Abstract
This study aimed to compare the failure rates of two different sizes of plates and screws to stabilize critical-sized (7 mm) femoral defects in male Sprague‒Dawley rats (aged 10 weeks). Femoral defects were stabilized with either a 4-hole plate (length 29 mm, thickness 1 mm, 10 rats, Group 1) and 4 cortical screws (diameter 2 mm) or with a 6-hole plate (length 30 mm, thickness 0.6 mm, 9 rats, Group 2) and 4 cortical screws (diameter 1.5 mm). A polymethylmethacrylate spacer was inserted into the defects to reproduce the first stage of the induced membrane technique. Radiographic evaluations, macroscopic and histologic assessments of the induced membranes were conducted at 1 week and 4 weeks. No implant failure occurred in Group 1 whereas in Group 2, 4/9 (44.4%) implant failures occurred during the follow-up (p = 0.03). On histomorphometry, cell density was higher in Group 1 (4996 ± 716 cells / mm²) than in Group 2 (3500 ± 728 cells/mm²) (p = 0.0195) but the membrane thickness in Group 1 (735 ± 44 μm) was non-significantly lower than in Group 2 (979 ± 165 μm) (p = 0.4). This study suggests that, in rat models of critical-sized femoral defects (7 mm) to study the induced membrane technique, fixation plates with a thickness of 1 mm and four screws (2 mm in diameter) provide stable fixation without implant failure. In contrast, thinner plates (< 1 mm) combined with screws of smaller diameter (1.5 mm) result in a high rate of implant failure.
Similar content being viewed by others
Introduction
Critical bone defects in orthopedic and trauma surgery can be managed by the induced membrane technique (IMT), developed by A-C Masquelet1. It consists of placing a PMMA spacer into the bone defect. A foreign-body reaction leads to the formation of an induced membrane (IM) around the spacer that serves as a receptacle for a bone autograft. Despite the success rate of this technique, the IM is formed over a fairly long period (usually from 6 weeks to 3 months) before bone grafting, and two separate surgeries are needed, each necessitating an extended hospitalization. The relative immobility of the patient for several weeks has physical consequences (amyotrophy, joint stiffening) and socioeconomic consequences, with a delay in returning to personal and professional activities. The formation and quality of the IM depend on the patient (age, smoking status, associated comorbidities) and the pathological conditions (associated infectious diseases, localization of the bone defect, loss of soft tissue coverage)2. Moreover, to optimize fracture healing in clinical practice, several studies confirmed the need for maximum mechanical stability. Giannoudis et al. have established the “Diamond Concept”, comprising 4 major factors contributing to bone consolidation. Mechanical stability is one of these key factors. Mechanical bone stabilization enables the formation of a high-quality callus that will progressively absorb mechanical stresses3. The importance of this mechanical stability is also crucial in the induced membrane technique, as it seems to be linked to the success of this technique for several authors4,5,6,7,8. To optimize the IMT, animal models are widely used to understand and enhance the highly complex biological and cellular mechanisms that lead to IM formation and to test the efficacy of biomaterials or bone tissue engineering techniques. In vivo rat femoral defects are the most frequently studied model in this field9. Compared with those of larger models, the breeding, husbandry, and surgical procedures for rats are faster, easier, and more cost-effective. Compared with the tibia, the cylindrical shape of the femur allows more secure osteosynthesis and better soft tissue coverage10,11. Plate and screw fixation is the most frequent femoral osteosynthesis method12. Several studies in this field reported femoral defects no longer than 5–6 mm without any implant failures13,14,15. However, for longer segmental defects, the reported implant failure rates increase from 4.2%16 to 8.3%17. Plates associated with screws of 1.5 mm diameter have a variable thickness ranging from 0.6 mm to 1.3 mm and are commonly used in humans for hand and maxillofacial surgery. They are also commonly used in rat femoral defect models. Thin plates could have advantages as they are lighter than thicker ones, they could reduce stress shielding on the femur. Moreover, they could reduce soft tissue and muscle irritation, thus improving pain control and the rat’s natural locomotion. However, the thickness of osteosynthesis plates is the most influential parameter for bending strength18. No in vivo study has compared plates of various thicknesses, which are subject to continuous full weight bearing and consequently to bending stress in a rat femoral defect model. A biomechanical study on the internal fixation of mandibular condylar fractures showed that, in a finite element model, fixation was more stable with a 2.0 mm plate than with 3 types of 1.5 mm plates19. In another biomechanical study on bone substitutes, Watrous et al. found that plates with 1.5 mm screws were less stiff than plates with 2.0 mm screws in a 1 mm fracture gap model20. Finally, a study by Xie et al. on a rabbit radius bone defect model seemed to confirm in vivo that rigid plate fixation, versus no fixation of the defect, positively affected the quality of the induced membrane, notably in terms of osteogenic and angiogenic potential21. These findings call into question the feasibility of stabilizing longer femoral defects with plates less than 1 mm thick and screws 1.5 mm diameter without failures in a rat femoral defect model. Thus, the objective of this study was to compare the osteosynthesis of a rat femoral critical-sized bone defect model by using two different-sized plates and screws in the first stage of the induced membrane technique. We hypothesized that there was no difference in the rate of implant failure 4 weeks after this procedure between the two sizes of plate/screw.
Methods
Animal care and experimental procedure
All experimental animal procedures were carried out in the Animal Facility of the University of Lille (Agreement F5935010) according to the current European regulations regarding the protection of animals used for scientific purposes (Directive 2010/63/EU). The protocols and surgical procedures used for animal use were evaluated and approved by the French Minister of Research and the Ethical Committee for Animal Experimentation (APAFIS Project No. 46969). The study followed the ARRIVE guidelines (Animal Research: Reporting In Vivo Experiments)22. Nineteen male Sprague‒Dawley rats (Janvier Labs™, Le Genest-Saint-Isle, France), aged 10 weeks and weighing 450–500 g, were housed separately in a 12 h light/12 h dark, temperature-controlled environment (21 °C) with proper airflow and were given food and water ad libitum. Rats were acclimatized for a minimum of 7 days before any procedure. The rats were monitored daily during the post-operative period for signs of pain and complications. They were weighed twice a week during the first post-operative week and then once a week thereafter.
Sample size determination
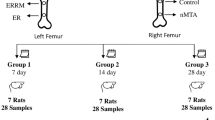
The number of rats per group was determined according to the principles of the 3Rs (Replacement, Reduction, and Refinement)23. The determination of group size was based on previous studies on the same animal model, which presented an a priori power analysis. These were based on quantitative histological variables with a pooled standard deviation of 25% and a power of 80%17,24. The estimated level of difference was 45%, and the required sample size was between 8 and 10 per group. A high effect size was proposed by Ziroglu et al. (Cohen’s f = 0.60), who determined a priori 7 animals per group25. The study by Xie et al. on the comparison of plate fixation versus no fixation of rabbit radius defects was closer to our model, since it included a biomechanical analysis. Each group included 6 rabbits and a mean maximum compressive strength of 110 Newtons versus 32 Newtons (p < 0.05). The effect size was large (greater than 2)21. The a priori power analysis of our study was based on these previous studies and was performed using the G-Power program version 3.1.9.7. In our study, the comparison of the number of failures between the 2 types of osteosynthesis was carried out using a Fisher’s exact test. For a power of 0.80, an alpha value of 0.05 and an effect size of 1, the number of animals required was 8 per group. To consider the risk of complications, one group (1 mm thick plates) had 10 rats, and the second group (0.6 mm thick plates) had 9 rats.
Surgery
All surgical procedures were performed under aseptic conditions. General anesthesia was induced in a chamber delivering a 4–5% isoflurane/O2 gas mixture, and the anesthetic state was maintained with a 1.5–2.5% isoflurane/O2 gas mixture with an adapted ventilation facemask. Each animal received an intramuscular injection of buprenorphine hydrochloride (0.05 mg/kg) pre-operatively. Rats were placed in the left lateral decubitus position. The right leg of each rat was shaved and scrubbed twice with chlorhexidine solution. The femur was exposed through a lateral approach. A longitudinal incision was made along the femur to expose the fascia lata. Then, the space between the vastus lateralis and the biceps femoris was developed. A plate was placed on the anterolateral aspect of the exposed femur. Two types of osteosynthesis (MODUS®, Medartis™, Switzerland) were performed: group one (10 rats) had 1 mm-thick 4-hole titanium plates (29 mm in length) and 4 cortical screws 2 mm in diameter (6 mm in length), and the group two (9 rats) had 0.6 mm-thick 6-hole titanium plates (30 mm in length) and 4 cortical screws 1.5 mm in diameter (6 mm in length) (2 for proximal and 2 for distal holes) (Fig. 1). After verification of placement, bicortical holes were drilled, and the plate was secured with the screws. Then, a 7 mm femoral defect was made in the diaphysis of the femur underneath the plate using a Gigli saw. PMMA cement (Palacos® R + G, Heraeus Medical™) was mixed according to the manufacturer’s protocol and was molded into the femoral defect, which was slightly wider than the femoral diameter (Fig. 1). After full polymerization of the cement, the wound was irrigated with sterile saline, and the incision was closed in two layers (muscular and subcutaneous) with interrupted 4–0 sutures (Vicryl®, Ethicon™, Johnson & Johnson™, Somerville, NJ). The bioglue (Dermabond®, Ethicon™, Johnson & Johnson™) was subsequently applied to the closed skin incision. Post-operatively, the rats were allowed to return to full weight bearing immediately. Analgesia comprised subcutaneous injection of buprenorphine hydrochloride (0.05 mg/kg) and meloxicam (2 mg/kg) twice a day for 3 to 7 days. After the fourth-week examination, the rats in Group 1 underwent 2nd stage of IMT, as this protocol was part of a broader protocol involving femoral defect models. The rats in Group 2 were euthanized painlessly under general anaesthesia (5% isoflurane/O2 gas mixture) with an intracardiac injection of pentobarbital (500 mg/kg) (DOLETHAL®, Vetoquinol™, Lure, France).
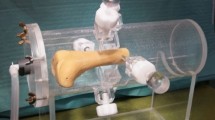
Intraoperative views and surgical steps of a 1 mm thick plate positioning in the induced membrane technique. (A) Circumferential exposition of the femoral shaft with a periosteal elevator protecting the medial structures. (B) Fixation of a 4-hole plate and screws. (C) The femoral defect was created with a Gigli saw and measured (7 mm). (D) Bone cement in place before closure.
Radiographic evaluation
All rats underwent two radiographs (anteroposterior and lateral of the femur) (BV Vectra®, Philips™, Amsterdam, Netherlands) under anesthesia to check the stability of the osteosynthesis (absence of femoral displacement, femoral fracture around a screw, plate breakage, screw loosening) at 1- and 4-weeks post-surgery.
Macroscopic and histologic evaluation of the induced membranes
Upon euthanizing the rats in Group 2, and executing the surgery of the second stage of IMT in the rats of Group 1, a sample of the induced membrane was then harvested and placed in 10% neutral buffered formalin for 24 h until ready for histological analysis. Briefly, the IM samples were dehydrated in a graded series of alcohol and embedded in paraffin. The paraffin blocks were cut into 5 μm slices with a microtome (Leica™ RM2245). Sections were deparaffinized in toluene and were colored with hematoxylin and eosin (HE) stain to detect cell types, and Masson-Trichrome (MT) stain to analyze the extracellular components. They were observed with a slide scanner (Zeiss™ Axioscan® Z1). Histomorphometry analyses were made on one slide per animal. The slides were analyzed to measure the thickness (in µm) of the IMs and the cell density (number of cells per mm²) (Zen® Blue edition software (Zeiss™, Oberkochen, Germany). Those measurements were made at three different areas per slide per rat and the respective mean values per slide per rat were calculated. The mean values of all slides of each group were calculated and compared. These measurements were randomly performed by an independent observer who was blinded to the osteosynthesis group to which each slide belonged.
Statistical analysis
Qualitative data are presented as numbers and percentages. A statistically significant difference in the failure rate of the procedures was analyzed using Fischer’s exact t-test. Quantitative variables are reported as the means with standard deviations. They have been rounded to the nearest integer. The normality of distributions was checked graphically using the Shapiro‒Wilk test. All quantitative data (pre and postoperative weights, induced membrane thickness, cell density) were compared with a Mann–Whitney nonparametric test as these variables were not normally distributed (BiostatGV, Institut Pierre Louis d’Epidémiologie et de Santé Publique affiliated with Sorbonne University, Paris). The significance level (α) was set at 5%.
Results
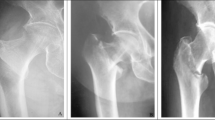
No animal died during the post-operative period. Weight did not differ between the two groups before surgery (486 ± 14 g for Group 1; 478 ± 12 g for Group 2) (p = 0.446) or 4 weeks after surgery (556 ± 20 g for Group 1; 541 ± 23 g for Group 2) (p = 0.470). Radiographic evaluations revealed that in Group 1, no implant failure occurred (Fig. 2); in Group 2, 4/9 rats exhibited implant-related failures during the follow-up (p = 0.03) (Fig. 3); No rats were euthanized before 4 weeks because they continued to gain weight and showed no signs of pain or suffering during the postoperative period. The failure cases were excluded from the histomorphology analysis. Macroscopic observation during induced membrane harvesting showed membranes lining the PMMA spacer when osteosynthesis did not fail. However, when osteosynthesis failed, there was a proliferation of fibrous pseudo-tumoral tissue with sero-hematic, compartmentalized content (Fig. 4). When the osteosynthesis was successful, histological analysis revealed (Fig. 5A) that the induced membranes were typically organized in 2 layers: the inner layer (in contact with the spacer) was rich in cells, rather round and with a developed, dense nucleus, similar to macrophages; the outer layer was rich in collagen, organized parallel to the PMMA spacer and crisscrossed by blood vessels. While histological analysis in the cases of osteosynthesis failure revealed a very large, completely disorganized tissue, without a clear distinction between different layers. Moreover, collagen and vessels were intermingled, and the cell population predominantly consisted of elongated, spindle-shaped fibroblasts with less dense nuclei (Fig. 5B). Histomorphometry was subsequently performed on the IM of the successful osteosynthesis cases (n = 10 for Group 1 and n = 5 for Group 2). The mean thickness of IM for Group 1 (735 ± 44 μm) showed no statistical difference (p = 0.4) from that of Group 2 (979 ± 165 μm). While the cell density in the IM of Group 1 (4996 ± 716 cells / mm²) was significantly higher (p = 0.0195) than that of Group 2 (3500 ± 728 cells / mm²) (Table 1).
Discussion
The main objective of this study was to compare two different-size plate/screw osteosynthesis to stabilize a 7 mm-long femoral segmental defect in Sprague‒Dawley rats. As a result, no implant failure occurred with 1 mm-thick plates/4 screws with Ø 2 mm compared to 4/9 (44.4%) failure rate for the group with 0.6 mm-thick plates/4 screws with Ø 1.5 mm (p = 0.03). These failures included plate breakage or screw loosening. It implies that to optimize the efficiency of a rat model of induced membrane technique, minimum 1-mm thick plates combined with larger screws than those reported in the literature seemed recommended. Most studies in rat femoral defect models have used plates with screws 1.5 mm in diameter and plates derived from human hand osteosynthesis, without reporting their thickness which could range between 0.6 mm and 1.3 mm16,17,26,27. For femoral defects shorter than 5–6 mm, almost no implant failures were reported14,15. Verboket et al. reported no implant failures in 5–6 mm femoral defects in 15 Sprague‒Dawley rats aged between 8 and 10 weeks13. However, another study reported failures on 2-stage IMT (3/65 rats, 4.6%) with this defect size27. Similar or greater implant failures were reported when femoral defects were 10 mm. Nau et al. reported 3 out of 72 (4.2%) loosening plates16 and Leiblein et al. reported 8 cases of screw loosening in 96 rats (8.3%)17. These femoral defects were longer than those created in our study (7 mm), but we reported a greater rate of implant failure (4/9 rats) in Group 2 (0.6 mm thick plates). Given the potential advantages of thinner plates, these results led us to analyze the reasons behind these implant failures thoroughly. However, no in vivo study has compared or reported the thickness of the plates in a rat femoral defect model. Only one study in a finite element model and another performed on bone substitutes seemed to favor thicker plates or larger screws19,20. Nevertheless, our study observed that higher implant failures could be attributed to the rats’ weight. Indeed, their weight (450 g at 10 weeks of age) was greater than those reported in the literature (ranging between 250 and 450 g)16,26. This difference could account for the higher rate of material breakage in heavier rats. We could have reduced the length of the femoral defect like previous studies13,14,15,28, which could have exposed to lesser loads on the osteosynthesis. However, a critical-sized defect, by definition, cannot heal by itself and must exceed 1.5-3.0 times the bone diameter10. A mature Sprague‒Dawley femur has a median diameter of 4.5 mm and a length of 35 mm. A femoral critical-size defect should then correspond to 6.75–13.5 mm. We chose a 7 mm femoral defect as it corresponds to 20% of the length of the femur29. These data, associated with our results, suggest that using 1-mm thick plates with 2 mm diameter screws in a rat femoral defect model could enhance the stability of the femoral fixation, and would allow resumption of walking without causing implant failures while respecting the definition of a critical-sized defect. Such refinement of applied implant materials could reduce the number of rats required for preclinical studies on the IMT concerning the reduction strategy of the 3Rs. Moreover, in studies reporting on the healing of femoral defects using biomaterials or bone-tissue engineering, more stable osteosynthesis could improve the results by decreasing their variability, as this also influences fracture healing in preclinical models30,31. Indeed, Kammerer et al. recently showed that bone union varied significantly between 5 mm and 10 mm femoral defects (90% versus 60%, p < 0.05) in 8–10 weeks-old SD rats, weighing 250–320 g. The union of the 10 mm femoral defect group varied from 35 to 82%, the highest variability reported in their study32. This recent result supported the hypothesis that the mechanical stability of the defect, more difficult to achieve when it was 10 mm versus 5 mm, could have a major impact on the outcome of studies designed to optimize the induced membrane technique in vivo.
Histomorphometry was performed on the IM of both groups of this study to provide detailed quantitative data on their structural and cellular characteristics. When no implant failure occurred, there was no statistical difference in IM thickness between the two groups and the measurements were consistent with values reported in the literature16,33. However, cell density was significantly higher in Group 1 (4996 ± 716 cells/mm²) compared to Group 2 (3500 ± 728 cells/mm²) (p = 0.0195). This could be attributed to the slightly reduced thickness of the IM in Group 1 (734.9 ± 44.3 μm) compared to Group 2 (978.9 ± 164.9 μm) which may have contributed to the increased cell density (p = 0.4). However, this result is in line with the study by Xie et al. which found higher cell proliferation by immunohistochemical measurement of Ki67-positive cells in the case of plate stabilization (33%) versus no stabilization (20%), in a rabbit radius bone defect model21. Our results for Group 1 were similar to those of Mathieu et al. (4923–4933 cells/mm²) but lower than the cell density reported by Toth et al. (5310–6300 cells/mm²), these studies used a rat model that used an external fixator to stabilize a 6 mm femoral defect34,35. Clinical studies in humans support the importance of mechanical stability for the success of the induced membrane technique. In a systematic review of the literature, Aurégan et al. studied the success rate and risk factors for failure of this technique in children. In their study, they found that unstable osteosynthesis accounted for 72% (out of 19 cases) of failures, compared with 16% of successes (out of 50 cases)4. In adults, Choufani et al. analyzed the results of the technique in a military setting, on average bone defects of 4.3 cm (extremes 2–10 cm) in 16 patients. They found that failure of the technique was linked to insufficient mechanical stability in 4 of the 8 patients (50%)5. The study by Mathieu et al. even proposed mechanical optimization by reinforcing the spacer with a centromedullary armature, resulting in 7 successes out of 8 cases of complex bone defects6. Other authors confirmed the need for maximum mechanical stability in this technique, notably Siboni et al. on 19 cases of critical tibial bone defects, and Wang et al. on 32 patients with post-traumatic osteomyelitis7,8. Finally, Wu et al. analyzed the factors influencing the success of this technique using a literature review and found that the local mechanical environment played an important role throughout the technique, affecting the quality of the induced membrane formed around the PMMA cement36.
This study has limitations. The number of rats included was low, but it corresponded to the average size of the groups in preclinical femoral defect models and was based on an a priori calculation of the sample size required. This could have led to a lack of statistical power, but the main result of this study reached statistical significance at 4 weeks. This study did not include other osteosynthesis methods that were also used, such as intramedullary nails28,37 or external fixators34,35 as plate/screw osteosynthesis was the most frequent in this preclinical model. The follow up period was short, but it corresponded to the mean time of the first stage in rat femoral defect models of IMT. The creation and stabilization of femoral defects in the rat model can be challenging and may introduce bias. However, all procedures were performed by the same operators who are experienced with this animal model. Furthermore, the positioning of the plates and screws was standardized to minimize failures due to technical difficulties.
Conclusion
This study suggests that, in rat models of critical-sized femoral defects (7 mm) to study the induced membrane technique, fixation plates with a thickness of 1 mm and four screws (2 mm in diameter) provide stable fixation without implant failure. In contrast, thinner plates (< 1 mm) combined with screws of smaller diameter (1.5 mm) result in a high rate of implant failure.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Masquelet, A. C. The induced membrane technique. Orthop. Traumatol. Surg. Res. 106, 785–787. https://doi.org/10.1016/j.otsr.2020.06.001 (2020).
Durand, M. et al. Towards understanding therapeutic failures in Masquelet surgery: first evidence that defective Induced membrane properties are associated with clinical failures. J. Clin. Med. 9, 450. https://doi.org/10.3390/jcm9020450 (2020).
Giannoudis, P. V., Einhorn, T. A. & Marsh, D. Fracture healing: the diamond concept. Injury 38 (Suppl 4). https://doi.org/10.1016/s0020-1383(08)70003-2 (2007).
Aurégan, J. C., Bégué, T., Rigoulot, G., Glorion, C. & Pannier, S. Success rate and risk factors of failure of the induced membrane technique in children: a systematic review. Injury 47 (Suppl 6), S62–S67. https://doi.org/10.1016/S0020-1383(16)30841-5 (2016).
Choufani, C. et al. Application of the Masquelet technique in austere environments: experience from a French forward surgical unit deployed in Chad. Eur. J. Trauma. Emerg. Surg. 48, 593–599. https://doi.org/10.1007/s00068-020-01471-5 (2022).
Mathieu, L. et al. Induced membrane technique with sequential internal fixation: use of a reinforced spacer for reconstruction of infected bone defects. Int. Orthop. 44, 1647–1653. https://doi.org/10.1007/s00264-020-04735-2 (2020).
Siboni, R. et al. Management of septic non-union of the tibia by the induced membrane technique. What factors could improve results? Orthop. Traumatol. Surg. Res. 104, 911–915. https://doi.org/10.1016/j.otsr.2018.04.013 (2018).
Wang, X., Luo, F., Huang, K. & Xie, Z. Induced membrane technique for the treatment of bone defects due to post-traumatic osteomyelitis. Bone Joint Res. 5, 101–105. https://doi.org/10.1302/2046-3758.53.2000487 (2016).
Sun, H. et al. The induced membrane technique in animal models: a systematic review. OTA Int. 5, e176. https://doi.org/10.1097/oi9.0000000000000176 (2022).
Garcia, P. et al. Rodent animal models of delayed bone healing and non-union formation: a comprehensive review. Eur. Cell. Mater. 26, 1–14. https://doi.org/10.22203/ecm.v026a01 (2013).
Klein, C. et al. The Masquelet technique: current concepts, animal models, and perspectives. J. Tissue Eng. Regen. Med. 14, 1349–1359. https://doi.org/10.1002/term.3097 (2020).
Saab, M., Zobrist, C., Blanchemain, N., Martel, B. & Chai, F. Systematic literature review of in vivo rat femoral defect models using biomaterials to improve the induced membrane technique: a comprehensive analysis. EFORT Open. Rev. 9, 138–145. https://doi.org/10.1530/EOR-23-0055 (2024).
Verboket, R. D. et al. From two stages to one: acceleration of the induced membrane (Masquelet) technique using human acellular dermis for the treatment of non-infectious large bone defects. Eur. J. Trauma. Emerg. Surg. 46, 317–327. https://doi.org/10.1007/s00068-019-01296-x (2020).
Sun, H. et al. The induced membrane technique: optimization of bone grafting in a rat model of segmental bone defect. Injury 53, 1848–1853. https://doi.org/10.1016/j.injury.2022.03.023 (2022).
Fenelon, M. et al. Comparison of amniotic membrane versus the induced membrane for bone regeneration in long bone segmental defects using calcium phosphate cement loaded with BMP-2. Mater. Sci. Eng. C Mater. Biol. Appl. 124, 112032. https://doi.org/10.1016/j.msec.2021.112032 (2021).
Nau, C. et al. Alteration of Masquelet’s induced membrane characteristics by different kinds of antibiotic enriched bone cement in a critical size defect model in the rat’s femur. Injury 47, 325–334. https://doi.org/10.1016/j.injury.2015.10.079 (2016).
Leiblein, M. et al. Introduction of a new surgical method to improve bone healing in a large bone defect by replacement of the Induced membrane by a human decellularized dermis repopulated with bone marrow mononuclear cells in rat. Materials (Basel) 13, 2629. https://doi.org/10.3390/ma13112629 (2020).
Gueorguiev, B. & Lenz, M. Why and how do locking plates fail? Injury 49 (Suppl 1), S56–S60. https://doi.org/10.1016/S0020-1383(18)30305-X (2018).
Aquilina, P., Parr, W. C. H., Chamoli, U., Wroe, S. & Clausen, P. A. Biomechanical comparison of three 1.5-mm plate and screw configurations and a single 2.0-mm plate for internal fixation of a mandibular condylar fracture. Craniomaxillofac. Trauma. Reconstr. 7, 218–223. https://doi.org/10.1055/s-0034-1375172 (2014).
Watrous, G. K., Moens, N. M. M., Runciman, J. & Gibson, T. W. G. Biomechanical properties of the 1.5 mm locking compression plate: comparison with the 1.5 and 2.0 mm straight plates in compression and torsion. Vet. Comp. Orthop. Traumatol. 31, 438–444. https://doi.org/10.1055/s-0038-1668084 (2018).
Xie, J. et al. Effects of topical mechanical stability on the formation of Masquelet membrane in a rabbit radial defect model. Sci. Rep. 10, 18939. https://doi.org/10.1038/s41598-020-76112-3 (2020).
du Sert, N. P. et al. Reporting animal research: explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 18, e3000411. https://doi.org/10.1371/journal.pbio.3000410 (2020).
Hubrecht, R. C. (ed Carter, E.) The 3Rs and humane experimental technique: implementing change. Animals (Basel) 9 754 https://doi.org/10.3390/ani9100754 (2019).
Leiblein, M. et al. Impact of scaffold granule size use in Masquelet technique on periosteal reaction: a study in rat femur critical size bone defect model. Eur. J. Trauma. Emerg. Surg. 48, 679–687. https://doi.org/10.1007/s00068-020-01516-9 (2022).
Ziroglu, N. et al. The antibiotics supplemented bone cement improved the masquelet’s induced membrane in a rat femur critical size defect model. Injury 54, 329–338. https://doi.org/10.1016/j.injury.2022.10.027 (2023).
Nau, C. et al. Influence of the induced membrane filled with syngeneic bone and regenerative cells on bone healing in a critical size defect model of the rat’s femur. Injury 49, 1721–1731. https://doi.org/10.1016/j.injury.2018.06.041 (2018).
Verboket, R. D. et al. The Induced membrane technique-the filling matters: evaluation of different forms of membrane filling with and without bone marrow mononuclear cells (BMC) in large femoral bone defects in rats. Biomedicines 10, 642. https://doi.org/10.3390/biomedicines10030642 (2022).
Bilal, Ö. et al. Epidermal growth factor or platelet-rich plasma combined with induced membrane technique in the treatment of segmental femur defects: an experimental study. J. Orthop. Surg. Res. 15, 601. https://doi.org/10.1186/s13018-020-02142-2 (2020).
Jäger, M., Sager, M., Lensing-Höhn, S. & Krauspe, R. The critical size bony defect in a small animal for bone healing studies (I): comparative anatomical study on rats’ femur. Biomed. Tech. (Berlin) 50, 107–110 (2005).
Histing, T. et al. Ex vivo analysis of rotational stiffness of different osteosynthesis techniques in mouse femur fracture. J. Orthop. Res. 27, 1152–1156. https://doi.org/10.1002/jor.20849 (2009).
Lienau, J. et al. Initial vascularization and tissue differentiation are influenced by fixation stability. J. Orthop. Res. 23, 639–645. https://doi.org/10.1016/j.orthres.2004.09.006 (2005).
Kammerer, A. et al. The impact of defect size on Bone Healing in critical-size bone defects investigated on a rat femur defect model comparing two treatment methods. Bioengineering (Basel) 11, 287. https://doi.org/10.3390/bioengineering11030287 (2024).
Henrich, D. et al. Establishment and characterization of the Masquelet induced membrane technique in a rat femur critical-sized defect model. J. Tissue Eng. Regen. Med. 10, E382–E396. https://doi.org/10.1002/term.1826 (2016).
Mathieu, L. et al. The Masquelet technique: can disposable polypropylene syringes be an alternative to standard PMMA spacers? A rat bone defect model. Clin. Orthop. Relat. Res. 479, 2737–2751. https://doi.org/10.1097/CORR.0000000000001939 (2021).
Toth, Z. et al. Masquelet technique: effects of spacer material and micro-topography on factor expression and bone regeneration. Ann. Biomed. Eng. 47, 174–189. https://doi.org/10.1007/s10439-018-02137-5 (2019).
Wu, J. H., Bao, Q. W., Wang, S. K., Zhou, P. Y. & Xu, S. G. Mechanisms of the Masquelet technique to promote bone defect repair and its influencing factors. Chin. J. Traumatol. S1008-1275 (24), 00054–00053. https://doi.org/10.1016/j.cjtee.2024.04.003 (2024).
DeBaun, M. R. et al. A bioactive synthetic membrane improves bone healing in a preclinical nonunion model. Injury 53, 1368–1374. https://doi.org/10.1016/j.injury.2022.01.015 (2022).
Acknowledgements
The authors would like to thank the Plateforme Ressources Expérimentales, D.H.U.R.E, University of Lille, for their support on animal studies, the Plateformes Lilloises en Biologie et Santé (PLBS) - UAR 2014 - US 41 for their support on histologic studies, and the Lille University Hospital for their funding support.
Funding
This research was supported by the CHU de Lille Fund 2023 for Research Activities and Teams.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Conceptualization, methodology, writing of original draft, data collecting and analysis were performed by MS. Methodology, data collection and analysis were performed by ASD. NB made critical revision and FC has supervised the study, performed data collection and analysis, and has edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was evaluated and approved by the French Minister of Research and the Ethical Committee for Animal Experimentation (APAFIS Project No. 46969).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Saab, M., Drucbert, AS., Blanchemain, N. et al. Comparison of two plates and screw osteosynthesis configurations in a rat model of critical sized femoral defects to reduce implant related failures. Sci Rep 15, 2796 (2025). https://doi.org/10.1038/s41598-025-85607-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-85607-w