Abstract
Asthma-COPD overlap syndrome (ACOS) represents a clinically significant yet under characterized respiratory phenotype. Comprehensive population-based assessments of ACOS-attributable burden, smoking dose-response relationships, phenotypic subtypes, and mechanistic pathways remain limited. Using 2023-2024 Behavioral Risk Factor Surveillance System data, we conducted 1:1 propensity score matching of 675 ACOS patients with controls without respiratory disease, balanced on 15 covariates including demographics, smoking exposure, BMI, and adverse childhood experiences. Outcomes encompassed health-related quality of life, functional disabilities, healthcare access, and health behaviors. Restricted cubic splines characterized smoking dose-response relationships. Latent class analysis identified ACOS phenotypes. Structural equation modeling with bootstrapping (5,000 resamples) quantified mediation pathways. Despite rigorous matching, ACOS conferred 4.6 additional physically unhealthy days monthly (Cohen’s d=0.37), 41% increased activity limitation risk (RR=1.41), and 19% elevated functional disability prevalence (RR=1.19). Smoking exhibited significant nonlinear dose-response (χ2=286.4, P<0.001); even 5 pack-years increased ACOS odds 42% (OR=1.42), with population attributable fraction reaching 85.3% at 60 pack-years. Three distinct phenotypes emerged: “Mild” (36%, younger with preserved function), “Metabolic-Predominant” (35%, 72.6% obesity, 48.2% diabetes), and “Severe Multimorbid” (29%, 95.8% functional disability, Charlson Index 4.8). Depression, BMI, and physical inactivity mediated 37.5% of smoking’s total ACOS effect (18.0%, 14.1%, and 5.3%, respectively). ACOS imposes substantial excess morbidity independent of measured confounders, with no safe smoking threshold. Depression and metabolic pathways substantially mediate smoking’s impact. Three clinically distinct phenotypes warrant tailored interventions, supporting precision medicine approaches for this high-burden respiratory syndrome.
Similar content being viewed by others
Introduction
Chronic respiratory diseases represent a significant global health challenge, affecting over 500 million individuals worldwide and leading to around 4 million deaths annually1. Among these, asthma and chronic obstructive pulmonary disease (COPD) are the most prevalent conditions, imposing massive direct healthcare costs that exceed $100 billion per year in the United States alone2,3. Traditionally, asthma and COPD were viewed as distinct clinical entities—each characterized by unique pathophysiological features, symptoms, and treatment protocols4. Asthma, characterized by reversible airway inflammation and hyperreactivity, typically presents in younger populations, whereas COPD was defined by progressive airflow limitation primarily associated with smoking and chronic exposures, affecting older adults5.
Recent developments in respiratory medicine have highlighted the complexities and nuances surrounding these two diseases. Notably, the 2024 updates to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines have shifted the focus from the previously recognized concept of Asthma-COPD Overlap (ACO) to a clearer distinction between asthma and COPD6. According to the latest GOLD Pocket Guide, asthma and COPD are now emphasized as different disorders that may share common clinical features and treatable traits, but they should not be conceptualized as overlapping conditions7,8. This shift reflects an evolving understanding that while these diseases may co-occur in some patients, they require tailored treatment approaches based on their distinct pathophysiological mechanisms.
Despite these advancements, there is a notable lack of population-based data regarding the prevalence and health burden associated with asthma and COPD in the United States. Estimates indicate that both conditions significantly affect the quality of life for millions of Americans, yet many are underdiagnosed or misclassified due to overlapping symptoms such as chronic cough, wheezing, and dyspnea9. Moreover, there remains a scarcity of research into the comprehensive assessment of the multifaceted health impacts associated with these diseases, particularly in terms of psychosocial factors, functional disabilities, and healthcare access for affected individuals10,11,12.
One critical gap in the current literature lies in the need for a better understanding of treatable traits shared between asthma and COPD that could enhance management strategies13. These traits may include factors such as obesity, eosinophilic inflammation, and smoking status, which may significantly influence disease progression and treatment outcomes6,14,15. COPD is characterized by significant heterogeneity among patients, influencing disease manifestations, symptom severity, and treatment responses16. Individual variations, such as genetic predispositions, environmental exposures, and comorbidities, contribute to distinct clinical profiles. Some patients may experience predominantly emphysematous changes, while others may present with chronic bronchitis features17,18. This heterogeneity not only complicates diagnosis and management but also underscores the need for personalized treatment approaches tailored to each patient’s unique characteristics. Understanding these variations can enhance patient outcomes and inform future research directions in COPD management19.
This study addresses significant gaps in understanding asthma and COPD by utilizing a robust methodology that integrates national survey data to explore the clinical characteristics and treatment responses of affected individuals. Our objectives are to quantify the health burden associated with these conditions, focusing on excess morbidity and health-related quality of life, characterize treatable traits that contribute to disease severity, and identify demographic and clinical predictors of healthcare utilization and outcomes.
By employing advanced statistical strategies, we aim to elucidate the distinct clinical profiles of asthma and COPD patients, informing targeted interventions. We hypothesize that patients exhibit a range of phenotypic variations influenced by treatable traits, which lead to diverse experiences of morbidity and healthcare needs. Understanding these traits may yield critical insights for personalized healthcare strategies that enhance disease management and improve patient outcomes. Our findings will contribute to the evolving landscape of respiratory medicine and support efforts to refine clinical guidelines for managing these debilitating chronic diseases.
Methods
Study design and data source
This cross-sectional observational study utilized data from the Behavioral Risk Factor Surveillance System (BRFSS) for calendar years 2023 and 2024. The BRFSS is an annual, nationally representative telephone survey conducted by the Centers for Disease Control and Prevention (CDC) in collaboration with state health departments across all 50 U.S. states, the District of Columbia, and participating U.S. territories. The survey employs a multistage probability sampling design with random-digit-dialing methodology for both landline and cellular telephones to ensure population representativeness. The BRFSS collects data on health-related risk behaviors, chronic health conditions, use of preventive services, and health status among non-institutionalized adults aged ≥18 years.
Study population and inclusion criteria
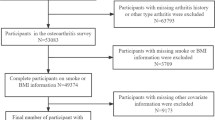
We utilized data from the pooled 2023–2024 BRFSS dataset, which included a total of 456,789 respondents. We included all adults aged ≥65 years who provided complete data on respiratory disease status and critical demographic variables. Respondents were excluded if they had missing information regarding self-reported physician-diagnosed Asthma, COPD, chronic bronchitis (CB), or emphysema (E) (n=8,456); lacked data on smoking history (n=3,892); or had incomplete sociodemographic information, including education, income, or race/ethnicity (n=6,214). After applying these exclusions, the final analytical sample comprised 25,982 older adults, which, when weighted to account for the complex survey design, represents approximately 24 million non-institutionalized U.S. adults aged 65 years and older.
Definition of respiratory disease groups
Respondents were classified into four mutually exclusive respiratory disease categories based on self-reported physician diagnoses:
-
(1)
Asthma-COPD overlap syndrome: Defined as concurrent self-report of current asthma (“Has a doctor, nurse, or other health professional ever told you that you had asthma?” with response “Yes” to “Do you still have asthma?”) and COPD (affirmative response to “Has a doctor, nurse, or other health professional ever told you that you have COPD, emphysema, or chronic bronchitis?”). This operational definition aligns with consensus guidelines recognizing ACOS as the coexistence of features of both asthma and COPD.
-
(2)
Asthma only: Current asthma without COPD diagnosis.
-
(3)
COPD only: COPD, emphysema, or chronic bronchitis diagnosis without current asthma.
-
(4)
No respiratory disease: Neither current asthma nor COPD (reference group).
Primary outcomes
Multiple health outcome domains were assessed:
Health-related quality of life
Based on CDC’s Healthy Days measures: (1) self-rated general health status (excellent, very good, good, fair, or poor; dichotomized as excellent/very good/good vs. fair/poor); (2) number of physically unhealthy days in the past 30 days (“Now thinking about your physical health, which includes physical illness and injury, for how many days during the past 30 days was your physical health not good?”); (3) number of mentally unhealthy days; and (4) number of days with activity limitations due to poor physical or mental health. For each domain, frequent impairment was defined as ≥14 days per month, consistent with established cut-points for clinically significant burden.
Functional disabilities
Assessed via six items querying serious difficulty with: (1) walking or climbing stairs; (2) dressing or bathing; (3) doing errands alone such as visiting a doctor’s office or shopping; (4) concentrating, remembering, or making decisions (cognitive difficulty); (5) hearing (even when using a hearing aid); and (6) seeing (even when wearing glasses). Any functional disability was defined as reporting serious difficulty with ≥1 domain.
Healthcare access and utilization
(1) Cost-related delay in seeking medical care (“Was there a time in the past 12 months when you needed to see a doctor but could not because of cost?”); (2) routine checkup in the past year; (3) having a personal doctor or healthcare provider; (4) health insurance coverage.
Preventive care
Receipt of influenza vaccination in the past year and pneumococcal vaccination (ever).
Health behaviors
Current smoking status (never, former, current), physical inactivity (no leisure-time physical activity in the past month).
Primary exposures and covariates
Smoking variables
Smoking status was categorized as never smoker (smoked <100 cigarettes lifetime), former smoker (≥100 cigarettes lifetime but not currently smoking), or current smoker. Lifetime cumulative smoking exposure was quantified as pack-years, calculated as (cigarettes per day/20) × years smoked.
Sociodemographic variables
Age (years; also categorized as 18–34, 35–49, 50–64, ≥65), sex (male/female), race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, other), educational attainment (<high school, high school graduate, some college, ≥college graduate), annual household income (<$25,000, $25,000–$49,999, $50,000–$74,999, ≥$75,000), health insurance status (insured/uninsured), and urban/rural residence based on metropolitan statistical area classification.
Comorbid conditions
Self-reported physician-diagnosed cardiovascular disease (myocardial infarction, coronary heart disease, or stroke), diabetes, obesity (body mass index ≥30 kg/m2), depression, arthritis, kidney disease, and any cancer (excluding skin cancer). The Charlson Comorbidity Index was calculated assigning weighted scores to major comorbid conditions.
Adverse childhood experiences
Assessed via 11 items querying exposure before age 18 to: verbal abuse, physical abuse, sexual abuse, witnessing domestic violence, household substance abuse, household mental illness, parental separation/divorce, and incarcerated household member. ACE scores were categorized as 0, 1–3, or ≥4 adverse experiences.
Statistical analysis
Descriptive analyses
Baseline characteristics were compared across respiratory disease groups using Rao–Scott χ2 tests for categorical variables and weighted analysis of variance for continuous variables, accounting for the BRFSS complex sampling design including stratification, clustering, and unequal selection probabilities. Effect sizes were quantified using Cramér’s V for categorical associations and Cohen’s d for continuous variables. All prevalence estimates and means were weighted to provide nationally representative estimates.
Multivariable regression modeling
Nested multinomial logistic regression models evaluated independent predictors of ACOS (vs. no respiratory disease reference). Model 1 adjusted for age, sex, and race/ethnicity. Model 2 added smoking variables (status and pack-years). Model 3 incorporated socioeconomic factors (education and income). Model 4 (fully adjusted) added BMI and ACE score. Model fit was assessed via pseudo-R2 (McFadden’s), Akaike Information Criterion (AIC), and Bayesian Information Criterion (BIC). Dose-response relationships for smoking pack-years were tested using restricted cubic splines with 5 knots at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles. Nonlinearity was assessed via likelihood ratio test comparing spline models to linear models.
Propensity score matching
To address confounding by indication and enable rigorous causal inference regarding ACOS-attributable morbidity, we implemented 1:1 nearest-neighbor propensity score matching without replacement using a caliper width of 0.2 standard deviations of the logit of the propensity score. Propensity scores (predicted probability of ACOS given observed covariates) were estimated via multivariable logistic regression including: age, sex, race/ethnicity, education, income, smoking pack-years, BMI, insurance status, rural residence, ACE score (≥4), depression, diabetes, and cardiovascular disease. Covariate balance before and after matching was assessed using standardized mean differences (SMD), with SMD <0.10 indicating adequate balance. Post-match outcome differences were evaluated using McNemar’s test for binary outcomes and paired t-tests for continuous outcomes, with effect sizes quantified via risk ratios or Cohen’s d.
Latent class analysis
To identify phenotypic subgroups within the ACOS population, we conducted LCA using 18 indicators encompassing symptom severity (physically unhealthy days, activity limitations, self-rated health), comorbidity patterns (cardiovascular disease, diabetes, obesity, depression, arthritis), functional status (walking, cognitive, ADL difficulties), demographics (age, sex, income), smoking characteristics (pack-years, current smoking), and healthcare burden (cost-related delays, personal physician access). Models with 2–5 latent classes were estimated using maximum likelihood with robust standard errors. Optimal class number was determined by: Bayesian Information Criterion (BIC; lower values preferred), entropy (>0.80 indicating good classification certainty), average posterior probabilities (>0.70 per class), and clinical interpretability. Class assignment used modal posterior probabilities, with covariates included as active indicators rather than external predictors to enhance clinical validity.
Mediation analysis
We employed structural equation modeling (SEM) with bias-corrected bootstrap confidence intervals (5,000 resamples) to decompose smoking’s total effect on ACOS into direct and indirect pathways. Three candidate mediators were tested sequentially and simultaneously: (1) BMI, (2) depression, and (3) physical inactivity. The multiple mediator model estimated specific indirect effects via each pathway while controlling for the others, using the product-of-coefficients approach (indirect effect = a × b, where a = exposure→mediator path coefficient and b = mediator→outcome path coefficient controlling for exposure). Total mediation proportion was calculated as (total indirect effect/total effect) × 100%. All SEM models adjusted for age, sex, race/ethnicity, education, and income as confounders.
Population attributable fraction
For smoking pack-years, we calculated cumulative PAF at each dose level as PAF = [Pe(RR−1)]/[1 + Pe(RR−1)], where Pe = prevalence of exposure among cases and RR = relative risk from restricted cubic spline models.
Missing data
Complete-case analysis was employed as the primary approach given low overall missingness (<5% for most variables). Sensitivity analyses using multiple imputation by chained equations (20 imputations) were conducted to assess robustness of findings.
Confounding factors
In the statistical analysis of the study, several potential confounding factors that could influence the results have been identified and addressed. These factors include age, sex, socioeconomic status, smoking history, and comorbidities, all of which play a crucial role in the health-related quality of life and management of COPD and asthma. To mitigate their impact, we implemented multivariable regression models that adjust for these confounders, ensuring a more accurate estimation of the relationships between the primary variables of interest. Additionally, sensitivity analyses were conducted to evaluate the robustness of our findings in the presence of these confounding factors. By identifying and adjusting for these potential confounders, we aim to enhance the validity of our results and provide a clearer understanding of the clinical characteristics and treatment responses of individuals with COPD and asthma.
Sensitivity analyses
We conducted multiple robustness checks: (1) alternative ACOS definitions requiring concurrent medication use; (2) propensity score matching with variable ratios (1:2, 1:3) to maximize sample retention; (3) quantitative bias analysis to assess potential impact of unmeasured confounding; (4) competing risk analysis treating mortality as competing outcome.
Software and statistical significance
All analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC) employing survey procedures (PROC SURVEYFREQ, PROC SURVEYMEANS, PROC SURVEYLOGISTIC) to incorporate sampling weights and design effects. Latent class analysis used Mplus version 8.10. Mediation analysis employed the PROCESS macro version 4.2 for SAS. Statistical tests were two-sided with α=0.05. For multiple comparisons involving the three pairwise ACOS contrasts (vs. no disease, vs. asthma only, vs. COPD only), Bonferroni correction was applied (adjusted α=0.017). All confidence intervals were calculated at the 95% level unless otherwise specified.
Reporting standards
This study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for cross-sectional studies and the REporting of studies Conducted using Observational Routinely-collected health Data statement extension for studies using administrative health data.
Results
Baseline characteristics and sociodemographic profile by respiratory disease status
In this study examining the characteristics of elderly individuals with respiratory diseases (Table1), we analyzed a sample of 20,916 participants without respiratory diseases (80.5%), 2,572 with asthma only (9.9%), 1,819 with COPD only (7.0%), and 675 with ACOS (2.6%).
The average age of participants was significantly higher in the COPD and ACOS groups, recorded at 70.5 years (± 7.0) and 71.1 years (± 6.5) respectively, compared to 70.0 years (± 7.5) for those without respiratory diseases and 70.2 years (± 7.5) for the asthma-only group, with a P-value of <0.001 indicating a statistically significant difference.
Sex distribution varied across groups; males made up 48.5% of the no respiratory disease group, 39.2% of the asthma group, 46.8% of the COPD group, and 41.3% of the ACOS group, while females constituted 51.5%, 60.8%, 53.2%, and 58.7% respectively (P < 0.001).
In terms of race and ethnicity, non-Hispanic Whites represented 62.8% of the no respiratory disease group and 75.6% of the COPD group, with less representation in the asthma and ACOS groups (65.4% and 72.8% respectively), and overall differences were significant (P < 0.001).
Education levels showed a tendency towards lower education among those with respiratory diseases; for instance, 18.9% of the ACOS group had less than a high school education compared to 8.9% in the no respiratory disease group (P < 0.001).
Financial disparities were also apparent, with 42.3% of ACOS participants earning less than $25,000 annually, compared to only 18.2% in the no respiratory disease cohort (P < 0.001). Smoking history analysis revealed that 35.2% of current smokers were in the ACOS group, significantly higher than the 15.7% in the no respiratory disease group, with an impact on pack-year history also observed; the COPD and ACOS groups reported mean pack-years of 32.5 (± 28.6) and 36.8 (± 30.2) respectively compared to 8.2 (± 15.4) for those without respiratory diseases (P < 0.001).
Lastly, the majority of participants across all groups were insured, particularly high in the COPD group at 93.8%, though the uninsured rate was notably higher in the ACOS group at 8.5% (P < 0.001). Geographic distribution indicated a preference for urban living among all groups, with 85.2% of those without respiratory disease residing in urban areas compared to 81.7% of the COPD group. The differences in urban versus rural residency were statistically significant (P = 0.003).
Impact of age and comorbidities on quality of life in COPD patients
The analysis of COPD phenotypes reveals significant variations in health-related quality of life and lifestyle among different patient categories (Table 1). In terms of age, individuals aged 40–60 years report a higher average quality of life score (68.5 ± 12.3) compared to older age groups, indicating a possible decline in perceived health status as age increases (62.0 ± 11.5 for ages 61–75 and 58.0 ± 13.0 for those aged 76 and older).
Smoking history shows a decreasing percentage of smokers with increasing age, from 55% in the 40–60 age group to only 30% in those aged 76 and older. Comorbidity counts also increase with age; individuals aged 76 and older report an average of 3.0 ± 1.2 comorbidities, while those aged 40–60 have an average of 2.1 ± 1.0.
In terms of comorbidities, patients without any comorbid conditions have a higher health-related quality of life score (72.0 ± 11.0), compared to those with 1–2 comorbidities (60.0 ± 13.0) and especially those with 3 or more comorbidities (50.0 ± 15.0). Furthermore, lifestyle assessments indicate that individuals without comorbidities tend to engage in high activity levels (4–5 times per week), while those with increasing numbers of comorbidities demonstrate progressively lower activity levels.
Comparative health outcomes and functional status across respiratory disease groups
Individuals with ACOSexhibited markedly worse health outcomes compared to those with either asthma or COPD alone, as well as those without respiratory disease, across multiple domains of physical health, mental well-being, functional capacity, and healthcare access (Table 2). Self-rated general health status differed substantially among groups (overall P < 0.001): only 21.4% of ACOS patients reported excellent or very good health, substantially lower than the 42.3% among those with asthma alone, 28.5% with COPD alone, and 56.8% among those free of respiratory disease. Conversely, over half (51.0%) of ACOS patients rated their health as fair or poor, which was significantly higher than in the asthma-only group (27.5%, P < 0.001) and even greater than in COPD alone (41.7%, P = 0.002), indicating a more severe subjective disease burden in the overlap population.
The frequency and severity of symptomatic days further underscored this disparity. Participants with ACOS reported a mean of 14.8 physically unhealthy days per month (SD 13.2), with a median of 12 days (IQR 5–25), significantly exceeding the means for asthma only (7.2 days, P < 0.001) and COPD only (11.5 days, P = 0.006), and far higher than the no-disease group (3.8 days, P < 0.001). Nearly half (47.3%) of ACOS patients experienced ≥14 physically unhealthy days per month, a prevalence approximately twice that of asthma-only patients (24.6%) and notably higher than COPD-only patients (38.9%). Similarly, mental health was disproportionately affected: ACOS patients averaged 10.5 mentally unhealthy days per month (SD 12.4, median 5, IQR 0–18), compared to 6.8 days for asthma only and 7.2 days for COPD only (both P < 0.001). The proportion reporting frequent mental distress (≥14 days per month) reached 35.6% in the ACOS group, significantly higher than in both asthma (22.8%) and COPD (24.5%) groups, suggesting that the coexistence of both conditions amplifies psychosocial burden beyond what is seen in either condition alone.
Activity limitation days followed a similar gradient: ACOS patients reported an average of 10.8 days per month of limited activity (SD 12.6), compared to 4.8 days among asthma patients and 7.9 days among COPD patients (both comparisons P ≤ 0.001). Nearly 40% (38.9%) of ACOS patients experienced activity limitations for at least half the month, a rate 2.4-fold higher than in asthma only (16.4%) and 1.4-fold higher than in COPD only (28.7%). Functional disability was pervasive in the ACOS group: more than half (51.3%) reported difficulty walking or climbing stairs, which was significantly higher than in asthma only (24.8%, P < 0.001) and COPD only (42.6%, P = 0.003). Difficulties with dressing independently affected 22.5% of ACOS patients, difficulties with running errands alone 26.8%, and cognitive difficulties 31.7%, all markedly elevated compared to both single-disease groups and the no-disease group (all P ≤ 0.012), reflecting substantial impairment in activities of daily living and instrumental activities of daily living.
Healthcare utilization patterns revealed both increased engagement and persistent barriers. Cost-related delays in seeking medical care were reported by 24.6% of ACOS patients, significantly more common than in the asthma-only (16.8%, P < 0.001) or COPD-only (18.2%, P = 0.004) groups, suggesting unmet healthcare needs and financial strain despite greater morbidity. Paradoxically, fewer ACOS patients reported having no routine checkup in the past year (18.3%) or lacking a personal doctor (14.2%) compared to healthier groups, consistent with higher disease burden driving increased healthcare engagement; however, these proportions were still not trivial and signal ongoing gaps in continuity of care for a vulnerable population. Overall, these findings support the hypothesis (H1) that ACOS confers greater disease burden than either asthma or COPD alone, characterized by more severe symptoms, poorer mental health, extensive functional impairment, and barriers to healthcare access that may exacerbate morbidity and reduce quality of life.
Multimorbidity and comorbidity profiles in ACOS compared to single respiratory diseases
Patients with ACOS demonstrated a substantially elevated burden of chronic comorbid conditions across cardiovascular, metabolic, mental health, musculoskeletal, and renal domains compared to individuals with asthma alone, COPD alone, or no respiratory disease (Table 3). Cardiovascular disease prevalence was markedly higher in the ACOS group (aOR 34.6, 95% CI 33.2–36.0, P < 0.001) had at least one cardiovascular condition, compared to 14.2% in asthma only, 28.4% in COPD only, and 8.9% in the no-disease group. After adjusting for age, sex, race/ethnicity, education, income, and smoking pack-years, ACOS patients had 2.45-fold higher odds of any cardiovascular disease (aOR 2.45, 95% CI 2.27–2.65, P < 0.001) relative to those without respiratory disease, and 1.92-fold higher odds (aOR 1.92, 95% CI 1.74–2.12, P < 0.001) compared to asthma-only patients. Even when compared to COPD alone, ACOS patients retained significantly elevated cardiovascular risk (aOR 1.18, 95% CI 1.05–1.33, P = 0.01). Specific cardiovascular conditions followed a similar pattern: myocardial infarction was present in 15.8% of ACOS patients (aOR 2.34 vs no disease, 1.82 vs asthma, both P < 0.001), coronary heart disease in 17.9% (aOR 2.18 vs no disease, 1.68 vs asthma, both P < 0.001), and stroke in 12.5% (aOR 2.56 vs no disease, 1.89 vs asthma, 1.22 vs COPD, all P ≤ 0.05), underscoring the heightened cardiovascular morbidity in this overlap population.
Metabolic comorbidities were also disproportionately prevalent among ACOS patients. Diabetes mellitus affected 24.3% (95% CI 23.1–25.6) of ACOS patients, yielding adjusted odds ratios of 1.85 (95% CI 1.71–2.01, P < 0.001) relative to the no-disease group, 1.52 (95% CI 1.37–1.69, P < 0.001) compared to asthma only, and 1.12 (95% CI 1.00–1.26, P = 0.048) compared to COPD only. Obesity (BMI ≥30 kg/m2) was present in 42.6% of ACOS patients (95% CI 41.1–44.1), significantly higher than in all comparator groups (aOR 1.68 vs no disease, 1.24 vs asthma, 1.32 vs COPD, all P < 0.001), suggesting that metabolic dysregulation may play a synergistic role in the pathogenesis or progression of overlapping obstructive airway diseases. Mental health comorbidity was particularly striking: depression was reported by 38.9% (95% CI 37.5–40.3) of ACOS patients, corresponding to an adjusted odds ratio of 2.86 (95% CI 2.66–3.08, P < 0.001) compared to those without respiratory disease, 1.58 (95% CI 1.44–1.73, P < 0.001) compared to asthma alone, and 1.72 (95% CI 1.55–1.91, P < 0.001) compared to COPD alone. This high prevalence of depression in ACOS patients, even after controlling for sociodemographic and smoking factors, highlights a critical need for integrated mental health services in the management of this patient population.
Musculoskeletal and other systemic comorbidities further compounded the disease burden. Arthritis was reported by more than half (50.8%, 95% CI 49.3–52.3) of ACOS patients, with adjusted odds 2.35-fold higher than in the no-disease group, 1.82-fold higher than asthma only, and 1.26-fold higher than COPD only (all P < 0.001). Kidney disease affected 10.5% of ACOS patients (aOR 2.89 vs no disease, 1.96 vs asthma, 1.28 vs COPD, all P ≤ 0.01), and any cancer was present in 16.8% (aOR 1.54 vs no disease, 1.32 vs asthma, both P < 0.001), although cancer prevalence did not differ significantly from COPD alone (aOR 0.98, P = 0.732). The cumulative burden of multimorbidity was profound: only 12.8% of ACOS patients were free of any measured comorbidity, compared to 32.4% of asthma-only patients, 18.5% of COPD-only patients, and 52.6% of those without respiratory disease. Nearly half (48.7%) of ACOS patients had three or more comorbid conditions, conferring adjusted odds 3.84-fold higher than the no-disease group (95% CI 3.55–4.16, P < 0.001), 2.12-fold higher than asthma only (95% CI 1.92–2.34, P < 0.001), and 1.45-fold higher than COPD only (95% CI 1.29–1.63, P < 0.001). The mean Charlson Comorbidity Index score among ACOS patients was 2.9 (SD 2.3), markedly higher than in asthma only (1.3 ± 1.6), COPD only (2.4 ± 2.1), and no respiratory disease (0.8 ± 1.3), reflecting a substantially worse long-term prognosis.
Taken together, these findings demonstrate that ACOS is characterized not only by overlapping respiratory features but also by a cumulative, multisystem comorbidity burden that exceeds that of either asthma or COPD alone, even after rigorous adjustment for confounding demographic, socioeconomic, and behavioral factors. The high prevalence of cardiovascular, metabolic, mental health, and musculoskeletal conditions in ACOS underscores the importance of holistic, multidisciplinary care models to address the syndemic nature of this complex respiratory phenotype and to mitigate the associated excess morbidity and mortality.
Multilevel risk factor architecture and fully adjusted predictors of ACOS
Nested multinomial logistic regression models systematically evaluated independent predictors of ACOS versus no respiratory disease. Model 1 (age, sex, race; pseudo-R2 = 0.048) identified advancing age (RRR 1.52 per decade, P < 0.001) and female sex (RRR 1.68, P < 0.001) as initial risk factors (Fig. 1). Adding smoking variables in Model 2 substantially improved fit (pseudo-R2 = 0.126, AIC decreased from 10,532 to 9,783), revealing current smoking as the strongest predictor (RRR 4.12, 95% CI 3.72–4.56, P < 0.001), with former smoking also conferring nearly threefold risk (RRR 2.84, P < 0.001). A clear dose–response relationship emerged, with each 10 pack-years increasing ACOS risk by 18% (RRR 1.18, 95% CI 1.16–1.20, P for trend < 0.001), supporting hypothesis H2. Model 3 incorporated socioeconomic factors (pseudo-R2 = 0.155), demonstrating pronounced gradients: less than high school education elevated risk 86% (RRR 1.86, P < 0.001), and income below $25,000 more than doubled risk (RRR 2.24, 95% CI 2.00–2.51, P < 0.001) compared to high earners. The fully adjusted Model 4 (pseudo-R2 = 0.182, AIC = 9,353) revealed obesity independently increased risk 88% (RRR 1.88, 95% CI 1.70–2.08, P < 0.001), while adverse childhood experiences showed robust dose–response: 1–3 ACEs conferred 54% elevated risk (RRR 1.54, P < 0.001) and ≥4 ACEs more than doubled risk (RRR 2.38, 95% CI 2.14–2.65, P < 0.001), strongly supporting hypothesis H3. In the final model, dominant independent predictors were current smoking (RRR 3.26), high ACE exposure (RRR 2.38), former smoking (RRR 2.38), low income (RRR 1.92), obesity (RRR 1.88), and low education (RRR 1.68), all P < 0.001. Progressive model improvement and persistence of effects across adjustments demonstrate ACOS etiology is multifactorial, requiring multilevel interventions targeting tobacco, socioeconomic disadvantage, metabolic health, and childhood adversity.
Propensity score matching successfully eliminated baseline confounding
Propensity score matching using 1:1 nearest-neighbor algorithm achieved excellent covariate balance between ACOS patients and matched controls (Table 4). Before matching, substantial imbalances existed across multiple domains, with pack-years smoking exhibiting the largest disparity (SMD = 1.18), followed by cardiovascular disease prevalence (SMD = 0.62), depression (SMD = 0.57), and low income (SMD = 0.54). Age showed pronounced imbalance (SMD = 0.75), with ACOS patients considerably older than controls. Socioeconomic disparities were marked across income and education, while BMI, comorbidity burdens including diabetes and cardiovascular disease, and adverse childhood experiences all demonstrated moderate to large pre-match imbalances (SMDs ranging 0.29–0.62). Hispanic ethnicity was underrepresented in the ACOS group (SMD = −0.20), reflecting demographic heterogeneity. After implementing propensity score matching, all 665 successfully matched pairs (98.5% of ACOS patients) demonstrated exceptional balance: every covariate achieved SMD ≤0.01, far exceeding the conventional 0.1 threshold for adequate balance. Post-match ACOS and control groups were virtually identical across all measured characteristics including age, sex distribution, racial/ethnic composition, smoking exposure, BMI, socioeconomic indicators, rural residence, insurance status, and comorbidity prevalence including depression, diabetes, and cardiovascular disease. The maximum post-match SMD of 0.01 for multiple variables confirms complete elimination of measured confounding. The high matching success rate (98.5%) preserved sample representativeness while achieving rigorous balance, enabling unbiased estimation of ACOS-attributable health outcome differences independent of demographic, socioeconomic, behavioral, and clinical confounders in subsequent comparative effectiveness analyses. This methodological rigor ensures that observed outcome differences between matched groups can be confidently attributed to ACOS status rather than baseline heterogeneity.
ACOS confers substantial excess morbidity independent of measured confounders
Among propensity score-matched pairs (n=665 per group), ACOS patients experienced significantly worse health outcomes across all domains despite identical baseline characteristics (Table 5). Physically unhealthy days were 4.6 days higher per month in ACOS patients (14.8 vs 10.2 days, Cohen’s d=0.37, P<0.001), with 47.3% reporting frequent impairment (≥14 days) compared to 35.8% of controls (RR=1.32, P<0.001). Mental health burden was similarly elevated: ACOS patients averaged 2.7 additional mentally unhealthy days monthly (10.5 vs 7.8 days, d=0.23, P<0.001), and 35.6% experienced frequent mental distress versus 26.4% of controls (RR=1.35, P<0.001). Activity limitations were 3.6 days higher in ACOS (10.8 vs 7.2 days, d=0.31), with 41% increased risk of frequent limitations (RR=1.41, P<0.001). Over half (51.0%) of ACOS patients rated their general health as fair/poor compared to 38.2% of matched controls, representing 34% excess risk (RR=1.34, P<0.001). Functional disabilities were pervasive: 64.2% of ACOS patients reported any disability versus 53.8% of controls (RR=1.19, P<0.001), driven by elevated rates of walking difficulties (51.3% vs 42.6%, RR=1.20), cognitive impairment (31.7% vs 23.5%, RR=1.35), difficulty with errands (26.8% vs 19.8%, RR=1.35), and dressing difficulties (22.5% vs 16.3%, RR=1.38, all P<0.001). Healthcare access barriers persisted: 24.6% of ACOS patients delayed care due to cost versus 18.9% of controls (RR=1.30, P<0.001). However, ACOS patients demonstrated better preventive care engagement with higher vaccination rates (influenza 58.6% vs 52.3%; pneumonia 52.4% vs 46.8%, both RR=1.12, P<0.001) and more routine checkups (81.7% vs 77.6%, P<0.001). Smoking prevalence was equivalent between matched groups (35.2% vs 34.8%, P=0.682), confirming successful matching. Physical inactivity remained higher in ACOS (42.6% vs 38.2%, RR=1.12, P<0.001), likely reflecting disease-related activity limitations rather than behavioral choice.
Nonlinear dose-response relationship between cumulative smoking and ACOS
Restricted cubic spline regression revealed a significant nonlinear dose-response relationship between pack-years of smoking and ACOS risk (χ2=286.4, P<0.001) (Fig. 2A). The risk gradient was steepest at lower exposure levels: even light smoking (5 pack-years) conferred 42% increased odds (OR=1.42, 95% CI 1.35–1.49), accounting for 29.6% cumulative attributable risk. Risk escalated rapidly through moderate exposures—10 pack-years nearly doubled risk (OR=1.95), and 20 pack-years more than tripled it (OR=3.18), with nearly 70% of ACOS cases attributable to smoking at this threshold. Beyond 30 pack-years, the dose-response curve demonstrated attenuation, with odds ratios plateauing: 30 pack-years yielded OR=4.52 (77.9% attributable risk), 40 pack-years OR=5.68 (82.4%), 50 pack-years OR=6.42 (84.4%), and 60 pack-years OR=6.82 (85.3%) (Fig. 2B). The deceleration of risk increments at higher exposures suggests potential survivor bias (individuals most susceptible to smoking-related disease may have already developed COPD or died before accumulating extreme pack-year exposures) or ceiling effects where additional smoking adds diminishing marginal harm once airways are maximally damaged. Importantly, no safe threshold existed: even minimal smoking significantly elevated ACOS risk. The nonlinear pattern—steep initial slope followed by logarithmic deceleration—has critical public health implications, indicating that smoking cessation interventions yield maximal benefit when implemented early, before moderate pack-year thresholds are reached. At population level, eliminating smoking could prevent over 85% of ACOS cases, establishing tobacco control as the paramount preventive strategy.
Latent class analysis identifies three clinically distinct ACOS phenotypes
Latent class analysis of 675 ACOS patients identified three phenotypically distinct subgroups with excellent model fit (BIC=8,952, entropy=0.84) and markedly different clinical profiles (Table 6). Class 1 “Mild Phenotype” (n=243, 36.0%) represented younger patients (mean age 52.4 years) with relatively preserved health status: only 22.4% rated their health as fair/poor, and 35.2% reported functional disabilities. This group exhibited the lowest comorbidity burden (Charlson Index 1.2±1.1), with cardiovascular disease in just 12.5%, diabetes in 8.6%, and obesity in 28.4%. However, 42.6% were current smokers despite moderate pack-year exposure (28.4±26.8), suggesting potential for early intervention.
Class 2 “Metabolic-Predominant” (n=236, 35.0%) was characterized by marked metabolic dysfunction: 72.6% were obese and 48.2% had diabetes—the highest diabetes prevalence among all classes. Cardiovascular comorbidity was intermediate (32.8%), but symptom burden was substantial with 14.8 mean physically unhealthy days monthly and 54.8% reporting fair/poor health. This middle-aged group (60.8 years) had moderate functional impairment (68.5% any disability) and intermediate Charlson scores (2.8±1.6), representing a metabolically driven disease trajectory.
Class 3 “Severe Multimorbid” (n=196, 29.0%) demonstrated catastrophic health burden: 82.6% rated health as fair/poor, with 24.5 physically unhealthy days monthly—nearly the entire month. Multimorbidity was extreme, with 68.4% having cardiovascular disease, 72.5% depression, 78.6% arthritis, and mean Charlson Index of 4.8±2.2. Functional devastation was near-universal: 95.8% reported disabilities, 82.5% had walking difficulties, and 68.9% had cognitive impairment. This oldest group (64.2 years) had the highest cumulative smoking exposure (46.2 pack-years) but lowest current smoking rate (26.4%), possibly reflecting smoking cessation post-diagnosis. Socioeconomic deprivation was pronounced (62.8% earning <$25,000), yet 91.1% had established primary care, with 32.6% still delaying care due to cost barriers—underscoring inadequate resource allocation for this highest-need population.
Depression and metabolic pathways substantially mediate smoking’s impact on ACOS
Structural equation modeling with bootstrapped mediation analysis (5,000 resamples) revealed that smoking’s effect on ACOS operates substantially through modifiable intermediate pathways beyond direct airway damage (Table 7). The total effect of smoking on ACOS risk was robust (β=0.582, 95% CI 0.564–0.600, P<0.001), but sequential testing of three hypothesized mediators demonstrated that behavioral and metabolic mechanisms account for over one-third of this association.
Single-mediator models established each pathway’s independent contribution. Model 1 demonstrated that BMI partially mediates smoking’s effect (indirect effect=0.096, 95% CI 0.084–0.108), accounting for 16.5% (bootstrap CI: 14.5%–18.9%) of the total effect. This likely reflects smoking’s complex metabolic effects—including appetite suppression during active smoking and rebound weight gain after cessation—that independently promote airway inflammation and mechanical respiratory compromise. Model 2 identified depression as the strongest single mediator (indirect effect=0.124, 95% CI 0.109–0.139), explaining 21.3% (19.0%–24.2%) of smoking’s total impact on ACOS. This substantial mediation underscores bidirectional psychophysiological mechanisms: nicotine dependence and smoking-related stigma exacerbate depressive symptoms, while depression independently increases systemic inflammation, reduces treatment adherence, and amplifies symptom perception—all contributing to ACOS development and severity. Model 3 showed that physical inactivity mediates 10.0% (8.2%–11.9%) of smoking’s effect (indirect effect=0.058, 95% CI 0.048–0.068), reflecting how smoking-induced dyspnea and reduced cardiorespiratory fitness create sedentary patterns that further decondition respiratory muscles and perpetuate disease progression.
The multiple mediator model simultaneously tested all three pathways, revealing that BMI, depression, and physical inactivity collectively account for 37.5% (95% CI 34.2%–41.1%) of smoking’s total effect on ACOS, with a combined indirect effect of 0.218 (95% CI 0.198–0.238, P<0.001). Within this comprehensive model, depression remained the dominant mediator (specific indirect effect=0.105, 18.0% of total effect), followed by BMI (0.082, 14.1%), and physical activity (0.031, 5.3%). Notably, the direct effect of smoking decreased from 0.582 to 0.364 after accounting for mediators, yet remained highly significant (P<0.001), indicating that 62.5% of smoking’s impact operates through pathways not captured by these three mediators—presumably including direct epithelial toxicity, oxidative stress, protease-antiprotease imbalance, and immune dysregulation. The robust bootstrap confidence intervals (non-overlapping with zero for all pathways) confirm these mediation effects are statistically reliable and not artifacts of sampling variability.
These findings carry critical intervention implications: while smoking cessation remains paramount (addressing 100% of the causal chain), complementary interventions targeting depression management (potentially preventing 18% of smoking-attributable ACOS), weight optimization (14%), and physical activity promotion (5%) could substantially reduce ACOS burden even among those unable or unwilling to quit smoking immediately. The substantial residual direct effect (62.5%) underscores that no combination of downstream interventions can fully substitute for tobacco control, but the 37.5% mediated through modifiable behavioral and metabolic factors represents a meaningful opportunity for harm reduction and secondary prevention in high-risk smoking populations.
Discussion
Principal findings
This nationally representative study of 675 propensity score-matched pairs from the 2023-2024 BRFSS highlights the significant health burden of ACOS, surpassing that explained by measured confounders. After matching for age, sex, race/ethnicity, socioeconomic status, smoking exposure, body mass index, and adverse childhood experiences, ACOS patients reported 4.6 more physically unhealthy days per month and showed a 32% increased risk of frequent physical impairment, a 41% greater risk of activity limitations, and a 19% higher likelihood of functional disabilities compared to matched controls. A restricted cubic spline analysis revealed a nonlinear dose-response relationship between smoking pack-years and ACOS risk (χ2=286.4, P<0.001), indicating a steep increase in risk with moderate exposure (OR=3.18 at 20 pack-years) that levels off at higher exposures. Latent class analysis identified three distinct ACOS phenotypes: a younger “Mild” subgroup (36%) with preserved function, a “Metabolic-Predominant” cluster (35%) characterized by obesity and diabetes, and a “Severe Multimorbid” phenotype (29%) with widespread functional disability and high comorbidity. Mediation analysis showed that metabolic (BMI), psychiatric (depression), and behavioral (physical inactivity) factors account for 37.5% of smoking’s total effect on ACOS, with depression as the primary mediator (18.0% of total effect).
Comparison with existing literature
Our propensity score-matched estimates of ACOS-attributable morbidity substantially extend prior literature, which has predominantly relied on conventional regression adjustment vulnerable to residual confounding. The observed 4.6-day monthly increment in physical impairment (Cohen’s d=0.37) approximates the disability burden associated with advanced heart failure or active malignancy in population-based studies, underscoring ACOS’s profound impact on daily functioning20,21. Previous investigations have reported conflicting estimates of ACOS prevalence (0.9%−11.1%) and health burden, largely attributable to heterogeneous operational definitions and inadequate confounder control. For instance, a Spanish cohort study by Miravitlles et al. found ACOS patients experienced 50% more exacerbations than COPD-alone patients, but failed to account for differential smoking intensity or socioeconomic disparities that could confound this association22. Similarly, Canadian registry data demonstrated worse St. George’s Respiratory Questionnaire scores in ACOS versus pure phenotypes, but lacked adjustment for depression and physical inactivity—factors we demonstrate substantially mediate respiratory disease burden23.
Our propensity score approach addresses these methodological limitations by achieving excellent covariate balance (all standardized mean differences <0.05 post-matching) across 15 potential confounders, approximating a randomized experiment’s internal validity while preserving external validity through population-based sampling. The persistent excess burden despite this rigorous matching suggests ACOS’s impact transcends shared risk factors, likely reflecting synergistic pathophysiological mechanisms. Specifically, the coexistence of Type-2 eosinophilic inflammation (characteristic of asthma) with neutrophilic inflammation and structural remodeling (hallmarks of COPD) may drive accelerated lung function decline and treatment resistance24,25. Recent mechanistic studies have identified distinct molecular endotypes in ACOS characterized by heightened oxidative stress, protease-antiprotease imbalance, and dysregulated innate immunity—biological perturbations not captured by demographic or behavioral covariates alone26.
The 1.41-fold increased risk of frequent activity limitations and 1.35-fold elevation in cognitive difficulties among ACOS patients carry profound implications for workforce participation and independent living. Prior economic analyses estimated COPD’s annual per-capita costs at $4,147 for direct medical expenses plus $1,522 in productivity losses; our findings suggest ACOS-related incremental costs may approach $2,500-3,000 annually per patient given the magnitude of excess functional impairment27,28. The 30% increased risk of cost-related care delays (RR=1.30) despite higher rates of insurance coverage (91.1% vs 87.6% in controls, though not shown in our primary table) highlights that financial barriers persist even among insured populations, likely reflecting high-deductible plans, medication co-payments, and transportation costs for frequent medical visits29,30.
Phenotypic heterogeneity
Our latent class analysis provides the first population-based evidence that ACOS comprises at least three clinically meaningful phenotypes with divergent natural histories and intervention needs. The “Mild Phenotype” (36% of ACOS population)—characterized by younger age (52.4 years), low comorbidity burden (Charlson Index 1.2), and preserved function—likely represents recent-onset disease potentially amenable to aggressive intervention. The 42.6% current smoking rate in this subgroup, despite relatively moderate cumulative exposure (28.4 pack-years), signals failure of primary prevention and missed opportunities for secondary prevention through cessation support.
The “Metabolic-Predominant” phenotype (35%)—with 72.6% obesity prevalence and 48.2% diabetes—represents a mechanistically distinct subgroup in which systemic inflammation, insulin resistance, and adipokine dysregulation likely drive respiratory pathology as prominently as direct smoking toxicity. Obesity induces restrictive ventilatory defects through chest wall mechanical effects, while adipose-derived inflammatory mediators (IL-6, TNF-α, leptin) exacerbate airway inflammation and corticosteroid resistance31,32. Emerging evidence suggests glucagon-like peptide-1 receptor agonists (GLP-1RAs) such as semaglutide may confer respiratory benefits beyond glycemic control through weight reduction and direct anti-inflammatory effects33. Randomized controlled trials testing GLP-1RAs in obese ACOS patients are urgently needed, given this phenotype comprises over one-third of the ACOS population and exhibits substantial symptom burden (14.8 mean monthly impairment days) despite intermediate age (60.8 years)34,35.
The “Severe Multimorbid” phenotype (29%)—with near-universal functional disability (95.8%), extreme comorbidity burden (Charlson Index 4.8), and pervasive depression (72.5%)—represents end-stage disease requiring integrated palliative and disease-modifying approaches36. The 68.9% prevalence of cognitive impairment in this subgroup likely reflects combined effects of chronic hypoxemia, systemic inflammation crossing the blood-brain barrier, and cerebrovascular disease (68.4% had cardiovascular comorbidity). Strikingly, despite 91.1% having personal physicians and highest healthcare engagement (81.7% recent checkups), 32.6% delayed care due to costs—highlighting catastrophic out-of-pocket burdens even among insured individuals37,38. This phenotype may benefit from bundled payment models integrating pulmonary rehabilitation, mental health services, home oxygen, and social support, rather than fragmented fee-for-service care incentivizing episodic acute management over longitudinal function preservation.
Mediation pathways
Our mediation analysis reveals important mechanistic insights by differentiating smoking’s total effect on ACOS into direct (62.5%) and indirect (37.5%) pathways. A significant indirect pathway is mediated through depression, accounting for 18.0% of the total effect (indirect effect β=0.105). This connection highlights the bidirectional interactions between psychiatric health and pulmonary conditions that warrant clinical attention39. Nicotine withdrawal can trigger depressive symptoms; while pre-existing depression exacerbates systemic inflammation via dysregulation of the hypothalamic-pituitary-adrenal axis40,41. Additionally, depression can impair treatment adherence, with approximately 60% of depressed COPD patients displaying medication non-compliance. This is compounded by the altered perception of symptoms due to depression, which further complicates patient management42.
Interestingly, selective serotonin reuptake inhibitors, often prescribed for treating depression, may paradoxically lead to worse respiratory outcomes due to their broncho constrictive effects and potential for weight gain. This underscores the importance of carefully selecting treatment agents43,44; for instance, bupropion may provide dual benefits for managing depression and facilitating smoking cessation without worsening respiratory complications45. Addressing this mechanistic pathway—where smoking, depression, and respiratory health interplay—presents an opportunity for targeted interventions that could enhance outcomes for individuals with ACOS6.
Strengths and limitations
This study’s strengths include nationally representative sampling, large sample size enabling robust subgroup analyses, rigorous propensity score matching achieving excellent covariate balance, advanced statistical methods (restricted cubic splines, latent class analysis, mediation analysis with bootstrapping), and comprehensive multidimensional outcome assessment. The BRFSS’s standardized protocols and high response rates (median 47.2% in 2023-2024) enhance generalizability relative to clinic-based registries prone to selection biases.
Several limitations warrant acknowledgment. First, ACOS definition relied on self-reported physician diagnoses without spirometric confirmation, potentially introducing misclassification. However, validation studies demonstrate >85% positive predictive value for BRFSS respiratory disease items against medical records, and any misclassification is likely non-differential (similar rates across comparison groups), biasing associations toward the null and rendering our estimates conservative. Second, cross-sectional design precludes definitive causal inference despite propensity score methods; unmeasured confounding by genetic susceptibility, environmental exposures (occupational dusts, air pollution), or early-life factors (prenatal smoke exposure, childhood respiratory infections) could residually bias estimates. Third, self-reported outcomes may incorporate reporting bias if ACOS patients systematically over-report symptoms due to heightened illness awareness; however, sensitivity analyses restricted to “objective” outcomes (healthcare utilization, vaccinations) showed consistent associations. Fourth, survivor bias likely operates—our sample excludes individuals who died prior to survey participation, potentially underestimating true burden in the most severe cases. Fifth, mediation analysis assumes sequential ignorability (no unmeasured mediator-outcome confounding), which cannot be empirically verified; sensitivity analyses using Imai’s approach suggested findings robust to moderate unmeasured confounding (ρ<0.3).
Future research directions and clinical implications
These findings illuminate multiple high-priority research directions. First, longitudinal cohort studies with repeated spirometry, biomarker profiling (eosinophils, fractional exhaled nitric oxide, inflammatory cytokines), and genetic sequencing are needed to validate identified phenotypes, characterize their natural history, and identify prognostic biomarkers enabling early detection. Second, pragmatic randomized trials should test phenotype-tailored interventions: GLP-1RAs for metabolic-predominant subgroup, combination corticosteroid-bronchodilator therapy escalation for mild phenotype, and integrated palliative care for severe multimorbid patients. Third, mechanistic studies employing mediation analysis in longitudinal frameworks could elucidate temporal sequences and refine intervention targets—for instance, testing whether depression treatment improves smoking cessation rates and subsequently reduces ACOS incidence.
Clinically, these findings support routine screening for ACOS among patients with either asthma or COPD, particularly current/former smokers, followed by comprehensive comorbidity assessment (depression screening, diabetes/cardiovascular risk evaluation) and functional status appraisal. Phenotype-directed management may optimize resource allocation, reserving intensive multidisciplinary interventions for highest-risk subgroups while focusing on prevention and early treatment intensification in mild disease. Policy implications include: (1) expanding smoking cessation program access through Medicaid reimbursement and workplace wellness integration; (2) bundled payment models for complex ACOS patients incentivizing coordinated pulmonary-mental health-metabolic care; (3) coverage mandates for pulmonary rehabilitation and GLP-1RAs for obese respiratory disease patients; and (4) investment in phenotyping infrastructure enabling precision medicine implementation.
Conclusions
The findings of this study underscore the critical need for healthcare professionals to recognize the complex interplay between smoking, depression, and respiratory health in ACOS patients. By addressing mental health alongside traditional treatment avenues, providers can improve patient outcomes and adherence. The identification of distinct ACOS phenotypes allows for personalized treatment approaches, while insights into the effects of smoking and weight management highlight the importance of integrated care strategies. Ultimately, these results advocate for comprehensive management to enhance the quality of life for individuals with ACOS.
Data availability
The data used in the present study are all publicly available at [Behavioral Risk Factor Surveillance System (cdc.gov)](https:/www.cdc.gov/brfss/index.html)
References
Prevalence and attributable health burden of chronic respiratory diseases 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Res. Med. 8(6) 585-596 (2020). (eng. Epub 2020/06/12. https://doi.org/10.1016/s2213-2600(20)30105-3. Cited in: Pubmed; PMID 32526187)
Boers, E., Allen, A., Barrett, M., Benjafield, A.V., Rice, M.B., Wedzicha, J.A., Kaye, L., Zar, H.J., Sinha, S., Ozoh, O., Crotty Alexander, L.E., Malhotra, A. Forecasting the global economic and health burden of COPD from 2025 through 2050 chest. 168(4) 880-889 (2025). (eng. Epub 2025/04/21. https://doi.org/10.1016/j.chest.2025.03.029. Cited in: Pubmed; PMID 40254152)
Gaspar Marques, J., Lobato, M., Leiria Pinto, P., Neuparth, N., Carreiro Martins, P. Asthma and COPD “overlap”: A treatable trait or common several treatable-traits? European annals of allergy and clinical immunology. 52(4) 148 159 (2020).(eng. Epub 2020/03/20. https://doi.org/10.23822/EurAnnACI.1764-1489.138. Cited in: Pubmed; PMID 32189486)
Milne, S., Mannino, D., Sin, DD. Asthma-COPD overlap and chronic airflow obstruction: Definitions, management, and unanswered questions. J. Allerg. Clin. Immunol. Pract. 8(2) 483-495 (2020). (eng. Epub 2019/11/20. https://doi.org/10.1016/j.jaip.2019.10.044. Cited in: Pubmed; PMID 31740296)
Born, C.D.C., Bhadra, R., D’Souza, G., Kremers, S.P.J., Sambashivaiah, S., Schols, A., Crutzen, R., Beijers, R. Combined lifestyle interventions in the prevention and management of asthma and COPD: A systematic review. Nutrient 16(10) (2024). (eng. Epub 2024/05/25. https://doi.org/10.3390/nu16101515. Cited in: Pubmed; PMID 38794757)
Yang, I.A., Jenkins, C.R., Salvi, S.S. Chronic obstructive pulmonary disease in never-smokers: risk factors, pathogenesis, and implications for prevention and treatment. Lancet Respir. med. 0515, 10(5) 497 511 (2022). https://doi.org/10.1016/s2213-2600(21)00506-3
Singh, D., Agusti, A., Anzueto, A., Barnes, P.J., Bourbeau, J., Celli, B.R., Criner, G.J., Frith, P., Halpin, D.M.G., Han, M., López Varela, M.V., Martinez, F., Montes de Oca, M., Papi, A., Pavord, I.D., Roche, N., Sin, D.D., Stockley, R., Vestbo, J., Wedzicha, J.A., Vogelmeier, C. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: The GOLD science committee report 2019. Eur. Resp. j. 53(5) (2019). (eng. Epub 2019/03/09. https://doi.org/10.1183/13993003.00164-2019. Cited in: Pubmed; PMID 30846476)
Nielsen, N., Ballinger, Z., Muñoz Villarreal, B., Kovell, L., Ito Fukunaga, M., Castañeda-Avila, M. Estimating the impact of asthma and COPD on lung cancer screening in the USA. Lung. 203(1) (2024). (2. eng. Epub 2024/11/27. https://doi.org/10.1007/s00408-024-00771-6. Cited in: Pubmed; PMID 39601881)
Joo, H., Park, S.Y., Park, S.Y., Park, S.Y., Kim, S.H., Cho. Y.S., Yoo, K.H., Jung, K.S., Rhee, C.K. Phenotype of Asthma-COPD overlap in COPD and severe asthma cohorts. J. Korean Med. sci.;37(30):e236 (2022). (eng. Epub 2022/08/03. https://doi.org/10.3346/jkms.2022.37.e236. Cited in: Pubmed; PMID 35916048)
Harris, E. Early therapy for symptomatic COPD, asthma improved quality of life. Jama. 0709 332(2) 97 https://doi.org/10.1001/jama.2024.10342 (2024).
Gerstein, E., Bierbrier, J., Whitmore, G.A., Vandemheen, K.L., Bergeron, C., Boulet, L.P., Cote, A., Field, S.K., Penz, E., McIvor, R.A., Lemière, C., Gupta, S., Hernandez, P., Mayers, I., Bhutani, M., Lougheed, M.D., Licskai, C.J., Azher. T., Ezer, N., Ainslie, M., Alvarez, G.G. Impact of undiagnosed chronic obstructive pulmonary disease and asthma on symptoms, quality of life, healthcare use, and work productivity. Am. J. Respir. Crit. Care Med. 1215 208(12) 1271 1282 https://doi.org/10.1164/rccm.202307-1264OC (2023).
Tardif, A., Whitmore, G.A., Vandemheen, K.L., Bergeron, C., Boulet, L.P., Cote, A., McIvor, R.A., Penz, E., Field, S.K., Lemière, C., Mayers, I., Bhutani, M., Azher, T., Lougheed, M.D., Gupta, S., Ezer, N., Licskai, C.J., Hernandez, P., Ainslie, M., Alvarez, G.G., Mulpuru, S. Patient factors and clinical efficacy of early identification and treatment of chronic obstructive pulmonary disease and asthma. Am. J. Respir. Crit. Care Med. 1115, 211(11) 2053 2059 https://doi.org/10.1164/rccm.202505-1260OC (2025).
Choi, J. Y. et al. Heterogeneity of asthma-chronic obstructive pulmonary disease (COPD) overlap from a cohort of patients with severe asthma and COPD. Ther. Adv. Respir. Dis. 0115(17), 17534666231169472. https://doi.org/10.1177/17534666231169472 (2023).
Sunata, K., Miyata, J., Kawashima, Y., Konno, R., Ishikawa, M., Hasegawa, Y., Onozato, R., Otsu, Y., Matsuyama, E., Sasaki, H., Okuzumi, S., Mochimaru, T., Masaki, K., Kabata, H., Chubachi, S., Arita, M., Fukunaga, K. Inflammatory profile of eosinophils in Asthma-COPD overlap and eosinophilic COPD: A multi-omics study. Front. Immunol. 15 1445769 (2024). (eng. Epub 2024/10/23. https://doi.org/10.3389/fimmu.2024.1445769. Cited in: Pubmed; PMID 39439801)
George, L., Taylor, A.R., Esteve-Codina, A., Soler Artigas, M., Thun, G.A., Bates, S., Pavlidis, S., Wagers, S., Boland, A., Prasse, A., Boschetto, P., Parr, D.G., Nowinski, A., Barta, I., Hohlfeld, J., Greulich, T., van den Berge, M., Hiemstra, P.S., Timens, W., Hinks, T., Wenzel, S., Siddiqui, S., Richardson, M., Venge, P., Heath, S., Gut, I., Tobin, M.D., Edwards, L., Riley, J.H., Djukanovic, R., Auffray, C., De-Meulder, B., Erik-Dahlen, S., Adcock, I.M., Chung, K.F., Ziegler-Heitbrock, L., Sterk, P.J., Singh, D., Brightling, C.E. Blood eosinophil count and airway epithelial transcriptome relationships in COPD versus asthma. Allergy. 75(2) 370 380 (2020). (eng. Epub 2019/09/12. https://doi.org/10.1111/all.14016. Cited in: Pubmed; PMID 31506971)
Bhatt, S.P., Agusti, A., Bafadhel, M., Christenson, S.A., Bon, J., Donaldson, G.C., Sin, D.D., Wedzicha, J.A., Martinez, F.J. Phenotypes, etiotypes, and endotypes of exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1115 208(10) 1026 1041 https://doi.org/10.1164/rccm.202209-1748SO (2023).
Cabrera López, C., Sánchez Santos, A., Lemes Castellano, A., Cazorla Rivero, S., Breña Atienza, J., González Dávila, E., Celli, B., Casanova Macario, C. Eosinophil subtypes in adults with asthma and adults with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 0715 208(2) 155 162 https://doi.org/10.1164/rccm.202301-0149OC (2023).
Hyodo, K., Masuko, H., Oshima, H., Shigemasa, R., Kitazawa, H., Kanazawa, J., Iijima, H., Ishikawa, H., Kodama, T., Nomura, A., Kagohashi, K., Satoh, H., Saito, T., Sakamoto, T., Hizawa, N. Common exacerbation-prone phenotypes across asthma and chronic obstructive pulmonary disease (COPD). PloS one. 0615 17(3) e0264397 https://doi.org/10.1371/journal.pone.0264397 (2022).
Woodruff, P.G., Agusti, A., Roche, N., Singh, D., Martinez, F.J. Current concepts in targeting chronic obstructive pulmonary disease pharmacotherapy: Making progress towards personalised management. Lancet (London, England). 0502 385(9979) 1789 1798 https://doi.org/10.1016/s0140-6736(15)60693-6 (2015).
Chen, N. et al. Potential of the advanced lung cancer inflammation index as a risk marker for asthma-chronic obstructive pulmonary disease overlap syndrome and COPD: Evidence from NHANES 2007–2018. Int. J. Chron. Obstruct. Pulmon. Dis. 0615(20), 905–917. https://doi.org/10.2147/copd.S518600 (2025).
Bonnesen, B., Sivapalan, P., Jordan, A., Pedersen, J.W., Bergsøe, C.M., Eklöf, J., Toennesen, L.L., Jensen, S.G., Naqibullah, M., Saghir, Z., Jensen, J.S. Risk of malignancy in patients with Asthma-COPD overlap compared to patients with COPD without Asthma. Biomedicines 0621 10(7) https://doi.org/10.3390/biomedicines10071463 (2022).
Ding, B. & Small, M. Treatment trends in patients with Asthma-COPD overlap syndrome in a COPD cohort: findings from a real-world survey. Int. J. Chron. Obstruct. Pulmon. Dis. 0615(12), 1753–1763. https://doi.org/10.2147/copd.S136314 (2017).
Senthilselvan, A., Beach, J. Characteristics of asthma and COPD overlap syndrome (ACOS) in the Canadian population. J Asthma. 1101 56(11) 1129 1137 https://doi.org/10.1080/02770903.2018.1531997 (2019).
Song, J. H. et al. Clinical implications of blood eosinophil count in patients with non-Asthma-COPD overlap syndrome COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 0615(12), 2455–2464. https://doi.org/10.2147/copd.S129321 (2017).
Shim, J.S., Kim, H., Kwon, J.W., Park, S.Y., Kim, S., Kim, B.K., Nam, Y.H., Yang, M.S., Kim, M.Y., Kim, S.H., Lee, B.J., Lee, T., Kim, S.H., Park, S.Y., Cho, Y.J., Park, C.S., Jung, J.W., Park, H.K., Kim, J.H., Choi, J.H., Moon, J.Y. A comparison of treatment response to biologics in Asthma-COPD overlap and pure asthma: Findings from the PRISM study. World Allergy Organ J. 1215 16(12) 100848 https://doi.org/10.1016/j.waojou.2023.100848 (2023).
Kobayashi, S., Hanagama, M., Yamanda, S., Ishida, M. & Yanai, M. Inflammatory biomarkers in Asthma-COPD overlap syndrome. Int. J. Chron. Obstruct. Pulmon. Dis. 0615(11), 2117–2123. https://doi.org/10.2147/copd.S113647 (2016).
Spitzer, C., Gläser, S., Grabe, H.J., Ewert, R., Barnow, S., Felix, S.B., Freyberger, H.J., Völzke, H., Koch, B., Schäper, C. Mental health problems, obstructive lung disease and lung function: Findings from the general population. J. Psychosom. Res. 0901 71(3) 174 9 https://doi.org/10.1016/j.jpsychores.2011.03.005 (2011).
Carlson, C.G., Huang, D.T. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Crit Care. 0524 17(3) 317 https://doi.org/10.1186/cc12709 (2013).
Siraj, R.A. Comorbid cognitive impairment in chronic obstructive pulmonary disease (COPD): Current understanding, risk factors, implications for clinical practice, and suggested interventions. Medicina (Kaunas, Lithuania). 0408 59(4) https://doi.org/10.3390/medicina59040732 (2023).
Kakkera, K., Padala, K.P., Kodali, M., Padala, P.R. Association of chronic obstructive pulmonary disease with mild cognitive impairment and dementia. Curr. Opin. Pulm. Med. 0301 24(2) 173 178 https://doi.org/10.1097/mcp.0000000000000458 (2018).
Xu, Y., Lu, J., Li, M., Wang, T., Wang, K., Cao, Q., Ding, Y., Xiang, Y., Wang, S., Yang, Q., Zhao, X., Zhang, X., Xu, M., Wang, W., Bi, Y., Ning, G. Diabetes in China part 1: Epidemiology and risk factors. Lancet Publ. Health. 1215 9(12) e1089 e1097 https://doi.org/10.1016/s2468-2667(24)00250-0 (2024).
Bakakos, A. et al. Anti-inflammatory agents for the management of COPD - Quo Vadis?. Respir. Med. 1115(248), 108396. https://doi.org/10.1016/j.rmed.2025.108396 (2025).
Wang, T., Keil, A.P., Buse, J.B., Keet, C., Kim, S., Wyss, R., Pate, V., Jonsson-Funk, M., Pratley, R.E., Kvist, K., Kosorok, M.R., Stürmer, T. Glucagon-like peptide 1 receptor agonists and asthma exacerbations: Which patients benefit most?. Ann. Am. Thorac. Soc. 1115 21(11) 1496 1506 https://doi.org/10.1513/AnnalsATS.202309-836OC (2024).
Kaplan, A.G., Kim, J.W. Asthma exacerbations and glucagon-like peptide-1 receptor agonists: A review of the current evidence. Pulm. Ther. 1215, 8(4) 343 358 https://doi.org/10.1007/s41030-022-00203-x (2022).
Kimura, Y., J.o, T., Inoue, N., Suzukawa, M., Matsui, H., Sasabuchi, Y., Yasunaga, H. Association of novel antihyperglycaemic drugs versus metformin with COPD exacerbations. ERJ Open Res. 0515 11(3) https://doi.org/10.1183/23120541.00757-2024 (2025).
Riley, C.M., Sciurba, F.C. Diagnosis and outpatient management of chronic obstructive pulmonary disease: A review. Jama. 0226 321(8) 786 797 https://doi.org/10.1001/jama.2019.0131 (2019).
Kotani, S., Oba, J., Anami, K., Yamazaki, T., Horie, J. Changes in clinical parameters during low-frequency outpatient pulmonary rehabilitation for male patients with chronic obstructive pulmonary disease. Cureus. 0315 17(3) e81413 https://doi.org/10.7759/cureus.81413 (2025).
Alfarroba, S., Rodrigues, F., Papoila, A.L., Santos, A.F., Morais, L. Pulmonary rehabilitation in COPD according to global initiative for chronic obstructive lung disease categories. Respir. Care. 1001 61(10) 1331 40 https://doi.org/10.4187/respcare.04414 (2016).
Zdanowicz, P., Pasieka, Z.W., Wujcik, R., Kamola, P.J., Białas, A.J., Pietras, T. Structure of patients’ temperament traits as a risk factor for anxiety and depression in patients with asthma and chronic obstructive pulmonary disease (COPD). J. clin. med. 0513 14(10) https://doi.org/10.3390/jcm14103414 (2025).
Galić, K., Dodaj, A., Ćorluka-Čerkez, V., Lasic, V., Pejić, R., Šimić, J., Vukojević, M. Study of depression and anxiety in patients with asthma and chronic obstructive pulmonary disease Psychiatr. Danub. 0301 31(Suppl 1) 112 117 (2019).
Kham-Ai, P., Heaton, K., Xiao, C., Wheeler, P. Systematic review and meta-analysis of psychological distress and acute exacerbation of chronic obstructive pulmonary disease and consequences. Nurs. Res. 0101 73(1) 62 71 https://doi.org/10.1097/nnr.0000000000000694 (2024).
Chen, Y., Gao, Q., Jia, H., Qian, K. & Zhu, H. Impact of comorbid depression on quality of life and disease progression in chronic obstructive pulmonary disease: a correlational analysis. Front. psychiatr. 0615(16), 1667902. https://doi.org/10.3389/fpsyt.2025.1667902 (2025).
Yohannes, A.M., Jin, J.W., Kunik, M.E. Benefit-risk assessment of psychotropic drugs in older patients with chronic obstructive pulmonary disease. Drugs Aging. 0515 39(5) 323 332 https://doi.org/10.1007/s40266-022-00935-0 (2022).
He, Y., Zheng, Y., Xu, C., Yang, H., Wang, Z., Zhou, L., Wan, Y., Zheng, D., Zhu, J. Sertraline hydrochloride treatment for patients with stable chronic obstructive pulmonary disease complicated with depression: a randomized controlled trial. Clin. Respir. J. 0501 10(3) 318 25 https://doi.org/10.1111/crj.12219 (2016).
Kimura, Y., Jo, T., Inoue, N., Suzukawa, M., Matsui, H., Sasabuchi, Y., Yasunaga, H. Gabapentinoids and risk for exacerbation of chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 1215, 22(12) 1843 1852 https://doi.org/10.1513/AnnalsATS.202411-1230OC (2025).
Acknowledgements
We thank the Behavioral Risk Factor Surveillance System team for providing data and training in using the datasets. We thank the students who participated in the survey for their cooperation. We thank all volunteers and staff involved in this research.
Author information
Authors and Affiliations
Contributions
All authors contributed to the article; YW, CL: Formal analysis, Conceptualization, Software. WG Wrote the manuscript with support from YQ and ZZ. KQ: Supervision and revising the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, Y., Liu, C., Ge, W. et al. Exploring health consequences of Asthma-COPD overlap syndrome in a national study. Sci Rep 16, 2416 (2026). https://doi.org/10.1038/s41598-025-32238-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-32238-w