions are included in the PCA, clear clustering among mice with DSS colitis vs. control emerged (p = 0.0022). Further, when PCA loadings were restricted to transcript expression within specific brain regions, there were significant differences between control and DSS animals in the CA1 (p = 0.0022) and NAc (p = 0.015). When only transcripts related to mitochondrial function (Drp1, Mfn2, Mt-Co1) were loaded into the PCA analysis, there was a significant difference in PC1 between control and DSS mice among all brain regions; and upon separation of structure in the analysis, there were significant differences between principal components of control and DSS mice in the CA1 (P = 0.015), and PC2 NAc (p = 0.0043) (Fig. 4B). Similarly, PCA of transcripts related to inflammation regulation (Gpx1, Nrf2) revealed significant differences between the PC1 of controls and DSS among all brain regions (p = 0.0022; Fig. 4C). This significance persisted when the PCA only included transcript expression from the CA1 (p = 0.0022) and the NAc (p = 0.026). The expression of transcripts related to pre- and post-synaptic density proteins did not reveal any significant clustering of principal components (Fig. 4D).
Principal component analysis (PCA) of mRNA transcript expression (row labels) from brain samples (column labels) All genes include Mfn2, Drp1, Mtco1, CFos, Gad1, Gpx1, Nrf2, Snap25, and Psd95. Genes related to mitochondrial function include Drp1, Mfn2, Mt-Co1. Genes related to antioxidant regulation include Nrf2 and Gpx1. Pre and post-related synapse genes include post-synpatic (Psd95) and pre-synaptic (Snap25) mechanisms. The percentage of variation explained by the principal component is indicated on the axis. N = 6 male mice per treatment group. Ellipses indicate significant differences between the first or second principal component (*p < 0.05) between treatment groups.
Correlations between transcript expression within brain regions among healthy and gut-inflamed animals
We next tested if gut inflammation affects the relationships (i.e. correlations) between expression among transcripts within each brain structure. DSS treatment did have structure-dependent effects on the correlation among transcript levels (Fig. 5).
Animals with gut inflammation displayed significantly different relationships between several transcripts in the NAc relative to healthy controls (Table 3). Interestingly, the expression of MtCo-1 was a common denominator for all these relationships. We observed a significant positive correlation with Mt-Co1 and Drp1 (p = 0.048, r2 = 0.67; Fig. 6A), Mfn2 (p = 0.015, r2 = 0.81; Fig. 6B) and Gad1 (p = 0.030, r2 = 0.73, Fig. 6C), with a similar trend observed with Psd95 (p = 0.067; Fig. 6D). A negative relationship between Mt-co1 and Drp1, Mfn2, Gad1, and Psd95 was observed among controls, with the latter reaching statistical significance (p = 0.016, r2 = 0.80; Fig. 6).
Gut inflammation is associated with an inverse in the relationships between multiple transcripts in the NAc, relative to controls Expression of (A) Drp1, (B) Mfn2, (C) Gad1, and (D) Psd95 as a function of Mt-Co1 in the NAc among mice with gut inflammation and healthy controls. Transcript expression normalized to expression of Hprt. N = 6 mice/group. Linear regression and Pearson R coefficients are provided in Table 3.
Within the CA1, expression of Psd95 was positively correlated with Nrf2 among controls (p = 0.012, r = 0.91), but not DSS animals (p = 0.64, r=−0.24; Fig. 7). No significant relationship between Psd95 and Nrf2 was observed for any other regions, among control of DSS mice. We observed a significant correlation between the expression of Psd95 and Gad1 among control and DSS animals in the M1 and the NAc, among DSS animals in the ACC, and among controls in the CA1 (Supplementary Table 2). However in the CA1, among animals with DSS, the positive relationship between these genes was lost, and trended towards a negative correlation, although this did not reach statistical significance. The expression of Drp and Gad1 were positively correlated with each other among controls, in all limbic regions, but not M1 (Supplementary Table 3). This positive correlation was maintained in mice with DSS in the ACC, with similar trends in the NAc and the M1. Conversely, exposure to DSS was associated with a negative correlation between Drp and Gad1 in CA1 (Supplementary Table 3).
Correlation between Nrf2 and Psd95 expression among control and DSS animals, in the CA1. Expression of Psd95 as a function of Nrf2 among control and DSS animals in the CA1 N = 6 animals per group. Transcript expression normalized to expression of Hprt. Pearson R and p values for all comparisons can be found in Supplementary Table 4.
In the motor cortex and the NAc, DSS animals exhibited a significant positive correlation between MtCo1 and Gad (p = 0.030 r = 0.85); this relationship did not exist among controls, trending towards a negative correlation in the NAc (p = 0.065, r=−0.78) (Table 3). No such relationships between transcript expression in the ACC were affected by gut inflammation.
Correlations of transcript expression between brain structures, among control and DSS animals
To examine the possibility that gut inflammation affects the way the expression of a given transcript between two structures are related, we analyzed the relationship between transcript expression among different brain structures. Correlations of the same gene transcript between different regions are listed in Supplementary Table 5. Treatment status affected the correlation of transcripts related to GABA synthesis, mitochondrial function, and synapse function between brain structures. For example, correlations between the expression of Drp1, Gad1, and Psd95 in the ACC and the NAc were only present in healthy controls (Supplementary Table 5). CFos expression in the CA1 and NAc was negatively correlated only among DSS-treated animals. Further, Psd95 expression in the CA1 and ACC were correlated only among mice exposed to DSS. Interestingly, many correlations between gene expression were observed between the M1 and ACC, and the M1 and the CA1 (Supplementary Table 5).
Correlations between fecal Lcn-2 and transcript expression within DSS animals
To determine if there was a relationship between the severity of disease among mice with DSS and mRNA transcript expression, we produced a correlation matrix between transcript expression and levels of Lcn-2 from fecal samples of DSS exposed mice that were collected the same day as euthanasia.
Pearson correlation coefficients relating levels of fecal Lcn-2 with expression of transcripts in different brain regions reveals that ACC transcript expression was most strongly correlated with fecal Lcn-2 levels, with a positive correlation between 4 transcripts (Fig. 8). Specifically, the expression of Drp1 (p = 0.033, r = 0.849), Gad1 (p = 0.029, r = 0.858), Psd95 (p = 0.049, r = 0.813), and Snap25 (p = 0.003, r = 0.957) in the ACC were all positively correlated with fecal Lcn-2. CA1 expression of Psd95 (p = 0.018, and M1 expression of Snap25 (p = 0.019, r = 0.0886) were also positively correlation with fecal Lcn-2. No significant relationships between transcripts in the NAc and fecal Lcn-2 were observed, although Gad1 and Psd95 approached significance (p = 0.062 and p = 0.064, respectively).
Discussion
In this study we investigated the effects of chronic gut inflammation on transcript expression in four neural structures, using the DSS-colitis model of IBD in mice. We report here that chronic gut inflammation is associated with changes in the expression of several transcripts related to mitochondrial function and antioxidant responses. The CA1 of the hippocampus, and secondarily the nucleus accumbens were most affected. Several interesting relationships between transcript expression emerged in mice with chronic gut inflammation that suggest impaired mitochondrial metabolism in the CA1 and NAc. Reduced transcript expression of Mt-co1, indicative of diminished mitochondrial output, was also observed in the ACC. In contrast to these limbic structures, the expression of transcripts in the primary motor cortex (M1) were not affected by DSS. Combined, these results suggest (i) effects of chronic gut inflammation are heterogeneous among brain structures, and (ii) that limbic structures may be more susceptible to such inflammation.
Chronic gut inflammation is associated with regional changes in mitochondrial transcripts
Mitochondria play a key role in brain health, and their impairment is hypothesized as a key feature of psychiatric diseases including anxiety, depression, and cognitive dysfunction56,57,58,59. Inflammation can produce mitochondrial dysfunction through several mechanisms. For example, pro-inflammatory mediators can promote oxidative damage to mitochondrial components, leading to impaired energy production60,61. Further, inflammation can generate alterations in calcium homeostasis, in turn disrupting mitochondrial dynamics and promoting fragmentation, which can exacerbate inflammation, and reduce energy capacity62,63. The DSS colitis model has been previously shown to evoke systemic inflammatory responses, such as upregulation of inflammatory mediators like TNF-ɑ, IL-1β and IL-6, and to evoke microglial activation in brain regions including the medial prefrontal cortex, cingulate cortex, hippocampus, amygdala, hypothalamus, nucleus accumbens64,65,66. Considering the high basal metabolic rate of the brain, colitis may generate exacerbated metabolic consequences, particularly in neural structures with high metabolic activity. This is supported by studies showing that DSS administration increased the neuronal degeneration evoked by chemically-induced mitochondrial dysfunction67,68.
The bulk of ATP production in the brain is accomplished by mitochondrial oxidative phosphorylation, by which electrons are transported through a series of protein complexes to generate ATP. The final complex of the electron transport chain is cytochrome oxidase c, which is comprised of several subunits, including mt-co169. Thus, Mt-co1 gene expression plays a role in regulating energy production, and can be used as an indicator for ATP production70. Indeed, previous studies have reported a relationship between low Mt-co1 gene expression and mitochondrial dysfunction and oxidative stress71. Our results show that chronic gut inflammation is associated with significant reduction in Mt-Co1 expression relative to control animals in the NAc and ACC. The reduced Mt-Co1 expression was most pronounced in the NAc, with a 30% decrease relative to healthy controls; comparatively, the ACC exhibited a 21% reduction in Mt-Co1 expression. Within the CA1, there was a trend towards reduced Mt-co1 expression relative to controls, and significantly increased expression of Drp1, which suggests increased mitochondrial fission72. Enhanced fission can lead to increased levels of mitochondrial damage associated molecular patterns (DAMPS; e.g. ROS, mtDNA) in the cytoplasm, inducing the production of the proinflammatory cytokine IL-1β via inflammasome activation73. Increased expression of IL-1β has been reported in the HPC following gut inflammation74. We speculate that the emergence of DAMPS within the brain as a consequence of chronic gut inflammation may be a mechanism by which neuroinflammation persists during periods of disease remission, mirroring the role of systemic mitochondrial DAMPS in promoting inflammation in IBD62.
Chronic gut inflammation also transformed the relationship between Mt-Co1 and other transcripts in the NAc. Among control mice, we observed a negative relationship between expression of Mt-Co1 and Drp1, Mfn2, Gad1, and Psd95. These relationships were consistently shifted to the left and inverted to positive correlations among mice with chronic gut inflammation. The physiological significance of this finding is unclear; however, the fact that this transformation was only observed in the NAc, and all correlated with expression with Mt-Co1, suggests chronic gut inflammation may promote changes in mitochondrial function that impact neurotransmitter signaling and neuronal spine structure in this region. In our study, the NAc had significantly highest expression of Mt-Co1 relative to other regions among control animals. This may reflect a higher basal metabolic requirement in the NAc, which could render this region especially vulnerable to the metabolic demands associated with gut inflammation. Research indicates that there are regional differences in mitochondrial bioenergetics within the brain38,75 and evidence suggests mitochondria within the striatum may be particularly active. For example, mitochondria from striatal neurons exhibited highest rates of oxygen consumption and ATP production in the presence of some respiratory substrates, relative to mitochondria from cerebral cortex and hippocampal neurons75. Indeed, striatal mitochondria are more susceptible to calcium-induced activation of the permeability transition pore (leading to cell damage or death) than cortical mitochondria76, and evidence suggests the striatal mitochondria are particularly sensitive to defects in oxidative phosphorylation relative to mitochondria in the cortex and hippocampus77. Together, these findings reveal region-specific transcriptional changes in mitochondrial genes during gut inflammation, leading us to hypothesize that energy production capacity may be impaired. Future studies are needed to directly test this hypothesis and to establish how such mitochondrial dysfunction might influence neural physiology.
The CA1 displays increased sensitivity to chronic gut inflammation
Our study revealed that out of the structures examined, the CA1 of the hippocampus appeared to be the most sensitive to gut inflammation; the expression of 6 out of 9 of the selected transcripts were significantly altered. As well, our PCA revealed that the principal components that included the expression for all transcripts, antioxidant-related transcripts, and mitochondria function related transcripts in the CA1 was consistently different between treatment groups. The apparent sensitivity of the HPC to gut inflammation is supported by the literature, where the reported effects of gut-inflammation in the brain are dominated by the HPC16. For example, mice administered chemically-induced models of colitis demonstrated increased expression of proinflammatory cytokines (e.g. IL-6, IL-1β, TNF-α) and a variety of markers of oxidative stress in the HPC34,74,78. Our previous research has revealed that acute exposure to DSS is associated with increased proportion of immature spines in the apical dendrites of CA1 pyramidal neurons, and reduced neural activation in the CA1, based on reduced immunostaining of cFos8. The specific upregulation of CA1 Snap25, critical for synaptic vesicle release and plasticity, raises the question of whether this region’s sensitivity to gut inflammation involves altered synaptic signaling. One testable hypothesis is that this transcriptional change reflects an adaptive, or maladaptive, engagement of synaptic mechanisms in response to the systemic inflammatory state.
In the present study we also observed that chronic gut inflammation is associated with elevated cFos mRNA expression in the CA1. This contrasts the effects of acute DSS exposure, which is associated with reduced immunostaining of cFos in the CA1 of mice8. Interestingly, CA1 hypoactivity was correlated with increased swimming time in the FST in mice exposed to a single 5-day exposure of DSS; here we report increased cFos expression in mice with three cycles of 5-day exposures to DSS, which was accompanied by swimming in the FST equivalent to controls. This suggests neural activity in the CA1 may contribute to mobility in this task.
We took two measures to confirm that the CA1 activity was associated with stress coping rather than motor output: first, we compared cFos in M1, a region dedicated to movement, and found no difference between chronic colitis and control animals. Second, we ensured animals were not habituated to the FST, confirming that the mobility times reflected response to a novel stressor. With equivalent motor activity (as indicated by M1 activation and mobility times), the selective increase in CA1 activity strongly implicates this region in the stress-coping behavior itself.
Taken together, the results from these studies suggest that the duration of inflammation differentially modulates neural activity in the CA1 hippocampus.
Chronic gut inflammation is associated with reduced expression of antioxidant-related genes
Reactive oxygen species (ROS) play an essential role in normal physiological processes, including cell signaling, immune responses, and mitochondrial function. However, the accumulation of ROS can lead to cellular damage, a pathological state termed oxidative stress79,80. Antioxidant responses play an important role in maintaining the balance between ROS production and its elimination. The brain is a huge consumer of oxygen; estimates suggest 20% of the body’s oxygen supply is consumed by this organ to support the ATP intensive activities of neurons, despite only accounting for 2% of the body weight81. As such, neurons are especially vulnerable to oxidative damage due to their high oxygen demand, combined with their relatively weak antioxidant defense system82. Inflammation is energy-intensive; it is estimated that chronic inflammatory diseases can increase energy costs by 10–15%23,24. Inflammation drives a metabolic switch in astrocytes and microglia towards glycolysis, increasing the production of ATP and the consumption of glucose83. Further, inflammation can lead to impairments in glutamate clearance, leading to neuronal excitotoxicity and metabolic dysfunction84.Thus, neuroinflammation places an additional metabolic demand on an already highly metabolic organ, leading to increased risk of oxidative stress, both of which are hypothesized to play a role in the pathophysiology of anxiety, depression, and cognitive dysfunction13,85,86,87. Chronic gut inflammation was associated with reduced mRNA expression for Gpx1, the enzyme that contributes to the potent antioxidant activity of glutathione, in the CA1 and the NAc. Reduced expression of the transcription factor Nrf2, which regulates antioxidant expression, was also observed in the CA1. These findings suggest impaired antioxidant responses in the CA1 and NAc of mice with chronic gut inflammation.
Mice with acute DSS colitis have increased levels of reactive oxidant-induced protein and lipid modifications in the hippocampus and striatum, respectively78. The effects of chronic gut inflammation on antioxidant systems in the brain has not been reported. However, chronic unpredictable stress, which can produce neuroinflammation and similar behavioural phenotypes as chronic inflammation in animal models, was found to reduce activity of glutathione peroxidase in the brain of rats88. Interestingly, we observed a positive correlation between Nrf2 and Psd95 among control mice; chronic gut inflammation decoupled this relationship. Further, both Nrf2 and Psd95 were reduced in a mouse model of chronic stress, and treatment with the Nrf2 activator oltipraz restored Psd95 expression in the hippocampus90.
Research suggests the brain possesses regional vulnerability to oxidative stress. For example, the literature paints the CA1 as a region that is highly vulnerable to insult. Within the hippocampus, the CA1 and CA3 subfields lie adjacent to each other, and both possess a high density of pyramidal neurons. However, despite these similarities, studies conducted in a diversity of mammalian species (rats, gerbils, primates, humans) using a variety of different methods to generate oxidative stress in the brain demonstrate that CA1 neurons are much more sensitive to oxidative stress relative to their CA3 neuronal counterparts91,92,93,94. Molecular mechanisms that ascribe such vulnerability are not completely understood; it has been suggested that high baseline levels of ROS-generating activities (e.g. cellular respiration) within neuronal populations render those neurons more vulnerable to oxidative stress95. Extending from this, regions with greater basal levels of oxidative stress should also possess higher basal expression of genes involved in antioxidant responses, such as Nrf2. Indeed, Wang et al.. report higher basal expression of Nrf2 in the CA1 than that of the CA396. In the present study, basal levels of Nrf2 in control mice were significantly higher in the CA1 and NAc compared to the ACC and M1. Further, transcript expression of Gpx1 was significantly higher in the NAc relative to other regions in control mice. Gut inflammation in rodent models of colitis have been demonstrated to induce oxidative stress and impaired mitochondrial function34,97.
Combined, these data support previous assertations that the CA1 maybe especially vulnerable relative to other structures. Our results extend upon these studies and suggest the NAc may similarly possess intrinsic vulnerability to the costs incurred due to gut inflammation. Both the CA1 and NAc represent brain regions involved in anxiodepressive phenotypes, which are well-established comorbidities in preclinical and clinical studies of IBD16. The relative vulnerability of neural regions involved in threat-coping and motivation behaviours in the face of chronic inflammation may explain why anxiety and depression emerge in such a wide range of chronic inflammatory diseases, despite their diverse etiology. Further studies are necessary to confirm the oxidative stress vulnerability transcript responses to chronic gut inflammation in other regions known to be involved in anxiety and depression, such as the basal ganglia, prefrontal cortex, and amygdala.
Chronic gut inflammation is associated with altered Gad1 expression in the CA1
Disturbed modulation of excitatory circuits by gamma-aminobutyric acid (GABA), the primary inhibitory neurotransmitter in the CNS, is implicated in psychological processes such as anxiety and depression98,99. GABA synthesis is in part regulated by the rate-limiting enzyme glutamate decarboxylase isoform Gad67, which is encoded by Gad1 mRNA. We report the expression of Gad1 mRNA is significantly increased in the CA1 of mice with chronic DSS exposure. Further, chronic gut inflammation transformed the relationship between the expression of Gad1 and Psd95, and Drp1 in the CA1. The functional significance of these relationships is not clear; however, it suggests an intriguing relationship between post-synaptic density, mitochondrial dynamics, and the GABAergic system in our model.This correlation warrants future investigation to determine if it reflects an adaptive or pathological response.
To our knowledge, the effects of gut inflammation on hippocampal GABA expression have not been previously reported. However, research suggests chronic stress100 and toxin exposure101 can alter GABA signaling in the hippocampus by increasing GABA receptor expression and GABA levels, respectively.Whether the increased Gad1 mRNA expression observed in our study translates to functional changes in GABAergic neurotransmission requires direct assessment through protein-level validation and functional assessments.
In vitro studies indicate GABA contributes to neuroinflammation by stimulating the secretion of pro-inflammatory cytokines from microglia100. Using the TNBS model of colitis in rats, Riazi et al. revealed that gut inflammation is associated with increased neuronal excitability in the HPC, and increased susceptibility to seizure that was dependent on microglial activation9. Specifically microglial activation was increased in the entorhinal cortex, and activation of this region has been shown to promote increased Gad1 mRNA in the CA19,102. The entorhinal cortex was not investigated in the present study, but is involved in processing fear responses and anxiety, which we have shown are modulated as a consequence of chronic gut inflammation41.
Fecal Lcn2 expression is correlated with transcript expression in the ACC, and other regions
The ACC, positioned at the interface between the ‘emotional’ limbic system, and the ‘cognitive’ prefrontal cortex, is heavily involved in affective processing. Neuroinflammatory remodeling of the ACC is a key driver of anxiety and depression, and this region is among the most consistently altered neural structures in patients with gastrointestinal diseases and disorders, displaying cortical thinning and hyperactivity16. In our model, the primary transcriptional change in the ACC of mice with chronic gut inflammation was the reduced expression of Mt-co1. Furthermore, we observed that fecal Lcn2, a robust biomarker of gut inflammation severity42,103,104, was positively correlated with transcript expression (4 out of 9) in the ACC.
These correlations generate testable hypotheses about how gut inflammation may be linked to brain changes. For instance, the correlation between fecal Lcn-2 and Drp1 could suggest a relationship between the severity of gut inflammation and increased mitochondrial fission, which aligns with reduced Mt-co1 expression in this region. GABAergic neurons regulate neuronal activity, and their activity in the ACC has been shown to attenuate anxiety in a rodent model of inflammatory pain105.Therefore, we cautiously propose that the observed correlation between fecal Lcn2 and ACC Gad1 could be interpreted as a compensatory inhibitory control in our animals, representing a testable hypothesis for future work. The physiological significance of changes in pre and postsynaptic protein transcripts in the ACC, CA1 and M1 as they relate to fecal Lcn-2 levels are not clear. The correlation between fecal Lcn-2 and Psd95 mRNA may reflect visceral pain among more severely inflamed animals; DSS-induced colitis promotes visceral hyperalgesia in mice106,107,108, and Psd95 in the ACC has been shown to contribute to neuropathic pain109. Stress and inflammation have also been shown to alter spine morphology in both the ACC and CA1110,111. However, it is critical to emphasize that these correlations are strictly associative. A key limitation of this analysis is that it cannot establish causality between gut inflammation and brain transcription; the observed associations may reflect a gut-to-brain signal, independent effects of systemic inflammation, or a bidirectional loop. This study did not assess post-transcriptional modifications, protein expression, or the specific cell phenotypes involved, which limits functional interpretations. Therefore, these correlations should be viewed as generating hypotheses for future research that can experimentally manipulate these pathways to establish causality.
Conclusions
Our results indicate chronic gut inflammation is associated with heterogenous changes in the brain, particularly the limbic system. Changes in transcript expression suggest prolonged colitis promotes reduced oxidative phosphorylation, and impaired antioxidant defense systems in the ACC, CA1, and NAc. Our data suggest chronic gut inflammation may alter mitochondrial function and dynamics in regions of the brain that are known to be affected in patients with symptoms of anxiety and depression. This lends support to the literature that proposes a role of altered bioenergetics and mitochondrial dysfunction in the brain of patients with chronic inflammatory diseases, which may play a role in mood disorders that are comorbid in these illnesses.
Data availability
The datasets generated and analyzed for the current study are included in the supplementary materials.
References
Castellano-Guerrero, A. M. et al. Prevalence and predictors of depression and anxiety in adult patients with type 1 diabetes in tertiary care setting. Acta Diabetol. 55, 943–953 (2018).
Isik, A., Koca, S. S., Ozturk, A. & Mermi, O. Anxiety and depression in patients with rheumatoid arthritis. Clin. Rheumatol. 26, 872–878 (2007).
Celano, C. M., Daunis, D. J., Lokko, H. N., Campbell, K. A. & Huffman, J. C. Anxiety disorders and cardiovascular disease. Curr. Psychiatry Rep. 18, 101–101 (2016).
Barberio, B., Zamani, M., Black, C. J., Savarino, E. V. & Ford, A. C. Prevalence of symptoms of anxiety and depression in patients with inflammatory bowel disease: a systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 6, 359–370 (2021).
Emge, J. R. et al. Modulation of the microbiota-gut-brain axis by probiotics in a murine model of inflammatory bowel disease. Am. J. Physiology-Gastrointestinal Liver Physiol. 310, G989–G998 (2016).
Jain, P. et al. Behavioral and molecular processing of visceral pain in the brain of mice: impact of colitis and psychological stress. Front. Behav. Neurosci. 9, 177–177 (2015).
Matisz, C. E., Vicentini, F. A., Hirota, S. A., Sharkey, K. A. & Gruber, A. J. Behavioral adaptations in a relapsing mouse model of colitis. Physiol. Behav. 216, 112802–112802 (2020).
Matisz, C. E. et al. Acute gut inflammation reduces neural activity and spine maturity in hippocampus but not basolateral amygdala. Sci. Rep. 12, 20169–20169 (2022).
Riazi, K. et al. Microglia-Dependent alteration of glutamatergic synaptic transmission and plasticity in the hippocampus during peripheral inflammation. J. Neurosci. 35, 4942–4952 (2015).
Zonis, S. et al. Chronic intestinal inflammation alters hippocampal neurogenesis. J. Neuroinflamm. 12, 65–65 (2015).
Salim, S. Oxidative stress and psychological disorders. Curr. Neuropharmacol. 12, 140–147 (2014).
Liu, Y., Zhao, J. & Guo, W. Emotional roles of Mono-Aminergic neurotransmitters in major depressive disorder and anxiety disorders. Front. Psychol. 9, 2201–2201 (2018).
Guo, B. et al. Neuroinflammation mechanisms of neuromodulation therapies for anxiety and depression. Translational Psychiatry. 13, 5–5 (2023).
Allen, J., Romay-Tallon, R., Brymer, K. J., Caruncho, H. J. & Kalynchuk, L. E. Mitochondria and mood: mitochondrial dysfunction as a key player in the manifestation of depression. Front. NeuroSci. 12, 386–386 (2018).
Rezin, G. T., Amboni, G., Zugno, A. I., Quevedo, J. & Streck, E. L. Mitochondrial dysfunction and psychiatric disorders. Neurochem. Res. 34, 1021–1029 (2009).
Matisz, C. E. & Gruber, A. J. Neuroinflammatory remodeling of the anterior cingulate cortex as a key driver of mood disorders in Gastrointestinal disease and disorders. Neurosci. Biobehavioral Reviews. 133, 104497–104497 (2022).
Picard, M. & McEwen, B. S. Psychological stress and mitochondria: A conceptual framework. Psychosom. Med. 80, 126–140 (2018).
Courchet, J. et al. Terminal axon branching is regulated by the LKB1-NUAK1 kinase pathway via presynaptic mitochondrial capture. Cell 153, 1510–1525 (2013).
Gebara, E. et al. Mitofusin-2 in the nucleus accumbens regulates anxiety and Depression-like behaviors through mitochondrial and neuronal actions. Biol. Psychiatry. 89, 1033–1044 (2021).
Kwon, S. K. et al. Correction: LKB1 regulates Mitochondria-Dependent presynaptic calcium clearance and neurotransmitter release properties at excitatory synapses along cortical axons. PLoS Biol. 16, e3000040–e3000040 (2018).
Sun, T., Qiao, H., Pan, P. Y., Chen, Y. & Sheng, Z. H. Motile axonal mitochondria contribute to the variability of presynaptic strength. Cell. Rep. 4, 413–419 (2013).
Castrillon, G. et al. An energy costly architecture of neuromodulators for human brain evolution and cognition. Sci. Adv. 9, eadi7632–eadi7632 (2023).
Lacourt, T. E., Vichaya, E. G., Chiu, G. S., Dantzer, R. & Heijnen, C. J. The high costs of Low-Grade inflammation: persistent fatigue as a consequence of reduced Cellular-Energy availability and Non-adaptive energy expenditure. Front. Behav. Neurosci. 12, 78–78 (2018).
Straub, R. H. The brain and immune system prompt energy shortage in chronic inflammation and ageing. Nat. Rev. Rheumatol. 13, 743–751 (2017).
Morava, É. & Kozicz, T. Mitochondria and the economy of stress (mal)adaptation. Neurosci. Biobehavioral Reviews. 37, 668–680 (2013).
Picard, M., McEwen, B. S., Epel, E. S. & Sandi, C. An energetic view of stress: focus on mitochondria. Front. Neuroendocr. 49, 72–85 (2018).
Lei, W. et al. Gut microbiota-driven neuroinflammation in alzheimer’s disease: from mechanisms to therapeutic opportunities. Front. Immunol. 16, 1582119 (2025).
Bellavite, P. Neuroprotective potentials of flavonoids: experimental studies and mechanisms of action. Antioxidants 12, 280 (2023).
Lee, K. H., Cha, M. & Lee, B. H. Crosstalk between neuron and glial cells in oxidative injury and neuroprotection. IJMS 22, 13315 (2021).
Minghetti, L. & Giulio, L. Microglia as effector cells in brain damage and repair: focus on prostanoids and nitric oxide. Prog. Neurobiol. 54, 99–125 (1998).
Qin, P., Sun, Y. & Li, L. Mitochondrial dysfunction in chronic neuroinflammatory diseases (Review). Int. J. Mol. Med. 53, 47 (2024).
Clemente-Suárez, V. et al. Mitochondria and brain disease: A comprehensive review of pathological mechanisms and therapeutic opportunities. Biomedicines 11, 2488 (2023).
Bagot, R. C. et al. Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat. Commun. 6, 7062–7062 (2015).
Haj-Mirzaian, A. et al. Anxiety- and Depressive-Like behaviors are associated with altered hippocampal energy and inflammatory status in a mouse model of crohn’s disease. Neuroscience 366, 124–137 (2017).
Wang, J. et al. Astrocytic l -Lactate signaling facilitates Amygdala-Anterior cingulate cortex synchrony and decision making in rats. Cell. Rep. 21, 2407–2418 (2017).
Strasser, A., Xin, L., Gruetter, R. & Sandi, C. Nucleus accumbens neurochemistry in human anxiety: A 7 T 1H-MRS study. Eur. Neuropsychopharmacol. 29, 365–375 (2019).
Duman, R. S., Aghajanian, G. K., Sanacora, G. & Krystal, J. H. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat. Med. 22, 238–249 (2016).
Rosenberg, A. M. et al. Brain mitochondrial diversity and network organization predict anxiety-like behavior in male mice. Nat. Commun. 14, 4726–4726 (2023).
Zhao, W. et al. Elamipretide (SS-31) improves mitochondrial dysfunction, synaptic and memory impairment induced by lipopolysaccharide in mice. J. Neuroinflamm. 16, 230–230 (2019).
Culmsee, C. et al. Mitochondria, Microglia, and the immune System—How are they linked in affective disorders? Front. Psychiatry. 9, 739–739 (2019).
Matisz, C. E., Patel, M., Hong, N. S., McDonald, R. J. & Gruber, A. J. Chronic gut inflammation impairs contextual control of fear. Sci. Rep. 12, 20586–20586 (2022).
Chassaing, B., Aitken, J. D., Malleshappa, M. & Vijay-Kumar, M. Dextran sulfate sodium (DSS)‐Induced colitis in mice. Current Protocols Immunology 4, 104 (2014).
Eichele, D. D. & Kharbanda, K. K. Dextran sodium sulfate colitis murine model: an indispensable tool for advancing our Understanding of inflammatory bowel diseases pathogenesis. WJG 23, 6016–6029 (2017).
Han, Y. et al. Cortical inflammation is increased in a DSS-Induced colitis mouse model. Neurosci. Bull. 34, 1058–1066 (2018).
Wager-Miller, J., Green, M., Shafique, M., Mackie, K. & H. & Collection of frozen rodent brain regions for downstream analyses. J. Visualized Experiments. https://doi.org/10.3791/60474-v (2020).
Paxinos, G. & Franklin, K. B. J. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates. (2013).
Akbarian, S. & Huang, H. S. Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain Res. Rev. 52, 293–304 (2006).
Antonucci, F. et al. SNAP-25, a known presynaptic protein with emerging postsynaptic functions. Front Synaptic Neurosci 8, 23 (2016).
Brandes, M. S., Zweig, J. A., Tang, A. & Gray, N. E. NRF2 activation ameliorates oxidative stress and improves mitochondrial function and synaptic Plasticity, and in A53T α-Synuclein hippocampal neurons. Antioxidants 11, 26 (2021).
Filadi, R., Pendin, D. & Pizzo, P. Mitofusin 2: from functions to disease. Cell. Death Dis. 9, 330 (2018).
Herrero-Martín, M. D. et al. A new pathologic mitochondrial DNA mutation in the cytochrome oxidase subunit I (MT-CO1). Hum. Mutat. 29, E112–E122 (2008).
Keith, D. Excitation control: balancing PSD-95 function at the synapse. Front Mol. Neurosci 28(1), 4 (2008).
Kovács, K. J. Invited review c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem. Int. 33, 287–297 (1998).
Meng, Q. et al. GPx1 is involved in the induction of protective autophagy in pancreatic cancer cells in response to glucose deprivation. Cell. Death Dis. 9, 1187 (2018).
Sbai, O. et al. Is Drp1 a link between mitochondrial dysfunction and inflammation in alzheimer’s disease? Front. Mol. Neurosci. 16, 1166879 (2023).
Giménez-Palomo, A. et al. The role of mitochondria in mood disorders: from physiology to pathophysiology and to treatment. Front. Psychiatry. 12, 546801–546801 (2021).
Liu, L. et al. Mitochondria-wide association study observed significant interactions of mitochondrial respiratory and the inflammatory in the development of anxiety and depression. Translational Psychiatry. 13, 216–216 (2023).
Picard, M. & S McEwen, B. Mitochondria impact brain function and cognition. Proc. Natl. Acad. Sci. 111, 7–8 (2014).
Khacho, M. et al. Mitochondrial dysfunction underlies cognitive defects as a result of neural stem cell depletion and impaired neurogenesis. Hum. Mol. Genet. 26, 3327–3341 (2017).
Brown, G. C. & Borutaite, V. Inhibition of mitochondrial respiratory complex I by nitric oxide, peroxynitrite and S-nitrosothiols. Biochim. Et Biophys. Acta (BBA) - Bioenergetics. 1658, 44–49 (2004).
Al-Mehdi, A. B. et al. Perinuclear mitochondrial clustering creates an Oxidant-Rich nuclear domain required for Hypoxia-Induced transcription. Sci. Signal. 5, ra47–ra47 (2012).
Boyapati, R. K. et al. Mitochondrial DNA is a Pro-Inflammatory Damage-Associated molecular pattern released during active IBD. Inflamm. Bowel Dis. 24, 2113–2122 (2018).
Irazoki, A. et al. Disruption of mitochondrial dynamics triggers muscle inflammation through interorganellar contacts and mitochondrial DNA mislocation. Nat. Commun. 14, 108–108 (2023).
Lee, K., Kumazoe, M., Marugame, Y., Fujimura, Y. & Tachibana, H. Dextran sulfate sodium-induced mild chronic colitis induced cognitive impairment accompanied by Inhibition of neuronal maturation in adolescent mice. Biochem. Biophys. Res. Commun. 669, 46–53 (2023).
Reichmann, F., Painsipp, E. & Holzer, P. Environmental enrichment and gut inflammation modify Stress-Induced c-Fos expression in the mouse corticolimbic system. PLoS ONE. 8, e54811 (2013).
Sroor, H. M. et al. Experimental colitis reduces microglial cell activation in the mouse brain without affecting microglial cell numbers. Sci. Rep. 9, 20217 (2019).
Sharma, N. et al. Chronic DSS-Induced colitis exacerbates parkinson’s disease phenotype and its pathological features following intragastric rotenone exposure. ACS Pharmacol. Transl Sci. 8, 346–367 (2025).
Yang, M. et al. Mild Chronic Colitis Exacerbates Intracerebral Inflammation in Mice with Parkinson’s Disease Through LRRK2-Mediated Regulation of NF-κB Activation and Inhibition of Nrf2. JIR Volume 18, 8493–8507 (2025).
Rak, M. et al. Mitochondrial cytochrome c oxidase deficiency. Clin. Sci. 130, 393–407 (2016).
Nagai, N. et al. Changes in mitochondrial cytochrome c oxidase mRNA levels with cataract severity in lens epithelia of Japanese patients. Mol. Med. Rep. 19, 5464–5472 (2019).
Holvoet, P. et al. Low cytochrome oxidase 1 links mitochondrial dysfunction to atherosclerosis in mice and pigs. PLOS ONE. 12, e0170307–e0170307 (2017).
Wang, N., Wang, X., Lan, B., Gao, Y. & Cai, Y. DRP1, fission and apoptosis. Cell. Death Discov. 11, 150 (2025).
Park, S. et al. Defective mitochondrial fission augments NLRP3 inflammasome activation. Sci. Rep. 5, 15489–15489 (2015).
Gadotti, V. M. et al. Neuroimmune Responses Mediate Depression-Related Behaviors following Acute Colitis. iScience 16, 12–21 (2019).
Andersen, J. V., Jakobsen, E., Waagepetersen, H. S. & Aldana, B. I. Distinct differences in rates of oxygen consumption and ATP synthesis of regionally isolated non-synaptic mouse brain mitochondria. J. Neurosci. Res. 97, 961–974 (2019).
Brustovetsky, N. et al. Increased susceptibility of striatal mitochondria to Calcium-Induced permeability transition. J. Neurosci. 23, 4858–4867 (2003).
Pickrell, A. M., Fukui, H., Wang, X., Pinto, M. & Moraes, C. T. The striatum is highly susceptible to mitochondrial oxidative phosphorylation dysfunctions. J. Neurosci. 31, 9895–9904 (2011).
Hilel, A. S. et al. Dextran sulphate of Sodium-induced colitis in mice: antihyperalgesic effects of ethanolic extract of citrus reticulata and potential damage to the central nervous system. An. Acad. Bras. Cienc. 90, 3139–3145 (2018).
Checa, J. & Aran, J. M. Reactive oxygen species: drivers of physiological and pathological processes. JIR Volume. 13, 1057–1073 (2020).
Schieber, M. & Chandel, N. S. ROS function in redox signaling and oxidative stress. Curr. Biol. 24, R453–R462 (2014).
Rolfe, D. F. & Brown, G. C. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 77, 731–758 (1997).
Cobley, J. N., Fiorello, M. L. & Bailey, D. M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 15, 490–503 (2018).
Wang, L. et al. Glucose transporter 1 critically controls microglial activation through facilitating Glycolysis. Mol. Neurodegeneration. 14, 2–2 (2019).
Haroon, E., Miller, A. H. & Sanacora, G. Inflammation, Glutamate, and glia: A trio of trouble in mood disorders. Neuropsychopharmacology 42, 193–215 (2017).
Black, C. N., Bot, M., Scheffer, P. G., Cuijpers, P. & Penninx, B. W. J. H. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology 51, 164–175 (2015).
Bhatt, S., Nagappa, A. N. & Patil, C. R. Role of oxidative stress in depression. Drug Discovery Today. 25, 1270–1276 (2020).
Hovatta, I., Juhila, J. & Donner, J. Oxidative stress in anxiety and comorbid disorders. Neurosci. Res. 68, 261–275 (2010).
Che, Y. et al. Chronic unpredictable stress impairs endogenous antioxidant defense in rat brain. Neurosci. Lett. 584, 208–213 (2015).
Ansari, M. A., Roberts, K. N. & Scheff, S. W. Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free Radic. Biol. Med. 45, 443–452 (2008).
Zeng, T. et al. Nrf2 regulates iron-dependent hippocampal synapses and functional connectivity damage in depression. J. Neuroinflamm. 20, 212–212 (2023).
Bartsch, T. et al. Selective neuronal vulnerability of human hippocampal CA1 neurons: lesion Evolution, Temporal Course, and pattern of hippocampal damage in Diffusion-Weighted MR imaging. J. Cereb. Blood Flow. Metabolism. 35, 1836–1845 (2015).
Kirino, T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 239, 57–69 (1982).
Pulsinelli, W. A., Brierley, J. B. & Plum, F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann. Neurol. 11, 491–498 (1982).
Tabuchi, E. et al. Hippocampal neuronal damage after transient forebrain ischemia in monkeys. Brain Res. Bull. 29, 685–690 (1992).
Wang, X. & Michaelis, E. K. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci. 2, 12–12 (2010).
Wang, X. et al. High intrinsic oxidative stress May underlie selective vulnerability of the hippocampal CA1 region. Mol. Brain Res. 140, 120–126 (2005).
Craig, C. F. et al. Neuroinflammation as an etiological trigger for depression comorbid with inflammatory bowel disease. J. Neuroinflammation. 19, 4 (2022).
Möhler, H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology 62, 42–53 (2012).
Nuss, P. Anxiety disorders and GABA neurotransmission: a disturbance of modulation. Neuropsychiatr. Dis. Treat. 11, 165–175 (2015).
Lang, L. et al. GABA-mediated activated microglia induce neuroinflammation in the hippocampus of mice following cold exposure through the NLRP3 inflammasome and NF-κB signaling pathways. Int. Immunopharmacol. 89, 106908–106908 (2020).
Nascimento, T. S. et al. Chronic Methylmercury intoxication induces systemic Inflammation, Behavioral, and hippocampal amino acid changes in C57BL6J adult mice. Int. J. Mol. Sci. 23, 13837–13837 (2022).
Falkenberg, T. et al. GABA release and GAD67 mRNA expression in rat hippocampus following entorhinal cortex activation. Mol. Brain Res. 48, 413–416 (1997).
Hsieh, H. et al. Fecal Lipocalin-2 as a sensitive and noninvasive biomarker in the TNBS crohn’s inflammatory bowel disease model. Toxicol. Pathol. 44, 1084–1094 (2016).
Buisson, A. et al. Sa1216 faecal lipocalin-2 as biomarker in the evaluation of Crohn’s disease activity. Gastroenterology 146, S-233 (2014).
Shao, F. et al. Anxiolytic effect of GABAergic neurons in the anterior cingulate cortex in a rat model of chronic inflammatory pain. Mol. Brain. 14, 139–139 (2021).
Lapointe, T. K. et al. TRPV1 sensitization mediates postinflammatory visceral pain following acute colitis. Am. J. Physiology-Gastrointestinal Liver Physiol. 309, G87–G99 (2015).
Defaye, M. et al. Gut-innervating TRPV1 + Neurons drive chronic visceral pain via microglial P2Y12 receptor. Cell. Mol. Gastroenterol. Hepatol. 13, 977–999 (2022).
Esquerre, N. et al. Colitis-Induced microbial perturbation promotes postinflammatory visceral hypersensitivity. Cell. Mol. Gastroenterol. Hepatol. 10, 225–244 (2020).
Li, A. et al. PSD-95 in the anterior cingulate cortex contributes to neuropathic pain by interdependent activation with NR2B. Sci. Rep. 12, 17114–17114 (2022).
Metz, A. E., Yau, H. J., Centeno, M. V., Apkarian, A. V. & Martina, M. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc. Natl. Acad. Sci. U S A. 106, 2423–2428 (2009).
Shi, H. J., Wang, S., Wang, X. P., Zhang, R. X. & Zhu, L. J. Hippocampus: Molecular, Cellular, and circuit features in anxiety. Neurosci. Bull. 39, 1009–1026 (2023).
Acknowledgements
We are grateful for Natural Sciences and Engineering Research Council (CEM; PDF, AJG and RJS, discovery grant), and Canada Research Chairs program and Genome Canada BioNet grant (AZ) for financial support of this work. We also extend our gratitude to the Alberta Children’s Research Institute, Azrieli Foundation, and Hotchkiss Brain Institute (AJG) for their contribution to this work.
Funding
AJG and RJS received an NSERC Discovery Grant from the National Science and Engineering Research Council. AJG is also supported by the Alberta Children’s Research Institute, Azrieli Foundation, and Hotchkiss Brain Institute. AZ is a recipient of the Canada Research Chairs program and Genome Canada BioNet grant. CEM was supported by an NSERC PDF.
Author information
Authors and Affiliations
Contributions
CEM and AJG conceptualized the study; CEM, VL, KB, and TH collected, curated and analyzed data; CEM and AJG drafted the manuscript; all authors critically revised and approved the final manuscript for submission. AJG, AZ, and RJS obtained funding and provided oversight and supervision of the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All experiments were carried out in accordance with the guidelines of the Canadian Council of Animal Care and were approved by the University of Lethbridge Animal Care Committee (Protocol #2018).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Matisz, C., Lapointe, V., Beekman, K. et al. Chronic gut inflammation differentially modulates mitochondrial and antioxidant transcriptional programs in limbic brain structures. Sci Rep 16, 1829 (2026). https://doi.org/10.1038/s41598-025-31573-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-31573-2