Abstract
To assess the efficacy and safety of LiWei Capsule (LWC) in the treatment of chronic non-atrophic gastritis (CNG) with erosions and damp-heat stasis syndrome, based on Traditional Chinese Medicine (TCM) principles. This phase II, multicenter, randomized, double-blind, placebo- and positive-controlled trial enrolled patients diagnosed with CNG with erosions and damp-heat stasis syndrome. Participants were allocated to LWC, Sanjiu Weitai Capsule (SJWT, positive control), and placebo groups (2:1:1 ratio) and received corresponding treatment for 4 weeks, followed by a 16-week follow-up. The primary outcome was the curative rate of epigastric pain/bloating. Secondary outcomes included improvement in endoscopic examination, histopathological examination, and TCM symptom scores. Of 301 enrolled participants from five centers, 189 completed the study (95, 45, 49 cases in LWC, SJWT, and placebo groups, respectively). After 4 weeks of treatment, the curative rates of epigastric pain/bloating were 76.84%, 42.22%, and 22.45% in LWC, SJWT, and placebo groups, respectively (P < 0.001). The healing rates of endoscopic erosions were 65.3%, 46.7%, and 30.6% in LWC, SJWT, and placebo groups, respectively (P < 0.001). LWC effectively and safely alleviates epigastric pain/bloating and promotes endoscopic erosion healing in CNG patients with erosions (damp-heat stasis syndrome). LWC may be a promising treatment option for this condition.
Trial registration: ChiCTR2100052010, assigned by the Chinese Clinical Trial Registry, registration date 13/10/2021.
Similar content being viewed by others
Introduction
Chronic gastritis, a multifactorial, progressive, and chronic inflammatory condition, is a prevalent finding in upper gastrointestinal endoscopy. It is estimated that, on average, over half of the global population may have chronic gastritis, with its prevalence increasing with age1. Chronic gastritis can present as non-atrophic or atrophic forms. It commonly begins in childhood as superficial gastritis, characterized by the coexistence of chronic and active inflammation. This condition may progress sequentially to atrophic gastritis, which is epidemiologically associated with an increased risk of gastric cance2.
Chronic non-atrophic gastritis (CNG) is the predominant endoscopic diagnosis among Chinese patients presenting with upper gastrointestinal symptoms. A multi-center national survey, conducted by the Digestive Endoscopy Branch of the Chinese Medical Association, revealed that among the 8,892 patients surveyed, 4,389 were diagnosed with CNG, and 3,760 with CNG accompanied by erosions3. Patients with CNG and erosions may experience nonspecific symptoms, including epigastric pain, bloating, belching, loss of appetite, dyspepsia, and acid regurgitation. These symptoms can persist or recur due to factors such as repeated or prolonged Helicobacter pylori (HP) infection, heightened psychological stress, and poor dietary habits.
The primary goals of treating CNG include addressing its underlying etiology, alleviating symptoms, improving gastric mucosal histology, and adhering to individualized treatment principles4. For CNG patients with erosions, HP eradication is recommended for those with HP-positive CNG. Prokinetics and/or gastric protective agents may be administered for patients experiencing upper abdominal symptoms. The use of antacids, H2-receptor antagonists, or proton pump inhibitors (PPIs) is considered based on clinical manifestations1,4. However, the complex pathogenesis and etiology of CNG, rising HP drug resistance, and intolerable side effects of medications can lead to continued suffering for some patients even after receiving these treatments5,6. Consequently, there is an increasing and urgent need for convenient and reliable medications for CNG management.
Traditional Chinese Medicine (TCM), a medical system rooted in Chinese culture and philosophy, has been practiced for millennia and demonstrates significant preventive and therapeutic effects for various diseases. Evidence suggests that TCM may be more effective than conventional pharmacotherapies for chronic gastritis7,8. The Chinese Society of Gastroenterology recommends TCM for CNG treatment, with a consensus rate of 89.8%4. Several Chinese herbal medicine (CHM) formulations have been proven to alleviate dyspeptic symptoms and potentially improve gastric mucosal pathology. Chinese patent medicines for CNG are widely accepted due to their convenience and efficacy. Previous studies have shown that QZWT granules alleviate CNG in mice by modulating gut microbiota and bile acid metabolism9, WZYD effectively treats CNAG by inhibiting inflammation and regulating related metabolic processes10, and Xiangsha Liujunzi decoction is safe and effective for CNG patients11. However, it is crucial to note that multicenter, placebo-controlled, large-sample, and long-term follow-up clinical studies are still lacking, which hinders the broader clinical application of TCM in CNG treatment.
LiWei Capsule (LWC) is a proprietary herbal formulation developed by Guang’anmen Hospital of the China Academy of Chinese Medical Sciences. It contains four key components: Panax notoginseng, Rhubarb, Alum, and Stamen stone. LWC is designed to address blood stasis, dampness, and food stagnation, while clearing heat, promoting tissue regeneration, and alleviating pain. This formulation has been utilized for over two decades in the treatment of CNG with erosions, demonstrating both efficacy and safety. LWC is particularly indicated for patients with CNG and erosions diagnosed with damp-heat stasis syndrome, especially those experiencing epigastric pain and/or bloating. Preclinical pharmacological studies indicate that LWC protects gastric mucosa by promoting erosion healing, exerting anti-inflammatory effects, and inhibiting Helicobacter pylori (HP) proliferation in rats (unpublished data). No animal deaths or overt toxic reactions were observed.
Consequently, this phase II, randomized, controlled, double-blind clinical trial was designed to assess the efficacy and safety of LWC for the treatment of CNG with erosions (damp-heat stasis syndrome) compared with a positive control drug and placebo. The study aimed to provide additional evidence to support the initiation of a phase III clinical trial in the future.
Methods
This is a Phase II, multicenter, double-blind, randomized, positive- and placebo-controlled trial. The study protocol was approved by the Ethics Committee of the Affiliated Hospital of Nanjing University of Chinese Medicine (Approval No. 2011 NL-041-01). It was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation’s Good Clinical Practice (GCP) guidelines12. Additionally, the trial adhered to the Standards for the Quality Control of Clinical Trials of Drugs, as approved by the State Drug Administration (SDA, version September 2003). Written informed consent was obtained from all participants prior to their enrollment. The CONSORT checklist was utilized for the preparation of this manuscript13. The trial is registered with the Chinese Clinical Trial Registry under the identifier ChiCTR2100052010.
Patients
A total of 301 patients were enrolled between October 16, 2012, and March 23, 2015, across five participating centers: (1) Gansu Provincial Hospital of Traditional Chinese Medicine, (2) Hebei Province Hospital of Chinese Medicine, (3) Affiliated Hospital of Changchun University of Chinese Medicine, (4) Jiangsu Province Hospital of Chinese Medicine, and (5) The First Affiliated Hospital of Tianjin University of Chinese Medicine.
Diagnostic criteria of CNG accompanied with erosions
The diagnosis of the disease should be established based on a combination of clinical symptoms, endoscopic findings, and pathological examination14,15,16, We adhered to the Sydney Protocol for biopsy sampling17. A patient was classified as having chronic non-atrophic gastritis (CNG) with erosive changes if they presented with epigastric pain or discomfort and met the specified endoscopic and histopathological diagnostic criteria. The endoscopic and histopathological diagnostic criteria and grading scales are detailed in Tables 1 and 2.
Diagnostic Criteria for Damp-Heat Stagnation Syndrome in Traditional Chinese Medicine
The diagnostic criteria for the Damp-Heat Stagnation Syndrome associated with Chronic Non-atrophic gastritis featuring erosions are delineated as follows: the presence of at least one primary symptom and two secondary symptoms, concurrently accompanied by specific tongue and pulse manifestations18,19,20. The scoring criteria for Traditional Chinese Medicine (TCM) symptoms are detailed in Table 3.
Primary Symptoms:
-
1.
Epigastric Pain: Characterized by a painful sensation localized to the upper abdomen or below the breastbone.
-
2.
Epigastric Bloating: Encompassing various degrees of abdominal discomfort, this may manifest as epigastric bloating.
Secondary Symptoms:
-
1.
Bitter Taste, Halitosis, or Sticky Mouth: Presence of an unpleasant taste, foul breath, or a feeling of stickiness in the oral cavity.
-
2.
Loss of Appetite: Manifested as a reduced or complete lack of interest in food, leading to an absence of desire to eat.
-
3.
Belching: The involuntary expellation of gas from the mouth due to spasms of the diaphragm.
-
4.
Gastric Discomfort: Experiencing one or more gastrointestinal sounds and a sense of discomfort in the stomach, often in the absence of food intake and typically without severe pain.
-
5.
Acid Regurgitation: The backward flow of stomach acid or contents into the esophagus.
-
6.
Tongue and Pulse Manifestations: Presentation with a dark purple tongue with petechiae, a yellow and greasy tongue coating, and a pulse that is either wiry and rough or wiry and slippery.
Inclusion and exclusion criteria
Patients were eligible for inclusion if they met the following criteria:
-
1.
A confirmed diagnosis of chronic non-atrophic gastritis (CNG) complicated by erosions, with a score of ≥ 3 for epigastric pain or bloating.
-
2.
A diagnosis of damp-heat stasis syndrome according to traditional Chinese medicine criteria.
-
3.
Age range between 18 and 65 years.
-
4.
Provision of written informed consent before participation in the study.
Patients were excluded from the study based on the following criteria:
-
1.
Diagnosis of secondary gastritis or concurrent conditions such as gastric and duodenal ulcers, deep ulcers, arterial bleeding, severe gastric mucosal dysplasia, or suspected malignancy as indicated by pathological examination.
-
2.
Previous treatment with other medications or traditional Chinese medicines for CNG within a specified period before enrollment.
-
3.
Presence of severe comorbidities affecting the heart, liver, lungs, kidneys, blood system, or other life-threatening conditions, including tumors, AIDS, or disorders of blood coagulation.
-
4.
Elevated serum creatinine (Cr) levels exceeding the upper limit of normal, alanine aminotransferase (ALT)/aspartate aminotransferase (AST) levels greater than 1.5 times the upper limit of normal, or a serum white blood cell count greater than 1.0 × 10^9/L.
-
5.
Female patients who were planning to conceive, pregnant, or lactating.
-
6.
Known hypersensitivity or allergy to the trial medications.
-
7.
Psychiatric disorders that impaired the ability to cooperate with the study procedures.
-
8.
Participation in other clinical trials within the past 3 months.
Drugs
LWC (batch numbers 161201 and 180701) and placebo capsules (batch numbers 120501 and 130701) were both produced by Jianmin Pharmaceutical Group Co., Ltd, located in Wuhan, China. The SJWT capsules were manufactured by China Resources Sanjiu Pharmaceutical Co., Ltd, based in Shenzhen, China, with batch numbers 1,612,007 H and 1,803,011 H.
Each capsule contained 0.5 g of the active ingredient. The medication regimen consisted of oral administration of 4 capsules 30 min before each meal (breakfast and dinner) of 4 weeks, resulting in a twice-daily dosage.
To ensure a comprehensive characterization and stringent control of drug quality, as well as to guarantee the efficacy and safety of the medication, an Ultra-high Performance Liquid Chromatography-Mass Spectrometry (UPLC-MS) fingerprinting method was developed for LWC. This method provided excellent separation and identified 66 chemical components, predominantly saponins (including 20®-Notoginsenoside R2, Gypenoside XVII, Ginsenoside Rf, Ginsenoside rg2, Ginsenoside rg3, Ginsenoside F2, Ginsenoside Rb1, Ginsenoside Rb3, Ginsenoside Rd, Ginsenoside Re, Ginsenoside Rg1, Ginsenoside Rh1, (S)-Ginsenoside Rh2, 3-[(Carboxycarbonyl)amino]-L-alanine, Notoginsenoside R1, Uvaol, Protopanaxatriol, Protopanaxadiol), anthraquinones (such as 3,8-Dihydroxy-1-methylanthraquinone-2-carboxylic acid, 3-Hydroxymorindone, Emodic acid, Chrysophanol 8-O-β-D-glucoside, Emodin, Emodin 8-glucoside, Physcion, Rhein, Danthron, Erythrodiol, Lucidin, Aloe emodin, Citreorosein), and flavonoids (including 4,6,3’,4’,5’-Pentahydroxyauron, Epicatechin, Epicatechin gallate, Liquiritin, (-)-Homoeriodictyol, Quercetin-3-O-.beta.-D-xylopyranosyl (1->6)-.beta.-D-glucopyranoside, Flavanone 7-O-.beta.-D-glucoside, Norwogenin, Apigenin 7-glucoside, Leucoside, Salicylic acid, Procyanidin B2, Butin), along with minor amounts of amino acids and organic acids. The saponins were primarily derived from Panax notoginseng, while the anthraquinones were sourced mainly from rhubarb.
The LWC capsules were manufactured in strict compliance with Good Manufacturing Practices (GMP). Prior to the clinical trial, the stability of the drug and the impact of packaging materials on stability were assessed using 11 batches of samples, with all quality indicators meeting the specified standards. Each capsule had a loading capacity of 0.5 g, and the content of ginsenoside Rg1 (C42H72O14) from Panax notoginseng in each capsule was required to be within the range of 4.0 to 9.0 mg. The detailed methodology and results, including the classification of chemical components and UPLC-MS profiles of LWC, are presented in Table 4; Fig. 1.
LC-MS chromatograms (positive ion and negative ion) of the LW, SQ and DH extract combination. *Assigned compounds: (1) Sucrose; (2) L-Tryptophan; (3) Epicatechin; (4) 3-O-p-Coumaroylquinic acid; (5) 7-Hydroxy-4-methylcoumarin-3-acetic acid; (6) Ginsenoside Rg1; (7) Ginsenoside Rg3; (8) 6-Gingerol; (9) Butin; (10) Physcion; (11) 2-Linoleoylglycerol; (12) Protopanaxadiol; (13)Norwogenin; (14)Soyacerebroside II; (15) Stigmastadienone.Positive ion(a) and negative ion(b) is LW; positive ion(c) and negative ion(d) is DH; positive ion(e) and negative ion(f) is SQ.
Randomization, control, and blinding procedures
Participants were randomly allocated to the LWC, SJWT, and placebo groups in a 2:1:1 ratio using a stratified randomization technique, with stratification by study center. The randomization sequence was generated using SAS 9.2 software to assign the appropriate randomization segment for each statistical center.
SJWT and placebo capsules were designated as the positive control and placebo control, respectively. LWC, SJWT, and the placebo were all formulated as capsules. To maintain the double-blind design, the three types of capsules were manufactured to have an identical appearance in color and size. They were packaged in uniform, sealed polyethylene plastic bottles and labeled similarly, ensuring that the participants, investigators, and outcome assessors remained blinded to the treatment allocation.
Study assessment items and time points
Participant evaluations were scheduled at the following time points: prior to treatment (baseline, 0 week) and at 1, 2, 4, 8, and 16 weeks post-treatment. At each visit, the following procedures were conducted: collection of demographic data, assessment of symptoms, and physical examination. Endoscopic and biopsy procedures, Helicobacter pylori (HP) C13 or C14 urea breath tests, and safety assessments were performed before and after the treatment period. A urine pregnancy test was administered to women of childbearing age prior to the initiation of treatment.
Outcome measures
Primary outcome
The primary endpoint of the study was the curative rate of epigastric pain/bloating, defined as a reduction in the epigastric pain score to zero. In cases where participants did not experience epigastric pain, the epigastric bloating score was used for outcome evaluation.
Secondary outcomes
Secondary outcomes assessment included endoscopic and histopathological evaluation (improvement of erosion, overall endoscopic lesions, active inflammation, and chronic inflammation, the evaluation criteria were listed in Table 5), and TCM symptoms (the evaluation criteria were presented in Table 3).
Safety evaluation
The safety assessment comprised laboratory tests including blood, urine, and stool routine analyses, fecal occult blood test, liver function tests (AST, ALT, ALP, γ-GT, T-BIL), renal function tests (BUN, Scr), and electrocardiogram (ECG). Gastrointestinal adverse reactions and all other adverse events were meticulously documented (including type, severity, and incidence) to evaluate the safety profile of the treatment.
The severity grading of adverse events is as follows: Mild – the event is tolerable to the participant, does not interfere with treatment, requires no specific intervention, and has no adverse effect on the participant’s health; Moderate – the event is intolerable to the participant, necessitating drug withdrawal or special treatment, with a direct impact on the participant’s health; Severe – the event poses a threat to the participant’s life, potentially resulting in death or disability, and requires immediate withdrawal of the drug or emergency intervention.
Statistical analysis
Sample size determination
This Phase II clinical trial is designed as a three-armed study, incorporating a placebo group and a positive drug control group. The primary outcome is the curative rate of epigastric pain/bloating. Drawing on data from previous studies, the expected efficacy rate is 43.1% for the SJWT group and 70% for the LWC group (based on previous clinical experiences and unpublished data). Utilizing a superiority test with a significance level of α = 0.025 (one-sided test) and a test power (1-β) of 80%, the case allocation ratio between the LWC and SJWT groups is set at 2:1. The calculated sample size is 38 for the LWC group and 19 for the SJWT group. In accordance with the “Measures for the Administration of Drug Registration” (2007) (available at www.gov.cn/zhengce/zhengceku/2020-04/01/content_5498012.htm), a Phase II clinical trial should include no fewer than 100 subjects in the trial group. Accounting for a potential 20% dropout rate and using a 2:1:1 allocation ratio, the study requires 100, 50, and 50 patients in the LWC, SJWT, and placebo groups, respectively.
All statistical analyses were conducted using SAS 9.2, assuming a two-sided test with a significance level of 0.05. Descriptive statistics for continuous variables will be presented as means ± standard deviations (SD), medians, minimum and maximum values, and 95% confidence intervals (CIs). Categorical variables will be described using frequencies and percentages. Chi-square analysis or the Fisher exact probability test will be used to compare the efficacy rates and adverse events across groups. Analysis of variance (ANOVA) will be applied for comparing syndrome scores (for non-normally distributed data, the Wilcoxon test will be used). The least significant difference (LSD)-t test will be employed for pairwise comparisons between groups, with results reported only for significant differences. The Cochran-Mantel-Haenszel (CMH) Chi-square analysis method will be used to compare single symptom scores, single endoscopy scores, and single scores for chronic and active inflammation.
Results
Baseline characteristics of the patients
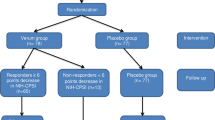
A total of 231 participants were randomized into the study (Full Analysis Set, FAS), comprising 116 cases in the LWC group, 58 in the SJWT group, and 57 in the placebo group. Ultimately, 189 participants completed the entire study and were included in the Per Protocol Set (PPS), with 95 cases in the LWC group, 45 in the SJWT group, and 49 in the placebo group. The patient flow diagram is presented in Fig. 2.
The baseline characteristics of the patients are detailed in Table 6. No significant differences were observed among the three groups for most characteristics, except for the score of active inflammation, which was statistically significant (P < 0.05).
Primary outcome evaluation: curative rate of Epigastric Pain/Bloating in each group
he efficacy rates for the relief of epigastric pain/bloating increased progressively throughout the treatment period within each group, as depicted in Tables 7 and Fig. 3.
At the 1-week, 2-week, and 4-week post-treatment follow-up time points, statistically significant differences in the efficacy rates for epigastric pain/bloating were observed among the three groups (P < 0.05). LSD multiple comparison tests revealed that the efficacy rates for epigastric pain/bloating in the LWC group were significantly higher than those in the placebo group at 1-week, 2-week, and 4-week post-treatment. Additionally, the LWC group exhibited higher efficacy rates than the SJWT group at the 2-week and 4-week post-treatment time points (P < 0.05). These findings suggest that LWC is more effective in alleviating epigastric pain/bloating in patients with chronic non-atrophic gastritis accompanied by erosions compared to SJWT and placebo.
During the follow-up period after treatment discontinuation, the efficacy rates for epigastric pain/bloating in the LWC group remained above 65%, despite a slight decline, and were higher than those in the SJWT and placebo groups. However, no significant differences were noted among the groups at this stage (P > 0.05).
Evaluation of secondary outcomes: improvement in endoscopic erosions, endoscopic and histopathological assessments, and Symptom Relief following treatment in each group
The outcomes of the secondary endpoints are detailed in Table 8, with endoscopic findings illustrated in Fig. 4.
Typical endoscopic results before and after treatment in the three groups. *(1) Before treatment in LWC group: extensively fused gastric antrum erythema, with more than 6 multiple extensive erosions, the gastroscopy score = 6; (2) After treatment in LWC group: scattered erythema, and less than 5 erosions the gastroscopy score = 3; (3)Before treatment in SJWT group: about 0.3 cm dense erythema on gastric antrum, with more than 6 multiple extensive erosions, the gastroscopy score = 5; (4) After treatment in SJWT group: scattered erythema and single erosion on mucous membrane, the gastroscopy score = 2; (5) Before treatment in Placebo group: more than 6 patchy erosions with 0.2-0.3cmin the gastric antrum, the gastroscopy score = 6; (6) After treatment in Placebo group: still more than 6 extensive erosions with scattered hemorrhagic spots in the gastric antrum, the gastroscopy score = 6.
Impact on endoscopic erosions The rates of resolution of endoscopic erosions were 65.3% in the LWC group, 46.7% in the SJWT group, and 30.6% in the placebo group, respectively, showing significant differences among the three groups (P < 0.05). The LWC group exhibited a higher resolution rate compared to both the SJWT and placebo groups (P < 0.05) as determined by multiple comparison analysis. No significant difference was observed between the SJWT and placebo groups (P > 0.05).
Impact on overall endoscopic lesions The overall efficacy rates for endoscopic lesions, which included patients classified as clinically cured, significantly improved, and improved, were 76.8% for the LWC group, 55.6% for the SJWT group, and 53.1% for the placebo group, respectively, with significant differences observed among the three groups (P < 0.05). The LWC group demonstrated a higher efficacy rate than both the SJWT and placebo groups (P < 0.05) based on multiple comparison analysis. No significant difference was found between the SJWT and placebo groups (P > 0.05).
Impact on active inflammation The resolution rates for active inflammation were 23.2% in the LWC group, 8.9% in the SJWT group, and 18.4% in the placebo group, with no significant differences observed among the three groups (P > 0.05).
Impact on chronic inflammation The rates of resolution and significant improvement for chronic inflammation were relatively low across all groups. The overall efficacy rates were 20% for the LWC group, 17.8% for the SJWT group, and 24.5% for the placebo group, respectively, with no significant differences among the three groups (P > 0.05).
Impact on Symptom Improvement The rates of symptom resolution were 27.37% in the LWC group, 13.33% in the SJWT group, and 10.20% in the placebo group, respectively, revealing significant differences among the three groups. Multiple comparison analysis indicated significant differences between any two groups (P < 0.05). Considering symptom improvement, the LWC group showed superior effects compared to both the SJWT and placebo groups; the SJWT group also demonstrated better outcomes than the placebo group.
Safety evaluation
No serious adverse events were reported throughout the study across the three groups. A total of seven adverse events were documented, all of which were classified as mild. Three cases were deemed to have an improbable association with the study drugs, three were considered to have a possible but unconfirmed association, and two cases reported a mild increase in menstrual volume, which was judged as potentially related to the study drug. Following a one-week discontinuation of the drug, the patients recovered, and the study was resumed.
One case reported altered liver function after the conclusion of the observation period. The patient had not been taking any other medications during the treatment, had no history of fatty liver disease, and denied alcohol use, suggesting that the liver injury might be medication-related. A follow-up liver function test was recommended, but the patient was non-compliant. There were no significant differences in adverse events or adverse drug reactions among the three groups (P > 0.05), indicating the favorable safety profile of both LWC and SJWT. The findings are presented in Table 9.
Discussion
To the best of our knowledge, this is the first clinical trial to assess the efficacy and safety of a Chinese herbal medicine in the treatment of patients with chronic non-atrophic gastritis (CNG) complicated by erosions, utilizing both positive and placebo control interventions. The findings revealed that LWC demonstrated superior therapeutic outcomes in alleviating symptoms, as well as in reducing endoscopic and histopathological scores, in patients with CNG and erosions when compared to both the placebo and SJWT treatments. Additionally, we were encouraged to find that LWC exerted a therapeutic effect on HP infection, with improvement rates of 52.7%, 34.6%, and 22.6% in the LWC, SJWT, and placebo groups, respectively. However, due to the lack of long-term follow-up data, we are unable to definitively confirm its eradicative effects.
Control drugs design
LWC is an in-hospital medication with a well-defined formula. Drawing on previous clinical experience, LWC has exhibited comprehensive therapeutic efficacy in patients with chronic non-atrophic gastritis (CNG) complicated by erosions. This includes not only the alleviation of clinical symptoms but also the enhancement of endoscopic evaluation outcomes and anti-Helicobacter pylori (HP) activity. In this Phase II clinical trial, we aim to underscore this therapeutic advantage of LWC. However, the standard treatments for CNG with erosions, including triple or quadruple anti-HP infection regimens, prokinetics, gastric protectants, antacids, H2-receptor antagonists, and proton pump inhibitors (PPIs), tend to demonstrate either a relatively narrow spectrum of efficacy or significant side effects, rendering them insufficient as positive control agents for this study. Consequently, SJWT, a renowned Chinese proprietary medicine with analogous therapeutic properties, was selected as the positive control.
SJWT capsules (Registration Number zz-5012, approved by the Guangdong Food and Drug Administration in 1994, No. 904050) are a widely utilized and accepted proprietary medicine for chronic gastritis in China. This preparation consists of eight herbal components: trigeminal bitter, Scutellaria baicalensis, jiulixiang, Nitraria lanceolata, wood incense, Poria cocos, white peony, and Rehmannia glutinosa. The primary functions of SJWT are to clear heat, dry dampness, promote qi circulation, activate blood flow, reduce inflammation, and alleviate pain.
In accordance with authoritative consensus and guidelines for chronic gastritis, SJWT can be administered as a standalone treatment for chronic gastritis accompanied by erosions, gastric mucosal red plaques, mucosal bleeding, and bile reflux21,22. Evidence suggests that SJWT’s efficacy on endoscopic mucosal lesions is comparable to that of teprenone and superior to placebo21. Considering these factors, SJWT was chosen as the positive control in this study. To ensure a more rigorous and compelling conclusion, a placebo control arm was also incorporated into the trial design.
Potential underlying mechanism
In this clinical trial, the assessment of efficacy encompasses improvements in clinical symptoms, endoscopic findings, and histopathology. The alleviation of epigastric pain/bloating was selected as the primary outcome measure, as these symptoms are the most commonly reported complaints in patients with CNG complicated by erosions. LWC demonstrated a superior effect on reducing epigastric pain/bloating compared to placebo after just one week of treatment. Following two weeks of treatment, the LWC group exhibited a higher cure rate for epigastric pain/bloating than both the placebo and SJWT groups. This superiority was sustained throughout the four-week treatment period and was still evident during the 16-week follow-up period. This outcome may be partially attributed to the significant amount of missing data during the follow-up phase. The rates of missing data were 16.84%, 24.44%, and 53.06% in the LWC, SJWT, and placebo groups, respectively, at the 8-week follow-up, and 18.95%, 31.11%, and 61.22% at the 16-week follow-up. The high rates of missing data may have contributed to an apparent reduction in the overall efficacy rates.
The findings of this study suggest that LWC possesses antibacterial and analgesic properties. Notable among the constituents of LWC are Panax notoginseng (San Qi) and Rhubarb (Da Huang). Quercetin, a key compound in Panax notoginseng, has been shown to enhance gastrointestinal motility by modulating the peristaltic function of gastrointestinal smooth muscle cells through acetylcholine receptors (mAChRs) and by stimulating mucin secretion23. Additionally, quercetin is known for its ability to regulate inflammatory responses and inhibit bacterial growth24. Furthermore, Panax notoginseng saponins have been found to increase vitamin C levels in the adrenal gland, decrease plasma corticosteroid levels, hinder receptor activation, and exert a potent anti-inflammatory effect25,26.
Emodin methyl ether, a primary constituent of wine-treated rhubarb, is recognized as a potent anti-inflammatory agent. It effectively mitigates the inflammatory response in macrophages by decreasing interleukin-1, inhibiting the activity of nitric oxide synthase β (IL-1β), and tumor necrosis factor-α (TNF-α)27,28. Additional research has demonstrated that emodin can disrupt the permeability of bacterial cell membranes, inhibit bacterial protein synthesis, and exert a bactericidal effect29. Moreover, its free anthraquinone derivatives are also capable of inhibiting HP30,31. These two herbal components contribute to the alleviation of epigastric pain and bloating through their anti-inflammatory, sterilizing, and gastrointestinal motility-enhancing actions.
LWC demonstrated superior performance in the improvement of endoscopic erosions and overall endoscopic lesions when compared to both the placebo and SJWT. These beneficial effects may be attributed to the anti-inflammatory and antibacterial properties, as well as the acid suppression and gastric mucosal protection afforded by the two mineral components in the formulation: Alum and Stamen Stone. However, the precise mechanisms underlying these effects warrant further investigation.
No significant differences were observed among the three groups with respect to the improvement of endoscopic active inflammation and chronic inflammation. Across all groups, the curative rates for endoscopic active inflammation were notably low, indicating that chronic inflammation is persistent and challenging to resolve. This suggests that a longer treatment duration may be essential for effectively improving the chronic inflammation associated with chronic non-atrophic gastritis accompanied by erosions.
Limitations
This study is not without its limitations. Firstly, constrained by financial resources, the sample size for both the placebo and SJWT groups was relatively modest, which may have impaired the study’s ability to detect significant differences in outcomes between the SJWT and placebo groups. Secondly, the duration of the treatment course may not have been sufficient to fully ascertain the curative effects on endoscopic chronic inflammation and the eradication of HP across all groups. Furthermore, the high incidence of missing data throughout the follow-up period likely influenced the assessment of the treatment’s long-term efficacy. Consequently, future research, such as a Phase III clinical trial, should be contemplated with an enlarged sample size and an extended treatment duration to address these issues.
Conclusion
LWC has demonstrated efficacy and safety in mitigating epigastric pain/bloating and facilitating the endoscopic healing of erosions in patients with CNG complicated by erosions, characteristic of the damp-heat stasis syndrome. The therapeutic benefits of LWC are notable, outperforming both placebo and SJWT. LWC emerges as a promising therapeutic alternative for the management of CNG with erosions, potentially expanding the array of treatment strategies for CNG within the framework of TCM.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
Abbreviations
- LWC:
-
LiWei Capsule
- CNG:
-
Chronic non-atrophic gastritis
- TCM:
-
Traditional Chinese medicine
- SJWT:
-
Sanjiu Weitai Capsule
- HP:
-
Helicobacter pylori
- CHM:
-
Chinese herbal medicine
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- ALP:
-
A Lkaline Phosphatase
- r-GT:
-
Gamma-glutamyl transpeptidase
- T-BIL:
-
Total bilirubin
- BUN:
-
Urea nitrogen
- SCR:
-
Serum creatinine
- ECG:
-
Electrocardiograph
- FAS:
-
Full analysis set
- PPS:
-
Per protocol set
References
Sipponen, P. & Maaroos, H. I. Chronic gastritis. Scand. J. Gastroenterol. 50(6), 657–667 (2015).
de Vries, A. C. et al. Gastric cancer risk in patients with premalignant gastric lesions: A nationwide cohort study in the Netherlands. Gastroenterology 134(4), 945–952 (2008).
Du, Y. et al. Chronic gastritis in China: A national multi-center survey. BMC Gastroenterol. 14, 21 (2014).
Fang, J. Y. et al. Chinese consensus on chronic gastritis (2017, Shanghai). J. Dig. Dis. 19(4), 182–203 (2018).
Flores-Trevino, S., Mendoza-Olazaran, S., Bocanegra-Ibarias, P., Maldonado-Garza, H. J. & Garza-Gonzalez, E. Helicobacter pylori drug resistance: Therapy changes and challenges. Expert Rev. Gastroenterol. Hepatol. 12(8), 819–827 (2018).
Sugano, K. et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 64(9), 1353–1367 (2015).
Yan, Z. X. et al. Efficacy of traditional Chinese medicine for chronic gastritis: A meta-analysis of randomized controlled trials. Med. (Baltim). 98(20), e15710 (2019).
Qin, F., Liu, J. Y. & Yuan, J. H. Chaihu-Shugan-San, an oriental herbal preparation, for the treatment of chronic gastritis: A meta-analysis of randomized controlled trials. J. Ethnopharmacol. 146(2), 433–439 (2013).
Chen, M. et al. Qi-Zhi-Wei-Tong granules alleviates chronic non-atrophic gastritis in mice by altering the gut microbiota and bile acid metabolism. J. Ethnopharmacol. 319(Pt 3), 117304 (2024).
Hu, Q. et al. Metabolomics profiles reveal the efficacy of Wuzhuyu Decoction on patients with chronic non-atrophic gastritis. Drug Des. Devel Ther. 17, 3269–3280 (2023).
Yue, P., Zhong, J., Huang, J., Lan, Z. & Zhong, S. The efficacy and safety of Xiangsha Liujunzi decoction in the treatment of chronic non-atrophic gastritis: A protocol for a systematic review and meta-analysis. Med. (Baltim). 100(4), e24504 (2021).
Vijayananthan, A. & Nawawi, O. The importance of good clinical practice guidelines and its role in clinical trials. Biomed. Imaging Interv J. 4(1), e5 (2008).
Cheng, C. W. et al. CONSORT extension for Chinese Herbal Medicine Formulas 2017: Recommendations, explanation, and Elaboration. Ann. Intern. Med. 167(2), 112–121 (2017).
Endoscopy CMAoD. Endoscopic classification and trial standards on treatment of chronic gastritis (Dalian, 2003). Chin. J. Dig. Endosc. 21, 77–78 (2004).
Fang, J. Y., Liu, W. Z., Shi, Y., Ge, Z. Z. & Xiao, S. D. Consensus on chronic gastritis in China–Second National Consensus Meeting on Chronic Gastritis (14–16 September 2006 Shanghai, China). J. Dig. Dis. 8(2), 107–119 (2007).
Fulian, H. et al. The third national consensus report on several issues of Helicobacter pylori infection. Chin. J. Gastroenterol. 13(1), 42–46 (2008) [in Chinese].
Sipponen, P. & Price, A. B. The Sydney System for classification of gastritis 20 years ago. J. Gastroenterol. Hepatol. 26(Suppl 1), 31–34 (2011).
XY Z. Guidelines for Clinical Research on Chinese New Herbal Medicines (Trial Implementation) (Medical Science and Technology Publishing House of China, 2002).
Medicine DDBoCAfTC. Consensus on diagnosis and treatment of chronic superficial gastritis with TCM (Shenzhen 2009). Chin. J. Integr. Trad West. Med. Dig. 18, 207–209 (2010). [in Chinese].
Medicine DsdCoCsoitaW. Schedule for diagnosis and treatment of chronic gastritis with integrative Chinese and western medicine (draft). CJITWM 25(2), 172–175 (2005). [in Chinese].
diseases SptocgfCpmittod. Clinical guidelines of Chinese patent medicines in the treatment of chronic gastritis (2020). Chin. J. Integr. Traditional Chin. Western Med. 41(2), 157–163 (2021) [in Chinese].
medicine SasdboCsotC. Experts consensus in TCM diagnosis and treatment of epigastric pain. J. Tradit. Chin. Med. 58(13), 1166–1670 (2017) [in Chinese].
JE, K., MR, L., JJ, P., JY, C. & BR, S. Quercetin promotes gastrointestinal motility and mucin secretion in loperamide-induced constipation of SD rats through regulation of the mAChRs downstream signal. Pharm. Biol. 56 (1), 309–317 (2018).
Anand David, A. V., Arulmoli, R. & Parasuraman, S. Overviews of Biological Importance of Quercetin: A bioactive flavonoid. Pharmacogn Rev. 10(20), 84–89 (2016).
Xu, C. et al. Analytical methods and biological activities of Panax notoginseng saponins: Recent trends. J. Ethnopharmacol. 236, 443–465 (2019).
Wang, W. et al. Combination of Panax notoginseng saponins and aspirin potentiates platelet inhibition with alleviated gastric injury via modulating arachidonic acid metabolism. Biomed. Pharmacother. 134, 111165 (2021).
Tu, Y. et al. Dahuang Fuzi Decoction ameliorates tubular epithelial apoptosis and renal damage via inhibiting TGF-beta1-JNK signaling pathway activation in vivo. J. Ethnopharmacol. 156, 115–124 (2014).
Kim, B. J., Kim, H., Lee, G. S., So, I. & Kim, S. J. Effects of San-Huang-Xie-Xin-tang, a traditional Chinese prescription for clearing away heat and toxin, on the pacemaker activities of interstitial cells of Cajal from the murine small intestine. J. Ethnopharmacol. 155(1), 744–752 (2014).
Chen, J. et al. Emodin targets the beta-hydroxyacyl-acyl carrier protein dehydratase from Helicobacter pylori: enzymatic inhibition assay with crystal structural and thermodynamic characterization. BMC Microbiol. 9, 91 (2009).
Huang, Y. Q. et al. Inhibitory effects of emodin, baicalin, schizandrin and berberine on hefA gene: Treatment of Helicobacter pylori-induced multidrug resistance. World J. Gastroenterol. 21(14), 4225–4231 (2015).
Wang, H. H. & Chung, J. G. Emodin-induced inhibition of growth and DNA damage in the Helicobacter pylori. Curr. Microbiol. 35(5), 262–266 (1997).
Acknowledgements
Thank all subjects and investigators of the 5 sites that participated in this clinical trial. This clinical trial was supported by the Jianmin Pharmaceutical Group Co., LTD. The sponsor provided funding for trial drugs and research cost of clinical trials.
Funding
National Science and Technology Major Special Project of “Major New Drug Innovation” (National Key New Drug Creation and Manufacturing Program, Ministry of Science and Technology(CN)), 2018ZX09731-004.
Author information
Authors and Affiliations
Contributions
Each author have made substantial contributions to the conception. Y.G. designed of the work; X.G., X.H. were responsibled for analysis, J.W., Z.Z.H. interpretated of data; D.Y.C. have drafted the work and M.J. substantively revised it.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
The manuscript has been approved by all authors for publication.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cui, D., Geng, X., Wang, J. et al. Efficacy and safety of LiWei capsule in chronic non-atrophic gastritis with erosions: a randomized controlled trial. Sci Rep 15, 1620 (2025). https://doi.org/10.1038/s41598-025-85122-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-85122-y