Abstract
To investigate temporal changes in the relationship between central venous pressure (CVP) and B-line scores through dynamic monitoring of the two parameters in patients undergoing transurethral resection of the prostate (TURP). A total of 101 patients who underwent TURP were enrolled in the study, with the procedure being performed under general anesthesia. B-line scores (quantified via transthoracic lung ultrasound), CVP, PaO₂/FiO₂ ratio, hemoglobin (Hb), peak airway pressure (Ppeak), arterial sodium (Na⁺), and potassium (K⁺) concentrations were measured at the following time points: baseline at the start of surgery (T0) and upon irrigation fluid volumes of 5000 mL (T1), 10,000 mL (T2), 15,000 mL (T3), and 20,000 mL (T4). Generalized additive mixed models (GAMMs) were used to analyze the longitudinal data. In the longitudinal study of 101 patients undergoing TURP, 90 were included in the final analysis. Poisson GAMM analyses revealed a significant time effect on B-line scores (F = 62.029–69.486, P < 0.001 across models), showing progressive increases from T0 to T4. CVP demonstrated a modest main effect (F = 3.156–4.026, P = 0.045–0.076) and a non-significant interaction with time (F = 1.441–1.967, P = 0.161–0.231), persisting after adjustments for age, weight, height, effective tidal volume, and intraoperative use of furosemide (adjusted pseudo-R2 = 0.553). Quantitative and percentile analyses showed only small and uncertain associations between CVP and B-line scores, with confidence intervals consistently crossing the null. Across multiple sensitivity analyses, the estimated CVP effect remained minimal and statistically non-significant regardless of modeling approach. B-line scores increased progressively during TURP, whereas the association between CVP and B-line scores remained small and statistically non-significant across all analyses. These findings suggest that intraoperative B-line dynamics primarily reflect temporal changes rather than CVP fluctuations.
Trial registration
The trial registration number: ChiCTR2200065753, 14/11/2022. Title: “Application of transthoracic lung ultrasound in patients undergoing prostatectomy”. Website: https://www.chictr.ogr.cn.
Similar content being viewed by others
Introduction
Transurethral resection of the prostate (TURP) would potentially damage the prostatic venous plexus, thereby permitting irrigation fluid to enter the systemic circulation via disrupted blood vessels1. Excessive absorption of irrigation fluid into the bloodstream may result in circulatory overload, pulmonary edema, and various other complications, thereby posing a substantial risk to patient safety2. Consequently, close monitoring of irrigation fluid absorption is imperative.
Central venous pressure (CVP) is an indicator of venous return and right heart preload3,4. Although the accuracy of CVP is influenced by numerous factors and its performance in predicting fluid responsiveness is limited4, CVP remains widely used for routine volume monitoring in patients undergoing TURP. However, the implementation of CVP monitoring requires central venous catheterization. This technique is an invasive procedure that relies on specific technical skills and equipment, while carrying inherent risks of complications5.
A plethora of studies had previously corroborated the notion that the transthoracic lung ultrasound B-line scores were capable of reflecting interstitial lung pathology with a high degree of sensitivity, whilst concurrently providing intuitive, real-time monitoring of dynamic changes in pulmonary congestion and edema6,7. In patients exhibiting critical illness, B-line scores exhibited a weak yet statistically significant correlation with CVP. Conversely, lung ultrasound demonstrated higher sensitivity than CVP in assessing pulmonary fluid status8. As a non-invasive and safe monitoring technique, transthoracic lung ultrasound is simple to learn and suitable for clinical practice9,10.
The objective of this study was to dynamically monitor the CVP and B-line scores in patients undergoing TURP, with a view to investigating the relationship between CVP and B-line scores.
Materials and methods
Study design
This prospective, single-center observational study was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (2022-K124-01) and registered in the Chinese Clinical Trial Registry (ChiCTR2200065753).
Participants
All eligible patients were scheduled for TURP at the First Affiliated Hospital of Guangxi Medical University. The inclusion criteria encompassed patients between the ages of 60 and 85 years, with a physical status classified as I–III according to the American Society of Anesthesiologists (ASA) grading system, prostate hyperplasia grade ≥ III, and surgical duration ≥ 1 h.
The exclusion criteria comprised the following: severe cardiac diseases (e.g. heart failure, severe arrhythmias); pulmonary diseases (e.g. pneumonia, pleural effusion, pulmonary tumours, history of lung surgery); liver or kidney dysfunction; electrolyte imbalances; and chest skin infections, defects, or severe scarring. All patients were informed of the study details and procedures and provided written informed consent prior to enrolment.
Anesthesia management
Patients underwent a series of standard monitoring procedures, including electrocardiography, blood pressure, pulse oximetry, and bispectral index (BIS) monitoring before receiving anesthesia. Anesthesia was induced with target-controlled infusion (TCI) of propofol (2.0–4.0 μg/ml) and remifentanil (2.0–4.0 ng/ml). Following the onset of loss of consciousness, 0.2 mg/kg of cisatracurium and 0.3 μg/kg of sufentanil were administered intravenously. Three to five minutes later, endotracheal intubation was performed, followed by mechanical ventilation. The ventilator settings employed in this study were volume-controlled, with a tidal volume of 6–8 ml/kg based on ideal body weight, a respiratory rate of 10–15 breaths/min, and a fraction of inspired oxygen of 50%–60%. Anesthesia was sustained through the administration of TCI of propofol (1.0–3.0 μg/ml) and remifentanil (1.0–3.0 ng/ml), with the adjunctive use of sevoflurane (1%–2%), while maintaining BIS values within the range of 40 and 60.
Following the induction of anesthesia, ultrasound guidance was utilized to navigate a 7F double-lumen catheter (Arrow, Teleflex, Wayne, PA, USA) into the right internal jugular vein. The catheter depth was calculated as follows: depth (cm) = [patient height (cm) ÷ 10] − 111. The pressure transducer was zeroed at the midaxillary line at the fourth intercostal space and connected to the central venous catheter for continuous CVP monitoring. Patients were positioned in the lithotomy position for surgical procedures, with the irrigation bag positioned at a distance of 70 cm above the bladder and an irrigation flow rate of 200–300 ml per minute. In instances where CVP reached at least 7.4 mmHg intraoperatively, furosemide (10 mg) was administered intravenously.
Transthoracic lung ultrasound examination method
All patients underwent lung ultrasound performed by a senior anesthesiologist using a portable convex array probe (FUJIFILM Sonosite M-Turbo, L38xi, 3–8 MHz), with a second operator responsible for image review. Patients were positioned supine, and the classic 8-zone method was applied to systematically evaluate bilateral lung fields12. Each hemithorax was divided longitudinally into anterior and lateral regions by the parasternal, anterior axillary, and posterior axillary lines, and transversely into upper and lower zones by the nipple line (approximately the 5th intercostal space), yielding a total of eight assessment zones per patient.
The probe was initially placed perpendicular to the ribs, and B-lines were counted after identification of the “bat sign.” It was then oriented parallel to the intercostal spaces, and the maximum number of B-lines was recorded on two occasions. In each zone, the probe was adjusted up to three times to obtain the maximal B-line count. B-lines were defined as comet-tail artifacts arising from the pleural line, extending vertically to the bottom of the screen without attenuation, and moving synchronously with lung sliding. Multiple confluent B-lines appeared as diffuse subpleural hyperechoic shadows.
A semi-quantitative B-line score was assigned for each zone as follows: no B-line or a single A-line, 0 points; each discrete B-line, 1 point; confluent B-lines occupying 50% of the screen, 5 points; 75% of the screen, 8 points; and full screen, 10 points. The total B-line score was calculated by summing all zones (range, 0–80 points)13.
Data collection
Lung ultrasound B-line scores, CVP, and physiological Parameters—including the PaO₂/FiO₂ ratio, hemoglobin (Hb), peak airway pressure (Ppeak), and arterial sodium (Na⁺) and potassium (K⁺) concentrations, as well as effective tidal volume and positive end-expiratory pressure (PEEP) during mechanical ventilation—were measured at the following time points: baseline at the start of surgery (T0) and upon irrigation fluid volumes of 5000 mL (T1), 10,000 mL (T2), 15,000 mL (T3), and 20,000 mL (T4).
Sample size calculation
The required sample size was estimated using a simulation-based approach for a Poisson generalized additive mixed model (GAMM) with random intercepts for repeated B-line measurements. We assumed a mean B-line score increase of 0.48 points per 1 mmHg increase in CVP, five repeated measurements per patient, and a within-subject correlation of 0.48. Simulations were conducted by generating repeated B-line scores under these assumptions, fitting the Poisson GAMM, and calculating the proportion of simulations in which the CVP effect was statistically significant at a two-sided α = 0.05. A sample of 72 patients achieved approximately 80% power. Accounting for a 15% dropout rate, the target sample size was increased to 85 patients.
Statistical analysis
This study employed a longitudinal design to collect B-line scores, CVP, and physiological parameters—including the PaO₂/FiO₂ ratio, Hb, Ppeak, Na⁺, and K⁺—from perioperative patients at five measurement time points (T0 to T4). All continuous variables were described using means ± standard deviations. Participants with incomplete data were excluded from the analysis.
The primary outcome of the study was the longitudinal effect of CVP on B-line scores. We used Poisson GAMMs to deal with the count nature of B-line scores and mild underdispersion (variance-to-mean ratio = 0.924). The models incorporated time as a smooth term (using thin plate regression splines, with degrees of freedom selected via generalized cross-validation), CVP as a fixed effect, and a time × CVP interaction term to evaluate time-dependent associations.
The secondary outcomes were the longitudinal effects of B-line scores and CVP on physiological parameters. To accommodate the non-normal distribution, strictly positive values, and skewness of these parameters, we applied Gamma GAMMs. Each physiological parameter was modeled separately.
To evaluate the robustness of the primary results, we performed sensitivity analyses using an expanded dataset (n = 98, including the 8 patients with intraoperative irrigation volume < 20,000 mL), Quasi-Poisson GAMMs, and Poisson models with robust standard errors (SE). To ensure that the observed CVP–B-line association was not confounded by acute diuretic effects, we additionally adjusted for current furosemide use and preceding-time-point furosemide exposure (lag 1).
All analyses were performed using R software version 4.5.1 (R Foundation for Statistical Computing, Vienna, Austria), with two-sided P-values < 0.05 deemed significant. Model fitting was conducted using the mgcv package (The exact formula of the final model for the primary outcome was provided in the Supplementary Materials).
Result
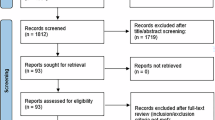
Between December 2022 and October 2024, 106 patients scheduled for TURP were screened for eligibility. Among them, 5 were excluded due to meeting exclusion criteria or declining participation, 3 were excluded for having a surgery duration of less than 1 h, and 8 were excluded despite adequate surgical duration because their intraoperative irrigation volume did not reach 20,000 mL. Ultimately, 90 patients with complete data were included in the final statistical analysis (Fig. 1).
Flowchart of patient inclusion in the prospective observational study. Notes: A total of 106 potential eligible patients were screened, and ultimately 90 patients were included in the final analysis. After enrollment, 11 patients were excluded due to not meeting the predetermined surgical conditions during surgery (3 cases with surgery duration < 1 h, 8 cases with Intraoperative irrigation fluid volume < 20,000 mL). All 11 cases were excluded intraoperatively, none were lost to follow-up.
The demographic and procedural characteristics of the study were as follows: the mean age 69.4 ± 7.8 years, mean body weight 64.6 ± 10.5 kg, and mean height 165.6 ± 6.7 cm, mean effective tidal volume 418.4 ± 55.3 mL, mean duration of surgery anaesthesia 145.5 ± 41.1, mean duration of anaesthesia 131.7 ± 40.1 min, mean intraoperative infusion volume 697.2 ± 276.3 mL, and mean irrigation volume 32,527.8 ± 9017.4 mL. In addition, we compared baseline characteristics between included patients (intraoperative irrigation volume ≥ 20,000 mL, n = 90) and those excluded due to an irrigation volume < 20,000 mL (n = 8). The results showed that patients with irrigation volume < 20,000 mL had significantly shorter duration of surgery than those with ≥ 20,000 mL (P < 0.001) (Table S1 in Supplementary Materials).
Longitudinal effects of CVP on B-line scores
To assess the longitudinal effect of CVP on B-line scores, a Poisson GAMM was constructed, with covariates—including age, weight, height, effective tidal volume, and intraoperative use of furosemide—progressively incorporated for adjustment (Table 1). Since all patients received mechanical ventilation without the use of PEEP, this variable was not included as a covariate. After adjustment, all models achieved deviance-explained values (all pseudo-R2 = 0.553), indicating strong model fit and substantial explanatory power for B-line scores.
Model analysis demonstrated a highly significant time effect across all models (all P < 0.001), indicating a marked increase in B-line scores over time. The main effect of CVP varied among the adjusted models: in Model 3 (adjusted for age, weight, height, and effective tidal volume), CVP was significantly associated with B-line scores (F = 4.026, P = 0.045). However, in the final model (Model 4, further adjusted for intraoperative use of furosemide), the association lost statistical significance (F = 3.156, P = 0.076). The interaction between time and CVP was not statistically significant in any model (all P > 0.05).
Predicted trajectories further clarified the longitudinal association between CVP and B-line scores. As illustrated in Fig. 2, B-line scores increased exponentially throughout the observation period (T0–T4). Patients with higher CVP levels (upper 75th percentile) consistently exhibited greater predicted B-line scores at all time points than those with lower CVP levels (lower 25th percentile).
Poisson GAMM–adjusted temporal trends of predicted B-line scores stratified by CVP levels. Notes: Predicted B-line score over five measurement time points (T0–T4) was shown for three CVP levels [low (25th percentile), median, and high (75th percentile)], adjusted for age, weight, height, actual tidal volume, and intraoperative use of furosemide. Solid lines represent predicted means, and shaded areas indicate 95% confidence intervals for each CVP level. CVP, central venous pressure. Measurement time points were defined as baseline at the start of surgery (T0), and after intraoperative irrigation volumes of 5000 mL (T1), 10,000 mL (T2), 15,000 mL (T3), and 20,000 mL (T4).
Based on Model 4 predictions, Fig. 3 (interaction contour plot) visualizes the combined effects of CVP and time on B-line scores. The transition of B-line scores from low (lower left) to high (upper right) regions indicates that elevated CVP levels and advanced time points jointly contribute to higher B-line scores. The nearly parallel diagonal contour lines corroborate the statistical finding that the interaction between CVP and time was not significant.
Poisson GAMM-adjusted predicted B-line score as a function of time and CVP. Notes: The contour plot shows predicted B-line score across five measurement time points (T0–T4) and CVP levels, adjusted for age, weight, height, actual tidal volume, and intraoperative use of furosemide. Filled colors indicate predicted scores (cool = low, warm = high), and white contour lines mark specific score boundaries. CVP, central venous pressure. Measurement time points were defined as baseline at the start of surgery (T0), and after intraoperative irrigation volumes of 5000 mL (T1), 10,000 mL (T2), 15,000 mL (T3), and 20,000 mL (T4).
Quantitative analysis (Table 2) showed a positive association between CVP and B-line scores, with rate ratios exceeding 1.0 across all time points and CVP levels (range: 1.017–1.075). However, the 95% confidence intervals crossed 1.0, indicating uncertainty in the effect size. Complementary percentile analysis (Table 3) revealed a progressive increase in B-line scores from T0 to T4, while the spread between the 75th and 25th percentiles remained relatively stable (0.331–0.470). Confidence intervals for these differences consistently crossed zero, suggesting no significant change in interindividual variability.
Across all sensitivity analyses, the estimated effect of CVP on B-line scores remained consistently small and statistically non-significant. The primary Poisson GAMM yielded a rate ratio (RR) of 1.046 (95% CI: 0.895–1.221), which was virtually unchanged in the alternative Poisson GAMM including all 98 patients (RR = 1.036). The quasi-Poisson GAMM (RR = 1.070) and the Poisson model with robust standard errors (RR = 1.025) likewise produced null results (Table S2 in Supplementary Materials).
To further evaluate potential confounding from intraoperative diuretic administration, furosemide use was incorporated as a time-varying binary covariate, modeled as both a current and lagged exposure. Neither specification was associated with B-line scores (current: RR = 0.981; lag1: RR = 0.965; both P > 0.78), and model fit indices (adusted pseudo-R2 and REML) remained unchanged (Table S3 in Supplementary Materials).
Together, these analyses demonstrate that the absence of a detectable CVP effect is robust across modeling approaches, dataset specifications, and adjustment for intraoperative furosemide administration.
Longitudinal changes in physiological parameters
We employed gamma GAMMs to analyze the temporal dynamics of B-line scores or CVP on physiological parameters (PaO₂/FiO₂ ratio, Hb, Ppeak, Na⁺, and K⁺). All models adjusted for age, weight, height, effective tidal volume, and intraoperative use of furosemide.
Longitudinal effects of B-line scores on physiological parameters
As shown in Table 4, The main time effect was significant in all models (F values ranging from 25.132 to 368.848, all P < 0.001), confirming that physiological parameters changed over time. The B-line score effect was observed in the PaO₂/FiO₂ ratio (F = 8.460, P = 0.004), Hb (F = 1.170, P = 0.280) , Ppeak (F = 2.066, P = 0.151), Na⁺ (F = 3.765, P = 0.053), and K⁺ (F = 1.487, P = 0.223), with PaO₂/FiO₂ ratio being the only significant effect, suggesting B-line score independently influences oxygenation indicators. Interaction effects (time × B-line score) were statistically significant in most models: PaO₂/FiO₂ ratio (F = 0.657, P = 0.409); Hb (F = 5.338, P < 0.001); Ppeak (F = 3.107, P = 0.016); Na⁺ (F = 6.229, P < 0.001); K⁺ (F = 0.007, P = 0.933). This indicated that the B-line scores modulates the temporal trajectories of Hb, Ppeak, and Na⁺.
These findings were visualized in Fig. 4, which depicted the adjusted time trend of predicted physiological parameters stratified by B-line score level (low: 25th percentile; median; high: 75th percentile). The stratified curves show: PaO₂/FiO₂ ratio decreased from approximately 340 mmHg at T0 to approximately 240 mmHg at T4 across all groups, with a slower decline in the high B-line score group; Hb decreased from approximately 115 g/L to approximately 105 g/L, starting at a lower baseline in the high group; Ppeak increased from approximately 18 cmH₂O to approximately 22 cmH₂O, with the steepest rise in the high group; Na⁺ rose slightly from approximately 137 mmol/L to approximately 140 mmol/L; K⁺ decreased from approximately 4.4 mmol/L to approximately 3.8 mmol/L. The 95% confidence intervals indicated increased variability in the later stages, particularly in the high B-line score group. Overall, GAMM results and stratified trends highlighted a time-dependent association between higher B-line scores and deteriorating trajectories of physiological parameters such as Hb and Ppeak.
Poisson GAMMs–adjusted temporal trends of predicted PaO₂/FiO₂ ratio, Hb, Ppeak, Na+, and K+ Stratified by B-line score levels. Notes: The fitted plot illustrated the temporal trends of the predicted B-line Score over the five measurement time points (T0–T4), stratified by three key B-line score levels [low (25th percentile), median, and high (75th percentile)]. The predictions were adjusted for age, weight, height, actual tidal volume, and intraoperative use of furosemide. The solid lines and shaded areas represented the predicted means and their respective 95% confidence intervals,respectively. Hb, hemoglobin; Ppeak, peak airway pressure; Na⁺, arterial sodium concentration; K⁺, arterial potassium concentration. Measurement time points were defined as baseline at the start of surgery (T0), and after intraoperative irrigation volumes of 5000 mL (T1), 10,000 mL (T2), 15,000 mL (T3), and 20,000 mL (T4).
Longitudinal effects of CVP on physiological parameters
As shown in Table 5, the main time effect was highly significant (F values ranging from 38.033 to 166.789, all P <0.001), confirming parameter changes over time. The CVP effect was not statistically significant for PaO₂/FiO₂ ratio (F = 2.875, P = 0.096), Hb (F = 3.811, P = 0.052) , and Na⁺ (F = 0.001, P = 0.980), while it reached statistical significance for Ppeak (F = 4.002, P = 0.046), and K⁺ (F = 7.419, P = 0.007). These findings suggest that CVP may independently influence both Ppeak dynamics and serum K⁺ levels. Interaction effects (time × CVP) were significant in most models: PaO₂/FiO₂ ratio (F = 6.322, P = 0.012); Hb (F = 10.229, P < 0.001); Ppeak (F = 3.660, P = 0.008); Na⁺ (F = 5.528, P < 0.001); K⁺ (F = 2.446, P = 0.119). This indicates that CVP significantly modulates the temporal trajectories of most parameters.
These findings were visualized in Fig. 5, illustrating the adjusted trends of predicted physiological parameters stratified by CVP level (low: 25th percentile; median; high: 75th percentile). The curves indicated: PaO₂/FiO₂ ratio decreased from approximately 340 mmHg at T0 to approximately 240 mmHg at T4, with the steepest decline in the high CVP group; Hb decreased from approximately 115 g/L to approximately 105 g/L, with a lower trajectory in the high group; Ppeak increased from approximately 18 cmH₂O to approximately 22 cmH₂O, with a faster rise in the high group; Na⁺ increased from approximately 137 mmol/L to approximately 140 mmol/L; K⁺ decreased from approximately 4.4 mmol/L to approximately 3.8 mmol/L, with a greater decline in the high group. The 95% confidence intervals widened in the later stages, particularly in the high CVP group. Overall, GAMM analysis and trend plots highlighted the temporal association between elevated CVP and adverse changes in physiological parameters with this association persisting after adjustment.
Poisson GAMMs–adjusted temporal trends of predicted PaO₂/FiO₂ ratio, Hb, Ppeak, Na+, and K+ stratified by CVP levels. Notes: The fitted plot illustrated the temporal trends of the predicted CVP over the five measurement time points (T0–T4), stratified by three key CVP levels [low (25th percentile), median, and high (75th percentile)]. The predictions were adjusted for age, weight, height, actual tidal volume, and intraoperative use of furosemide. The solid lines and shaded areas represented the predicted means and their respective 95% confidence intervals,respectively. Hb, hemoglobin; Ppeak, peak airway pressure; Na⁺, arterial sodium concentration; K⁺, arterial potassium concentration. Measurement time points were defined as baseline at the start of surgery (T0), and after intraoperative irrigation volumes of 5000 mL (T1), 10,000 mL (T2), 15,000 mL (T3), and 20,000 mL (T4).
Discussions
This prospective observational study investigated the temporal dynamics of B-line scores and their relationship with CVP during TURP procedures associated with substantial irrigation fluid absorption. Consistent with the expected physiological impact of progressive fluid uptake, B-line scores increased markedly over time. In contrast, the association between CVP and B-line scores was small and statistically non-significant across all modeling approaches, and no time-dependent interaction was detected. These findings indicate that intraoperative B-line progression primarily reflects temporal fluid accumulation rather than changes in CVP, supporting the utility of dynamic lung ultrasound as a sensitive marker for early pulmonary fluid shifts during fluid-intensive urological procedures.
The observed gradual rise in B-line scores aligns with the underlying pathophysiology of irrigation fluid absorption during TURP. Irrigation fluid enters the systemic circulation through the prostatic venous sinuses at rates of approximately 10–30 mL/min, with total absorption potentially exceeding 1.5 L during a single procedure1. This process can lead to hypervolemia, dilutional hyponatremia, and elevated pulmonary capillary hydrostatic pressure, facilitating transudation of fluid into the lung interstitium and resulting in the appearance of B-lines on lung ultrasound12. By excluding cases with irrigation volumes < 20,000 mL, we specifically targeted high-risk situations in which fluid absorption is closely associated with prolonged surgical duration (> 1 h), as supported by the significantly shorter procedures observed in the excluded low-volume group14.
Although point estimates from Poisson and quasi-Poisson GAMMs suggested a numerical trend toward higher B-line scores with increasing CVP, all confidence intervals crossed unity, indicating substantial uncertainty and the absence of a statistically reliable association. The lack of a CVP–time interaction further suggests that CVP does not meaningfully modify the temporal trajectory of pulmonary congestion. This pattern aligns with previous evidence showing that static CVP is an imprecise indicator of intravascular volume status or extravascular lung water, particularly under conditions of fluid loading. In contrast, lung ultrasound–derived B-line scores remain a more responsive and physiologically direct marker of interstitial fluid accumulation15.
Adjustment for intraoperative furosemide administration—modeled as both current and lagged exposure—did not materially alter the association between CVP and B-line scores, as neither form of furosemide use was significantly related to B-line outcomes. Model fit indices also remained unchanged, reinforcing that the null CVP effect was robust to the inclusion of time-varying diuretic use. The wider confidence intervals observed toward T3–T4 likely reflect increased interindividual variability during ongoing fluid absorption rather than any CVP-mediated phenomenon.
Regarding secondary outcomes, we examined the bidirectional relationships between B-line scores and CVP with key physiological parameters, including the PaO₂/FiO₂ ratio, Hb, Ppeak, and Na⁺ and K⁺. Gamma GAMM analyses revealed significant interaction effects for most variables under both stratifications (P < 0.05 for Hb, Ppeak, and Na⁺ with B-lines; P < 0.05 for PaO₂/FiO₂, Hb, Ppeak, and Na⁺ with CVP), indicating that higher B-line scores and a lesser extent higher CVP levels modulate the temporal trajectories of these parameters.
The PaO₂/FiO₂ ratio declined across all groups (from approximately 340 mmHg at T0 to 240 mmHg at T4), suggesting impaired oxygenation likely due to ventilation–perfusion mismatch secondary to pulmonary edema16,17. Similarly, Hb levels decreased (from ~ 115 g/dL to ~ 105 g/dL), with a more pronounced decline in patients with higher B-line scores or CVP, possibly reflecting hemodilution from fluid overload or blood loss exacerbated by venous congestion18. Ppeak increased progressively (from 18 cmH₂O to 22 cmH₂O), with the steepest rise in high-risk strata, suggesting reduced pulmonary compliance due to interstitial edema—consistent with critical care literature linking B-lines to ventilator demand and impaired mechanics19,20.
Electrolyte changes were more subtle. Serum Na⁺ levels showed a mild increase (from ~ 137 to 140 mmol/L), possibly attributable to the use of normal saline as the irrigation solution or other hypertonic effects, whereas K⁺ levels decreased (from ~ 4.4 to 3.8 mmol/L), with CVP demonstrating a significant independent association. This finding aligns with prior reports of perioperative hypokalemia in cardiac surgery, potentially related to fluid shifts or diuretic use21,22.
Collectively, these secondary associations highlight the systemic consequences of pulmonary congestion and elevated CVP, extending beyond the lungs to influence gas exchange, hematologic parameters, and electrolyte homeostasis—factors that collectively shape perioperative morbidity risk.
Our results align with growing evidence supporting the prognostic utility of lung ultrasound in perioperative care, where B-lines have been shown to predict postoperative pulmonary complications (PPCs) and correlate inversely with the PaO₂/FiO₂ ratio in mechanically ventilated patients19,23. Clinically, these findings support the integration of serial lung ultrasound assessments into TURP management protocols for high-risk patients (e.g., those undergoing prolonged procedures or with hypertension), as B-line scores exceeding 5–8 have demonstrated approximately 80% sensitivity for predicting PPCs24. Moreover, the use of generalized additive mixed models (GAMMs), which capture nonlinear temporal dynamics beyond the capability of traditional linear mixed models, enhances analytical precision in longitudinal perioperative studies and offers a framework for developing individualized, physiology-guided fluid management strategies.
This study has several limitations. First, its observational design precludes causal inference; randomized trials evaluating CVP-guided interventions are warranted to confirm these associations. Second, the predominantly elective surgical population limits generalizability to emergency or non-urological settings. The eight-zone lung ultrasound protocol may have missed subtle interstitial changes and is sensitive to patient positioning. Additional limitations include the single-center design and exclusion of short or low-irrigation procedures, which restricts applicability to low-risk TURP but enhances relevance for high-absorption cases. Moreover, the study did not include postoperative clinical outcomes—such as pulmonary complications or respiratory support requirements—which limits the ability to determine whether the observed changes in B-line scores or CVP translate into clinically meaningful differences. As such, the predictive clinical value of the observed physiological variations remains uncertain. Finally, the semi-quantitative nature of B-line scoring and the cohort’s older, male demographic may limit broader extrapolation. Future studies should integrate multimodal monitoring—combining ultrasound with bioimpedance or biomarkers—to define actionable thresholds for CVP and B-line scores and guide timely, individualized interventions.
Conclusions
B-line scores increased progressively during TURP, driven predominantly by a strong temporal effect rather than by CVP. Across all analyses, the association between CVP and B-line scores was small, statistically non-significant, and remained unstable in sensitivity models. In contrast, B-line scores more consistently tracked dynamic changes in Hb, Ppeak, and Na+, highlighting their sensitivity to evolving physiological status. These findings support the role of lung ultrasound as a responsive, non-invasive tool for perioperative fluid assessment, whereas CVP alone showed limited value in detecting dynamic pulmonary congestion in this setting.
Data availability
All data generated or analyzed except anonymized aggregated data and the R code used for the statistical analyses during this study are included in this published article and its supplementary information files. Anonymized aggregated data and the R code used for the statistical analyses are available from the corresponding author upon reasonable request. The detailed trial protocol and the CONSORT 2025 checklist, can be found in the ‘Related manuscript files’ section.
The detailed trial protocol and the CONSORT 2025 checklist, can be found in the ‘Related manuscript files’ section.
References
Hahn, R. G. Fluid absorption in endoscopic surgery. Br. J. Anaesth. 96(1), 8–20 (2006).
Hawary, A. et al. Transurethral resection of the prostate syndrome: Almost gone but not forgotten. J. Endourol. 23(12), 2013–2020 (2009).
Magder, S. Central venous pressure: A useful but not so simple measurement. Crit. Care Med. 34(8), 2224–2227 (2006).
Marik, P. E., Baram, M. & Vahid, B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest 134(1), 172–178 (2008).
Parienti, J. J. et al. Intravascular complications of central venous catheterization by insertion site. N. Engl. J. Med. 373(13), 1220–1229 (2015).
Picano, E. & Pellikka, P. A. Ultrasound of extravascular lung water: A new standard for pulmonary congestion. Eur. Heart J. 37(27), 2097–2104 (2016).
Falcetta A, et al. The role of lung ultrasound in the diagnosis of interstitial lung disease. Shanghai Chest. 2(5). (2018).
Mayr, U. et al. B-lines scores derived from lung ultrasound provide accurate prediction of extravascular lung water index: An observational study in critically III patients. J. Intensive Care Med. 37(1), 21–31 (2022).
Lichtenstein, D. Lung ultrasound in the critically ill. Curr. Opin. Crit. Care 20(3), 315–322 (2014).
Volpicelli, G. et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 38(4), 577–591 (2012).
Czepizak, C. A., O’Callaghan, J. M. & Venus, B. Evaluation of formulas for optimal positioning of central venous catheters. Chest 107(6), 1662–1664 (1995).
Gargani, L. Lung ultrasound: A new tool for the cardiologist. Cardiovasc. Ultrasound 9(1), 6 (2011).
He, Y. et al. Prognostic value of the early lung ultrasound B-line score for postoperative pulmonary insufficiency in patients undergoing thoracic surgery: an observational study. Eur. J. Med. Res. 28(1), 160 (2023).
Issa, M. M. et al. Dilutional hyponatremia of TURP syndrome: A historical event in the 21st century. Urology 64(2), 298–301 (2004).
Enghard, P. et al. Simplified lung ultrasound protocol shows excellent prediction of extravascular lung water in ventilated intensive care patients. Crit. Care 19(1), 36 (2015).
Koh, J. C. et al. Relationship between PaO2/FiO2 and number of regions with B-line on transthoracic lung ultrasound: a prospective, observational study. Anesth. Pain Med. 14(2), 187–192 (2019).
Theerawit, P. et al. Transthoracic ultrasound assessment of B-lines for identifying the increment of extravascular lung water in shock patients requiring fluid resuscitation. Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 18(4), 195–199 (2014).
Polderman, K. H. & Girbes, A. R. Severe electrolyte disorders following cardiac surgery: a prospective controlled observational study. Crit. Care 8(6), R459–R466 (2004).
Alexander, E. C. et al. Serial fluid overload assessment in critically ill children using chest ultrasound (FOCUS): a prospective cohort study. Intensive Care Med. Paediatr Neonatal 3(1), 23 (2025).
Ciumanghel, A. et al. B-lines score on lung ultrasound as a direct measure of respiratory dysfunction in ICU patients with acute kidney injury. Int. Urol. Nephrol. 50(1), 113–119 (2018).
Young, R. Perioperative fluid and electrolyte management in cardiac surgery: A review. J. Extra Corpor. Technol. 44(1), P20–P26 (2012).
Ahmadi, A., Dawlaty, B. & Haidary, A. M. Electrolyte imbalance in pediatric patients following cardiac surgery with CPB: Experience from a single institution in Afghanistan. Prog. Pediatr. Cardiol. 77, 101807 (2025).
Fan, G. et al. Association of point-of-care lung ultrasound findings with 30-day pulmonary complications after cardiac surgery: A prospective cohort study. Heliyon 10(10), e31293 (2024).
Jambrik, Z. et al. Usefulness of ultrasound lung comets as a nonradiologic sign of extravascular lung water. Am. J. Cardiol. 93(10), 1265–1270 (2004).
Acknowledgements
The study was approved by the ethics committee of the First Affiliated Hospital of Guangxi Medical University (approval number: 2022-K124-01).
Funding
Qing Liu was supported by the Health Commission of Guangxi Zhuang Autonomous Region Self-financing (No. Z-A20220430). No other external funding or competing interests declared.
Author information
Authors and Affiliations
Contributions
Qing Liu and Chaoxiu Jiang wrote the main manuscript text. Qing Liu prepared Tables 1–5, Figs. 1–5, Table S1-S3, Figures S1-S2, and revised the manuscript. Qing Liu, Chunyi Yang, and Lizhen Wu contributed to data collection. Jingwen Wei revised the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
The study had been performed in accordance with the Declaration of Helsinki. The study protocol was conducted with the consent of the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University in China on 14 November 2022 (approval number: 2022-K124-01). Informed consents to participate in the study had been obtained from all participants or their legal guardian(s). The study was registered at the Chinese Clinical Trial Registry (ChiCTR2200065753).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, Q., Wei, J., Yang, C. et al. Intraoperative B-line scores increase over time and are minimally affected by central venous pressure during transurethral resection of the prostate. Sci Rep 16, 4001 (2026). https://doi.org/10.1038/s41598-025-34114-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-34114-z