Abstract
Diabetes mellitus is a major global health burden characterized by hyperglycemia, oxidative stress, and progressive pancreatic β-cell dysfunction. Despite the availability of conventional therapies, the need for safer and more effective alternatives persists. Citrullus lanatus (African watermelon) is a fruit rich in bioactive compounds with reported antihyperglycemic and antioxidant properties. This study investigated the modulatory effects of C. lanatus juice on pancreatic and duodenal homeobox 1 (PDX1) gene expression and glycemic control in fortified-diet-fed streptozotocin-induced diabetic Wistar rats. Thirty-six rats weighing 90–110 g, were randomized into six groups: normal control (NMC), diabetic control (DBC), metformin-treated diabetic rats (DBM, 500 mg/kg), and diabetic rats treated with C. lanatus juice at 200 mg/kg (DBCL1), 500 mg/kg (DBCL2), and 1000 mg/kg (DBCL3). Animals were pre-fed either normal or fortified diets prior to diabetes induction with streptozotocin (35 mg/kg). Following 14 days of treatment, blood and pancreatic tissues were analyzed for glycemic, antioxidant, and molecular parameters. C. lanatus juice significantly (p < 0.05) reduced fasting blood glucose in a dose-dependent manner, with the greatest effect observed in DBCL3. Antioxidant enzymes (catalase, superoxide dismutase, and glutathione peroxidase) were significantly increased, while malondialdehyde levels were reduced in all treated groups. Notably, PDX1 gene expression was markedly upregulated (p < 0.05) in C. lanatus-treated rats, accompanied by improved insulin, C-peptide, and HDL levels. These findings suggest that C. lanatus juice exerts antidiabetic effects that are associated with increased PDX1 mRNA expression, improved glycemic control, and reduced oxidative stress, supporting a potential role for transcriptional modulation of β-cell function, highlighting its potential as a functional dietary intervention for type 2 diabetes management.
Similar content being viewed by others
Introduction
Diabetes mellitus remains one of the foremost causes of global morbidity and mortality. It contributes significantly to complications such as cardiovascular diseases, renal failure, blindness, and lower limb amputations1,2. According to the International Diabetes Federation (IDF) Diabetes Atlas, 2025, approximately 643 million adults (aged 20–79 years) are currently living with diabetes worldwide, and this number is projected to rise to 783 million by 2045. The highest growth is expected in low- and middle-income countries, particularly in sub-Saharan Africa, where prevalence is estimated to increase by over 129% by 2045. These alarming statistics underscore the urgent need for innovative therapeutic and preventive strategies to mitigate the global diabetes burden3.
Type 2 diabetes mellitus (T2DM) accounts for more than 90% of all diabetes cases and is characterized by insulin resistance, impaired insulin secretion, and progressive β-cell dysfunction. It does not segregate between race, age, gender or status of the individual4,5. Chronic hyperglycemia, oxidative stress, and lipotoxicity contribute to the decline in β-cell mass and insulin production6. Sustained high glucose and fatty acids promote insulin resistance, decreased pancreas mass, and dysfunctional insulin7,8. Central to maintaining β-cell identity and insulin gene transcription is the pancreatic and duodenal homeobox 1 (PDX1) gene—a key transcription factor whose expression is suppressed under diabetic oxidative conditions. PDX1 downregulation has been linked to impaired insulin biosynthesis and β-cell apoptosis, making it a potential therapeutic target in T2DM management9.
The PDX1 gene is a protein encoding gene recognised for its regulatory role in the development and maintenance of pancreatic β-cell identity10,11. As a potential target for T2DM, PDX1 gene expression is significantly down-regulated by oxidative stress12. Oxidative stress downregulation in the management of T2DM has now gained more research focus and attention. Current T2DM drugs, including metformin, with adverse side effects, remained prominent in the face of the yet-unresolved increase in T2DM cases. This led researchers to seek out natural alternatives.
Despite the availability of synthetic antidiabetic agents like metformin, adverse effects and the rising incidence of diabetes have sparked interest in plant-derived therapeutics13. Natural products rich in antioxidants have shown promise in attenuating oxidative stress and restoring pancreatic function. C. lanatus (African watermelon), a fruit abundant in bioactive compounds like lycopene and citrulline, has been previously reported to possess antihyperglycemic and antioxidant properties. However, its potential to modulate PDX1 gene expression remains largely unexplored.
Studies have identified bioactive compounds from plants for their possible ameliorating in vivo and in vitro prospects in antidiabetic activities13. Lycopene and citrulline, found abundantly in Citrullus lanatus, have been shown to possess antihyperglycemic and antilipidemic properties14,15,16,17. C. lanatus, a tropical to moderate climate fruit, has over the centuries served many therapeutic purposes, including anticancer, anti-inflammation, anti-vascular diseases, and anti-diabetes17,18,19. In light of these discoveries, the anti-diabetic activities of C. lanatus on PDX1 gene expression have not been well explored. The PDX1 gene, a key transcriptional regulator of pancreatic β-cell identity, has been identified as a prospective molecular target in T2DM, although functional outcomes depend on regulation at multiple levels beyond mRNA expression.
Although numerous studies have established the antioxidant and antihyperglycemic activities of C. lanatus, there is limited understanding of its molecular mechanism of action, particularly concerning genes involved in β-cell function and insulin biosynthesis9,12,20,21. Oxidative stress is a central contributor to the development and complications of diabetes mellitus. Excessive production of reactive oxygen species (ROS) and inadequate antioxidant defense disturb cellular redox balance, resulting in lipid peroxidation, protein oxidation, and DNA damage. In pancreatic β-cells, which possess inherently low antioxidant capacity, oxidative stress leads to mitochondrial dysfunction, apoptosis, and impaired insulin secretion. In peripheral tissues, it contributes to insulin resistance by disrupting insulin receptor signaling and glucose uptake mechanisms. Consequently, regulating oxidative stress has become a vital therapeutic target in preventing β-cell failure and metabolic complications associated with diabetes (Robertson, 2020; Matough et al., 2022).
Beyond its metabolic consequences, oxidative stress also influences gene regulatory networks crucial for β-cell identity and function. Elevated ROS suppress the transcriptional activity of PDX1, a master regulator of insulin gene expression and β-cell maintenance. Mechanistically, oxidative stress activates stress-responsive pathways such as JNK and FoxO1, leading to PDX1 nuclear exclusion, post-translational modification, and degradation. Sustained downregulation of PDX1 under these conditions results in β-cell dysfunction and apoptosis, contributing to progressive hyperglycemia. Understanding the association between oxidative stress and altered PDX1 transcription provides a strong rationale for investigating antioxidant interventions such as C. lanatus juice in diabetes management12,20,21. The PDX1 gene is a critical transcription factor responsible for maintaining β-cell identity and regulating insulin gene expression. Downregulation of PDX1 under diabetic oxidative conditions contributes to β-cell dysfunction and apoptosis. Despite this, no previous study has examined whether C. lanatus can modulate PDX1 expression. Addressing this gap, the present study explores the potential of C. lanatus juice to modulate PDX1 gene expression and related β-cell functional markers, thereby providing insight into possible molecular associations, offering new mechanistic insight into its antidiabetic action.
This study, therefore, investigates the effect of C. lanatus juice on PDX1 gene expression and glycemic control in fortified diet-fed streptozotocin-induced diabetic Wistar rats. By evaluating antioxidant status, lipid profiles, and β-cell functional markers, the study aims to assess whether C. lanatus can serve as a functional dietary intervention for T2DM through molecular and metabolic modulation.
Materials and methods
Plant materials
The plant C. lanatus (Thunb.) Matsum. and Nakai (watermelon) was purchased from Ilishan-Remo Market, Ogun State, Nigeria, which were all obtained directly from a single local farm during the same harvest period (to minimize variability in watermelon composition due to cultivar and seasonal factors), and taken to the University of Ibadan, Oyo State, Nigeria, for botanical identification and authentication with the Forest Herbarium, Ibadan (FHI). The voucher specimen No. 113,954 was deposited. The fruits were processed under uniform laboratory conditions to ensure chemical consistency and reproducibility. The reddish-pink, watery pulp of C. lanatus was sliced, and the seeds removed. Four litres of juice were extracted using an electric blender and oven-dried at 50 ℃ to obtain a semisolid paste (215.32 g). The drying step effectively reduced moisture and enzyme activity, enhancing product stability. The paste was then stored at 10–20 ℃ in airtight containers, a condition under which previous studies have shown minimal degradation of bioactive compounds14,16. Although refrigeration at 4 ℃ is typically optimal, the paste remained stable at 10–20 ℃ due to its low water content and short-term storage period.
Phytochemical characterization of the C. lanatus juice extract was not performed in this study. Consequently, no assumptions are made regarding the presence, concentration, or relative abundance of specific phytochemical constituents in the extract used. References to previously reported constituents of C. lanatus are provided solely for contextual background and do not constitute compositional confirmation of the extract evaluated in this work that contribute to its antioxidative and antihyperglycemic properties14,16,19. These established findings provided the biochemical rationale for evaluating its potential modulation of PDX1 gene expression. Future studies will incorporate GC–MS or HPLC-based profiling to identify and quantify the specific bioactive constituents responsible for the observed effects.
Experimental animals
The acute toxicity of C. lanatus juice was not re-assessed in this study, as prior investigations have demonstrated its high safety margin. Studies by Ajiboye et al.22 and Jibril et al.23 reported no mortality or adverse behavioral changes in Wistar rats administered up to 5000 mg/kg of C. lanatus extracts or juice. Given its wide traditional dietary use and previously confirmed non-toxic profile, the present study focused on evaluating its antidiabetic and molecular effects rather than acute toxicity testing. To closely replicate the pathophysiology of human type 2 diabetes, this study employed a combination of a fortified (high-fat/fructose) diet to induce insulin resistance and a low-dose STZ injection to trigger partial β-cell dysfunction. This dual model effectively captures the metabolic and pancreatic impairments characteristic of T2DM, providing a more physiologically relevant framework for assessing the therapeutic effects of C. lanatus24.
Animals comprising 36 male Wistar rats weighing 90–110 g, were obtained from the Babcock University Animal Facility. These animals were acclimatised for two weeks in a well-ventilated room at 20–25 °C, with 12 h of daylight and 12 h of darkness (OECD and WHO laboratory standards). During this period, animals were given normal rat feed and tap water. After the acclimatisation period, animals were assigned to two feeding groups: normal diet-fed (NDF) and fortified diet-fed (FDF). This protocol was adopted from the work of Okoduwa et al.24. Normal diet-fed (crude protein 16.0% min, fat 3.0% min, fibre 8.0% max, calcium 1.0% min, available phosphorus 0.4% min, and energy 2600 kcal/kg min), and fortified diet-fed (NDF, margarine, plus 20% fructose drink). The FDF was in the ratio of 5 g of NDF to 1 g of Simas margarine (fat 99.9%, emulsifier E471, E322, antioxidant E304, E306, beta-carotene C175130, Vitamin A 30,000 IU/kg, Vitamin D 30,000 IU/kg). After five weeks of a dietary regimen and a 14-day C. lanatus administration, animals were sacrificed by cervical dislocation without prior anaesthesia and/or analgesia, minimising potential chemical interference and suffering for the animals25. For biochemical evaluation, terminal blood from a cardiac puncture and pancreatic tissue were obtained.
Ethical considerations
Ethical approval for the study was obtained from the Babcock University Health Research Ethics Committee (BUHREC 048/24). All methods were carried out in accordance with the relevant guidelines and regulations of the institution and national standards for the care and use of laboratory animals. Furthermore, all methods are reported in accordance with the ARRIVE guidelines (https://arriveguidelines.org) for animal research. All experimental procedures were conducted in compliance with the Nigerian National Code of Health Research Ethics (NNCHRE, 2019) and the institutional guidelines of the University Animal Research Ethics Committee (UAREC/2024/012). Animals were humanely sacrificed by cervical dislocation performed by trained personnel without prior anesthesia, as permitted under local and OECD guidelines for small rodents when executed rapidly and skillfully to minimize pain and distress. The method was approved as part of the ethical clearance for this study.
Chemicals and reagents
Streptozotocin was purchased from Nanjing Forever Pharmacy Co., Ltd. (Nanjing City, China). Fructose (MOLYCHEM, India), Simas Margarine (PT Salim Ivomas Pratama Tbk, Indonesia), Normal Diet Feed (Animal Care Services Konsult Ltd., Nigeria); Lipid profile and oxidative stress biomarkers kits (Randox Laboratories, UK); ultra-sensitive rat insulin ELISA kit (Elabscience Biotechnology Inc., USA); rat C-peptide ELISA kit (Elabscience Biotechnology Inc., USA); RNA extraction kits (Direct-zol RNA MiniPrep, Zymo Research, USA); Primers and reagents (Inqaba Biotec West Africa Ltd., Nigeria); and all other reagents were of analytical grade and purchased from recognised and credible companies and laboratories in Nigeria.
Equipment
On Call Plus glucometer and strips (ACON Biotech Hongzhou Co., Ltd., China). Real-time polymerase chain reaction (RT-PCR) machine for gene expression analysis (LineGene 9600 Plus Fluorescent Quantitative Detection System, BIOER). An electronic weighing scale, hot-air oven, incubator, ELISA microplate reader, and microscope were among the items of equipment used.
Induction of diabetes
After five weeks of a diet regimen, animals were fasted overnight for 10–12 h, weighed, and fasting blood glucose measured. All experimental animals, except the normal control, were administered 35 mg/kg per body weight of streptozotocin in a citrate buffer with a pH of 4.5 intraperitoneally and were provided a 5% glucose solution in the first 24 h.
The combination of fortified diet feeding and STZ administration (35 mg/kg) was used to establish a reliable model of type 2 diabetes, as the diet induces insulin resistance while STZ causes partial β-cell damage. This approach produces a stable diabetic phenotype resembling human T2DM, as previously validated by Okoduwa et al.24.
Diabetes confirmation and validation
Confirmation and validation of diabetes were done after 72 h and one week of post-STZ induction, respectively24. Fasting blood glucose was measured using a glucose metre and strips. Rats considered diabetic (> 200 mg/dl fasting blood glucose or > 300 mg/dl non-fasting blood glucose) were selected for the study24. All 36 rats survived the STZ induction and treatment periods, with no mortality recorded. Mild, transient hyperglycemic symptoms (polyuria and lethargy) were observed within 48 h post-induction, confirming successful diabetes establishment. All animals resumed normal activity before treatment initiation.
Experimental design
Thirty-six (36) experimental animals weighing 90–110 g, were randomly assigned to six groups (n = 6): normal control (no diabetes, no treatment), negative control (diabetes, no treatment), positive control (diabetes, 500 mg/kg metformin-treated), experimental test 1 (diabetes, 200 mg/kg watermelon juice-treated), experimental test 2 (diabetes, 500 mg/kg watermelon juice-treated), and experimental test 3 (diabetes, 1000 mg/kg watermelon juice-treated). The doses of 200, 500, and 1000 mg/kg body weight of C. lanatus juice were selected based on previously reported efficacious ranges in diabetic Wistar rats22,23. These doses also align with traditional use levels when scaled from human consumption. Furthermore, the doses have been shown to produce significant improvements in glycemic control and antioxidant parameters without inducing toxicity. A preliminary laboratory screening further confirmed their safety and dose-dependent biological activity, justifying their use for evaluating both metabolic and molecular responses in this study.
Animals were treated for two weeks. The treatment period of 14 days was selected in accordance with previous studies demonstrating that C. lanatus and other phytotherapeutic interventions elicit significant glycemic and molecular responses within 10–21 days of administration in rodent models of type 2 diabetes22,23. This duration was deemed adequate to evaluate the acute modulatory effects on PDX1 gene expression and oxidative stress markers. However, longer-term studies are warranted to assess the sustained antidiabetic efficacy and potential cumulative effects of C. lanatus juice.
Observation of experimental animals
Daily fluid and feed intake were measured using a measuring cylinder and an electronic weighing scale, respectively. The animals were weighed weekly.
Biochemical analysis
Fasting blood glucose.
The fasting blood glucose (FBG) level was measured using a glucose metre and strips. Before and after 72 h of STZ induction, after a week of STZ induction, and subsequently, weekly FBG measured during the two weeks of treatment. Blood was obtained from animals’ tails.
Lipid profile determination.
Total cholesterol, high-density lipoprotein (HDL), and triglycerides were analysed using Randox kits in accordance with the manufacturer’s manual. Low-density lipoprotein (LDL) was determined from values obtained from total cholesterol and HDL. These assays were carried out using animals’ serum.
Serum insulin determination.
The serum insulin and C-peptide levels were determined using ultra-sensitive rat insulin and rat C-peptide ELISA kits, according to the manufacturer’s procedures.
Homeostatic model assessment (HOMA) score.
The scores for insulin resistance and beta-cell function (HOMA-IR and HOMA-β) were calculated using the formula described by Matthews et al.26.
Measurement of oxidative stress biomarkers.
Malondialdehyde (MDA), catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) were measured using procedures outlined in their respective assay kits. Animals’ pancreatic tissues were used in analysing the oxidative stress biomarkers.
Pancreatic duodenal homeobox-1 gene analysis.
Primers by Babaiedarzi et al.27, which matched the primers designed, were used. Total RNA was extracted from the pancreas using RNA extraction kits following the manufacturer’s procedures. The extracted RNA was quantified using a nanodrop spectrophotometer at A260/A280 nm. A one-step real-time polymerase chain reaction was carried out to evaluate the level of PDX-1 gene expression. PDX1 expression was assessed at the mRNA level only; protein expression and functional activity were not evaluated in this study.
Histopathological analysis
Pancreatic tissues were dehydrated by immersing them in ascending concentrations of alcohol solutions (70–100%) and in paraffin. Slices of 4–5 μm of pancreas slides were prepared and stained with hematoxylin and eosin (H&E). Slides were analysed under a light microscope at 100X and photographed with a Zeiss Axio photomicroscope.
Statistical analysis
All statistical analyses were performed using SPSS version 21, Excel 2019, and GraphPad Prism 10. Data were expressed as mean ± standard deviation (SD). Group differences were evaluated using one-way analysis of variance (ANOVA). Following one-way ANOVA, post hoc multiple comparison testing was performed using Duncan’s Multiple Range Test (DMRT) for biochemical and oxidative stress parameters and Tukey’s test for gene expression analyses. Superscript letters in tables denote statistically homogeneous subsets, where identical letters indicate no significant difference and different letters indicate significant difference at P < 0.05.
Results and discussion
Pretreatment feed and fluid consumption
During the pretreatment period with normal diet feed (NDF) and fortified diet feed (FDF), animals showed higher consumption of NDF and tap water compared to FDF and fructose drink (Fig. 1). This difference may be attributed to the higher palatability of the NDF compared with the fat-enriched fortified diet24. Consumption gradually increased and stabilized by day 21. Previous findings suggest that sugar and salt are more effective in promoting feed intake than lipid enrichment28.
Daily feed and fluid intake are presented as “mean ± SD” (n = 6).
Change in animals’ weight, feed and fluid intake
Body weight remained comparable between NDF and FDF groups prior to induction (Fig. 2). One week after STZ induction, a significant weight reduction and increase in feed and fluid intake were observed (Figs. 2, 3 and 4), confirming hyperglycemic symptoms characteristic of diabetes29. Treatment with C. lanatus juice for 14 days gradually normalized feed and fluid intake and increased body weight, suggesting improvement in glycemic control and energy metabolism, consistent with earlier reports23.
All animals were fed normal diet feed during acclimatisation (weeks 0–2). At weeks 3 through 10, animals were divided into two feeding groups (normal-diet-fed and fortified-diet-fed). The data is represented by the mean ± SD of each experimental group, with n = 6. Watermelon juice (WMJ).
Daily feed intake by six groups of experimental animals after streptozotocin induction at week 8 and through week 10 is presented as “mean ± SD” (n = 6). Watermelon juice (WMJ).
Daily fluid intake by six groups of experimental animals after streptozotocin induction at week 8 and through week 10 is presented as “mean ± SD” (n = 6). Watermelon juice (WMJ).
Effects of C. lanatus on fasting blood glucose
Following STZ induction, fasting blood glucose (FBG) levels increased significantly in all diabetic groups. After 14 days of treatment, fasting blood glucose levels decreased significantly (p < 0.05) in all C. lanatus-treated groups in a clear dose-dependent pattern (Tables 1 and 2). This reduction aligns with previous reports that C. lanatus juice and leaf extracts improve glycemic control by enhancing insulin secretion and reducing oxidative stress in diabetic rodents22,23. The dose-dependent pattern observed here further indicates that the C. lanatus juice extract may enhance β-cell responsiveness. The observed normalization of glucose levels in the 1000 mg/kg group suggests an improvement in β-cell functional status, which may be associated with increased PDX1 gene expression, although direct mechanistic links require further validation.
Fasting blood glucose (FBG) was recorded at the major stages of the experiment:
-
1.
After five weeks of a dietary regimen at week 7;
-
2.
After 72 h of streptozotocin (STZ) induction;
-
3.
One-week post-STZ induction at week 8;
-
4.
One-week treatment at week 9; and.
-
5.
Two-week treatment at week 10.
Effect of C. lanatus juice on serum insulin and C-peptide of experimental animals
C. lanatus treatment improved insulin and C-peptide levels in a dose-dependent manner (Figs. 5 and 6). The 500 mg/kg and 1000 mg/kg groups exhibited significantly higher insulin levels compared to the diabetic control. These findings are consistent with reports that C. lanatus enhances insulin secretion through antioxidant protection of β-cells and modulation of key metabolic genes22,30. The concurrent rise in C-peptide further confirms improved endogenous insulin synthesis rather than external modulation.
The data represents the mean ± SD of all experimental groups. The different letters attached to each bar indicate a significant difference with p < 0.05. (n = 6). Watermelon juice (WMJ).
The data represents the mean ± SD of all experimental groups (n = 6). The different letters attached to each bar indicate a significant difference with p < 0.05. Watermelon juice (WMJ).
Effects of C. lanatus on insulin resistance and β-cell function
Homeostasis model assessment of insulin resistance (HOMA-IR) and homeostasis model assessment for beta-cell function (HOMA-β) results are recorded in Table 3. C. lanatus juice-treated groups showed significant (p < 0.05) beneficial effects on beta-cell function in a dose-dependent manner. Also, insulin resistance was significantly (p < 0.05) decreased in all C. lanatus-treated groups compared with the negative control group. Animals’ fasting blood glucose after two weeks of treatment with C. lanatus juice and animals’ terminal insulin levels were used in deriving HOMA scores26,31. Findings are indicative of type-2 diabetes, but none of these (HOMA-IR or HOMA-β) alone is sufficient to predict prediabetes and diabetes32,33. Also, C. lanatus was effective in reducing insulin resistance and improving beta-cell function in STZ-induced diabetes rats.
Effects of C. Lanatus juice on serum lipid profile of experimental animals
C. lanatus administration improved serum lipid profiles in diabetic rats (Table 4). HDL increased while LDL, triglycerides, and total cholesterol decreased in a dose-dependent manner. The 1000 mg/kg group showed lipid levels approaching those of metformin-treated animals. These findings corroborate previous studies reporting lipid-lowering and cardioprotective properties of C. lanatus34. The mechanism likely involves enhanced antioxidant activity, leading to reduced lipid peroxidation and improved lipid metabolism35.
Effect of C. Lanatus on oxidative stress in pancreatic tissues of experimental animals
Oxidative stress markers revealed substantial differences among groups (Table 5). STZ-induced diabetic rats showed elevated MDA and decreased antioxidant enzyme levels (CAT, SOD, GPx), confirming oxidative damage. C. lanatus juice significantly reversed these effects in a dose-dependent manner, restoring antioxidant defense comparable to metformin. Reduced levels of catalase, superoxide dismutase, and glutathione peroxidase, along with higher levels of malondialdehyde, were indicative of significant oxidative stress in the negative control group13,36. On the other hand, C. lanatus juice administration improved oxidative stress biomarkers, indicative of its antioxidative qualities, as previously noted18,22. It can be said that C. lanatus has antioxidative actions necessary for ameliorating oxidative stress in STZ-induced diabetic rats.
Effects of C. Lanatus juice on PDX1 gene expression in experimental animals
C. lanatus juice significantly upregulated PDX1 gene expression in a dose-dependent manner (Figs. 7 and 8). The highest increase occurred in the 1000 mg/kg group, approaching normal control levels. STZ-induced rats showed marked PDX1 suppression, consistent with oxidative stress–mediated β-cell dysfunction. Decreased expression of the PDX1 gene was significantly (p < 0.05) seen in the negative control group. The increase in PDX1 mRNA expression following C. lanatus treatment suggests a transcriptional response consistent with improved β-cell integrity, although this does not directly confirm protein-level restoration or β-cell regeneration. This aligns with studies linking antioxidant therapy to preservation of β-cell transcription factors20,37,38. A quantitative study also confirmed the relationship between elevated oxidative stress and type 2 diabetes as well as hypertension39.
The observed upregulation of PDX1 mRNA expression following C. lanatus treatment may reflect transcriptional responses associated with the administration of the crude juice extract. While antioxidant-related mechanisms are plausible, definitive attribution to specific phytochemical constituents cannot be made in the absence of extract-level quantitative characterization, which are known to exert potent antioxidant and cytoprotective effects. Oxidative stress has been shown to downregulate PDX1 expression through suppression of pancreatic transcriptional activity and activation of apoptotic cascades20. Lycopene, a carotenoid abundant in C. lanatus, can activate the Nrf2/Keap1 signaling pathway, enhancing the expression of endogenous antioxidant enzymes (SOD, CAT, GPx), thereby protecting β-cells from oxidative DNA and protein damage. Similarly, citrulline supports nitric oxide homeostasis and mitochondrial function, reducing oxidative burden and sustaining PDX1 transcriptional activity. These observations suggest that the increased PDX1 mRNA expression observed in this study may reflect antioxidant-mediated modulation of redox-sensitive transcriptional pathways, although confirmation at the protein and signaling levels is required, contributing to β-cell regeneration and improved insulin synthesis9,13,16,20.
The observed upregulation of PDX1 mRNA expression aligns with emerging evidence that antioxidant interventions can counteract oxidative suppression of β-cell transcription factors, primarily at the transcriptional level9,16. These recent findings collectively support our hypothesis that C. lanatus bioactives act via redox-sensitive transcriptional regulation pathways.
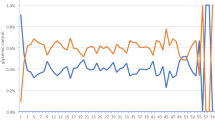
Pancreatic duodenal homeobox 1 (PDX1) gene expression is shown here by a real-time polymerase chain reaction (RT-PCR) graph. The Y axis is plotted with the frequencies of the fluorescence dye. The X axis is plotted with the number of RT-PCR cycles per gene expressed. The lower the cycle number above the threshold (62.29), the higher the number of DNA in solution.
The data represent the mean ± SD of each experimental group (n = 6). The letters attached to the bar indicate a significant difference (p < 0.05). Watermelon juice (WMJ) and pancreatic duodenal homeobox 1 (PDX1).
Histopathological effects of C. Lanatus juice on the pancreas of experimental animals
Histopathological examination of pancreatic sections revealed qualitative differences in overall islet architecture among experimental groups (Fig. 9). Diabetic control rats exhibited distorted pancreatic morphology with apparent islet disruption compared to normal controls, whereas C. lanatus–treated groups showed partial preservation of general islet structure. However, due to the absence of high-magnification images and quantitative morphometric analysis, these observations are limited to descriptive, low-resolution structural features and do not permit detailed assessment of β-cell mass, shrinkage, or cellular regeneration. These structural improvements corroborate biochemical and molecular findings, indicating that C. lanatus mitigates oxidative injury and supports β-cell structural preservation, with concurrent increases in PDX1 mRNA expression30.
Representative histopathological sections of pancreatic tissues (H&E stain). Yellow arrows indicate islet lesions and necrotic β-cells. Images illustrate overall pancreatic and islet architecture across experimental groups. Due to image resolution and magnification constraints, the figure is intended for qualitative comparison only and does not support quantitative evaluation of β-cell mass or cellular morphology. (1) Normal control; (2) Diabetic control; (3) Metformin-treated (500 mg/kg); (4–6) C. Lanatus juice–treated groups (200, 500, and 1000 mg/kg), showing progressive regeneration and improved cellular architecture. *Images are presented at low magnification to illustrate overall pancreatic architecture.
Taken together, these findings demonstrate that C. lanatus juice effectively ameliorates hyperglycemia, dyslipidemia, and oxidative stress in STZ-induced diabetic rats through both biochemical and molecular mechanisms. The observed restoration of PDX1 expression and preservation of pancreatic histoarchitecture suggest a potential β-cell–protective role of C. lanatus. These integrated results form the basis for the concluding remarks presented in this study.
The histopathological findings presented in this study are qualitative and supportive in nature. In the absence of high-magnification imaging and quantitative histological scoring, pancreatic histology was not used to infer β-cell mass, cellular regeneration, or precise architectural remodeling. Instead, histology was included to provide contextual structural observations that complement the biochemical and transcriptional findings.
A limitation of the present study is the absence of quantitative phytochemical characterization of the C. lanatus juice extract. Plant-derived products are known to exhibit batch-to-batch variability in phytochemical composition due to factors such as cultivar, maturity, processing, and storage conditions. Therefore, while prior studies have identified various bioactive compounds in C. lanatus, the present findings should be interpreted strictly at the level of the administered crude extract. Future studies incorporating HPLC-, LC–MS-, or GC–MS–based profiling are required to identify, quantify, and standardize the constituents responsible for the observed biological and transcriptional effects. A limitation of this study is the absence of higher-magnification histopathological images (40× or 100×), which restricted detailed cellular-level assessment of pancreatic islet morphology. Consequently, histological interpretations were based on overall structural preservation rather than fine β-cell ultrastructure. Future studies incorporating higher-resolution imaging and quantitative morphometric analysis would strengthen histopathological evaluation.
Another limitation of the present study is that only PDX1 mRNA expression was analyzed as a molecular endpoint. While this provides preliminary evidence of transcriptional modulation, further validation through upstream (FoxO1) and downstream (GLUT2, INS) gene analysis would better define the regulatory network underlying β-cell protection. Moreover, protein-level confirmation of PDX1 expression through Western blot or immunohistochemical analysis would strengthen the mechanistic interpretation of the observed effects. Future studies are therefore planned to integrate these approaches to provide a comprehensive understanding of how C. lanatus bioactives modulate β-cell function and glycemic regulation.
A further limitation of this study is that molecular interpretation was based solely on PDX1 mRNA expression. While changes in mRNA levels provide valuable insight into transcriptional regulation, they do not necessarily reflect corresponding changes in protein abundance, localization, or functional activity. Consequently, the present findings should be interpreted as supportive of transcriptional modulation rather than definitive mechanistic evidence. Future studies incorporating protein-level validation (e.g., Western blotting or immunohistochemistry) and analysis of upstream and downstream signaling pathways are required to confirm the functional role of PDX1 in mediating the observed β-cell effects.
Conclusion
This study demonstrates that C. lanatus juice significantly ameliorates hyperglycemia, dyslipidemia, and oxidative stress while upregulating PDX1 gene expression in STZ-induced type 2 diabetic rats. These effects suggest that C. lanatus may support pancreatic β-cell function and insulin biosynthesis, with effects associated with antioxidant activity and increased PDX1 gene expression, although definitive molecular mechanisms require further investigation. However, as these findings were obtained from an animal model, they should be interpreted with caution when considering human relevance. Differences in metabolism, dosage scaling, and long-term responses between species limit direct extrapolation. Therefore, further studies—including isolation of active compounds, mechanistic exploration in human cell models, and controlled clinical trials—are required to validate the therapeutic potential of C. lanatus juice in human diabetes management. Nonetheless, this study provides novel mechanistic insight into the role of C. lanatus in modulating PDX1 expression and oxidative stress, offering a strong foundation for future translational research.
Data availability
The datasets used and/or analysed during the current study are available from the first author on reasonable request.
References
Diabetes [Internet]. Who.int. [cited 2023 Nov 15]. Available from: https://www.who.int/news-room/fact-sheets/detail/diabetes
Okoduwa, S. I. R., Igiri, B. E., Tagang, J. I., Okoduwa, U. J. & Adeyi, A. O. Therapeutic smart-footwear approach for management of neuropathic diabetic foot ulcers: current challenges and focus for future perspective. Med. Nov Technol. Devices. 23, 100311. https://doi.org/10.1016/j.medntd.2024.100311 (2024).
IDF diabetes atlas [Internet]. International Diabetes Federation. IDF Diabetes Atlas, 11th Edition. (2025). Available at: https://www.diabetesatlas.org
Okoduwa, S. I. R., Umar, I. A., Ibrahim, S., Bello, F. & Habila, N. Age-dependent alteration of antioxidant defense system in hypertensive and type-2 diabetes patients. J. Diabetes Metab. Disord. 14 (32). https://doi.org/10.1186/s40200-015-0164-z (2015).
Okoduwa, S. I. R., Umar, I. A., Ibrahim, S., Bello, F. & Ndidi, U. S. Socio-Economic status of patients with type 2 diabetes and hypertension attending the Ahmadu Bello university teaching Hospital, Zaria, North-West Nigeria. Glob J. Health Sci. 7 (1), 280–287. https://doi.org/10.5539/gjhs.v7n1p280 (2014).
Galicia-Garcia, U. et al. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 21 (17), 6275. https://doi.org/10.3390/ijms21176275 (2020).
Marselli, L. et al. Persistent or transient human β cell dysfunction induced by metabolic stress: specific signatures and shared gene expression with type 2 diabetes. Cell. Rep. 33 (9), 108466. https://doi.org/10.1016/j.celrep.2020.108466 (2020).
Vilas-Boas, E. A., Almeida, D. C., Roma, L. P., Ortis, F. & Carpinelli, A. R. Lipotoxicity and β-Cell failure in type 2 diabetes: oxidative stress linked to NADPH oxidase and ER stress. Cells 10 (12), 3328. https://doi.org/10.3390/cells10123328 (2021).
Ebrahim, N., Shakirova, K. & Dashinimaev, E. PDX1 is the cornerstone of pancreatic β-cell functions and identity. Front. Mol. Biosci.. https://doi.org/10.3389/fmolb.2022.1091757 (2022).
McKinnon, C. M. & Docherty, K. Pancreatic duodenal homeobox-1, PDX-1, a major regulator of beta cell identity and function. Diabetologia 44 (10), 1203–1214. https://doi.org/10.1007/s001250100628 (2001).
Holland, A. M., Hale, M. A., Kagami, H., Hammer, R. E. & MacDonald, R. J. Experimental control of pancreatic development and maintenance. Proc. Natl. Acad. Sci. USA. 99 (19), 12236–12241. https://doi.org/10.1073/pnas.192255099 (2002).
Baumel-Alterzon, S. & Scott, D. K. Regulation of Pdx1 by oxidative stress and Nrf2 in pancreatic beta-cells. Front. Endocrinol. 13, 1011187. https://doi.org/10.3389/fendo.2022.1011187 (2022).
Barakat, A. Z., Bassuiny, R. I., Abdel-Aty, A. M. & Mohamed, S. A. Diabetic complications and oxidative stress: the role of phenolic‐rich extracts of saw palmetto and date palm seeds. J. Food Biochem. 44 (11). https://doi.org/10.1111/jfbc.13416 (2020).
Yin, Y., Zheng, Z. & Jiang, Z. Effects of lycopene on metabolism of glycolipid in type 2 diabetic rats. Biomed. Pharmacother. 109, 2070–2077. https://doi.org/10.1016/j.biopha.2018.07.100 (2019).
Danboyi, T. et al. Antioxidant effects of L-citrulline supplementation in high-fat diet- and dexamethasone-induced Type-2 diabetes mellitus in Wistar rats (Rattus norvegicus). Nigerian J. Exp. Clin. Biosci. 9 (2), 95. https://doi.org/10.4103/njecp.njecp_4_21 (2021).
Leh, H. E., Lee, L. K. & Lycopene A potent antioxidant for the amelioration of type II diabetes mellitus. Molecules 27 (7), 2335. https://doi.org/10.3390/molecules27072335 (2022).
Okoduwa, S. I. R. et al. Phytomedicine approach for management of diabetes mellitus: an overview of scientifically confirmed medicinal plants with hypoglycaemic properties and their probable mechanism of action. Phytochem Rev. 22. https://doi.org/10.1007/s11101-024-09984-2 (2024 June).
Oseni, O., Odesanmi, O. & Oladele, F. Antioxidative and antidiabetic activities of watermelon (Citrullus lanatus) juice on oxidative stress in alloxan-induced diabetic male Wistar albino rats. Nigerian Med. J. 56 (4), 272. https://doi.org/10.4103/0300-1652.169707 (2015).
Gautam, K., Kumar, S., Srivastava, S. & Singh, P. Pharmacological properties of citrullus lanatus. Perspect. Recent. Adv. Med. Res. 6, 10–30. https://doi.org/10.9734/bpi/pramr/v6/4105b (2023).
Zhang, Y. et al. PDX-1: A promising therapeutic target to reverse diabetes. Biomolecules 12 (12), 1785. https://doi.org/10.3390/biom12121785 (2022).
Scully, T. et al. New insights into PDX1 signaling and β-cell plasticity under metabolic stress. Trends Endocrinol. Metabol. 34 (4), 270–284 (2023).
Ajiboye, B. O., Shonibare, M. T. & Oyinloye, B. E. Antidiabetic activity of watermelon (Citrullus lanatus) juice in alloxan-induced diabetic rats. J. Diabetes Metab. Disord. 19 (1), 343–352. https://doi.org/10.1007/s40200-020-00515-2 (2020).
Jibril, M. M. et al. Watermelon (Citrullus lanatus) leaf extract attenuates biochemical and histological parameters in high‐fat diet/streptozotocin‐induced diabetic rats. J. Food Biochem. 46 (2). https://doi.org/10.1111/jfbc.14058 (2022).
Okoduwa, S. I. R., Umar, I. A., James, D. B. & Inuwa, H. M. Appropriate insulin level in selecting fortified Diet-Fed, Streptozotocin-Treated rat model of type 2 diabetes for Anti-Diabetic studies. PloS One. 12 (1), e0170971. https://doi.org/10.1371/journal.pone.0170971 (2017).
Binter, M. et al. A simple dissection method for the isolation of mouse trabecular meshwork cells. PloS One. 18 (12), e0296124. https://doi.org/10.1371/journal.pone.0296124 (2023).
Matthews, D. R. et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28 (7), 412–419. https://doi.org/10.1007/bf00280883 (1985).
Babaiedarzi, A., Ghanbari, S., Seresht, M. M. & Nasiri, M. Antidiabetic effects of scrophularia striata ethanolic extract via suppression of Pdx1 and Ins1 expression in pancreatic tissues of diabetic rats. Sci. Rep. 12 (1). https://doi.org/10.1038/s41598-022-13698-w (2022).
Oliva, L. et al. In rats fed high-energy diets, taste, rather than fat content, is the key factor increasing food intake: a comparison of a cafeteria and a lipid-supplemented standard diet. PeerJ 5 (e3697), e3697. https://doi.org/10.7717/peerj.3697 (2017).
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 32(Supplement_1), S62–S67. https://doi.org/10.2337/dc09-s062
Ahn, J., Choi, W., Kim, S. & Ha, T. Anti-diabetic effect of watermelon (Citrullus vulgaris Schrad) on Streptozotocin-induced diabetic mice. Food Sci. Biotechnol. 20 (1), 251–254. https://doi.org/10.1007/s10068-011-0034-5 (2011).
Thomas, M. K. et al. Dual GIP and GLP-1 receptor agonist Tirzepatide improves Beta-cell function and insulin sensitivity in type 2 diabetes. J. Clin. Endocrinol. Metab. 106 (2), 388–396. https://doi.org/10.1210/clinem/dgaa863 (2020).
Sama, S. et al. Quantifying the homeostatic model assessment of insulin resistance to predict mortality in Multi-organ dysfunction syndrome. Indian J. Crit. Care Med. 25 (12), 1364–1369. https://doi.org/10.5005/jp-journals-10071-24043 (2021).
Khalili, D. et al. Are HOMA-IR and HOMA-B good predictors for diabetes and pre-diabetes subtypes? BMC Endocri Disord. 23 (1). https://doi.org/10.1186/s12902-023-01291-9 (2023).
Connolly, M. et al. Effect of fresh watermelon consumption on risk factors for cardiovascular disease in overweight and obese adults (P06-102-19). Curr. Dev. Nutr. 3 (Suppl 1), nzz031–P06. https://doi.org/10.1093/cdn/nzz031.p06-102-19 (2019).
Kebede, M. et al. Glucose activates free fatty acid receptor 1 gene transcription via phosphatidylinositol-3-kinase-dependent O -GlcNAcylation of pancreas-duodenum homeobox-1. Proc. Natl. Acad. Sci. USA. 109 (7), 2376–2381. https://doi.org/10.1073/pnas.1114350109 (2012).
Tekin, S. & Seven, E. Assessment of serum catalase, reduced glutathione, and superoxide dismutase activities and malondialdehyde levels in keratoconus patients. Eye 36 (10), 2062–2066. https://doi.org/10.1038/s41433-021-01753-1 (2021).
Evans-Molina, C. et al. Glucose regulation of insulin gene transcription and Pre-mRNA processing in human Islets. Diabetes 56 (3), 827–835. https://doi.org/10.2337/db06-1440 (2007).
Leenders, F. et al. Oxidative stress leads to β-Cell dysfunction through loss of β-Cell identity. Front. Immunol. 12, 690379. https://doi.org/10.3389/fimmu.2021.690379 (2021).
Okoduwa, S. I. R., Umar, A., Ibrahim, S. & Bello, F. Relationship of oxidative stress with type 2 diabetes and hypertension. J. Diabetol. 4 (1), 4 (2013). https://journals.lww.com/jodb/abstract/2013/04010/relationship_of_oxidative_stress_with_type_2.4.aspx
Acknowledgements
Thanks and appreciation to the Babcock University administration for providing enabling facilities and staff in the conduct of this study. Special thanks to the laboratory scientists at the Department of Biochemistry, Babcock University, Ilishan-Remo, Nigeria, for their technical support.
Funding
declaration.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
The conceptualisation, study design, methodology, supervision, visualisation, and validation were undertaken by S.I.R.O. Investigation, formal analysis, resource provision, and funding acquisition were conducted by A.G.S.M. The original draft of the manuscript was written by A.G.S.M., while both S.I.R.O. and A.G.S.M. contributed to the review and editing of the final manuscript. Both authors approved the final version and consent for the publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
for the study was obtained from the Babcock University Health Research Ethics Committee (BUHREC 048/24). All methods were carried out in accordance with the relevant guidelines and regulations of the institution and national standards for the care and use of laboratory animals. Furthermore, all methods are reported in accordance with the ARRIVE guidelines (https://www.arriveguidelines.org) for animal research.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moore, A.G.S., Okoduwa, S.I.R. Modulation of PDX1 gene expression and glycemic control by Citrullus lanatus in experimental type 2 diabetes. Sci Rep 16, 3793 (2026). https://doi.org/10.1038/s41598-025-33982-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-33982-9