Abstract
Male patients undergoing urologic surgery under general anesthesia are more prone to developing catheter-related bladder discomfort (CRBD). Transcutaneous tibial nerve stimulation (TTNS) is an established intervention for lower urinary tract dysfunction. This study aimed to evaluate the impact of TTNS on the incidence of moderate-to-severe CRBD in male patients following urological surgery under general anesthesia. 124 male patients scheduled for urologic surgery from December 2023 to June 2024 were included and randomized to the TTNS or Control groups via stratified block group randomization. The TTNS group received 30 min of TTNS stimulation (200-μs pulses, 20/100 Hz alternating sparse-dense waves) in the post-anesthesia care unit, while the Control group received 30 min of sham stimulation. The degree of CRBD and VAS scores at 0 h, 1 h, 2 h, and 6 h after tracheal extubation, the Quality of Recovery-15 (QoR-15) score at 24 h postoperatively, length of hospitalization, rescue medication rates, and the incidence of adverse reactions were compared between the two groups. Compared with the Control group, a significant reduction was observed in the incidence of moderate-to-severe CRBD immediately after tracheal extubation (0 h) in the TTNS group [P = 0.002, CI 0.190–0.741, RR 0.375]. The incidence of moderate-to-severe CRBD in the TTNS group was significantly lower than that in the Control group at 1 and 2 h after extubation (P < 0.001, P = 0.002). Furthermore, the severity of CRBD at all time points was significantly lower than that in the TTNS group (P = 0.017, P < 0.001, P < 0.001, and P < 0.001, respectively). The VAS scores of patients in the TTNS group were notably lower than those in the Control group at 0, 1, and 2 h after tracheal extubation (P = 0.009, P = 0.012, P = 0.013, respectively). The QoR-15 scores at 24 h post-surgery in the TTNS group were significantly higher (P < 0.001). The incidence rates of postoperative nausea and medication rescue were lower in the TTNS group than in the Control group (P = 0.006, P < 0.001). Finally, the TTNS intervention was not associated with any adverse effects. TTNS effectively reduces the incidence of moderate-to-severe CRBD, enhances early postoperative analgesia, and improves recovery quality in male patients undergoing urologic surgery without significant safety concerns.
Trial registration: This study was retrospectively registered and reviewed by a principal investigator (Xiangying Zheng) in the Chinese Clinical Trials Registry (registration number: ChiCTR2300078536) on December 12, 2023.
Similar content being viewed by others
Introduction
Catheter-related bladder discomfort (CRBD) is a common adverse reaction following surgical procedures that require an indwelling catheter during or after the procedure1. CRBD occurs in 47–90% of patients after general anesthesia, and 44–67% of patients typically develop moderate-to-severe CRBD2,3,4. CRBD tends to trigger emergence agitation, which can result in pain and anxiety, an increased risk of postoperative complications, and prolonged hospitalization5,6. Currently, no optimal clinical solution for CRBD exists, and available medications are ineffective in preventing and treating CRBD, resulting in a high incidence of side effects such as dry mouth, nausea, and sedation2. While nerve blocks offer an alternative, they are invasive, technically complex, and carry risks of complications, including infection, nerve damage, and anaphylaxis to anesthetics7. Therefore, this study aims to explore a safe, effective, and simple new approach to managing CRBD.
Transcutaneous tibial nerve stimulation (TTNS) is a minimally invasive, safe, and well-tolerated neuromodulation technique for lower urinary tract dysfunction8. It has been shown to treat overactive bladder (OAB) by stimulating the tibial nerve to achieve a limiting effect on the overactivity of the detrusor muscle, relieving urinary frequency and urgency9,10,11,12. The symptoms of CRBD are similar to those of OAB13. Based on this mechanistic similarity, we hypothesize that TTNS may reduce the incidence of CRBD. However, this potential application remains unexplored in clinical studies.
In the present study, we sought to evaluate the preventive effect of TTNS on postoperative CRBD in patients who underwent urological surgery under general anesthesia.
Methods
This prospective, randomized controlled trial was conducted in a 1:1 allocation ratio between the control and TTNS groups, adhering to the CONSORT guidelines. The study was approved (approval number: KJ2023-437-01) by the Science and Technology Ethics Committee of the First Affiliated Hospital of Shihezi University and registered in the China Clinical Trial Registry (registration number: ChiCTR2300078536) by the primary investigator (Zheng Xiangying) on December 12, 2023. The methodology presented in the paper is consistent with the trial registration. Prior to their involvement in the study, written informed consent was obtained from all patients.
Study population
The patients were recruited at the First Affiliated Hospital of Shihezi University between December 2023 to June 2024. The inclusion criteria were as follows: male patients aged 18–70 years; an American Society of Anesthesiologists (ASA) physical status of I–III; scheduled for elective urological surgery under general anesthesia with intraoperative catheterization; and provision of written informed consent. The exclusion criteria were combined cardiac pacemaker implantation, severe arrhythmia, epilepsy and other contraindications to transcutaneous electrical stimulation. Besides, patients with a history of severe cardiovascular, renal, or liver diseases or cerebrovascular accidents, were excluded. An infection at the skin patch site, or a previous surgical scar or a history of an indwelling catheter. Trial termination criteria included: patient withdrawal without cause, post-enrollment discovery of exclusion criteria, or the occurrence of intraoperative adverse events such as significant hypertension, significant arrhythmia, or other patient discomfort.
Randomization, concealment, and blinding
In this study, stratified block randomization was employed. Initially, eligible participants were stratified into three categories based on the type of procedure: laparoscopic, transurethral, and percutaneous nephrolithotomy. These participants were then numbered by age in ascending order. Random numbers between 0 and 1 were then generated by an independent researcher through SPSS 22.0 software, with block size set to 4. The random numbers within the zones were ranked within SPSS, with the first half assigned to the Control group and the second half to the TTNS group. The allocation scheme was sealed in opaque, sequentially numbered envelopes. After patients were enrolled, the researcher (who was not involved in the intervention) opened the envelopes at the post-anesthesia care unit (PACU) to reveal the grouping assignment and then performed the intervention. The assessors were two experienced physicians who were blinded to the group assignments and were not involved in the treatment of either group. Data statistics were also obtained from personnel not involved in the study to reduce experimental bias and increase the power of the results. Furthermore, blinded outcome assessment was implemented for at least 10% of the cases to ensure that the assignment was correct. The stimulation intensity for the experimental intervention was individualized preoperatively to a level that caused visible muscle contraction without pain. The control group underwent an identical setup, with the stimulation circuit connected but no current delivered.
Anesthetic management
Upon arrival in the operating room, all patients underwent standard general anesthesia. Intravenous access was established, and noninvasive blood pressure (BP), heart rate (HR), saturation of peripheral oxygen (SpO2), electrocardiogram (EGG), and bispectral index (BIS) were monitored. Besides, oxygen was administered via a face mask with an oxygen flow rate of 5 L/min. Anesthesia was induced with 0.05 mg/kg midazolam, 0.25 mg/kg etomidate, 0.6 mg/kg rocuronium, and 0.5 µg/kg sufentanil. After intubation, the patient was mechanically ventilated. The respiratory parameters were adjusted to inspired oxygen flow rates of 1–2 L/min, tidal volumes of 6–8 mL/kg, respiratory rates of 12–14 times/min, end-tidal carbon dioxide concentrations of 35–45 mmHg, and peak airway pressures of no more than 30 cmH2O. Anesthesia was maintained with 4–12 mg/kg h propofol and 0.15–0.2 µg/kg·min remifentanil via a continuous intravenous pump. Neuromuscular blockade was monitored when the BIS value was < 70, the BIS value was maintained between 40 and 60 during the operation, and intermittent administration of rocuronium maintained a train-of-four (TOF) counts ≤ 2. Catheterization was performed under anesthesia, and noninvasive blood pressure and heart rate were maintained at baseline ± 20% by adjusting the infusion speed and using vasoactive drugs. At the end of the procedure, intravenous anesthetic drugs were stopped, the patient was admitted to the PACU for close observation, and sugammadex 2 mg/kg was administered to reverse neuromuscular blockade. After the patient’s level of consciousness and neuromuscular function (BIS > 90 and TOF ratio ≥ 0.9) were evaluated, extubation was conducted.
Transcutaneous tibial nerve stimulation
Before surgery, the patients were educated on the symptoms of CRBD, such as urinary urgency, pain or burning sensation in the urethra or suprapubic region. Patients in the TTNS group were familiarized with the target stimulation intensity (muscle contraction without pain). In the PACU, electrodes were placed on the tibial nerve region for all patients in both groups. The site was identified as approximately 3–4 cm proximal to the medial malleolus, posterior to the tibia. The TTNS group was subjected to TTNS stimulation for 30 min, with parameters set as follows: pulse 200 µs, frequency 20/100 Hz alternating sparse-dense waves, and the magnitude of the current that caused muscle contraction without pain. The control group underwent a sham procedure for 30 min, and the current circuit was normally connected, but no electrical stimulation was given. For postoperative analgesia, tramadol was used as a rescue medication. Tramadol was administered at a dose of 1 mg/kg if patients reported moderate-to-severe CRBD or a visual analog scale (VAS) pain score of 4 or more at the surgical site.
Outcome variables
Patient demographic and clinical characteristics were recorded, including age, body mass index (BMI), ASA classification and several comorbidities, such as hypertension and diabetes mellitus. The variables related to surgery and anesthesia included the duration of anesthesia, duration of surgery, size of catheter inserted and postoperative use of the analgesic pump.
CRBD severity was assessed at 0, 1, 2, and 6 h after tracheal extubation. The initial assessment was performed immediately after the patient was extubated to determine the classification of CRBD at 0 h after extubation. The severity of CRBD was categorized as follows: “none” if the patient did not report CRBD even when asked, “mild” if only disclosure was made when asked, “moderate” if the patient voluntarily mentioned it without any concomitant behavioral response, and “severe” if the patient voluntarily mentioned it without any concomitant behavioral response. These behavioral responses included attempts to remove the catheter, strong verbal responses, and flailing limbs4,14,15. The primary endpoint of this study was defined as the occurrence of moderate or severe CRBD immediately following tracheal extubation. The secondary endpoint was the presence of moderate or severe CRBD at 1, 2, and 6 h after extubation.
The severity of pain at the surgical site was evaluated at 0, 1, 2, and 6 h after tracheal extubation using a VAS ranging from 0 to 10, with 0 representing no pain and 10 representing the maximum pain imaginable. The quality of the patient’s postoperative recovery was assessed 24 h postoperatively via the Quality of Recovery-15 (QoR-15). The length of stay from admission to postoperative discharge was determined. The number of cases of dizziness, nausea, vomiting, agitation, delirium, and the use of pharmacologic remedies were recorded for postoperative patients. The occurrence of adverse effects related to TTNS, such as arrhythmias and allergies, was assessed.
Sample size calculation
This randomized controlled study trial was designed to compare the incidence of moderate-to-severe CRBD at 0 h after tracheal extubation between two independent samples. Based on previous clinical studies, the incidence of moderate-to-severe CRBD following urological surgery was estimated to be 60%. The study hypothesized that the TTNS intervention would reduce the incidence to 30%3,4,13. With a 2-sided significance level of 0.05 and a power of 0.9, the total sample size was calculated to be 106 cases via PASS2021 software. Considering a 10% loss to follow-up rate, a total of 118 study subjects were needed, which were randomly assigned at a 1:1 ratio, with 59 study subjects assigned to each group.
Statistical analysis
Data were expressed as the means ± standard deviations, medians (lower quartiles, upper quartiles), and numbers (proportions), and relative risks (RRs) and 95% confidence intervals (95% CIs) were determined.
The Shapiro–Wilk test was used to assess whether continuous data conformed to a normal distribution. Independent t-tests were used to test for normally distributed continuous variables. The Mann–Whitney U test was used to compare nonnormally distributed continuous variables. The categorical variables were compared via the chi-square test or Fisher’s exact test, and the relationships between ordered variables between two groups were analyzed via the Mann‒Whitney U test. Where applicable, the Bonferroni correction was applied, with an adjusted significance level of P < 0.0125. For all other analyses, a P value of less than 0.05 was considered statistically significant, and a P value of less than 0.001 was deemed highly significant. The data were analyzed using the Statistical Package for the Social Sciences (SPSS, Version 27.0, IBM Corp, USA).
Results
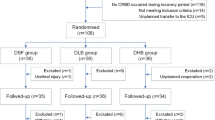
A total of 311 patients scheduled for urologic surgery were recruited for this study. Of these, 185 patients were excluded due to noncompliance with the inclusion and exclusion criteria, with the remaining 126 study subjects subsequently randomized. Following randomization, one patient was excluded due to a serious intraoperative cardiovascular event, and another was excluded due to postoperative refusal to participate in the trial. This resulted in a final sample of 62 patients in the TTNS group and 62 patients in the Control group, as illustrated in Fig. 1.
No significant differences were found in the comparative analysis of the clinical characteristics of the two groups of patients (P > 0.05), as outlined in Table 1. These characteristics included age, BMI, ASA classification, underlying disease, duration of anesthesia, duration of surgery, surgical approach, postoperative analgesic pump utilization rate, and catheter size.
As illustrated in Fig. 2, moderate to severe CRBD was observed at 0, 1, 2, and 6 h following tracheal extubation in both groups. TTNS stimulation resulted in a significant reduction in the incidence of moderate to severe CRBD at 0 h after tracheal extubation compared with the Control group [9 (14.5%) vs. 24 (38.7%), P = 0.002, 95% CI (0.190–0.741), RR 0.375]. A comparison of the incidence of moderate to severe CRBD at 1 h after extubation [2 (3.2%) vs. 18 (29.0%), P < 0.001, 95% CI (0.027–0.459), RR 0.111] revealed a significant decrease in the Control group. Similarly, a decrease in the incidence of moderate to severe CRBD at 2 h after tracheal extubation was observed [1 (1.6%) vs. 11 (17.7%), P = 0.002, 95% CI (0.012–0.683), RR 0.091]. However, the incidence of moderate or severe CRBD at 6 h following extubation between the two groups remained comparable [2 (3.2%) vs. 4 (6.5%), P = 0.403, 95% CI (0.095–2.631), RR 0.5].
The distribution of CRBD severity grades at 0, 1, 2, and 6 h following tracheal extubation in the two groups is illustrated in Fig. 3. A comparison of the two groups revealed that, in contrast to that in the Control group, the severity of CRBD at 0, 1, 2, and 6 h following tracheal extubation was significantly lower in the TTNS group. This reduction was significant at 1, 2, and 6 h (P = 0.017, P < 0.001, P < 0.001, P < 0.001, respectively).
The 0, 1, 2, and 6 h VAS scores following tracheal extubation were meticulously documented (Table 2). A comparative analysis of VAS scores revealed a decrease in pain levels in the TTNS group relative to the Control group at 0, 1, and 2 h following tracheal extubation, whereas no significant differences were observed at 6 h. The median [IQR] VAS scores were as follows: 0 h posttracheal extubation [2.05 (1.075–2.7) vs. 2.5 (1.7–2.8525), P = 0.009]; at 1 h [2.5 (1.275–2.9) vs. 2.85 (1.725–3.425), P = 0.012]; at 2 h [2.4 (1.15–2.7) vs. 3.0 (1.275–3.4), P = 0.013]; and at 6 h [2.9 (1.375–3.3) vs. 3.2 (1.275–3.625), P = 0.051].
A comprehensive evaluation was conducted to assess postoperative recovery and other pertinent aspects of the two groups (Table 2). Compared with the Control group, patients in the TTNS group exhibited significantly higher postoperative 24-h QoR-15 scores (135.44 ± 5.838 vs. 128.74 ± 7.038, P < 0.001). Furthermore, the incidence of postoperative nausea [6 (9.7%) vs. 18 (29%), P = 0.006] and the requirement for rescue medication [17 (27.4%) vs. 36 (58.1%), P < 0.001] were significantly lower than in the control group. Further analysis revealed no significant disparity in the duration of hospital stay between the two groups [9 (6–13) days vs. 10 (7–15) days, P = 0.231]. The incidence of dizziness [2 (3.2% vs. 4 (6.5%), P = 0.403], vomiting [4 (6.5%) vs. 10 (16.1%), P = 0.089], and agitation [1 (1.6% vs. 5 (8.1%), P = 0.094] did not significantly differ between the two groups. No cases of postoperative delirium or adverse events related to TTNS, such as arrhythmia, allergy, or skin discomfort at the electrode site, were observed in both groups.
A comparative analysis was conducted to ascertain the impact of the surgical approach on the incidence of moderate-to-severe CRBD following tracheal extubation (Fig. 4). The study revealed that in laparoscopic surgery, the observed difference in incidence between the TTNS group and the Control group did not reach statistical significance [3 (12.5%) vs. 5 (20.8%), P = 0.439]. However, when the transurethral and percutaneous nephrolithotomy cohorts were analyzed, significant differences were observed between the TTNS group and the Control group: transurethral [3 (15.0%) vs. 9 (45.0%), P = 0.038] and percutaneous nephrolithotomy [3 (16.7%) vs. 10 (55.6%), P = 0.015].
Discussion
In the present study, TTNS intervention was found to significantly reduce the incidence of moderate-to-severe CRBD at 0, 1, and 2 h after extubation. The intervention also provided superior early postoperative analgesia, as evidenced by significantly lower VAS scores at these time points. In addition, the VAS scores of patients in the TTNS group were significantly lower than those in the Control group at 0, 1, and 2 h after tracheal extubation, and the postoperative 24-h QoR-15 scores of patients in the TTNS group were significantly greater than those in the Control group. No adverse effects were associated with the TTNS intervention.
TTNS functions by modulation of the tibial nerve, which contains L4-S3 nerve fibers from the same segment as the S2-S4 nerve fibers that innervate the bladder16. It is well-established that the S3 nerve fibers contain sensory fibers from the pelvic floor and parasympathetic motor efferents to the detrusor muscle, as well as somatic motor fibers to the pelvic sphincter and pelvic floor muscles. These motor fibers primarily innervate the detrusor and levator muscles. The efficacy of TTNS in the treatment of lower urinary tract dysfunctions (LUTDs), such as OAB, has previously been documented17. Besides, a meta-analysis by Zifu Yu et al.18 evaluated the quality of evidence for the role of TTNS in improving maximal urethral pressure (MDP) in patients with neurogenic lower urinary tract dysfunction (NLUTD) after spinal cord injury (SCI). Randomized controlled studies by Ya-Xiong Xu et al.19 and Argyrios Stampas et al.20 reported that TTNS could treat the clinical symptoms of OAB with a favorable safety profile. Electrical stimulation of the afferent nerves of the posterior tibial nerve has been demonstrated to inhibit reflexive bladder activity. This inhibition is mediated by the suppression of afferent signaling to supraspinal centers involved in the voiding reflex and the periaqueductal gray‒pontine micturition center (PMC)21,22. Besides, electrical stimulation may directly inhibit the excitability of neurons in the sacral medullary pathway, thereby contributing to the inhibition of bladder activity23,24. Todd Yecies et al.24 also reported that these neuromodulatory effects can occur while stimulation is ongoing and continue to be suppressed after 30 min of stimulation. Research by Sibel Canbaz Kabay et al.25 and G Amarenco et al.26 found that tibial nerve stimulation could improve acute urodynamics instantly and thus be used to attenuate the onset of CRBD in the immediate postoperative period.
According to the gate control theory of pain, the application of high-frequency electrical stimulation initially activates myelinated A-β fibers. These fibers possess a lower threshold and exhibit faster conduction velocities. Besides, stimulation triggers the premature activation of glial cells, resulting in the inhibition of conduction in class C fibers27. Brian Wodlinger et al. reported that high-frequency stimulation could also reduce nociceptive sensitization for analgesia by blocking C nerve fiber activity28. Research conducted by Jason P Paquette et al.29 and Zhonghan Zhou et al.30 indicated that the activation of class C nerve fibers by low-frequency electrical stimulation could induce bladder inhibition. However, experiments by A Sato et al.31 and Michelle Yu et al.32 reported that repeated co-stimulation of both high-frequency and low-frequency tibial nerve stimulation produced superior inhibition and modulation of bladder function. Consequently, the alternating application of high- and low-frequency sparse wave stimulation of the tibial nerve may prove effective in the management of postoperative CRBD.
Recent studies have demonstrated that TENS facilitates the release of endogenous opioid peptides, reduces the level of inflammatory cytokines, and produces analgesia33,34. The activation of μ-opioid receptors is induced by low-frequency electrical stimulation, whereas the activation of δ-opioid receptors is induced by high-frequency stimulation35,36. Opioid medications, including tramadol and dizocine, have demonstrated clear efficacy in the treatment of CRBD14,37. Consequently, the potential of TTNS in mitigating the severity of CRBD is a promising avenue for further research.
The present study demonstrated that TTNS could significantly reduce the severity of postoperative CRBD in patients who underwent urologic surgery. Notably, there was no significant difference in the incidence of moderate-to-severe 6-h CRBD between the TTNS group and the Control group. However, a significant difference was observed in the overall severity of CRBD. This phenomenon can be attributed to the observed reduction in mild CRBD, resulting in a relative increase in the proportion of patients with no CRBD. Consequently, TTNS has been shown to play a beneficial role in mitigating the severity of CRBD. The attenuated efficacy at 6 h may be related to the following neurophysiological mechanisms: (1) Neural adaptation: repetitive or sustained neural stimulation may lead to a decrease in nerve fiber responsiveness, attenuating the initially potent inhibitory effect, consistent with the findings in an animal model of the spinal cord reported by Mayara Tavares Oliveira et al.38; (2) Limitations of stimulation parameters: a study by Gladys et al.39 showed that the half-life of the analgesic effect resulting from a single 40-min nerve electrical stimulation is approximately 6 h. Indeed, the selection of parameters in the present study may not have been optimal, resulting in an effect that did not last for 6 h.
The QoR-15 assessment revealed a substantial improvement in patient recovery quality following the TTNS intervention. The tibial nerve stimulation sites selected for this study (posterior to the medial ankle joint and approximately three transverse fingers above it) correspond to the Chinese medicine acupuncture points BL59 and BL60 on the Foot-Taiyang bladder meridian. The stimulation of these two points in acupuncture treatment studies has been shown to shorten the awakening time of patients, improve postoperative analgesia, and contribute to the rapid recovery of patients after surgery40. The present study revealed that the TTNS group presented reduced postoperative VAS scores and a diminished prevalence of nausea. Moreover, the QoR-15 assessment demonstrated a substantial improvement in the quality of patient recovery following TTNS intervention. This phenomenon may be attributed to a reduction in the incidence of postoperative complications, which is a hallmark of enhanced recovery. This finding is pivotal for achieving a favorable prognosis, suggesting that a reduction in CRBD severity after TTNS intervention may provide substantial benefits.
Research has demonstrated that TENS is associated with a low incidence of adverse effects41. Consistent with this, no adverse events unequivocally attributable to TTNS were observed in the present study. Consequently, 30 min of postoperative stimulation employing current sizes with pulses of 200 μs and alternating sparse-dense waves at a frequency of 20/100 Hz, designed to induce muscle contraction without pain, exhibits a good safety profile. However, the limited sample size of our trial precluded us from assessing the incidence of other serious events or generalizing to other intervention parameters.
The efficacy and safety of TTNS intervention in reducing moderate to severe CRBD following transurethral or percutaneous nephrolithotomy have been demonstrated. However, the intervention did not have a significant effect on the postoperative period after laparoscopic surgery. Using the Kruskal–Wallis rank-sum test, we compared the incidence of moderate-to-severe 0 h CRBD in the Control group for each surgical procedure separately and found that laparoscopic surgery yielded a lower incidence of moderate-to-severe CRBD at baseline compared with transurethral or percutaneous nephrolithotomy procedures (Laparoscopic vs. Transurethral = 20.8% vs. 45.0%, P = 0.090; Laparoscopic vs. Percutaneous Nephrolithotomy = 20.8% vs. 55.6%, P = 0.022). This may be due to the fact that laparoscopic surgery involves less mechanical stimulation of the bladder and urethra, a difference that may mask the effect of the TTNS intervention.
Indeed, the limitations of this study should be acknowledged. First, although the use of an alternating sparse-dense waves intervention with pulses of 200 µs and frequencies of 20/100 Hz resulted in moderate or greater reductions in CRBD, it cannot be asserted that these stimulation parameters are optimal to prevent CRBD. Besides, TTNS was conducted at the conclusion of the procedure; however, the optimal timing to prevent CRBD could not be determined. Consequently, further research is warranted to ascertain the most effective stimulation parameters and the optimal timing of TTNS intervention. Second, although sample size calculations were based on the primary outcome metrics, this may have limited the study’s sensitivity to rare safety events or minor differences in effect sizes in secondary endpoints. The generalizability of these findings is constrained by the single-center setting and the exclusive enrollment of male patients. Furthermore, the absence of long-term follow-up limits insights into the persistence of CRBD. Future studies should utilize large-scale, multicenter designs that include female participants and incorporate longitudinal assessments to validate and extend these results.
In conclusion, patients receiving TTNS presented a reduced incidence of moderate or severe CRBD following urologic surgery. Furthermore, patients who received TTNS exhibited increased postoperative analgesia and improved postoperative recovery. These findings suggest that the use of TTNS may be an effective strategy for mitigating postoperative CRBD in urologic surgery. Future studies should focus on expanding the sample size, improving the duration of intervention, and adjusting the stimulation parameters of TTNS to provide more comprehensive and reliable results.
Conclusions
The present study demonstrated that TTNS with pulses of 200 µs and alternating sparse-dense waves at a frequency of 20/100 Hz given for 30 min upon entering the recovery room can effectively reduce the occurrence of moderate-to-severe CRBD and its severity after general anesthesia in male patients with transurethral and percutaneous nephrolithotomy surgery. This approach also strengthens the effect of postoperative analgesia, reduces the occurrence of postoperative nausea, and improves the quality of postoperative recovery. Moreover, it does not increase the occurrence of postoperative adverse events.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available owing to the privacy of the patient data involved but are available from the corresponding author upon reasonable request.
Abbreviations
- CRBD:
-
Catheter-related bladder discomfort
- TTNS:
-
Transcutaneous tibial nerve stimulation
- OAB:
-
Overactive bladder
- ASA:
-
American Society of Anesthesiologists
- PACU:
-
Postanesthesia care unit
- BP:
-
Blood pressure
- HR:
-
Heart rate
- SpO2 :
-
Saturation of peripheral oxygen
- EGG:
-
Electrocardiogram
- BIS:
-
Bispectral index
- TOF:
-
Train-of-four
- VAS:
-
Visual analogue scale
- BMI:
-
Body mass index
- QoR-15:
-
Quality of recovery-15
- LUTD:
-
Lower urinary tract dysfunctions
- MDP:
-
Maximal urethral pressure
- NLUTD:
-
Neurogenic lower urinary tract dysfunction
- SCI:
-
Spinal cord injury
- PMC:
-
Periaqueductal gray-pontine micturition center
- TENS:
-
Transcutaneous electrical nerve stimulation
References
Chen, H. et al. Intravesical dexmedetomidine instillation reduces postoperative catheter-related bladder discomfort in male patients under general anesthesia: A randomized controlled study. BMC Anesthesiol. 20, 267 (2020).
Bai, Y. et al. Management of catheter-related bladder discomfort in patients who underwent elective surgery. J. Endourol. 29, 640–649 (2015).
Liang, D. et al. The effect of transcutaneous electrical acupoint stimulation on postoperative catheter-related bladder discomfort in patients undergoing transurethral resection of the prostate. Evid. Based Complement. Alternat. Med. 2021, 6691459 (2021).
Park, J.-Y. et al. Vitamin C and catheter-related bladder discomfort after transurethral resection of bladder tumor: A double-blind, randomized, placebo-controlled study. J. Clin. Anesth. 89, 111191 (2023).
Li, J. & Liao, R. Prevention of catheter-related bladder discomfort—Pudendal nerve block with ropivacaine versus intravenous tramadol: Study protocol for a randomized controlled trial. Trials 17, 448 (2016).
Bao, X. et al. The efficacy of peripheral nerve block on postoperative catheter-related bladder discomfort in males: A systematic review and meta-analysis. Front. Surg. 10, 1099628 (2023).
Li, S., Li, P., Wang, R. & Li, H. Different interventions for preventing postoperative catheter-related bladder discomfort: A systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 78, 897–906 (2022).
Agost-González, A. et al. Percutaneous versus transcutaneous electrical stimulation of the posterior tibial nerve in idiopathic overactive bladder syndrome with urinary incontinence in adults: A systematic review. Healthcare (Basel) 9, 879 (2021).
Kozma, B., Majoros, A., Pytel, Á., Póka, R. & Takács, P. Efficacy of the percutaneous tibial nerve stimulation in the treatment of lower urinary tract symptoms. Orv. Hetil. 159, 1735–1740 (2018).
Park, E. et al. The long-lasting post-stimulation inhibitory effects of bladder activity induced by posterior tibial nerve stimulation in unanesthetized rats. Sci. Rep. 10, 19897 (2020).
Al-Danakh, A. et al. Posterior tibial nerve stimulation for overactive bladder: Mechanism, classification, and management outlines. Parkinsons Dis. 2022, 2700227 (2022).
Abello, A. & Das, A. K. Electrical neuromodulation in the management of lower urinary tract dysfunction: Evidence, experience and future prospects. Ther. Adv. Urol. 10, 165–173 (2018).
Agarwal, A. et al. An evaluation of the efficacy of gabapentin for prevention of catheter-related bladder discomfort: A prospective, randomized, placebo-controlled, double-blind study. Anesth. Analg. 105, 1454–1457 (2007).
Agarwal, A., Yadav, G., Gupta, D., Singh, P. K. & Singh, U. Evaluation of intra-operative tramadol for prevention of catheter-related bladder discomfort: A prospective, randomized, double-blind study. Br. J. Anaesth. 101, 506–510 (2008).
Agarwal, A. et al. The efficacy of tolterodine for prevention of catheter-related bladder discomfort: A prospective, randomized, placebo-controlled, double-blind study. Anesth. Analg. 101, 1065 (2005).
Li, X., Li, X. & Liao, L. Mechanism of action of tibial nerve stimulation in the treatment of lower urinary tract dysfunction. Neuromodul. Technol. Neural Interface 27, 256–266 (2024).
Lightner, D. J., Gomelsky, A., Souter, L. & Vasavada, S. P. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline amendment 2019. J. Urol. https://doi.org/10.1097/JU.0000000000000309 (2019).
Yu, Z. et al. Effects of non-invasive or minimally invasive neuromodulation techniques on neurogenic lower urinary tract dysfunction after spinal cord injury: A network meta-analysis. Arch. Phys. Med. Rehabil. S0003–9993(24), 01420–01425. https://doi.org/10.1016/j.apmr.2024.12.016 (2024).
Xu, Y.-X. et al. Efficacy of the combination of transcutaneous tibial nerve stimulation and mirabegron in women with overactive bladder in a prospective randomized controlled trial. Sci. Rep. 14, 27248 (2024).
Stampas, A., Korupolu, R., Lee, K. H., Salazar, B. & Khavari, R. Reduction of overactive bladder medications in spinal cord injury with self-administered neuromodulation: A randomized trial. J. Urol. 212, 800–810 (2024).
Lyon, T. D. et al. Pudendal but not tibial nerve stimulation inhibits bladder contractions induced by stimulation of pontine micturition center in cats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310, R366–R374 (2016).
Xiao, Z. et al. Somatic modulation of spinal reflex bladder activity mediated by nociceptive bladder afferent nerve fibers in cats. Am. J. Physiol. Renal. Physiol. 307, 673–679 (2014).
Gupta, P., Ehlert, M. J., Sirls, L. T. & Peters, K. M. Percutaneous tibial nerve stimulation and sacral neuromodulation: An update. Curr. Urol. Rep. 16, 4 (2015).
Yecies, T. et al. Spinal interneuronal mechanisms underlying pudendal and tibial neuromodulation of bladder function in cats. Exp. Neurol. 308, 100–110 (2018).
Kabay, S. C., Kabay, S., Yucel, M. & Ozden, H. Acute urodynamic effects of percutaneous posterior tibial nerve stimulation on neurogenic detrusor overactivity in patients with Parkinson’s disease. Neurourol. Urodyn. 28, 62–67 (2009).
Amarenco, G. et al. Urodynamic effect of acute transcutaneous posterior tibial nerve stimulation in overactive bladder. J. Urol. 169, 2210–2215 (2003).
Mendell, L. M. Constructing and deconstructing the gate theory of pain. Pain 155, 210–216 (2014).
Wodlinger, B., Rashid, S. & Durand, D. M. Block of peripheral pain response by high-frequency sinusoidal stimulation. Neuromodulation 16, 312–317 (2013).
Paquette, J. P. & Yoo, P. B. Recruitment of unmyelinated C-fibers mediates the bladder-inhibitory effects of tibial nerve stimulation in a continuous-fill anesthetized rat model. Am. J. Physiol. Renal Physiol. 317, 163–171 (2019).
Zhou, Z., Wang, X., Li, X. & Liao, L. Transdermal tibial nerve optogenetic stimulation targeting C-fibers. Front. Physiol. 14, 1224088 (2023).
Sato, A., Sato, Y. & Schmidt, R. F. Reflex bladder activity induced by electrical stimulation of hind limb somatic afferents in the cat. J. Auton. Nerv. Syst. 1, 229–241 (1980).
Yu, M. et al. An excitatory reflex from the superficial peroneal nerve to the bladder in cats. Am. J. Physiol. Renal Physiol. 313, 1161–1168 (2017).
do Carmo Almeida, T. C. et al. Effects of transcutaneous electrical nerve stimulation on proinflammatory cytokines: Systematic review and meta-analysis. Mediat. Inflamm. 2018, 1094352 (2018).
Ulloa, L., Quiroz-Gonzalez, S. & Torres-Rosas, R. Nerve stimulation: Immunomodulation and control of inflammation. Trends Mol. Med. 23, 1103–1120 (2017).
Vance, C. G. T., Dailey, D. L., Rakel, B. A. & Sluka, K. A. Using TENS for pain control: The state of the evidence. Pain Manag. 4, 197–209 (2014).
Sluka, K. A., Bjordal, J. M., Marchand, S. & Rakel, B. A. What makes transcutaneous electrical nerve stimulation work? Making sense of the mixed results in the clinical literature. Phys. Ther. 93, 1397–1402 (2013).
Zhang, G.-F. et al. Effects of dezocine for the prevention of postoperative catheter-related bladder discomfort: A prospective randomized trial. Drug Des. Dev. Ther. 13, 1281–1288 (2019).
Tavares Oliveira, M., Maciel Santos, M., Lucas Mayara da Cruz Reis, K., Resende Oliveira, L. & DeSantana, J. M. Transcutaneous electric nerve stimulation in animal model studies: From neural mechanisms to biological effects for analgesia. Neuromodul. Technol. Neural Interface 27, 13–21 (2024).
Cheing, G. L. Y., Tsui, A. Y. Y., Lo, S. K. & Hui-Chan, C. W. Y. Optimal stimulation duration of tens in the management of osteoarthritic knee pain. J. Rehabil. Med. 35, 62–68 (2003).
Yang, Y. et al. Effects of transcutaneous electrical acupoint stimulation on recovery of patients undergoing robotic gynecologic surgery. J. Clin. Anesthesiol. 34, 11–15 (2018).
Chen, Y. et al. The effectiveness and safety of oral medications, onabotulinumtoxinA (three doses) and transcutaneous tibial nerve stimulation as non or minimally invasive treatment for the management of neurogenic detrusor overactivity in adults: A systematic review and network meta-analysis. Int. J. Surg. 109, 1430–1438 (2023).
Funding
This study was conducted at the First Affiliated Hospital of Shihezi University and was supported by the Science and Technology Program of the Corps (NO. 2022ZD077), and the Key Talent Projects (NO.TSYC202401B194).
Author information
Authors and Affiliations
Contributions
X.Z. helped in the conception and design of the work, acquired and analyzed the data, helped interpret the data, was a major contributor in writing the manuscript, revised the manuscript, and approved the final version of the manuscript. Y.L. helped in the conception and design of the work, acquired and analyzed the data, helped interpret the data, and had final approval of this version. T.W. was involved in the acquisition and analysis of the data, interpretation of the data, and final review of this version. C.D. helped in the conception and design of the work, helped interpret the data, helped revise the article, and had final approval of this version. Y.G. helped in the conception and design of the work, helped interpret the data, helped revise the article, and had final approval of this version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The present study was approved (approval number: KJ2023-437-01) by the Science and Technology Ethics Committee of the First Affiliated Hospital of Shihezi University, and written informed consent was obtained from all participants. This study was conducted in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zheng, X., Liu, Y., Wei, T. et al. Influence of transcutaneous tibial nerve stimulation on postoperative catheter-related bladder discomfort in urology: a prospective randomized controlled trial. Sci Rep 16, 2461 (2026). https://doi.org/10.1038/s41598-025-32254-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-32254-w