Abstract
We aimed to investigate prognostic determinants of extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). We analyzed 11,730 patients captured in the National cancer registry. Kaplan–Meier estimates compared survival by sex, age, and SEER stage, while multivariable Cox models identified mortality determinants. Women represented 54.6% of cases and had better survival than men. Age distribution peaked in the fifth–sixth decades. Survival declined with older age and advanced SEER stage. In multivariable models, male sex (hazard ratio [HR] 2.00, 95% confidence interval [CI] 1.48–2.70), advanced age (HR 33.92, 95% CI 18.23–63.10 for ≥ 80 years), and metastatic disease (HR 2.77, 95% CI 2.03–3.78), diabetes, cardiovascular, and cerebrovascular disease were associated with higher mortality. Conversely, higher household income (HR 0.63, 95% CI 0.45–0.88), physical activity (HR 0.60, 95% CI 0.42–0.86), and higher body mass index (HR 0.95, 95% CI 0.91–0.99) were associated with reduced mortality. Subgroup analyses showed consistently worse outcomes for men across age strata. Comorbidity effects were most pronounced at ages 60–79. Income, physical activity, smoking, and BMI have age-specific survival benefits. In conclusion, prognosis in MALT lymphoma is strongly influenced by sex, age, stage, comorbidities, and lifestyle.
Similar content being viewed by others
Introduction
Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) is an indolent non-Hodgkin lymphoma subtype that typically arises in extranodal sites, including the stomach, salivary glands, thyroid, lung, and ocular adnexa1,2. Incidence rate is low but increasing in Korea3. While MALT lymphoma is generally considered a low-grade malignancy with favorable outcomes, its clinical course is heterogeneous, with some patients experiencing aggressive progression or transformation (2–4%)2,4,5. Comorbidities, socioeconomic status, and lifestyle factors may influence outcomes, but prognostic factors in MALToma remain poorly understood.
Previous studies show that several factors predict prognosis in MALT lymphoma, including age, sex, tumor stage, and site of origin4,6. Advanced-stage disease and older age at diagnosis are consistently associated with poor survival, while localized disease often carries an excellent prognosis4. However, most prior investigations are limited to single institutions or small populations, which may reduce generalizability. Moreover, few studies comprehensively demonstrate how comorbidities such as hypertension, diabetes, and cardiovascular disease, alongside modifiable lifestyle factors, including smoking, alcohol consumption, physical activity, and body mass index (BMI), interact with traditional prognostic markers. Across hematologic malignancies, sex differences in lymphoma biology and outcomes are increasingly recognized7,8. While men often demonstrate more aggressive disease and higher comorbidity burdens, women may experience distinct patterns of incidence and survival8. Similarly, socioeconomic disparities, particularly household income, may influence access to timely diagnosis and treatment, ultimately affecting prognosis9,10. These factors remain understudied in MALT lymphoma, especially in large-scale Asian cohorts.
To address these gaps, a nationwide cohort of patients with MALT lymphoma was analyzed. This study aims to (1) describe baseline demographic, clinical, and lifestyle characteristics based on sex; (2) assess survival patterns based on sex, age, and disease stage; and (3) identify prognostic factors—including comorbidities, socioeconomic status, and lifestyle determinants—associated with mortality risk.
Method
Database and data contents
This study is cohort study and was conducted using the Korea Healthcare Bigdata system of the Ministry of Health and Welfare (approval no. 2022–00088). The dataset was developed by the Korean National Cancer Center (KNCC) through linkage with three nationwide resources: the National Cancer Registry, National Health Insurance Service (NHIS), and Health Insurance Review and Assessment Service (HIRA) (Fig. 1a). The National Cancer Registry provided data on International Classification of Diseases, 10th Revision (ICD-10) codes, age, sex, primary tumor site, pathology group, and Surveillance, Epidemiology, and End Results (SEER) stage. The SEER stage was categorized into four groups: localized (confined to the organ of origin), regional (involving adjacent tissues or regional lymph nodes), distant (metastasis to remote organs or lymph nodes), and unknown (insufficient information for staging). The HIRA database included diagnostic codes, inpatient and outpatient service claims, and prescription records. The NHIS database provided death certificate records, data on demographic information (age, sex, and income decile), biennial health examination results, self-reported information on chronic diseases and lifestyle factors (alcohol consumption, smoking status, and physical activity), and outcomes from the national cancer screening program for adults aged ≥ 40 years11. Income was originally provided in deciles (1–10). For this study, we grouped income into four categories based on the national income distribution: < 20%, < 40%, < 70%, and ≥ 70%. Moderate-intensity physical activity was classified as 0 day/week, 1–2 days/week, 3-5 days/week, and 6-7 days/week. Alcohol drinking frequency was categorized into none, 1/week, 2–3/week, 4–5/week, and ≥ 6/week. Laboratory and clinical variables extracted from the health examination database included BMI (weight/height2 [kg/m2]), blood pressure, fasting glucose levels, and lipid profiles. The Institutional Review Board of Kyungpook National University Hospital, Chilgok, approved the study (KNUCH 2023-01-020). Informed consent was waived, as the national organization provided anonymized data. All methods were performed in accordance with the relevant guidelines and regulations, and in compliance with the Declaration of Helsinki.
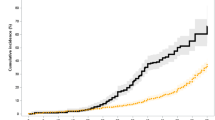
Study flow. (a) Study flow. (b) Annual trend of new extranodal marginal zone B-cell lymphoma. (c) Age distribution of new extranodal marginal zone B-cell lymphoma. C884 extranodal marginal zone B-cell lymphoma, HIRA Health insurance review and assessment service, NHIS national health insurance service, SEER surveillance, epidemiology, and end results.
Patient enrollment and follow-up
A total of 11,730 patients diagnosed with extranodal marginal zone B-cell lymphoma (ICD-10 code C884) between 2007 and 2019 were included in the study cohort (Fig. 1a). Follow-up commenced at the date of initial diagnosis and continued until death or the end of the observation period (December 31, 2022), based on the event that occurred first.
Statistical analysis
Given that the cohort was predefined, no sample-size calculation was performed. Continuous variables are presented as medians with interquartile ranges, and categorical variables as frequencies with percentages. Person-years were calculated from the date of cancer diagnosis until death or censoring on December 31, 2022, based on the event that occurred first. Age was categorized as < 50, 50–59, 60–69, 70–79, ≥ 80 years. Primary hypothesis is “Extranodal marginal zone B-cell lymphoma shows distinct prognostic outcomes by age, sex, and SEER.” Secondary hypotheses are “Survival outcomes differ according to comorbidities and life style factors.” Survival probabilities based on sex, age, and SEER stage were estimated using the Kaplan–Meier method, and group differences were assessed with the log-rank test. We assessed the proportional hazards assumption using Schoenfeld residuals, examining both global and covariate-specific tests. Visual inspection of Schoenfeld residual plots was also performed to confirm that no meaningful deviations from proportionality were present. All models satisfied the proportional hazards assumption, and no significant violations were observed.
Mortality among patients with extranodal marginal zone B-cell lymphoma was evaluated using Cox proportional hazards regression, yielding hazard ratios (HRs) with corresponding 95% confidence intervals (CIs). The selection of covariates for our multivariable models was based on a combination of clinical relevance, prior literature, and epidemiologic reasoning. Sequential multivariable models were constructed: Model I was adjusted for age, sex, and SEER stage, and Model IV was additionally adjusted for hypertension, diabetes mellitus, cardiovascular disease, and cerebrovascular disease. Model V was further adjusted for hypertension, diabetes mellitus, cardiovascular disease, cerebrovascular disease, income, physical activities, BMI, smoking, and alcohol consumption. In our dataset, the proportion of missing data from cancer registry and HIRA was minimal. However, the proportion of missing values for variables such as income, physical activity, smoking, alcohol use, and BMI, which are provided by NHISS, was high because the NHIS data were available from 2012 to 2019, whereas the cancer registry data were available from 2007 to 2019. Models I and IV, which did not include income, physical activity, smoking, alcohol use, or BMI variables, incorporated all cases. In contrast, Model V, which included income, physical activity, smoking, alcohol use, and BMI, was analyzed using a complete-case approach. We additionally performed a sensitivity analysis by treating missing values as ‘unknown’. Subgroup analyses were conducted based on sex and age. All statistical tests were two-sided, and a P-value < 0.05 was considered statistically significant. Statistical analyses and survival curves were generated using SAS software (version 9.4; SAS Institute, Cary, NC, USA), and annual trends and age distributions of extranodal marginal zone B-cell lymphoma were plotted with GraphPad Prism (version 10.0; GraphPad Software, Boston, MA, USA).
Results
Baseline characteristics
Among 11,730 patients with MALT lymphoma, baseline characteristics revealed distinct sex-specific patterns (Table 1). Incidence rates increased steadily over time in men and women, with women consistently exhibiting higher rates than men throughout the study period (Fig. 1b). Women accounted for a slightly larger proportion of cases than men (54.6 vs. 45.4%). The cohort accrued 47,119.7 person-years among men and 59,164.2 person-years among women. Comorbidities, including hypertension, diabetes, cardiovascular disease, and cerebrovascular diseases, were consistently more prevalent in men. Smoking and alcohol consumption were also markedly higher among men. The age distribution was broadly similar between sexes. Incidence gradually increased, peaked in the fifth and sixth decades of life, before subsequently declining in the oldest age categories (Fig. 1c).
Kaplan–Meier survival estimates for MALT lymphoma based on sex and age
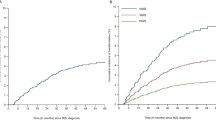
Women with MALT lymphoma exhibited slightly higher survival probabilities than those of men throughout the follow-up period (Fig. 2a). Kaplan–Meier survival analysis revealed a clear gradient in overall survival across various age categories (Fig. 2b). Younger patients (< 50 years) maintained the highest survival probabilities with only a modest decline over time, whereas older patients, particularly those ≥ 70 years, exhibited markedly lower survival probabilities with a steep decline evident from the early follow-up years. The Kaplan–Meier survival curves demonstrated clear differences in survival based on SEER stage at diagnosis (Fig. 2c). Patients with localized disease had the most favorable prognosis, maintaining the highest survival probabilities throughout follow-up. Conversely, patients with distant-stage disease exhibited the poorest survival, with a sharp decline evident within the first few years.
Kaplan–Meier survival estimates for MALT lymphoma. (a) Survival of extranodal marginal zone B-cell lymphoma based on sex. (b) Survival of extranodal marginal zone B-cell lymphoma based on age. (c) Survival of extranodal marginal zone B-cell lymphoma based on SEER. SEER surveillance, epidemiology, and end results.
Death risk of MALT lymphoma (overall and by sex)
In the adjusted model I, male sex was associated with a significantly higher mortality risk (HR 1.75, 95% CI 1.52–2.01) (Supplementary Table 1). Advancing age increased mortality, with HRs rising from 2.44 (95% CI 1.78–3.35) in the 50–59 year group to 35.44 (95% CI 25.85–48.6) among patients aged ≥ 80 years. Compared with localized disease, regional and metastatic SEER stages were associated with higher risks (HR 1.84 and 2.23, respectively). These associations were consistent in men and women. In the adjusted Model IV, the associations with male sex (HR 1.70, 95% CI 1.48–1.95; P < 0.0001), older age (HR, 30.08 for ≥ 80 years), and advanced SEER stage (HR, 1.82 for regional; HR 2.27 for metastatic) remained robust. Hypertension (HR 1.38, 95% CI 1.19–1.60) and cerebrovascular disease (HR 1.44, 95% CI 1.13–1.82) were also associated with increased mortality.
In the fully adjusted model V, the associations remained robust (Table 2). Male sex was associated with a two-fold higher risk of mortality (HR, 2.00, 95% CI 1.48–2.70). Age remained a strong predictor, with HRs increasing to 33.92 (95% CI 18.23–63.10) among individuals aged ≥ 80 years. Metastatic disease was consistently associated with the poorest prognosis (HR 2.77, 95% CI 2.03–3.78). Among comorbidities, diabetes (HR, 1.52, 95% CI 1.14–2.03), cardiovascular disease (HR 1.51, 95% CI 1.06–2.13), and cerebrovascular disease (HR 1.69, 95% CI 1.17–2.43) were significantly associated with elevated mortality risk. Higher household income was inversely associated with mortality, with individuals in upper 30% group exhibiting a 37% lower risk (HR 0.63, 95% CI 0.45–0.88). Frequent physical activity (3–5/weeks) (HR 0.60, 95% CI 0.42–0.86) and low frequency alcohol consumption (1/week) (HRs 0.59, 95% CI 0.38–0.92) were also associated with reduced mortality. A higher BMI conferred a modest protective effect (HR 0.95, 95% CI 0.91–0.99).
In subgroup analysis by sex, protective effect of frequent physical activity and low-to-moderate alcohol consumption on MALT lymphoma mortality was significant in men, but not in women (Fig. 3). Among women, former smokers showed a borderline increase in mortality risk, with an adjusted hazard ratio of 3.10 (95% CI 0.94–10.29; p = 0.064). Protective income of higher household income on MALT lymphoma mortality was significant in women. Increased mortality risk was associated with diabetes in men and heart diseases and cerebrovascular diseases in women (Fig. 4A).
Death risk of MALT lymphoma based on age
In subgroup analyses stratified based on age, the adverse prognostic effect of male sex was consistently observed across all age groups, although the magnitude varied (Table 3 and Supplementary Table 2). In Model I, male sex was associated with increased mortality risk in those aged < 50 (HR 2.24, 95% CI 1.30–3.87), 50–59 (HR 2.17, 95% CI 1.49–3.17), 60–69 (HR 1.88, 95% CI 1.45–2.45), and 70–79 years (HR 1.80, 95% CI 1.42–2.27). In adjusted Model IV (Supplementary Table 2), these associations remained robust, with hazard ratios ranging from 1.74 to 2.14 across age strata. In the fully adjusted Model V (Table 3), male sex remained significantly associated with mortality in the 50–59, 60–69, and 70–79-year groups, with the strongest effect observed in the oldest subgroup (HR 2.75, 95% CI 1.67–4.53). SEER stage was a key determinant of prognosis across age groups. Compared with localized disease, metastatic stage disease was associated with an increased risk of mortality in all age strata in Model IV. In the fully adjusted model V, this effect was most pronounced among individuals aged 70–79 years (HR 4.71, 95% CI 2.89–7.68).
Regarding comorbidities, hypertension was associated with a significant increase in mortality among middle-aged and older patients (Model IV: 50–59 years, HR, 1.57, 95% CI 1.01–2.43; 60–69 years, HR 1.44, 95% CI 1.09–1.90; 70–79 years, HR 1.31, 95% CI 1.03–1.67). Cerebrovascular disease demonstrated a strong association in patients aged 60–69 (HR 1.82, 95% CI 1.15–2.89) and 70–79 years (HR 1.44, 95% CI 1.02–2.03). In the fully adjusted Model V, diabetes (HR 2.85, 95% CI 1.74–4.66) and heart disease (HR 2.68, 95% CI 1.66–4.32) emerged as significant predictors of poor survival in the 70–79-year group (Fig. 4B). Cerebrovascular disease was associated with high mortality in patients aged 60–69 (HR 2.66, 95% CI 1.32–5.38). Hypertension was significantly associated with increased risk of high mortality in patients younger than 50 years (HR 4.08; 95% CI 1.15–14.45).
Economic and lifestyle factors showed heterogeneous associations across age strata. Higher household income was protective among individuals aged 60–69 years (HR 0.39, 95% CI 0.20–0.75 for the upper 30% versus the lowest group). Physical activity was associated with improved survival in the 60–79-year group. Higher BMI was inversely associated with mortality in the 50–59 (HR 0.88, 95% CI 0.78–1.00) and 70–79-year groups (HR 0.90, 95% CI 0.84–0.98). Current smoking was associated with increased mortality in younger patients aged < 50 years (HR 5.34, 95% CI 1.04–27.39). Moderate frequency alcohol consumption (2–3/week) demonstrated protective effect in individuals aged 50–59 years (HR 0.18, 95% CI 0.04–0.80).
Discussion
In this large, population-based cohort, 11,730 patients with MALT lymphoma provide key insights regarding incidence patterns, sex- and age-specific prognoses, and the effect of comorbidities, economic status, and lifestyle factors on survival outcomes. Our findings confirmed established predictors, including age and disease stage, while highlighting the prognostic significance of comorbid conditions and modifiable health behaviors, thereby advancing the current understanding of risk stratification in MALT lymphoma.
Across the study period, women consistently exhibited higher incidence rates and represented a slightly larger proportion of MALT lymphoma cases. This observation aligns with reports from European and Asian cohorts, indicating a female predominance in certain extranodal sites, particularly the stomach and ocular adnexa12. Sex-related differences in immune regulation, hormonal influences on lymphomagenesis13, or differential exposure to infectious agents such as Helicobacter pylori may explain these observations14. After adjustment for comorbidities and socioeconomic factors, women demonstrated a modest but consistent survival advantage. Across most age groups, male sex conferred up to a two-fold increased risk of mortality across models. Previous lymphoma studies show poorer survival in men, potentially owing to more aggressive disease biology and sex-related differences in immunochemotherapy pharmacokinetics (Supplementary Table 3)8,15. Previous studies have reported an increased mortality risk among male patients with MALT lymphoma6,16. This study adds to the literature by showing that such disparities persist even in indolent subtypes, including MALT lymphoma, highlighting the importance of considering sex as a biological and social determinant of outcomes.
Age was a strong prognostic determinant. The incidence of MALT lymphoma increased steadily through the fifth and sixth decades of life and declined thereafter. Survival probability decreased rapidly with advancing age, particularly among patients aged ≥ 70 years. Hazard ratios for mortality exceeded 30 in the oldest subgroup, underscoring the vulnerability of elderly patients. These findings are consistent with those of prior registry analyses demonstrating that advanced age is independently used to predict poorer outcomes in indolent lymphomas4,6,17,18. Mechanistically, advanced age may contribute through diminished physiologic reserve, higher comorbidity burden, and increased susceptibility to treatment-related toxicity. Furthermore, previous reported that old age (≥ 60 years) was associated with histological transformation in MALT lymphoma19. Subgroup analyses showed that the adverse prognostic influence of male sex was accentuated in older patients, particularly in those aged 70–79 years. This age-by-sex interaction suggests that biologic and behavioral differences accumulate over the life course, thereby compounding survival gaps. Therefore, tailored survivorship strategies, including closer monitoring and proactive management of comorbidities in elderly men, may be warranted.
The disease stage at diagnosis was a critical determinant of prognosis. Localized disease is associated with the most favorable survival, while metastatic disease conferred more than a two-fold increase in mortality risk, consistent with that of the natural history of indolent lymphomas. Stage dependent prognosis has been reported previously4,6,18,20. Stage-related survival disparities were most pronounced in patients aged 70–79 years, with metastatic disease conferring nearly a five-fold higher risk of mortality compared to that of localized disease. These findings indicate the importance of early detection and timely treatment initiation. In gastric MALT lymphoma, H. pylori eradication can achieve durable remission in localized disease, highlighting how early diagnosis directly translates into improved outcomes21,22. Conversely, patients with advanced disease often require systemic therapy, but its effectiveness may be limited by comorbidities and frailty, particularly in older adults23,24.
Our analysis highlighted the significant effect of comorbid conditions on survival. Hypertension, diabetes mellitus, cardiovascular, and cerebrovascular disease were associated with increased mortality risk, even after adjusting for demographic and clinical factors. Among these, cerebrovascular disease emerged as a strong predictor in middle-aged and older patients, while diabetes was significantly associated with mortality in the elderly.
These findings are consistent with prior studies demonstrating that comorbidity burden independently predicts survival in lymphoma25,26. However, evidence on the impact of comorbidities on mortality in MALT lymphoma remains scarce. Comorbidities may limit treatment options, impair tolerance to therapy, and increase the risk of competing causes of death24. In addition, they may interact with lymphoma-related inflammation to accelerate disease progression24,27. These findings underscore the need for comprehensive comorbidity assessment and multidisciplinary management, particularly in older patients with multiple chronic conditions.
Beyond clinical predictors, economic and lifestyle factors significantly influence outcomes. Highest income group was associated with reduced mortality in overall population (aHR 0.63), women (aHR 0.47), and patients aged 60–69 years (aHR 0.39). This observation is consistent with prior studies, demonstrating that socioeconomic deprivation adversely affects cancer survival through delays in diagnosis, reduced treatment intensity, and limited access to supportive care16,28,29. These findings underscore that income-related disparities persist even within a universal healthcare system. Lifestyle behaviors also contributed meaningfully to prognosis. Moderate-intensity physical activity of 3–5 days/week was associated with a 40% reduction in mortality in overall population, men, and age of 60–69 years. Low (1/week)-to-moderate (2–3/week) frequency alcohol consumption demonstrated protective effects in overall population, men, and age of 50-59 years. Conversely, current smoking substantially increased mortality risk, particularly among patients younger than 50 years (aHR, 5.34). A higher BMI exerted a modest protective effect, consistent with the “obesity paradox” described in other hematologic malignancies30. While causality cannot be inferred, these findings indicate that lifestyle modifications may meaningfully influence outcomes in MALT lymphoma, thereby complementing clinical management strategies.
This study demonstrates several important clinical implications. First, traditional prognostic factors such as age and stage remain critical, while comorbidities, socioeconomic factors, and lifestyle behaviors provide additional prognostic relevance. Integrating these dimensions into risk stratification models may improve prognostic accuracy. Second, the strong association between male sex and adverse outcomes warrants further mechanistic research. Third, the persistence of socioeconomic disparities despite universal healthcare access suggests that structural barriers beyond cost—including health literacy, social support, and geographic accessibility—require attention. Finally, the observed benefits of physical activity provide support for incorporating lifestyle-based survivorship strategies into MALT lymphoma care.
Key strengths of this study included its large sample size, nationwide coverage, and comprehensive assessment of demographic, clinical, socioeconomic, and lifestyle factors. The conducted multiple models offer robust estimates of MALT lymphoma–specific mortality. However, some limitations exist. First, residual confounding may persist despite extensive covariate adjustment. Second, lifestyle factors, including smoking, drinking, and physical activity, were self-reported and subject to misclassification. Alcohol consumption was classified by frequency rather than by the amount of alcohol intake, which may have limited our ability to fully capture dose–response relationships. Third, treatment details were unavailable, restricting evaluation of therapeutic effects relative to baseline prognostic factors. The possibility of Type I error due to multiple comparisons and clarifying that these findings should be interpreted carefully. Because treatment information was unavailable, differences in therapeutic strategies may have potentially influenced or partially confounded the observed survival patterns. Despite the inability to adjust for treatment variables, the large sample size and the consistently observed prognostic gradients across sensitivity analyses support the robustness of our findings. Finally, as an observational study, causal inference cannot be established.
In conclusion, this nationwide cohort of patients with MALT lymphoma demonstrates that survival is influenced not only by age and disease stage but also by sex, comorbidities, economic status, and lifestyle factors. Male sex, advanced age, and metastatic disease were consistently associated with poorer outcomes, whereas higher income, regular physical activity, and moderate alcohol intake conferred survival benefits. These findings highlight the importance of integrated care strategies that address clinical and social determinants of health to improve outcomes in patients with MALT lymphoma.
Data availability
This research used data from the Korea Healthcare Big Data Hub of the Ministry of Health and Welfare (No. 2022-00088). Raw data are unavailable because analyses may be performed only within secure ministry-regulated facilities. Researchers may request access from the ministry and conduct analyses on-site under the standard security constraints.
References
Zucca, E. & Bertoni, F. The spectrum of MALT lymphoma at different sites: biological and therapeutic relevance. Blood 127, 2082–2092. https://doi.org/10.1182/blood-2015-12-624304 (2016).
Cheah, C. Y., Zucca, E., Rossi, D. & Habermann, T. M. Marginal zone lymphoma: present status and future perspectives. Haematologica 107, 35–43. https://doi.org/10.3324/haematol.2021.278755 (2022).
Jeong, S. H., Hyun, S. Y., Choi, J. S. & Kim, H. M. Trends of Incidence and Survival Rates of Mucosa-associated lymphoid tissue lymphoma in the Korean population: Analysis of the Korea central cancer registry database. J. Korean Med. Sci. 35, e294. https://doi.org/10.3346/jkms.2020.35.e294 (2020).
Qi, S. et al. Predictors of survival in patients with MALT lymphoma: a retrospective, case-control study. Blood Adv. 7, 1496–1506. https://doi.org/10.1182/bloodadvances.2022007772 (2023).
Ishikawa, E., Nakamura, M., Satou, A., Shimada, K. & Nakamura, S. Mucosa-associated lymphoid tissue (MALT) Lymphoma in the gastrointestinal tract in the modern era. Cancers https://doi.org/10.3390/cancers14020446 (2022).
Sim, J. Y. et al. Long-term outcomes and prognostic factors of gastric MALT lymphoma. J. Gastric Cancer 24, 406–419. https://doi.org/10.5230/jgc.2024.24.e36 (2024).
Paltiel, O., Ratnasingam, S. & Lee, H. P. Are we ignoring sex differences in haematological malignancies? A call for improved reporting. Br. J. Haematol. 206, 1315–1329. https://doi.org/10.1111/bjh.20044 (2025).
Radkiewicz, C. et al. Sex differences in lymphoma incidence and mortality by subtype: A population-based study. Am. J. Hematol. 98, 23–30. https://doi.org/10.1002/ajh.26744 (2023).
Nielsen, L. H. et al. Socioeconomic status and overall survival among patients with hematological malignant neoplasms. JAMA Netw. Open 7, e241112. https://doi.org/10.1001/jamanetworkopen.2024.1112 (2024).
Frederiksen, B. L., Dalton, S. O., Osler, M., Steding-Jessen, M. & de Nully Brown, P. Socioeconomic position, treatment, and survival of non-Hodgkin lymphoma in Denmark–a nationwide study. Br. J. Cancer 106, 988–995. https://doi.org/10.1038/bjc.2012.3 (2012).
Nam, S. Y., Jo, J. & Cho, C. M. A population-based cohort study of longitudinal change of high-density lipoprotein cholesterol impact on gastrointestinal cancer risk. Nat. Commun. 15, 2923. https://doi.org/10.1038/s41467-024-47193-9 (2024).
Kiesewetter, B. et al. Gender aspects in extranodal marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue: Does sex matter?. Oncology 91, 243–250. https://doi.org/10.1159/000448218 (2016).
Nowak, T. J. & Muehlenbein, M. P. Toward understanding sexual immune dimorphism in humans. Front. Immunol. 16, 1570565. https://doi.org/10.3389/fimmu.2025.1570565 (2025).
Ibrahim, A., Morais, S., Ferro, A., Lunet, N. & Peleteiro, B. Sex-differences in the prevalence of Helicobacter pylori infection in pediatric and adult populations: Systematic review and meta-analysis of 244 studies. Dig. Liver Dis. 49, 742–749. https://doi.org/10.1016/j.dld.2017.03.019 (2017).
Delahousse, J. et al. Sex differences in the pharmacokinetics of anticancer drugs: a systematic review. ESMO Open 9, 104002. https://doi.org/10.1016/j.esmoop.2024.104002 (2024).
Bangolo, A. I. et al. Predictors of survival in gastric mucosa-associated lymphoid tissue lymphoma: An updated surveillance, epidemiology, and end results-based analysis of age and gender disparities. World J. Clin. Oncol. 16, 106408. https://doi.org/10.5306/wjco.v16.i6.106408 (2025).
Maartense, E. et al. Different age limits for elderly patients with indolent and aggressive non-hodgkin lymphoma and the role of relative survival with increasing age. Cancer 89, 2667–2676. https://doi.org/10.1002/1097-0142(20001215)89:12%3c2667::aid-cncr21%3e3.0.co;2-p (2000).
Thieblemont, C. et al. A MALT lymphoma prognostic index. Blood 130, 1409–1417. https://doi.org/10.1182/blood-2017-03-771915 (2017).
He, Y. & Gao, C. Risk and clinical implications of histological transformation in extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue: A population-based analysis. Sci. Rep. 15, 20407. https://doi.org/10.1038/s41598-025-07413-8 (2025).
Wen, Q. et al. A new prognostic nomogram in patients with mucosa-associated lymphoid tissue lymphoma: a multicenter retrospective study. Front. Oncol. 13, 1123469. https://doi.org/10.3389/fonc.2023.1123469 (2023).
Stathis, A. et al. Long-term outcome following Helicobacter pylori eradication in a retrospective study of 105 patients with localized gastric marginal zone B-cell lymphoma of MALT type. Ann. Oncol. 20, 1086–1093. https://doi.org/10.1093/annonc/mdn760 (2009).
Kim, J. S. et al. Helicobacter pylori eradication for low-grade gastric mucosa-associated lymphoid tissue lymphoma is more successful in inducing remission in distal compared to proximal disease. Br. J. Cancer 96, 1324–1328. https://doi.org/10.1038/sj.bjc.6603708 (2007).
Hormigo-Sanchez, A. I. et al. Frailty assessment to individualize treatment in older patients with lymphoma. Eur Geriatr. Med. 14, 1393–1402. https://doi.org/10.1007/s41999-023-00870-2 (2023).
Rodday, A. M. et al. Association of treatment intensity with survival in older patients with hodgkin lymphoma. JAMA Netw. Open 4, e2128373. https://doi.org/10.1001/jamanetworkopen.2021.28373 (2021).
van Spronsen, D. J., Janssen-Heijnen, M. L., Lemmens, V. E., Peters, W. G. & Coebergh, J. W. Independent prognostic effect of co-morbidity in lymphoma patients: results of the population-based Eindhoven cancer registry. Eur. J. Cancer 41, 1051–1057. https://doi.org/10.1016/j.ejca.2005.01.010 (2005).
Wieringa, A. et al. Comorbidity is an independent prognostic factor in patients with advanced-stage diffuse large B-cell lymphoma treated with R-CHOP: a population-based cohort study. Br. J. Haematol. 165, 489–496. https://doi.org/10.1111/bjh.12765 (2014).
Zhao, H. et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct. Targ. Ther. 6, 263. https://doi.org/10.1038/s41392-021-00658-5 (2021).
Bourgeois, A. et al. Barriers to cancer treatment for people experiencing socioeconomic disadvantage in high-income countries: a scoping review. BMC Health Serv. Res. 24, 670. https://doi.org/10.1186/s12913-024-11129-2 (2024).
Unger, J. M. et al. Persistent disparity: Socioeconomic deprivation and cancer outcomes in patients treated in clinical trials. J. Clin. Oncol. 39, 1339–1348. https://doi.org/10.1200/jco.20.02602 (2021).
Hontecillas-Prieto, L. et al. Obesity and overweight in R/R DLBCL patients is associated with a better response to treatment of R2-GDP-GOTEL trial. Potential role of NK CD8 + cells and vitamin D. Cancer Metab. 13, 12. https://doi.org/10.1186/s40170-025-00381-7 (2025).
Funding
This work was funded by the National Research Foundation of Korea (NRF-2022R1A2C2013044) and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (RS-2025-25410994).
Author information
Authors and Affiliations
Contributions
Specific author contributions: Conceptualization, study design, funding acquisition, and visualization: SY Nam. Data curation: SY Nam and JW Jo. Formal analysis and interpretation: SY Nam, JW Jo. Drafting the manuscript: SY Nam. Critical revision: SY Nam, JW Jo. Data accuracy verification and decision to submit: SY Nam. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The Institutional Review Board of Kyungpook National University Hospital, Chilgok, approved the study (KNUCH 2023-01-020). Informed consent was waived, as the national organization provided anonymized data.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nam, S.Y., Jo, J. Prognostic outcome of extranodal marginal zone B-cell lymphoma: a nationwide cohort. Sci Rep 16, 2425 (2026). https://doi.org/10.1038/s41598-025-32259-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-32259-5