Abstract
Herein, a novel amine-functionalized magnetic resorcinol-formaldehyde with a core-shell structure (Fe3O4@RF/Pr-NH2) is prepared through the chemical immobilization of (3-aminopropyl)trimethoxysilane over Fe3O4@RF composite. Characterization through FT-IR, EDX, PXRD, and TGA confirmed successful surface modification while preserving the crystalline structure of Fe3O4. The VSM analysis demonstrated excellent superparamagnetic properties, and SEM and TEM images revealed spherical particles for the designed nanocatalyst. The Fe3O4@RF/Pr-NH2 nanocomposite was employed as a robust nanocatalyst to promote the Knoevenagel condensation of benzaldehydes with ethyl cyanoacetate and malononitrile, resulting in the formation of substituted olefins. Various aromatic aldehydes were used as substrates in the presence of 0.01 g of Fe3O4@RF/Pr-NH2, achieving high to excellent yields (87–97%) within short reaction times (10–50 min) in EtOH at 60 °C. The high performance of Fe3O4@RF/Pr-NH2 is attributed to the hydrophobic nature of RF shell, which facilitates the accumulation of organic precursors around the catalytic active sites and enhances product yields. The designed magnetic catalyst could retain its high efficiency for at least ten runs. The metal-free, low-cost, and environmentally friendly attributes of the Fe3O4@RF/Pr-NH2 catalyst make it a promising alternative to traditional metal-based catalysts.
Similar content being viewed by others
Introduction
One of the biggest challenges in synthetic organic chemistry is the development of reactions that produce high-quality compounds in an environmentally friendly and sustainable manner1,2,3,4. Green chemistry, defined by principles that reduces or eliminates hazardous substances in chemical product design, manufacture, and use, is one of the most attractive concepts for sustainability5,6,7,8,9,10. Solvents, often constitute the majority of industrial waste, represent a critical area of focus in green chemistry. Efforts should focus on either the elimination of solvents or the substitution them with ecologically friendly alternatives in chemical processes. Moreover, many conventional solvents are toxic, flammable, and/or corrosive. To address these issues, alternative systems have been developed, such as solventless processes, water, supercritical fluids, and ionic liquids. One of the most important areas of green chemistry is catalysis, which offers selective, energy-efficient, and atom-economical solutions for various critical industrial concerns11,12,13,14,15,16,17,18. Therefore, there is a strong demand for the development of catalytic systems that provide an environmentally friendly and sustainable method to such chemical transformations19,20,21. The α, β unsaturated carbonyl compounds play a crucial role in several industries such as pharmaceuticals, agrochemicals, and fine chemicals manufacturing. The Knoevenagel condensation reaction is a highly effective method for preparation of these compounds. In this process, α, β-unsaturated carbonyl compounds are formed by a nucleophilic attack of a reactive methylene group on a carbonyl center, which is then followed by the removal of a water molecule22,23. Ionic liquids24,25, ammonium salts26,27, amino acids28, and amines29,30 are commonly employed as catalysts in a homogeneous environment to conduct the Knoevenagel condensation. However, these systems have several drawbacks, including elevated temperature requirements, challenges in catalyst separation and recovery, and excessive solvent usage, which contribute to environmental pollution31,32,33. Therefore, developing metal-free heterogeneous catalysts for the Knoevenagel condensation is an important challenge in this matter. Some recently reported catalysts in this matter are zeolites34,35, hydrotalcite36,37, metal-organic frameworks38,39, heteropoly acids40,41, and magnetic nanoparticles (MNPs)42,43. Among these, MNPs have attracted considerable interest due to their rapid recovery using an external magnet, which eliminates the need for tedious separation methods such as centrifugation, extraction, and filtration44,45,46. Additionally, MNPs exhibit high biocompatibility, unique magnetic properties and cost-effective preparation, making them suitable for large-scale production. Due to these advantages, the magnetic nanoparticles have been widely used in various areas such as catalysis, supercapacitors, therapy, diagnosis, drug delivery, etc47,48,49,50,51. Various types of shells, including silica, polymers, organic surfactants and carbon, can be employed to coat magnetic nanoparticles, thereby improving their chemical stability and inhibiting aggregation52,53,54,55,56. Soleiman‑Beigi et al. have reported the synthesis of Fe3O4 NPs coating with 3-propyltrimethoxysilane-natural asphalt sulfonic acid supported Pd and Zr, and investigate its catalytic activity in the preparation of symmetrical and unsymmetrical aryl/alkyl disulfides57, Esmaili et al. have synthesized silica sulfuric acid coated on SnFe2O4 MNPs and evaluated its catalytic activity in the asymmetric Hantzsch reaction58, ghobakhloo et al. have developed a zwitterionic HEPES catalyst on LDH modified γ-Fe2O3 MNPs and applied as a bifunctional catalyst for the synthesis of pyrazolo[3,4‐d]pyrimidines59. Some other recently reported nanocomposite in this context include ZrFe2O4@SiO2–N–(TMSP)–ASP–Pd(0)60, γ-Fe2O3@LDH/PCE-Mn(II)61, Cu2O/Fe3O4@C/Cu62, SiO2@MNP-A63, DETA-Fe3O464, Fe3O4@RF–Ag65. Among different shells, resorcinol-formaldehyde (RF) resin has gained significant attention due to its unique properties including a hydrophobic inner framework, an exterior surface rich in phenol hydroxyl groups, which promotes the accumulation of reactants around the catalytic active site, as well as its high biocompatibility, non-toxicity, and cost-effectiveness66,67,68. Therefore, coating magnetic nanoparticles with an RF shell not only enhances their chemical stability and prevents aggregation but can also takes advantage of the unique hydrophobic and biocompatible properties of RF, making these nanocomposites particularly effective in catalytic applications.
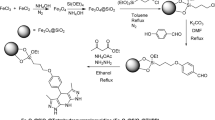
Considering all these aspects, in this work, a novel, metal-free and environmentally friendly magnetic nanocatalyst, denoted as Fe3O4@RF/Pr-NH2, is prepared, characterized and applied as a powerful catalyst for the Knoevenagel condensation under green and mild conditions (Fig. 1).
Experimental section
General
All chemicals and reagents such as iron (II) chloride tetrahydrate (99%), iron (III) chloride hexahydrate (99%), ammonia (25% wt), resorcinol (≥ 99%), formaldehyde (37% wt), (3-aminopropyl)trimethoxysilane (97%), benzaldehydes (97–99%), malononitrile (≥ 99%) and ethyl cyanoacetate (≥ 98%) were purchased from Fluka, Merck and Sigma-Aldrich companies. Solvents were dried and purified before use, according to standard procedures. The characterization of the materials was conducted using instruments previously reported69,70. The purity determination of the products and reaction monitoring were carried out by using TLC on silica gel polygram SILG/UV 254 plates.
Synthesis of Fe3O4@RF
First, Fe3O4 NPs were prepared according to our previous method71. To disperse 0.5 g of these NPs in deionized (DI) water, a volume of 150 mL was used during ultrasonic irradiation. This large solvent volume was employed to achieve a uniform dispersion and prevent nanoparticle aggregation. The high solvent volume reduces the concentration of nanoparticles in the solution, which helps minimize agglomeration and ensures effective energy transfer during sonication72. After 10 min of ultrasonic treatment, ammonia (0.3 mL, 25 wt%) and resorcinol (0.4 g) were added and the mixture was agitated at 30 °C for 1 h. Formaldehyde (0.6 mL) was then added drop by drop, and the mixture was stirred continuously for a duration of 6 h. Subsequently, the obtained mixture was subjected to heat treatment at 80 °C for a duration of 5 h. The magnetic material obtained was collected, rinsed with EtOH and DI water, and then subjected to drying at 70 °C for 6 h73.
Synthesis of Fe3O4@RF/Pr-NH2
For this purpose, the Fe3O4@RF NPs (0.5 g) were thoroughly dispersed in toluene (25 mL) at 25 °C for 20 min. Following the addition of (3-aminopropyl)trimethoxysilane (0.26 g), the reaction vessel was subjected to reflux at 100 °C for 24 h. After collecting the resultant magnetic material, it was rinsed with EtOH and DI water and dried at 70 °C for 6 h.
Procedure for the Knoevenagel coupling using Fe3O4@RF/Pr-NH2 nanocatalyst
For this, Fe3O4@RF/Pr-NH2 (0.01 g), ethyl cyanoacetate (0.113 g)/malononitrile (0.066 g) and aldehyde (1 mmol) were added into a reaction flask containing EtOH (5 mL) and stirred at 60 °C. After the process was finished, hot EtOH (10 mL) was added and the Fe3O4@RF/Pr-NH2 catalyst was separated using a magnet. Finally, the solvent was removed through evaporation to give crude products. Following the recrystallisation of crude products in EtOH and n-hexane solvents, the purified Knoevenagel products were obtained.
IR1, H NMR and13C NMR data of Knoevenagel products
(E)-ethyl 2-cyano-3-(4-nitrophenyl)acrylate
Pale yellow solid; yield: 94%; M. P.: 170–172 °C (ref: 172–174 °C74), IR (KBr, cm− 1): 3035 (= C-H, stretching vibration sp2), 2964 (C-H, stretching vibration sp3), 2223 (CN, stretching vibration), 1724 (C = O, stretching vibration), 1589, 1469 (C = C, Ar stretching sp2), 1243 (C-O, stretching vibration), 1510, 1350 (NO2, stretching vibration). 1H NMR (400 MHz, DMSO): δ (ppm) 1.45 (t, J = 7.0 Hz, 3 H), 4.43 (q, J = 7.0 Hz, 2 H), 8.17 (d, ArHmeta, J = 8.8 Hz, 2 H), 8.33 (d, ArHortho,J = 8.8 Hz, 2 H), 8.39 (s,1 H). 13C NMR (100 MHz, DMSO): δ (ppm) 14.4, 63.1, 106.0, 115.5, 125.4, 131.3, 136.9, 148.5, 153.2, 161.7.
(E)-ethyl 2-cyano-3-(4-bromophenyl)acrylate
Pale yellow solid; yield: 94%; M. P.: 92–94 °C (ref: 90–91 °C75), IR (KBr, cm− 1): 3035 (= C–H, stretching vibration sp2), 2985 (C–H, stretching vibration sp3), 2221 (CN, stretching vibration), 1727 (C = O, stretching vibration), 1593, 1484 (C = C, Ar stretching sp2), 1264 (C–O, stretching vibration). 1H NMR (400 MHz, DMSO): δ (ppm) 1.41 (t, J = 6.9 Hz, 3 H), 4.38 (q, J = 6.9 Hz, 2 H), 7.66 (d, J = 10.8 Hz, 2 H), 7.87 (d, J = 10.80 Hz, 2 H), 8.21 (s, 1 H). 13C NMR (100 MHz, DMSO): δ (ppm) 14.1, 62.9, 103.6, 115.2, 127.7, 128.3, 153.5, 133.2, 155.7, 163.1.
(E)-ethyl 2-cyano-3-(4-chlorophenyl)acrylate
Pale yellow solid; yield: 95%; M. P.: 91–93 °C (ref: 88–90 °C74), IR (KBr, cm− 1): 3035 (= C–H, stretching vibration sp2), 2985 (C–H, stretching vibration sp3), 2221 (CN, stretching vibration), 1727 (C = O, stretching vibration), 1593, 1484 (C = C, Ar stretching sp2), 1264 (C–O, stretching vibration). 1H NMR (400 MHz, DMSO): δ (ppm) 1.35 (t, J = 9.4 Hz, 3 H), 4.31 (q, J = 9.4 Hz, 2 H), 7.57 (d, J = 11.6 Hz, 2 H), 7.97 (d, J = 11.6 Hz, 2 H), 8.35 (s, 1 H). 13C NMR (100 MHz, DMSO): δ (ppm) 14.2, 63.3, 106.5, 114.9, 128.5, 130.5, 137.4, 139.7, 149.9, 161.8.
Results and discussion
Figure 1 illustrates the synthesis procedure of Fe3O4@RF/Pr-NH2 nanocomposite. First, the Fe3O4@RF NPs were prepared through interface polymerization of resorcinol and formaldehyde over the Fe3O4 NPs under alkaline conditions. Then, the RF shell was chemically modified with (3-aminopropyl)trimethoxysilane to give Fe3O4@RF/Pr-NH2 catalyst.
The FT-IR was used to demonstrate the functional moieties of the designed nanocatalyst (Fig. 2). Two peaks at 3409 and 578 cm− 1 were observed in all Fe3O4, Fe3O4@RF, and Fe3O4@RF/Pr-NH2 samples. These peaks are attributed to the O-H and Fe-O bonds, respectively. Signals at 2857–2932 and 1450 cm− 1 are respectively attributable to the vibrations of CH2 moieties of RF resin in Fe3O4@RF and Fe3O4@RF/Pr-NH2 samples. Moreover, the signals at 1025 and 1110 cm− 1 are related to the C–O–C methylene ether bridges between resorcinol moieties (Fig. 2b,c). Figure 2c demonstrated that the surface of Fe3O4@RF was effectively modified with (3-aminopropyl)trimethoxysilane, as evidenced by the Si-O-Si bonding peaks at 1029 and 806 cm− 1.
Also, the crystallinity of the Fe3O4@RF/Pr-NH2 nanocomposite was investigated by the powder XRD examination. As depicted in Fig. 3, the wide-angle powder X-ray diffraction (WAPXRD) indicates seven peaks at 2θ = 30.5°, 36.0°, 43.5°, 54.5°, 57.5°, 63.0°, and 75° corresponding to the crystal planes of (220), (311), (400), (422), (511), (440), and (533), respectively. These findings are consistent with the PXRD pattern of Fe3O476, confirming high stability of these NPs during modification steps.
The composition and thermal stability of materials are determined through the use of thermal gravimetric analysis (TGA). The TGA of the designed catalyst is shown in Fig. 4. The first weight reduction observed below 180 °C (about 3%) is attributed to the evaporation of adsorbed solvents on the material surface. With an increase in the temperature to a range between 200 and 380 °C, a weight loss of approximately 27% occurred that can be attributed to the decomposition of aminopropyl moieties and also surface parts of RF shell. The main weight loss of about 35% in the range of 420 to 780 °C is related to the elimination of the RF resin that is incorporated in the material structure. These results demonstrate the exceptional thermal stability of the Fe3O4@RF/Pr-NH2 nanocomposite.
Figure 5 displays the EDX analysis of the Fe3O4@RF/Pr-NH2 catalyst. Figure 5 displays the EDX analysis of the Fe3O4@RF/Pr-NH2 catalyst. The EDX pattern indicates the existence of C, N, O, Si and Fe in the sample. The finding aligns well with the FT-IR and TGA analyses, confirming the successful incorporation/immobilization of expected species in/on the Fe3O4@RF/Pr-NH2 nanocomposite.
The VSM analysis was utilized to determine the magnetic properties of the designed nanocomposite. A high saturation magnetization of 35 emu/g with zero coercivity (Hc) was observed for the Fe3O4@RF/Pr-NH2 composite indicating very good super paramagnetic properties of this material (Fig. 6). Therefore, in different chemical process, Fe3O4@RF/Pr-NH2 can be readily recovered due to its exceptional magnetic property.
The morphology of the Fe3O4@RF/Pr-NH2 nanocomposite was evaluated by SEM analysis (Fig. 7). The findings indicated that the catalyst possesses a spherical morphology with an average particle size of about 75 nm. These type particles possesses potential to be use in catalytic and adsorption processes.
The TEM image of the Fe3O4@RF/Pr-NH2 nanocatalyst also revealed spherical particles with a black core representing the magnetite NPs and a grey shell corresponding to the RF layer, as shown in Fig. 8. This observation is consistent with similar TEM images reported for other core-shell structured magnetic nanocomposites77,78,79.
After a comprehensive characterization of Fe3O4@RF/Pr-NH2, its catalytic performance was investigated in the Knoevenagel reaction. In order to find the best conditions for the reaction, we used the benzaldehyde and ethyl cyanoacetate reaction as a test model. Several parameters, such as the amount of catalyst, solvent, and temperature, were determined to find the ideal conditions (Table 1). Firstly, the effect of solvent-free media as well as H2O and EtOH solvents were screened (Table 1, entries 2–4). The findings indicated that the highest yield (95%) is achieved when using EtOH. The effect of catalyst amount was also evaluated and it was found that the use of 0.01 g of Fe3O4@RF/Pr-NH2 catalyst gives the best result (Table 1, entry 4). Notably, while the reaction yield dropped significantly when the catalyst amount was reduced to 0.005 g, raising it to 0.015 g had no effect on the yield (Table 1, entries 5, 6). The reaction rate was also significantly influenced by temperature, with the optimal result was obtained at 60 °C (Table 1, entry 4 vs. entries 7, 8). It should be noted that increasing the reaction temperature did not result in an enhanced yield of the product (Table 1, entry 4 vs. entry 9). Accordingly, the optimum conditions were selected as follows: 0.01 g of Fe3O4@RF/Pr-NH2 catalyst, EtOH solvent and 60 °C (Table 1, entry 4). In another study, to further validate the necessity of catalyst for the Knoevenagel reaction, a blank test without the catalyst was performed on the condensation between benzaldehyde and malononitrile (Table 1, entry 10). The result revealed that only 23% of the desired product is obtained in the absence of the Fe3O4@RF/Pr-NH2 catalyst, compared to a 96% yield when the catalyst was used (Table 1, entry 11). This significant difference confirms the key role of the designed catalyst in the reaction progress, which can be attributed to catalytic amine groups and also the hydrophobic nature of the catalyst shell that facilitates the accumulation of reactants around the catalytic active sites.
Following the optimization study of parameters, the substrate scope of this catalytic system was further investigated using both ethyl cyanoacetate and malononitrile as the active methylene compounds (Table 2). As demonstrated, the corresponding Knoevenagel products are delivered in high yields by all aldehydes that contain either electron-donating and/or electron-withdrawing groups. This highlights the exceptional efficiency of the Fe3O4@RF/Pr-NH2 catalyst in facilitating the reaction, thereby making it highly adaptable and suitable for a broad range of substrates.
The gram-scale experiment was also studied on entry 1 of Table 2. The results showed that using 10 mmol of substrates also produced the desired product in high yield, confirming the efficiency of the designed catalyst (Fig. 9).
In the subsequent investigation, the recoverability and reusability of the proposed catalyst were examined. To do this, after the process was finished, Fe3O4@RF/Pr-NH2 was magnetically isolated and reapplied in subsequent runs using identical circumstances as the initial run. It was found that the Fe3O4@RF/Pr-NH2 catalyst is able to maintain its effectiveness for at least ten runs, demonstrating its exceptional longevity in the given conditions (Fig. 10).
A leaching test was performed to investigate the nature of Fe3O4@RF/Pr-NH2 under applied conditions. To do this, the catalyst was removed from the reaction mixture after achieving a 50% conversion. Then, the reaction of the catalyst-free residue was then left to continue for 60 min under applied conditions. Notably, no significant increase in conversion was observed, confirming the heterogenous nature of the designed catalyst.
The EDX-mapping analysis was also performed on the recycled catalyst to investigate its chemical and structural stability (Fig. 11). The result confirmed the uniform distribution of C, N, O, Si and Fe elements onto/into material network, indicating the high stability of the catalyst after reusing and recovering times.
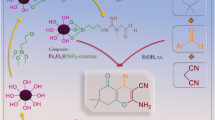
A plausible mechanism for the Knoevenagel condensation using Fe3O4@RF/Pr-NH2 catalyst is outlined in Fig. 12. At first, the amine groups on the catalyst deprotonate the hydrogen from ethyl cyanoacetate, forming an enolate ion. This enolate then nucleophilically attacks the carbonyl carbon of the aldehyde. The proton is subsequently re-captured from the protonated catalyst, leading to the formation of β-hydroxy compounds. After elimination of a water molecule from the β-hydroxy compounds, the desired Knoevenagel products are obtained84,85.
The performance of the Fe3O4@RF/Pr-NH2 catalyst was compared to various catalytic systems previously used in the Knoevenagel coupling reaction (Table 3). The study showed that the current catalyst outperforms previous catalytic systems in terms of mild reaction conditions, reaction rate, and recovery times.
Conclusion
In summary, a novel magnetic RF modified 3-aminopropyltrimethoxysilane (Fe3O4@RF/Pr-NH2) was successfully designed and synthesized. The FT-IR and EDX analyses clearly indicated that the RF moieties are successfully coated/immobilized on the magnetic NPs. Furthermore, TGA confirmed the remarkable thermal stability of the developed nanocomposites. The wide-angle PXRD analysis also demonstrated that the crystalline structure of the Fe3O4 NPs is extremely stable during the modification steps. In addition, the SEM and TEM image of Fe3O4@RF/Pr-NH2 showed well-defined spherical particles for this nanomaterial. The Fe3O4@RF/Pr-NH2 nanocomposite was effectively employed as a robust and highly recoverable catalyst for the Knoevenagel condensation providing the desired products in high yields at short times under mild conditions. Its advantages, including easy recoverability and high stability, suggest significant potential for use in a various of important organic reactions, such as aldol condensation, cyanation, O-arylation reactions, and multicomponent processes and so on.
Data availability
All data and materials are included in the manuscript.
References
Sharma, D., Choudhary, P., Kumar, S. & Krishnan, V. Transition Metal Phosphide Nanoarchitectonics for Versatile Organic Catalysis. Small 19, 2207053. https://doi.org/10.1002/smll.202207053 (2023).
Hosseini, S. & Azizi, N. New insight into highly efficient CSA@g-C3N4 for photocatalytic oxidation of benzyl alcohol and thioanisole: NAEDS as a promoter of photoactivity under blue LED irradiation. Photochem. Photobiol n/a. https://doi.org/10.1111/php.13883 (2023).
Yamaguchi, T. Application of ZrO2 as a catalyst and a catalyst support. Catal. Today. 20, 199–217. https://doi.org/10.1016/0920-5861(94)80003-0 (1994).
Zhang, R. et al. Energy-saving effect of low-cost and environmentally friendly sepiolite as an efficient catalyst carrier for CO2 capture. ACS Sustain. Chem. Eng. 11, 4353–4363 (2023).
Tandon, R., Tandon, N. & Patil, S. M. Overview on magnetically recyclable ferrite nanoparticles: synthesis and their applications in coupling and multicomponent reactions. RSC Adv. 11, 29333–29353 (2021).
Patil, S., Tandon, R. & Tandon, N. A current research on silica coated ferrite nanoparticle and their application. Curr. Res. Green. Sustain. 4, 100063 (2021).
Tandon, N., Patil, S. M., Tandon, R. & Kumar, P. Magnetically recyclable silica-coated ferrite magnetite-K 10 montmorillonite nanocatalyst and its applications in O, N, and S-acylation reaction under solvent-free conditions. RSC Adv. 11, 21291–21300 (2021).
Patil, S., Tandon, R. & Tandon, N. in Journal of Physics: Conference Series. 012107 (IOP Publishing).
Tandon, R., Patil, S., Tandon, N. & Kumar, P. Magnetically recyclable silica-coated Magnetite-Molybdate Nanocatalyst and its applications in N-Formylation reactions under Solvent-Free conditions. Lett. Org. Chem. 19, 616–626 (2022).
Pise, A., Patil, S. M. & Ingale, A. P. Malic acid as a Green Catalyst for the N-Boc Protection under Solvent-free Condition. Lett. Org. Chem. 21, 620–629 (2024).
Patil, S. M., Ingale, A. P., Pise, A. S. & Bhondave, R. S. Novel Cobalt-Supported Silica‐Coated Ferrite Nanoparticles Applicable for Acylation of Amine, Phenol, and Thiols Derivatives under Solvent‐Free Condition. ChemistrySelect 7, e202201590 (2022).
Patil, S. M. et al. Magnetite-supported montmorillonite (K 10)(nanocat-Fe-Si-K 10): an efficient green catalyst for multicomponent synthesis of amidoalkyl naphthol. RSC Adv. 13, 17051–17061 (2023).
Patil, S. M., Tandon, R. & Tandon, N. Magnetically recoverable silica-decorated ferromagnetic-nanoceria nanocatalysts and their use with O-and N-Butyloxycarbonylation reaction via Solvent-Free Condition. ACS Omega. 7, 24190–24201 (2022).
Patil, S. et al. One-pot protocol for the reductive amination of aldehydes using thiamine hydrochloride as a green catalyst under solvent-free condition. Synth. Commun. 53, 1545–1558 (2023).
Patil, S. M. Magnetically recoverable molybdate supported silica decorated ferrite nanocat (Fe–Si–Mo) and their utilization for reductive amination of carbonyl compound under benign condition. J. Indian Chem. Soc. 101, 101261 (2024).
Patil, S. M. Novel silica-coated magnetic nanoparticles and their synthetic applications. Iran. J. Catal. 13 (2023).
Pise, A. S., Ingale, A. P. & Patil, S. M. An efficient synthesis of 1, 3-oxazine derivatives catalyzed under ceric ammonium nitrate in an aqueous medium at ambient temperature. Polycycl. Aromat. Compd. 44, 5088–5098 (2024).
Ingale, A. P., Patil, S. M. & Shinde, S. V. Catalyst-free, efficient and one pot protocol for synthesis of nitriles from aldehydes using glycerol as green solvent. Tetrahedron Lett. 58, 4845–4848 (2017).
Kumar, A., Choudhary, P. & Krishnan, V. Selective and efficient aerobic oxidation of benzyl alcohols using plasmonic Au-TiO2: influence of phase transformation on photocatalytic activity. Appl. Surf. Sci. 578, 151953 (2022).
Verochkina, E. A., Vchislo, N. V. & Rozentsveig, I. B. α-functionally substituted α, β-unsaturated aldehydes as fine chemicals reagents: synthesis and application. Molecules 26, 4297 (2021).
Liandi, A. R. et al. Recent trends of spinel ferrites (MFe2O4: Mn, Co, Ni, Cu, Zn) applications as an environmentally friendly catalyst in multicomponent reactions: a review. Case Stud. Chem. Environ. Eng. 7, 100303 (2023).
Li, G., Xiao, J. & Zhang, W. Efficient and reusable amine-functionalized polyacrylonitrile fiber catalysts for Knoevenagel condensation in water. Green. Chem. 14, 2234–2242. https://doi.org/10.1039/C2GC35483G (2012).
Panchenko, V. N. et al. Catalytic behavior of metal–organic frameworks in the Knoevenagel condensation reaction. J. Catal. 316, 251–259. https://doi.org/10.1016/j.jcat.2014.05.018 (2014).
Hangarge, R. V., Jarikote, D. V. & Shingare, M. S. Knoevenagel condensation reactions in an ionic liquid. Green. Chem. 4, 266–268. https://doi.org/10.1039/B111634G (2002).
Player, L. C., Chan, B., Turner, P., Masters, A. F. & Maschmeyer, T. Bromozincate ionic liquids in the Knoevenagel condensation reaction. Appl. Catal. B: Environ. 223, 228–233 (2018).
van Schijndel, J. et al. Mechanistic considerations and characterization of ammonia-based catalytic active intermediates of the green Knoevenagel reaction of various benzaldehydes*. Green. Chem. Lett. Rev. 12, 323–331. https://doi.org/10.1080/17518253.2019.1643931 (2019).
Honarmand, M. & Givzad, M. An efficient and eco-friendly process for the Knoevenagel reaction using nano organosalt catalyst. Int. J. Environ. Sci. Technol. 15, 1551–1560 (2018).
Burate, P. A., Javle, B. R., Desale, P. H. & Kinage, A. K. Amino acid amide based ionic liquid as an efficient organo-catalyst for solvent-free Knoevenagel condensation at room temperature. Catal. Lett. 149, 2368–2375 (2019).
Hiba, K., Prathapan, S. & Sreekumar, K. Amine Functionalized Dendronized Polymer as a homogeneous Base Catalyst for the synthesis of polyhydroquinolines and 4-Arylidene-3-Methylisoxazol-5(4H)-Ones. Catal. Lett. 152, 2457–2469. https://doi.org/10.1007/s10562-021-03829-9 (2022).
Saha, E. et al. Amine-rich nickel (II)-Xerogel as a highly active Bifunctional Metallo-Organo Catalyst for Aqueous Knoevenagel Condensation and Solvent-free CO2 Cycloaddition. Inorg. Chem. 62, 14959–14970 (2023).
Kumar, A., Choudhary, P., Kumar, A., Camargo, P. H. C. & Krishnan, V. Recent advances in Plasmonic Photocatalysis based on TiO2 and Noble Metal nanoparticles for Energy Conversion, Environmental Remediation, and Organic synthesis. Small 18, 2101638. https://doi.org/10.1002/smll.202101638 (2022).
Speier, J. L. in Adv. Organomet. Chem. Vol. 17 (eds F. G. A. Stone & Robert West) 407–447Academic Press, (1979).
Bai, S. T. et al. Homogeneous and heterogeneous catalysts for hydrogenation of CO 2 to methanol under mild conditions. Chem. Soc. Rev. 50, 4259–4298 (2021).
Min, H. K. et al. Rational design of pomegranate-like base–acid bifunctional β Zeolite by Steam-assisted crystallization for the Tandem deacetalization–knoevenagel condensation. ACS Appl. Mater. Interfaces. 12, 57881–57887. https://doi.org/10.1021/acsami.0c17398 (2020).
Song, Q. et al. Zeolitic imidazolate framework (ZIF-8) based Polymer nanocomposite membranes for gas separation. Energy Environ. Sci. 5, 8359–8369. https://doi.org/10.1039/C2EE21996D (2012).
Devi, R., Begum, P., Bharali, P. & Deka, R. C. Comparative study of Potassium Salt-loaded MgAl hydrotalcites for the Knoevenagel Condensation reaction. ACS Omega. 3, 7086–7095. https://doi.org/10.1021/acsomega.8b00767 (2018).
Mancipe, S. et al. B-Containing hydrotalcites effectively catalyzed synthesis of 3-(Furan-2-yl) acrylonitrile derivatives via the Knoevenagel condensation. ACS Sustain. Chem. Eng. 10, 12602–12612 (2022).
Zhao, Z. S., Zhang, Y., Fang, T., Han, Z. B. & Liang, F. S. Chitosan-Coated Metal–Organic-Framework nanoparticles as catalysts for Tandem deacetalization–knoevenagel condensation reactions. ACS Appl. Nano Mater. 3, 6316–6320. https://doi.org/10.1021/acsanm.0c01486 (2020).
Li, J. et al. Triazole-directed fabrication of polyoxovanadate-based metal–organic frameworks as efficient multifunctional heterogeneous catalysts for the Knoevenagel condensation and oxidation of alcohols. Dalton Trans. 50, 10082–10091 (2021).
Jiang, L. et al. Acid–base bifunctional catalysis by a heteropolyacid and amines on the polyetheretherketone fiber for cleaner acceleration of the one-pot tandem reactions. J. Ind. Eng. Chem. 113, 439–449. https://doi.org/10.1016/j.jiec.2022.06.019 (2022).
Subrahmanyam, C. V. et al. Green synthesis of novel pyrano [2, 3-c] pyrazole-5-carbonitrile analogues by using Fe5 (PW10V2O40) 3 nanocatalyst through a one-pot knoevenagel condensation and Michael addition mechanism. Inorg. Chem. Commun. 159, 111906 (2024).
Chen, M. N., Mo, L. P., Cui, Z. S. & Zhang, Z. H. Magnetic nanocatalysts: synthesis and application in multicomponent reactions. Curr. Opin. Green. Sustain. 15, 27–37. https://doi.org/10.1016/j.cogsc.2018.08.009 (2019).
Kempasiddaiah, M. et al. Efficient and recyclable palladium enriched magnetic nanocatalyst for reduction of toxic environmental pollutants. J. Environ. Sci. 101, 189–204. https://doi.org/10.1016/j.jes.2020.08.015 (2021).
Karimi, B., Ghaffari, B. & Vali, H. Synergistic catalysis within core-shell Fe3O4@SiO2 functionalized with triethylene glycol (TEG)-imidazolium ionic liquid and tetramethylpiperidine N-oxyl (TEMPO) boosting selective aerobic oxidation of alcohols. J. Colloid Interface Sci. 589, 474–485. https://doi.org/10.1016/j.jcis.2020.12.111 (2021).
Mousavi, F., Elhamifar, D. & Kargar, S. Copper/IL-containing magnetic nanoporous MCM-41: a powerful and highly stable nanocatalyst. Surf. Interfaces. 25, 101225. https://doi.org/10.1016/j.surfin.2021.101225 (2021).
Polshettiwar, V. et al. Magnetically recoverable nanocatalysts. Chem. Rev. 111, 3036–3075. https://doi.org/10.1021/cr100230z (2011).
Mohammadi, M. & Ghorbani-Choghamarani, A. A novel Hercynite-supported tetradentate Schiff base complex of manganese catalyzed one-pot annulation reactions. Appl. Organomet. Chem. 36, e6905. https://doi.org/10.1002/aoc.6905 (2022).
Kumar, M. A. et al. Enhanced photocatalytic and electrochemical performance of TiO2-Fe2O3 nanocomposite: its applications in dye decolorization and as supercapacitors. Sci. Rep. 10, 1249 (2020).
Shende, P. & Shah, P. Carbohydrate-based magnetic nanocomposites for effective cancer treatment. Int. J. Biol. Macromol. 175, 281–293 (2021).
Fattahi Nafchi, R. et al. In vitro study: synthesis and evaluation of Fe3O4/CQD magnetic/fluorescent nanocomposites for targeted drug delivery, MRI, and cancer cell labeling applications. Langmuir 38, 3804–3816 (2022).
Mishra, A., Yadav, P. & Awasthi, S. K. Nitrogen-Enriched Biguanidine-Functionalized Cobalt Ferrite nanoparticles as a heterogeneous Base Catalyst for Knoevenagel Condensation under Solvent-Free conditions. ACS Org. Inorg. Au. 3, 254–265. https://doi.org/10.1021/acsorginorgau.3c00002 (2023).
Gong, M. et al. High-efficient and recoverable Mo72V30@ Fe3O4/C catalyst for oxidation of hydroxyfurfural. Fuel 332, 126050 (2023).
Tong, Q. et al. In-situ reduction-passivation synthesis of magnetic octahedron accumulated by Fe@ Fe3O4-C core@ complex-shell for the activation of persulfate. J. Environ. Chem. Eng. 10, 108116 (2022).
Elbarbary, A. M., Bekhit, M., Fadl, E., Sokary, R. & F. I. A. & Synthesis and characterization of magnetically retrievable Fe3O4/polyvinylpyrrolidone/polystyrene nanocomposite catalyst for efficient catalytic oxidation degradation of dyes pollutants. J. Inorg. Organomet. Polym. Mater. 32, 383–398 (2022).
Al-Zubaidi, U. Z. I., Bahrami, K. & Khodamorady, M. Fe3O4@ SiO2@ CSH + VO3 – as a novel recyclable heterogeneous catalyst with core–shell structure for oxidation of sulfides. Sci. Rep. 14, 8175 (2024).
Wang, F., Liu, D., Zheng, P. & Ma, X. Synthesis of rectorite/Fe3O4-CTAB composite for the removal of nitrate and phosphate from water. J. Ind. Eng. Chem. 41, 165–174 (2016).
Soleiman-Beigi, M., Noroozian, Z., Sarai, R., Kohzadi, H. & Naghipour, A. Palladium and zirconium nanoparticles immobilized on functionalized natural asphalt sulfonate as magnetically and recoverable nanocatalysts for the synthesis of symmetrical and unsymmetrical disulfides. Reaction Kinetics Mech. Catal. 136, 2465–2480 (2023).
Esmaili, S., Khazaei, A., Ghorbani-Choghamarani, A. & Mohammadi, M. Silica sulfuric acid coated on SnFe 2 O 4 MNPs: synthesis, characterization and catalytic applications in the synthesis of polyhydroquinolines. RSC Adv. 12, 14397–14410 (2022).
Ghobakhloo, F., Azarifar, D., Mohammadi, M. & Ghaemi, M. γ-Fe2O3@Cu3Al-LDH/HEPES a novel heterogeneous amphoteric catalyst for synthesis of annulated pyrazolo[3,4-d]pyrimidines. Appl. Organomet. Chem. 36, e6823. https://doi.org/10.1002/aoc.6823 (2022).
Mohammadi, M., Ghorbani-Choghamarani, A. & Ramish, S. M. [ZrFe2O4@SiO2–N–(TMSP)–ASP–Pd(0)] Complex: synthesis, characterizations, and its application as a nanomagnetic catalyst in cross-coupling and click reactions. J. Mol. Struct. 1292, 136115. https://doi.org/10.1016/j.molstruc.2023.136115 (2023).
Ghobakhloo, F., Azarifar, D. & Mohammadi, M. Macrocyclic pseudo crown-ether- manganese (II) complex coated on nanomagnetic LDH- catalyzed Biginelli annulation reactions. J. Phys. Chem. Solids. 175, 111222. https://doi.org/10.1016/j.jpcs.2023.111222 (2023).
Cui, L. et al. A recyclable photocatalyst Cu2O/Fe3O4@C/Cu nanocomposite for efficient photocatalytic reduction of 4-nitrophenol. Appl. Surf. Sci. 602, 154403. https://doi.org/10.1016/j.apsusc.2022.154403 (2022).
Ying, A., Wang, L., Qiu, F., Hu, H. & Yang, J. Magnetic nanoparticle supported amine: an efficient and environmental benign catalyst for versatile Knoevenagel condensation under ultrasound irradiation. C. R. Chim. 18, 223–232. https://doi.org/10.1016/j.crci.2014.05.012 (2015).
Shiri, L. & Kazemi, M. Magnetic Fe3O4 nanoparticles supported amine: a new, sustainable and environmentally benign catalyst for condensation reactions. Res. Chem. Intermed. 43, 4813–4832. https://doi.org/10.1007/s11164-017-2914-7 (2017).
Zhong, Y., Ni, Y., Li, S. & Wang, M. Chain-like Fe 3 O 4@ resorcinol-formaldehyde resins–ag composite microstructures: facile construction and applications in antibacterial and catalytic fields. RSC Adv. 6, 15831–15837 (2016).
Yu, L. et al. Nonsacrificial self-template synthesis of colloidal magnetic yolk–shell mesoporous organosilicas for efficient oil/water interface catalysis. Small 15, 1805465 (2019).
Yue, Q. et al. Plasmolysis-inspired nanoengineering of functional yolk–shell microspheres with magnetic core and mesoporous silica shell. J. Am. Chem. Soc. 139, 15486–15493 (2017).
Wang, M., Ni, Y. & Liu, A. Fe3O4@ resorcinol–formaldehyde resin/Cu2O composite microstructures: solution-phase construction, magnetic performance, and applications in antibacterial and catalytic fields. ACS Omega. 2, 1505–1512 (2017).
Elhamifar, D., Ramazani, Z., Norouzi, M. & Mirbagheri, R. Magnetic iron oxide/phenylsulfonic acid: a novel, efficient and recoverable nanocatalyst for green synthesis of tetrahydrobenzo [b] pyrans under ultrasonic conditions. J. Colloid Interface Sci. 511, 392–401 (2018).
Norouzi, M., Elhamifar, D., Mirbagheri, R. & Ramazani, Z. Synthesis, characterization and catalytic application of a novel ethyl and boron sulfonic acid based bifunctional periodic mesoporous organosilica. J. Taiwan. Inst. Chem. Eng. 89, 234–244 (2018).
Elhamifar, D., Ramazani, Z., Norouzi, M. & Mirbagheri, R. Magnetic iron oxide/phenylsulfonic acid: a novel, efficient and recoverable nanocatalyst for green synthesis of tetrahydrobenzo[b]pyrans under ultrasonic conditions. J. Colloid Interface Sci. 511, 392–401. https://doi.org/10.1016/j.jcis.2017.10.013 (2018).
Bang, J. H. & Suslick, K. S. Applications of ultrasound to the synthesis of nanostructured materials. Adv. Mater. 22, 1039–1059 (2010).
Zhang, X. B., Tong, H. W., Liu, S. M., Yong, G. P. & Guan, Y. F. An improved Stöber method towards uniform and monodisperse Fe3O4@C nanospheres. J. Mater. Chem. A. 1, 7488–7493. https://doi.org/10.1039/C3TA11249G (2013).
Mirbagheri, R., Elhamifar, D. & Norouzi, M. Propylamine-containing magnetic ethyl-based organosilica with a core–shell structure: an efficient and highly stable nanocatalyst. New. J. Chem. 42, 10741–10750 (2018).
Zhang, Q., Ma, X. M., Wei, H. X., Zhao, X. & Luo, J. Covalently anchored tertiary amine functionalized ionic liquid on silica coated nano-Fe 3 O 4 as a novel, efficient and magnetically recoverable catalyst for the unsymmetrical Hantzsch reaction and knoevenagel condensation. RSC Adv. 7, 53861–53870 (2017).
Liu, G., Wang, D., Zhou, F. & Liu, W. Electrostatic self-assembly of au nanoparticles onto Thermosensitive magnetic core-Shell microgels for thermally tunable and magnetically recyclable catalysis. Small 11, 2807–2816. https://doi.org/10.1002/smll.201403305 (2015).
Kargar, S. & Elhamifar, D. Magnetic mesoporous silica nanocomposite supported ionic Liquid/Cu as a powerful and highly stable Catalyst for Chan-Lam Coupling reaction. Silicon, 1–15 (2024).
Gong, C., Zhou, Z., Li, J., Zhou, H. & Liu, R. Facile synthesis of ultra stable Fe3O4@Carbon core-shell nanoparticles entrapped satellite Au catalysts with enhanced 4-nitrophenol reduction property. J. Taiwan. Inst. Chem. Eng. 84, 229–235. https://doi.org/10.1016/j.jtice.2018.01.026 (2018).
Shi, C. et al. Facile synthesis of magnetic resorcinol–formaldehyde resin Fe3O4@RF-Au composites for enhanced tetracycline photodegradation with simultaneous H2O2 production. J. Mater. Sci. : Mater. Electron. 35, 1070. https://doi.org/10.1007/s10854-024-12841-9 (2024).
Khoshnavazi, R., Bahrami, L. & Havasi, F. Organic–inorganic hybrid polyoxometalate and its graphene oxide–Fe 3 O 4 nanocomposite, synthesis, characterization and their applications as nanocatalysts for the Knoevenagel condensation and the synthesis of 2, 3-dihydroquinazolin-4 (1 H)-ones. RSC Adv. 6, 100962–100975 (2016).
Kolahdoozan, M., Kalbasi, R. J., Shahzeidi, Z. S. & Zamani, F. Knoevenagel Condensation of Aldehydes with Ethyl Cyanoacetate in Water Catalyzed by P4VP/Al2O3-SiO2. J. Chem. 496837 (2013). (2013).
Heravi, M. M., Tehrani, M. H., Bakhtiari, K. & Oskooie, H. A. A practical Knoevenagel condensation catalysed by imidazole. J. Chem. Res. 2006, 561–562 (2006).
Maleki, R., Kolvari, E., Salehi, M. & Koukabi, N. Fe3O4–cysteamine hydrochloride magnetic nanoparticles: New, efficient and recoverable nanocatalyst for Knoevenagel condensation reaction. Appl. Organomet. Chem. 31, e3795. https://doi.org/10.1002/aoc.3795 (2017).
Norouzi, M., Elhamifar, D. & Mirbagheri, R. Phenylene-based periodic mesoporous organosilica supported melamine: an efficient, durable and reusable organocatalyst. Microporous Mesoporous Mat. 278, 251–256 (2019).
Elhamifar, D., Kazempoor, S. & Karimi, B. Amine-functionalized ionic liquid-based mesoporous organosilica as a highly efficient nanocatalyst for the Knoevenagel condensation. Catal. Sci. Technol. 6, 4318–4326 (2016).
Karaoğlu, E., Baykal, A., Şenel, M., Sözeri, H. & Toprak, M. S. Synthesis and characterization of Piperidine-4-carboxylic acid functionalized Fe3O4 nanoparticles as a magnetic catalyst for Knoevenagel reaction. Mater. Res. Bull. 47, 2480–2486. https://doi.org/10.1016/j.materresbull.2012.05.015 (2012).
Zhang, Y., Zhao, Y. & Xia, C. Basic ionic liquids supported on hydroxyapatite-encapsulated γ-Fe2O3 nanocrystallites: an efficient magnetic and recyclable heterogeneous catalyst for aqueous knoevenagel condensation. J. Mol. Catal. Chem. 306, 107–112. https://doi.org/10.1016/j.molcata.2009.02.032 (2009).
Patel, D., Vithalani, R. & Modi, C. K. Highly efficient FeNP-embedded hybrid bifunctional reduced graphene oxide for Knoevenagel condensation with active methylene compounds. New. J. Chem. 44, 2868–2881. https://doi.org/10.1039/C9NJ05821D (2020).
Mondal, R. K. et al. Polymer immobilized [Mg@ PS-anthra] complex: an efficient recyclable heterogeneous catalyst for the incorporation of carbon dioxide into oxiranes at atmospheric pressure and knoevenagel condensation reaction under solvent free condition. J. Organomet. Chem. 880, 322–332 (2019).
Kim, H. C., Huh, S., Kim, S. J. & Kim, Y. Selective carbon dioxide sorption and heterogeneous catalysis by a new 3D Zn-MOF with nitrogen-rich 1D channels. Sci. Rep. 7, 17185 (2017).
Acknowledgements
The authors acknowledge Yasouj University and Iran National Science Foundation (INSF) for supporting this work.
Author information
Authors and Affiliations
Contributions
P. D.: Investigation, Resources, Formal analysis. D. E.: Conceptualization, Writing – review & editing, Visualization, Supervision. S. K.: Writing – original draft, Formal analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dehghani, P., Elhamifar, D. & Kargar, S. Amine functionalized magnetic resorcinol formaldehyde as a green and reusable nanocatalyst for the Knoevenagel condensation. Sci Rep 15, 2873 (2025). https://doi.org/10.1038/s41598-025-85921-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-85921-3
Keywords
This article is cited by
-
CdS/CeO2/Ag2CO3 nanocomposite as an efficient heterogeneous catalyst for Knoevenagel condensation and acetylation reactions
Scientific Reports (2025)
-
Magnetic resorcinol-formaldehyde supported-DABCO as an effective and recyclable nanocatalyst
Scientific Reports (2025)
-
Remarkable Catalytic Activity of Superparamagnetic Retrievable Mg-Doped Ferrite Nanoparticles for Knoevenagel Condensation
Topics in Catalysis (2025)
-
Zinc-polyurea-formaldehyde immobilized on magnetic nanoparticles: preparation, characterization and application in the synthesis of spirooxindoles
Research on Chemical Intermediates (2025)