Abstract
Cell-penetrating peptides (CPPs) have been shown to have superior material transport ability because poor infiltration of activated lymphocytes into tumors is one of the crucial factors limiting the therapeutic effect of tumor immunotherapy. Numerous studies have investigated the potential application of CPPs in tumor immunotherapy. This review delves into the crucial role that CPPs play in enhancing tumor immunotherapy, emphasizing their impact on various immunotherapy strategies, such as cytokine therapy, adoptive cell therapy, cancer vaccines, and immune checkpoint inhibitors. We also discuss the practical application challenges associated with enhancing the efficiency of CPPs in terms of their stability and targeting ability. In conclusion, the combination of CPPs with tumor immunotherapy is a promising strategy that has potential for precision administration and requires further research for optimal implementation.
Similar content being viewed by others
Introduction
Cancer, which is characterized by uncontrolled cell growth, is a challenge to global health. Traditional cancer treatments include surgery, radiotherapy, chemotherapy, targeted therapy, and groundbreaking immunotherapy. Immunotherapy utilizes the body’s immune system to combat and eliminate cancer cells, contributing to a paradigm shift in treatment. Advancements in medical technology and increased cancer awareness have significantly improved cure and survival rates. Immunotherapy, a recent breakthrough, plays a pivotal role in slowing cancer progression, particularly for challenging cancers1.
Immunotherapy efficacy is evident in experimental findings showing extended lifespans and reduced recurrence rates in responsive patients. Current traditional immunotherapy strategies include cytokine therapy, cell adoptive therapy, cancer vaccines, and immune checkpoint inhibitors2.
Galon et al. categorized tumor immune classification into four types: immune hot, altered-excluded, altered-immunosuppressed, and immune cold. Achieving an immune hot phenotype within tumors requires intricate modulation of T-cell condition, quantity, and distribution. However, challenges persist, notably in enhancing the quantity and spatial distribution of immune cells within the tumor microenvironment3.
Practical challenges in tumor immunotherapy include issues such as low fat solubility, limited bioavailability, and nonspecific targeting in vivo. Despite advancements, two common limitations in drug treatments persist: the absence of tumor cell-specific markers, leading to exposure to nonspecific targets and potentially toxic side effects, and the inability of drugs to precisely reach specific locations. Overcoming these challenges is crucial to avoid inadvertent toxicity in normal tissues.
Peptide drugs have a promising future due to their small size and advantages in large-scale synthesis. Among them, cell-penetrating peptides, small peptides with the ability to penetrate tissues and cell membranes, act as carriers under physiological conditions. They facilitate the transport of substances into cells, including nucleic acids, siRNAs, proteins, small-molecule therapeutics, and probes.
In 1988, Green and Frankel et al. highlighted the unique potential of transferring a functional protein (horseradish peroxidase) into cells using the transcriptional trans activator (Tat protein) derived from the HIV virus; this marked the advent of the first penetrating peptide, TAT4.Its discovery opened a series of subsequent research and application of CPPs, such as iRGD, and laid the foundation for CPPs to go to the clinic.
To date, there have been multiple clinical trials involving CPP-based macromolecular drug delivery types5,6,7, such as TAT-based class A botulinum toxin drugs has been extensively studied in clinical practice8, and the conversion rate of irinotexan to SN38 has been greatly improved through the binding of the penetrating peptide DTS-1089. The gastrointestinal cytotoxicity was significantly reduced. CPPs can be used not only as a therapeutic means that can cross cell membranes, but also as a promising tool for intracellular diagnosis and treatment of substances, including genes, nanocarriers, anticancer drugs, and imaging agents7. The use of CPPs for cancer drug delivery is considered a unique and most promising approach to the treatment of cancer and has great potential to transform the field of cancer treatment diagnostics in the near future.
CPPs efficiently facilitate substance transport, making them a recent focus in tumor therapy. Despite their robust penetration capabilities, these peptides lack specificity in selectively identifying tumor cells. The low number and poor availability of affinity-targeting receptors have been recognized as major bottlenecks in precision drug development. Consequently, one emphasis in tumor therapy has shifted towards modifying CPPs for specific targeting. For example, CPPs targeting tumor-associated extracellular matrixcan penetrate the tumor dense ECM, allowing therapeutic agents to reach their target cells10. Moreover, immune cells such as T cells, modified with CPPs, have also made significant progress in adoptive cell transfusions11.
Vaccines can synergistically stimulate T-cell-mediated immunity (T helper cell activity or cytotoxic T cell function) and immune memory, thereby providing effective treatments for a variety of diseases. CPPs has reported improvements in endosome escape efficiency, selective cell targeting, and antigen uptake, processing, and presentation by antigen presenting cells (APCs). The antigen epitope to APCs, and generate effective immune response, is vital for CPPs can promote an effective vaccine12. In the ongoing effort to provide these CPPs clinically, issues of in vivo stability, safety, improved cellular uptake, simplicity of synthesis, and manufacturing cost must also be considered13.
The application of CPP has deep exploration potential. iRGD is a CPP that has been widely studied and gradually deepened. iRGD, a cell peptide that has been proven to have penetrating ability14. iRGD-carrying drugs range from small molecules that are initially easy to attach such as DOX15, to subsequent use for binding large molecules such as antibodies16, to more recently being used in hot application of nanoparticles containing iRGD17, and even to the modification of lymphocytes11. As the technology advances, platforms equipped with iRGD vary.The structure and function of the ligands of iRGD are gradually improved, and the biological activity is gradually improved. (Fig. 1).This shows that CPP led by iRGD has broad application prospects.
Introduction to CPP: unraveling complexity
Classification of CPPs: decoding diversity
The diverse nature of CPPs necessitates comprehensive classification, often leading to overlapping outcomes. For example, hydrophobic CPP p28, which is 50–77 amino acids of azurin, azurin, a cupredoxin secreted by Pseudomonas aeruginosa, has the function of specifically recognizing preferential entry into tumor cells.
Classified based on physical and chemical properties
CPPs exhibit extensive diversity and are classified primarily by their physicochemical attributes. Three main categories have emerged: cationic, amphiphilic, and hydrophobic. Cationic CPPs, featuring positively charged amino acids such as arginine and lysine, include examples such as TAT4 and NLS18. At physiological pH, cationic CPP produces a net positive charge and exhibits a strong affinity to penetrate cells and avoid the need to interact with them through receptors13. Amphiphilic CPPs balance hydrophilic and hydrophobic amino acids, contain a combination of polar and nonpolar amino acid residues, and use polarity differences to penetrate cells through membranes5. Hydrophobic CPPs contain predominantly nonpolar amino acids.These peptide chains target and enter cells in a different way than cationic CPP. An illustrative example is p28(LSTAADMQGWTDGMASGLDKDYLKPDD) which has the ability preferentially enter cancer cells. P28 preferentially penetrate cancer cells via endocytotic, caveosome-directed, and caveosome-independent pathways. p28 significantly inhibits the binding of COP1 to DBD of wild-type or mutant p53, thereby inducing a post-translational increase in p53 levels and activity, thereby transcriptionally activating p21 and inhibiting G2/M cell cycle and reducing tumor cell growth19,20.
Classified based on origin
CPPs are classified into protein-derived, chimeric, and synthetic groups. Protein-derived CPPs utilize natural protein segments capable of membrane traversal, exemplified by TAT4. Chimeric CPPs merge peptides from different sources, serving as a bridge between natural and synthetic peptides. Synthetic CPPs, such as MAP (KLALKLALKALKAALKLA)21, are artificially designed. It can be specifically synthesized according to the different needs of the investigator.
Classified based on cell-specificity
Another crucial classification distinguishes CPPs as cell-specific or non-cell specific. Cell-specific CPPs selectively deliver cargo to specific cells, while non-cell-specific CPPs lack such selectivity. For example, PL1 peptide specifically binds to FN-EDB and TNC-C that are overexpressed in solid tumor ECM, and its targets FN-EDB and TNC-C exhibit similar low expression in normal tissues and violent upregulations in clinical and experimental solid tumors. Because most anticancer drugs play cytotoxic effects inside the cell. System affinity ligands for cancer are often selected based on their cellular internalization potential. PL1 peptide specifically targets tumor ECM tissue to improve its targeted penetration efficiency, and reasonable selection of targets is crucial in CPP research22. In the current research situation, due to the low targeting of traditional non-specific CPPs, it is impossible to accurately deliver drugs to tumors in tumor therapy, which is one of the difficulties of CPPs from experiment to clinical. Therefore, the research direction of CPPs is to give it the function of specific recognition of receptors while having the ability to penetrate.
However, due to the development of various complex internalization mechanisms, the heterogeneity of diseases, and the wide variety of cell lines available for in vitro studies, there is still no uniform standard to evaluate the improvement of internalization efficiency of one molecular design over another. Although great progress has been made in CPP design, there is undoubtedly still a lot of room for development in this field.
The mechanism of CPPs: navigating intricacies
The mechanism of CPPs internalization is complex and many biological and biophysical methods have been used to investigate internalization mechanisms, locate and quantify CPP and its cargo within cells. However, each approach has its drawbacks, and a combination of different approaches should be used in order to gain a global view. Intracellular studies are primarily aimed at tracking the absorption of CPPs and/or cargo, and can reveal the molecular mechanisms of internalization through fluorescein labeling23, determination of the biological activity of the cargo24, or direct quantification of the number of intracellular internalized peptides25.The internalization mechanism of CPPs involves energy-dependent and energy-independent pathways.
Endocytosis
Energy-dependent internalization, primarily through endocytosis, harnesses chemical energy to create vesicles known as endosomes from the cellular membrane. CPPs within these vesicles are conveyed to the interior of the cell, where they escape the endosome to play physiological roles26. The identified endocytosis pathways include macropinocytosis, clathrin-mediated endocytosis, caveolin-mediated endocytosis, and receptor-mediated/receptor-independent endocytosis. Most endocytosis processes occur within 20 min, but kinetics vary based on CPP concentration27.
It is well known that molecules left inside vesicles do not show their biological activity. In addition, as these molecules move from endosomes to eventually fuse with lysosomes, they are degraded by acidic pH or hydrolase28. Endosome release appears to be the limiting step in CPP endocytosis absorption—it determines how efficiently cargo reaches the cytoplasm. This challenge remains a key barrier to CPP-mediated drug delivery.
Below we summarize several ways to avoid endosomal entrapment:
-
(1)
The use of DOPE and other fusion promoting lipids can greatly enhance the release and activity of cargo molecules29.
-
(2)
Promote endosomal release by binding virus fusion sequences to nanocarriers and then fusing with endosomal membranes30.
-
(3)
Proton sponge effect: The “proton sponge” effect uses the buffering power of drugs to increase the osmotic pressure in the endosome, causing the endosome to swell, rupture and release its contents. This strategy has been successfully used to enhance gene expression of TAT/pDNA complexes31.
-
(4)
Use endosomal lysers such as chloroquine, which is a weak base that can enter cells after protonation and accumulate in endosomes and lysosomes32.
-
(5)
Photosensitizers can be accumulated in endosomes for endosomal release. When exposed to specific wavelengths of light, these molecules produce reactive oxygen species (ROS). These ROS can damage and destroy the endosomal membrane, resulting in the release of endosomal material28.
Direct penetration
Energy-independent direct penetration occurs under low temperature and endocytosis inhibitors. Positively charged CPPs interact with negatively charged cell membranes, inducing membrane instability 26. Three proposed mechanisms include pore formation, the carpet-like model, and the inverted micelle model. Small, hydrophobic CPP cargoes can permeate through energy-dependent pathways, whereas large, hydrophilic cargoes preferably utilize direct penetration (Fig. 2).
Nowadays, the exact way CPPs crosses the cell membrane is not clear. CPP enters cells in different ways under different conditions. Studies have shown that at low concentrations, arginine-rich CPP is mainly endocytosed, while at higher concentrations, it rapidly enters the cytoplasm33.
Application of CPPs in tumor immunotherapy
Immune checkpoint Inhibitors (ICIs)
Tumor immunotherapy, particularly immune checkpoint blocking (ICB) therapy, has garnered significant attention in cancer treatment. Currently, a Phase I clinical study of cell penetrating peptide p2820, the hydrophobic penetrating peptide mentioned above, which binds to the DNA binding domain of p53 to inhibit the proliferation of cancer cells in G2/M has been completed, and the results have confirmed that p28 is safe and well-tolerated in children with progressive central nervous system malignancies34. Clinical applications involving anti-cytotoxic T lymphocyte-associated antigen 4 (αCTLA-4), anti-programmed death 1 (αPD-1), and anti-programmed death ligand 1 (anti-PD-L1) antibodies have demonstrated success. Antibodies targeting the PD-1/PD-L1 signaling pathway, with applications across various clinical scenarios, have become particularly attractive35. The interaction between programmed cell death-1 (PD-1)/PD-L1 ligand 1 (PD-L1) in the tumor microenvironment forms a crucial checkpoint that inhibits T and B-cell activation36. Combining chemotherapy drugs with PD-1/PD-L1 pathway inhibitors represents a novel approach for cancer treatment37.
Fibrinogen-like protein 1 (FGL1) and programmed death ligand 1 (PD-L1) overexpression in tumor cells activates the FGL1/LAG-3 and PD-1/PD-L1 signaling pathways, negatively regulating immune responses. Dual blockade of these pathways enhances T-cell immunity against tumor growth, revealing a potential breakthrough in cancer therapy38. To address the inhibitory functions of FGL1 and PD-L1, a novel reactive oxygen-sensitive nanoparticle loaded with FGL1 siRNA (siFGL1) and PD-L1 siRNA combined with the tumor-penetrating peptide iRGD demonstrated enhanced delivery efficiency and downregulated the protein expression levels of FGL1 and PD-L1 in tumor cells both in vitro and in vivo. Double blockade of the PD-1/PD-L1 and FGL1/LAG-3 pathways significantly improved the immunosuppressive microenvironment39.
The tumor-penetrating peptide iRGD, which is widely used for drug delivery into tumors, demonstrated superior anti-tumor efficiency when combined with PD-1 knockout lymphocytes. iRGD modification coupled with PD-1 destruction has profound therapeutic effects, suggesting a promising avenue for antitumor therapy40.
Bispecific antibodies have emerged as an innovative and promising tumor immunotherapy approach. Previous experiments by our team have shown that iRGD-anti-CD3 promotes tumor-specific T-cell infiltration and activation41. In another follow-up experiment, due to the effective activation of T cells by iRGD-anti-CD3, PD-1 expression on T cells and PD-L1 expression on tumor cells were upregulated. Compared with iRGD-anti-CD3 alone, the addition of PD-1 blockade significantly enhanced the killing effect of T cells on monolayers of gastric cancer cells, and in vivo, synergistic enhancement of antitumor activity was observed with no significant side effects. These findings suggest that PD-1 blockade may be a potential combination approach with iRGD-antiCD3 therapy42.
In addition, recent studies suggest that binding peptides to polymers can significantly overcome the low affinity of receptors, so researchers prepared a bispecific peptide-polymer conjugate (octaPEG-PD1-PDL1) by simultaneously conjugating PD1-binding and PDL1-binding to connect T cells and cancer cells43. This enhances T cell-mediated cytotoxicity to cancer cells and increases cytotoxic T lymphocyte infiltration in tumors. Moreover, in the study of bispecific peptide drugs, some people use cyclic peptides in response to Redox to promote drug release in endosomes to overcome endosomal entrapment44.
Dual targeting can alleviate problems associated with the limited number of available receptors for affinity ligands. Tumor ECM expression is heterogeneous, and multi-targeting may result in a more uniform biological distribution of payloads45. A novel bispecific peptide (PL1; Amino acid sequence: PPRRGLIKLKTS) identifies overexpressed FN-EDB and TNC-C in ECM and suggests that this dual-targeted peptide can be used for robust and specific drug delivery to solid tumors10,22. This research direction is different from the previous target on cells, but to study the extracellular matrix, which is of great significance for the study of tumor microenvironment.
Additionally, tumors activate various other immune checkpoint receptors, including indoleamine 2,3-dioxygenase (IDO), which accelerates tumor migration46. A peptide-assembled tumor-targeting nanodelivery system combining a PD-L1 small interfering RNA (siRNA) and the IDO inhibitor 1-methyl-DL-tryptophan (1MT) demonstrated promising results as a double immune checkpoint blocker. The specific accumulation of the vector at the breast cancer tumor site improved the escape of the siRNA from endosomal vesicles, benefiting the survival, activation, and apoptosis of cytotoxic T lymphocytes (CTLs) in cancer cells. This combination achieved a synergistic enhancement of the anti-tumor immune response47.
In a study involving trichosanthin TCS as an adjuvant for anti-PD-1 therapy in a colon tumor model, recombinant cell-penetrating trichosanthin (rTCS-LMWP) overcame the challenge of intra-tumor penetration and intracellular delivery. The combination of rTCS-LMWP and anti-PD-1 exhibited strong anti-tumor activity, surpassing monotherapy efficacy48. The combined application of CPPs and ICI not covered in other articles can be seen in Table 2.
Cell adoptive therapy
In the context of cancer treatment, adoptive cell therapy (ACT) has emerged as a pivotal form of tumor immunotherapy49. Adoptive cell therapy involves the infusion of immune cells, such as tumor-infiltrating lymphocytes, T lymphocytes (T cells), and natural killer (NK) cells, to induce therapeutic effects.
Poor infiltration of activated lymphocytes into tumors is the crucial factor limiting the therapeutic effect of adoptive cellular tumor immunotherapy. Although iRGD has been shown to have strong penetration, unbound iRGD has a limited effect on lymphocyte transport. Previous studies by our team have used iRGD to modify T cells to functionalize them, which can enhance tumor-specific T-cell infiltration in multiple ways. More importantly, iRGD modification can synergistically exert antitumor effects with PD-1 destruction and prolong the survival of mouse models11. Therefore, modifying T cells with iRGD may be an innovative strategy that will ultimately improve the efficacy of adoptive cell therapy.
Effective T-cell infiltration, which is often associated with a favorable prognosis, is crucial for the success of expanded immunotherapy in solid tumors. The tumor-penetrating peptide iRGD has been known to enhance anticancer drug penetration, and recent research has shown that iRGD can also promote the infiltration of metastatic T cells into tumor cells11. A novel bifunctional protein, IRGD-anti-CD3, fixes iRGD to T-cell surfaces through CD3 binding. This modification promotes T-cell infiltration, induces activation and cytotoxicity in cancer cells, and significantly inhibits tumor growth in various xenograft mouse models. IRGD-anti-CD3 modification has proven to be an innovative strategy for overcoming a major bottleneck in adoptive cell therapy41.
Natural killer cells, which are effective innate defenders against tumor growth, exhibit a lower response rate in adoptive NK cell transfer immunotherapy for solid tumors. IRGD-modified NK cells, which induce cytotoxicity in target cancer cells, have promising results in inhibiting liver cancer growth. The combination approach, which involves the IRGD modification of IL-12-, IL-15-, and IL-18-induced peripheral blood mononuclear cells, enhances the effectiveness of adoptive NK cell immunotherapy for various solid tumors50.
Dendritic cells (DCs) form the core of effector cytotoxic CD8 + T lymphocytes, which specifically recognize tumor-specific antigens (TSAs). CPPs effectively increase the intracellular entry of TSAs, increasing IFN-γ secretion and cancer cell killing without altering DC function. This DC immunotherapy, alone or combined with other immune-boosting regimens, holds promise for enhancing overall tumor immunotherapy efficacy51.
Vaccines
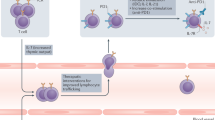
In tumor immunotherapy, inducing an immune response through vaccination is an important means of treating tumors. From initial antigen release to final cell death, vaccines can affect the immune circuit in a variety of ways to enhance the immune response (Fig. 3). CPPs can physically bind or covalently conjugate to antigen molecules, increasing their uptake by antigen-presenting cells and enhancing antigen-specific immune responses52. In contrast to current macromolecular delivery methods, such as nanoparticles, liposomes, virus-based vectors, microinjection, and electroporation, which frequently demonstrate significant toxicity, low specificity, immunogenicity, and inefficient delivery, CPPs offer a noninvasive means of cell entry with increased safety and effectiveness. CPPs have demonstrated the ability to promote the uptake of antigenic peptides by dendritic cells (DCs) in vitro, enhance vaccine immunogenicity, and improve the antitumor efficacy of cancer vaccines in mice53.
One explored approach to enhance the efficacy of peptide vaccines is to covalently couple antigens with CPPs, which are designed to facilitate the intracellular delivery of the cargo54. In the field of vaccine immunotherapy, CPPs are also used to achieve the intracellular delivery of antigens to antigen-presenting cells (APCs). In these studies, CPPs physically bind or covalently link to antigen molecules, increasing the absorption of antigens by APCs and further enhancing antigen-specific immune responses12.
Unexpectedly, in one study, the authors reported that many CPPs significantly enhanced antigen accumulation in the draining lymph nodes55. This effect is related to the ability of CPPs to bind lymphatic transport lipoproteins and protect CPP antigens from hydrolytic degradation by serum proteins; this results in prolonged presentation of CPP peptides in the draining lymph nodes, leading to T-cell initiation and expansion. Compared with free peptide antigens, CPPs promote antigen transport to lymph nodes, improve antigen stability, and prolong the antigen presentation time. These findings demonstrate the universality of CPPs as a strategy to improve the T-cell response to peptide vaccines, defining multiple mechanisms of action for in vivo antigen-CPP coupling potency.
In another experiment, researchers fused ST-456 (a variant of Staphylococcus enterotoxin C2) with the tumor-homing peptide iRGD. Staphylococcal enterotoxin C2 (SEC2), which functions as a bacterial superantigen, binds across the outer groove of major histocompatibility complex II (MHC II) on antigen-presenting cells (APCs) and specific regions of T cells. It possesses a notable capacity to directly activate T lymphocytes via the T-cell receptor (TCR). Consequently, SEC2 induces CD4 + helper T lymphocytes to secrete various cytokines abundantly while also prompting CTLs to engage in superantigen-dependent cell-mediated cytotoxicity against target cells, including tumor cells. Compared with ST-4 alone, the ST-4-IRGD fusion protein exhibits superior targeting of tumor cells and significantly enhances anti-tumor efficacy. Compared with ST-4 alone, ST-4-IRGD demonstrated improved tissue penetration, lymphocyte infiltration, and inhibition of solid tumors in vivo57.
Past research has demonstrated that penetratin binds to cytotoxic T lymphocyte epitopes originating from ovalbumin or mucin-1 tumor-associated antigens, promoting CD4 + and CD8 + T-cell activation in vitro. Furthermore, pre-immunization with penetratin-OVA shielded mice from subsequent tumor infiltration54. Wu et al. introduced an innovative cancer DNA vaccine integrating a unique CPP, cytosol-localizing internalizing peptide 6 (CLIP6), with the model antigen ovalbumin (OVA) alongside CpG as adjuvants. Compared with naked OVA, the obtained CLIP6-OVA conjugate showed greatly increased uptake by DCs and, more importantly, significantly enhanced antigen cross-presentation, triggering a more CTL-mediated immune response with the help of CpG. This CLIP6-OVA/CpG formulation provided effective protection against challenging B16-OVA tumors in mice and further functioned as a therapeutic vaccine. Additionally, when combined with PD-1 immune checkpoint blockade, this CLIP6-OVA/CpG formulation can act as a therapeutic vaccine, leading to significant regression of preexisting tumors58.
In vitro experiments have shown that CPPs can promote the uptake of antigenic peptides by DCs, improve the immunogenicity of vaccines in animal models, and enhance the anti-tumor effect of tumor vaccines in mice53. CPP-mediated DC nanovaccines are a promising strategy for combating various diseases. Penetratin (Antp), a 16-polymer peptide, is another DC vaccination candidate that has been used to enhance efficacy. It has been experimentally proven that Antp-OVA is internalized, processed and presented to CD8 + and CD4 + T cells by MHC Class I and Class II molecules, respectively. Furthermore, the administration of AntpOVA led to a significant delay in the progression of B16-OVA tumors in mice in a tumor therapy model59. In a particular investigation, researchers synthesized a tripartite CPP comprising a combination of multiple antigen peptide, a variable number tandem repeat encompassing the multiple T-cell epitope MUC1 (MUC1), and a tetanus toxoid universal T-helper epitope peptide (tetCD4) along with Antp (AntpMAPMUC1tet). They subsequently examined the immune response in mice. Compared with the AntpMAPMUC1tet vaccine alone, the AntpMAPMUC1tet + CpG vaccine resulted in enhanced antigen-specific interferon γ (IFN-γ) and IL-4 T-cell responses and induced a Th1 response, indicating that effective cellular and humoral immune responses could be induced. In addition, vaccination produced MUC1-specific antibodies and T-cell responses in mice and delayed the growth of MUC1 tumors60.
The packaging of antigens into nanoparticles to form nanovaccines has become an approach in cancer therapy, with several unique advantages. These benefits include achieving optimal strength or the desired immune response61.
One study reported the preparation of nanovaccines by chemically modifying OVA-encapsulated polylactic-glycolic acid nanoparticles with MPGΔNLS (a cell-penetrating peptide)62. MPGΔNLS is a mutant version of MPG that is artificially produced by chimeric fusion of a CPP of the HIV-1 gp41 protein with a nuclear localization signaling analog of the SV40 large T antigen. The experimental results revealed that CPP-modified antigens can mediate their escape from lysosomes to the cytosol and subsequently increase cross-presentation through MHC-I to enhance the CTL response. In vitro experiments revealed that the compound significantly increased the expression levels of the BMDC membrane co-stimulatory molecules CD40 and CD86. It also stimulates the secretion of Th1 cytokines (IL-12 and TNF-α), suggesting that it can promote the maturation of antigen-presenting cells and trigger the Th1 immune response. In addition, compared with unmodified OVA-PLGA NPs or naked OVA, PLGA NPs loaded with MPGΔNLS-OVA significantly inhibited tumor growth and prolonged survival in E·G7-OVA tumor-bearing mice.
Similarly, scientists have created nanovaccines by merging the CPP HIV-1 Tat49-57, which is fused with the CTL epitope HPV16 E749-57, alongside the granulocyte–macrophage colony-stimulating factor (GM-CSF) gene. It greatly improved epitope-specific immunity in vitro and in vivo, reduced tumor growth and improved long-term survival in mouse models63. This may be related to a strong memory CTL-mediated long-term response that improves survival.
GV1001 was originally developed as a vaccine against various cancers64 but has been reported to have unexpected CPP properties65. GV1001 polylysine is a protein or gene vaccine that facilitates intracellular delivery. While originally conceived as a cancer MHC class II binding epitope, GV1001’s CPP capability could explain its heightened anti-cancer immune response compared with alternative peptide-based vaccines66.Some examples of CPPs used in tumor vaccines are listed in Table 1.
Some tumor vaccines require potent adjuvants to overcome their poor immunogenicity, improve antigens, and be absorbed by APCs and stimulate antigen-specific cellular and humoral immune responses. Adjuvants commonly used today are often toxic and have limited effects67. CPP is generally a short peptide that has the ability to promote the translocation of antigens through cell membranes, thus enhancing the ability of antigens to be absorbed, processed, and presented by APCs. The effect of CPPs and adjuvant is not the same, generally not auxiliary function but play a co-stimulatory role. Nevertheless, adding CPP to a vaccine formulation often results in an improved immune response68. Therefore, even if CPPs does not replace traditional adjuvants, it still plays a key role in vaccine preparations.
Others
Interleukin-24 (IL-24) exhibits tumor suppressor activity and selectively induces apoptosis in many human cancer cells, with minimal harm to normal cells73,74. Previous studies have verified the ability of IL-24 to inhibit the migration and invasion of lung cancer cells, selectively impede the growth of cancer cells, and induce cell apoptosis75. However, the ineffective delivery of IL-24 to cancer cells has hindered its widespread use. iRGD (CRGDK/RGPDC) is a novel tumor-specific peptide with distinctive tumor penetration and cell internalization properties. To increase the tumor penetration ability of IL-24, the iRGD peptide was fused with the C-terminal domain of IL-24, resulting in a new recombinant protein, IL-24-iRGD. The results demonstrated that the IL-24-IRGD protein induced apoptosis, accelerated cell death, and inhibited tumor cell growth more effectively than IL-24 treatment alone; this is attributed to the enhanced tumor penetration mediated by IRGD in human A549 NSCLC tumors76.The CPPs mentioned above for tumor immunotherapy are summarized in Table 2 below.
Challenges and prospects
In clinical trials, in addition to efficacy, the feasibility of large-scale production, physicochemical properties, formulation, route of administration, stability, toxicity, and pharmacokinetics are crucial factors77.
Despite the ability of CPPs to transport cargo into cells, they have certain limitations, including a lack of specificity, restricted stability, and swift elimination from the body. Numerous approaches have been devised to enhance their pharmacological characteristics.
Binding with cationic CPPs can effectively improve cellular uptake of macrohydrophilic molecules (such as oligonucleotides and peptide nucleic acids), but cellular uptake is mainly through endosomal pathways, so mechanisms promoting endosomal escape are needed to improve bioavailability. The researchers show that binding lipid domains (fatty acids) to cationic peptides can enhance their biological effects. But as the length of the fatty acid (C8-C16) increases, this effect increases, but it also leads to increased cytotoxicity78.
Most studies of CPPs in vitro have shown that they are not immunogenic and have low toxicity. However, because the immunogenicity of most CPPs may be affected by their various physicochemical properties, including source, size, surface charge, amino acid sequence, morphology, and type of bound cargo, respectively, affecting the integrity of the cell membrane and the likelihood of triggering an immune response, comprehensive research is needed79,80. Although the fusion or combination of CPPs with vaccine/drug cargo significantly reduces the toxicity of CPPs, dose evaluation is important in drug and vaccine research81.
In this section, we explore several prevalent strategies employed in optimizing CPPs.
Improving CPP stability
The primary challenge affecting the stability of CPPs is their susceptibility to protease degradation upon exposure to cells or serum82; this heightened instability leads to degradation by various proteases in biological fluids, such as blood, gastric or intestinal fluid, extracellular fluid, and intracellular fluid, resulting in a restricted plasma half-life83. Additionally, the rapid clearance of positively charged substances by the reticuloendothelial system in the liver and spleen84 further limits the half-life period of many peptide-based treatments to just a few minutes after intravenous injection85. To address this, chemical modifications disrupting enzyme recognition can extend the CPP half-life and increase cellular entry. Terminal modifications, including N-terminal acetylation and C-terminal amidation, represent traditional strategies for improving peptide stability86. A more effective approach involves altering CPP stereochemistry, often using D-amino acids or non-natural amino acids, which are not recognized by metabolic enzymes and can lead to prolonged cytoplasmic presence87. Although replacing L-amino acids in bioactive peptides with D-amino acids or non-natural amino acids can effectively enhance proteolytic resistance, over-modification or replacement also has potential risks, such as cytotoxicity, immunogenicity and other toxic effects. For example, Pep05 did not show significant toxicity in the study, while DP06, which replaced all the original L-Lys and L-Arg residues with D-amino acids, showed very serious toxicity in vivo88.
Physical shielding using hydrophilic polymers, such as polyethylene glycol, can protect CPPs from enzymes, increase their metabolic stability, cycle half-life, and solubility, and reduce their immunogenicity89. Polyethylene glycol (PEG) has been classically used in peptide and protein drugs to improve pharmacokinetic properties (PEG-ization). However, polymer coupling does have one major drawback. It inhibits cellular uptake by reducing the interaction between the cell surface and CPPs90. Therefore, it is necessary to control the molecular weight or coupling density of the polymer, or to design additional strategies to separate the polymer from the coupling when near the target site. After years of research, the limitations of PEG (mainly non-degradability and immunogenicity) have emerged. Due to the limited chemical stability of PEG (mainly due to oxidative degradation and limited excretion in vivo), there is room for other polymers to be used in drug delivery. Polyglycerols (linear, dendritic, or hyperbranched) may be one option because they are less susceptible to oxidation or thermal stress than PEG91.
Several studies have indicated that cyclic CPPs display increased internalization and stability in contrast to linear CPPs, showing greater affinity for target receptors92.The paper explains why most CPPs have minimal toxicity to cultured mammalian cells, as they are released via vesicle budding without compromising the integrity of endosomal membranes. Peptides with higher binding affinity for endosomal membranes will have higher endosomal release efficiency, while the entropy advantage associated with the more rigid structure of cyclic peptides allows them to bind fluid membrane phospholipids more tightly than their linear counterparts, which is precisely why cyclic peptides are particularly potent CPPs.93.It has been confirmed that arginine side chains distributed on the peptide surface in annular TAT are less flexible, which, coupled with its disc-like appearance, leads to faster and denser occupying of the plasma membrane, resulting in faster transduction kinetics. Moreover, the increased distance between guanidine groups and structural rigidity dynamically improve the transduction efficiency of arginine-rich CPP94.
Additionally, various modifications, such as peptide suturing, involving covalent cross-linking between amino acid side chains, are effective for enhancing metabolic stability and permeability through conformational freezing95. (Several common methods mentioned above can be seen in Fig. 4).
Enhancing CPP selectivity
CPPs lack cell or tissue specificity from an osmotic perspective. In the context of tumor immunotherapy, a crucial development direction is improving the ability of CPPs to target tumor tissues or cells. Most membrane surfaces of cells contain negatively charged sialic acid, causing positively charged CPPs to bind indiscriminately to cell surfaces and transfer to the cell interior. Achieving a specific distinction between targeted and nontargeted cells, particularly in systemic applications, is challenging for most CPPs. Traditional strategies for enhancing drug carrier selectivity include the introduction of targeted ligands, with tumor-targeting peptides (TTPs) being a primary focus in polypeptide drug carrier development for tumor therapy. Some TTPs, such as iRGD and NGR, have been widely used for targeted delivery96,97. As mentioned above, the dual-targeting ECM-penetrating peptide PL1 can alleviate problems related to tumor heterogeneity and off-target effects precisely because it specifically recognizes overexpressed FN-EDB and TNC-C in ECM rather than cell surface ligands10,22. The blood–brain barrier formed by tight connections not only protects the brain from harmful substances, but also prevents therapeutic drugs from entering the central nervous system, which is one of the main reasons brain tumors are difficult to treat. Studies have shown that the brain-specific peptide Pep TGN can promote its modified complex to cross the blood–brain barrier and successfully deliver drugs to brain gliomas, which can be used for drug delivery in brain tumors98.
Another developmental direction involves the use of activated cell-penetrating peptides (ACPPs). In this strategy, CPP membrane permeability is masked by specific groups and then restored in tumor tissue. Activated cell-penetrating peptides utilize sectionable site-specific linkages or peptide sequences based on the tumor microenvironment. Various soluble ligands or peptide sequences, which are based on tumor characteristics, can be selected to break the joint or sequence near the tumor tissue, exposing CPPs to an environment that restores membrane permeability and facilitates cargo entry into tumor cells99. pH signals, due to the slightly acidic environment near cancer tissues and the ability to target enzymes, such as matrix metalloproteinases are popular activators of ACPPs100.
Improving CPP efficiency
CPPs can traverse the plasma membrane through endocytosis or direct osmosis. Even after overcoming pharmacokinetic challenges, high dosages are often required to maintain effective concentrations near target cells. Effective endosome escape is crucial for reducing the necessary dosage. Therefore, an effective method to promote endosome escape is one of the important measures to improve efficiency. Some peptides destabilize endosomal membranes at lower pH values without affecting other membranes, facilitating increased endosome escape during maturation101. Furthermore, the conjugation of fatty acid chains with CPPs has been utilized to augment their cellular uptake and escape from endosomes, whereby additional lipophilic domains bolster interactions between CPPs and membranes.102,103. More detailed approaches are referred to in the mechanism above.
Summary
While significant advancements in tumor immunotherapy through modalities such as cancer vaccines, ACT, and ICIs have been made in recent years, challenges persist. By facilitating the deep delivery of covalently coupled drug payloads into tumor tissue, CPPs show immense promise in cancer treatment and diagnosis. Since the discovery of the first CPP, researchers from diverse fields have recognized its potential as a delivery vehicle, spanning chemistry, biology, pharmaceutical science, and medicine.
This review underscores the versatility of CPPs across various applications, particularly in tumor immunotherapy. However, certain limitations, including the lack of cell specificity, short duration of action, and poor in vivo stability, hinder broader applications in tumor immunotherapy. Overcoming these challenges remains a crucial task for future clinical implementation. In conclusion, the concurrent use of CPPs with cancer immunotherapy has emerged as a novel strategy, offering fresh perspectives for subsequent treatment approaches.
Data availability
All data generated or analysed during this study are included in this published article.
References
Papaioannou, N. E. et al. Harnessing the immune system to improve cancer therapy. Ann. Transl. Med. 4(14), 261–261 (2016).
Cha, J.-H. et al. New approaches on cancer immunotherapy. Cold Spring Harbor Perspect. Med. 10(8), a036863 (2020).
Galon, J. & Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discovery 18(3), 197–218 (2019).
Frankel, A.D. and Pabo, C.O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell55(6), 1189–1193 (1988).
Bottens, R. A. & Yamada, T. Cell-penetrating peptides (CPPs) as therapeutic and diagnostic agents for cancer. Cancers 14(22), 5546 (2022).
Sun, Z. et al. Cell-penetrating peptide-based delivery of macromolecular drugs: development, strategies, and progress. Biomedicines 11(7), 1971 (2023).
Tripathi, P.P., et al., Cell penetrating peptides in preclinical and clinical cancer diagnosis and therapy. Oncotarget 9(98), 37252–37267 (2018).
Jankovic, J. et al. Injectable daxibotulinumtoxina in cervical dystonia: A phase 2 dose-escalation multicenter study. Mov. Disord. Clin. Pract. 5(3), 273–282 (2018).
Meyer-Losic, F. et al. DTS-108, a novel peptidic prodrug of SN38: In vivo efficacy and toxicokinetic studies. Clin. Cancer Res. 14(7), 2145–2153 (2008).
Lingasamy, P. et al. Bi-specific tenascin-C and fibronectin targeted peptide for solid tumor delivery. Biomaterials 219, 119373 (2019).
Ding, N., et al. iRGD synergizes with PD-1 knockout immunotherapy by enhancing lymphocyte infiltration in gastric cancer. Nat. Commun. 10(1) 2019.
Hasannejad-Asl, B. et al. Cell penetrating peptide: A potent delivery system in vaccine development. Front. Pharmacol. 13, 1072685 (2022).
Xie, J. et al. Cell-penetrating peptides in diagnosis and treatment of human diseases: From preclinical research to clinical application. Front. Pharmacol. 11, 697 (2020).
Yin, H. et al., iRGD as a tumor-penetrating peptide for cancer therapy. Molecular Medicine Reports 15(5),2925–2930 (2017).
Tian, Y. et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 35(7), 2383–2390 (2014).
Lingasamy, P., Laarmann, A.-H. & Teesalu, T. Tumor penetrating peptide-functionalized tenascin-C antibody for glioblastoma targeting. Curr. Cancer Drug Targets 21(1), 70–79 (2021).
Liu, C. et al. iRGD-mediated core-shell nanoparticles loading carmustine and O6-benzylguanine for glioma therapy. J. Drug Target. 25(3), 235–246 (2016).
Ponnappan, N. & Chugh, A. Cell-penetrating and cargo-delivery ability of a spider toxin-derived peptide in mammalian cells. Eur. J. Pharm. Biopharm. 114, 145–153 (2017).
Taylor, B. N. et al. Noncationic peptides obtained from azurin preferentially enter cancer cells. Cancer Res. 69(2), 537–546 (2009).
Yamada, T., Das Gupta, T. K. & Beattie, C. W. p28, an anionic cell-penetrating peptide, increases the activity of wild type and mutated p53 without altering its conformation. Mol. Pharm. 10(9), 3375–3383 (2013).
Silva, S. et al. A second life for MAP, a model amphipathic peptide. Int. J. Mol. Sci. 23(15), 8322 (2022).
Lingasamy, P. et al. PL1 peptide engages acidic surfaces on tumor-associated fibronectin and tenascin isoforms to trigger cellular uptake. Pharmaceutics 13(12), 1998 (2021).
Taylor, B.N., et al., Noncationic Peptides Obtained From Azurin Preferentially Enter Cancer Cells. Cancer Research. 69(2), 537–546 (2009).
Dietz, G.P.H., Cell-Penetrating Peptide Technology to Deliver Chaperones and Associated Factors in Diseases an Basic Research. Current Pharmaceutical Biotechnology 11(2), 167–174 (2010).
Burlina, F. et al. Quantification of the cellular uptake of cell-penetrating peptides by MALDI-TOF mass spectrometry. Angew. Chem. Int. Ed. 44(27), 4244–4247 (2005).
Futaki, S. & Nakase, I. Cell-surface interactions on arginine-rich cell-penetrating peptides allow for multiplex modes of internalization. Acc. Chem. Res. 50(10), 2449–2456 (2017).
Madani, F. et al. Mechanisms of cellular uptake of cell-penetrating peptides. J. Biophys. 2011, 1–10 (2011).
Erazo-Oliveras, A. et al. Improving the endosomal escape of cell-penetrating peptides and their cargos: Strategies and challenges. Pharmaceuticals 5(11), 1177–1209 (2012).
El-Sayed, A., Futaki, S. & Harashima, H. Delivery of macromolecules using arginine-rich cell-penetrating peptides: Ways to overcome endosomal entrapment. AAPS J. 11(1), 13–22 (2009).
El-Andaloussi, S. et al. Induction of splice correction by cell-penetrating peptide nucleic acids. J. Gene Med. 8(10), 1262–1273 (2006).
cationic cell penetrating peptides as source ther deliv 2015 apr 6 4 491 507.pdf.
Veldhoen, S. et al. Cellular delivery of small interfering RNA by a non-covalently attached cell-penetrating peptide: quantitative analysis of uptake and biological effect. Nucleic Acids Res. 34(22), 6561–6573 (2006).
Fretz, M. M. et al. Temperature-, concentration- and cholesterol-dependent translocation of L- and D-octa-arginine across the plasma and nuclear membrane of CD34+ leukaemia cells. Biochem. J. 403(2), 335–342 (2007).
Lulla, R. R. et al. Phase I trial of p28 (NSC745104), a non-HDM2-mediated peptide inhibitor of p53 ubiquitination in pediatric patients with recurrent or progressive central nervous system tumors: A pediatric brain tumor consortium study. Neuro-Oncology 18(9), 1319–1325 (2016).
Freeman-Keller, M. & Weber, J. S. Anti-programmed death receptor 1 immunotherapy in melanoma: Rationale, evidence and clinical potential. Ther. Adv. Med. Oncol. 7(1), 12–21 (2014).
Nixon, B. G. & Li, M. O. Satb1: Restraining PD1 and T Cell Exhaustion. Immunity 46(1), 3–5 (2017).
coadministration of a tumor penetrating peptide.pdf.
Wang, J. et al. Fibrinogen-like protein 1 is a major immune inhibitory ligand of LAG-3. Cell 176(1–2), 334-347.e12 (2019).
Wan, W.-J. et al. Coadministration of iRGD peptide with ROS-sensitive nanoparticles co-delivering siFGL1 and siPD-L1 enhanced tumor immunotherapy. Acta Biomater. 136, 473–484 (2021).
Zhang, M. et al. Recombinant cell-penetrating trichosanthin synergizes anti-PD-1 therapy in colorectal tumor. Int. J. Biol. Sci. 19(6), 1698–1712 (2023).
Zhou, S. et al. Bifunctional iRGD-anti-CD3 enhances antitumor potency of T cells by facilitating tumor infiltration and T-cell activation. J. ImmunoTher. Cancer 9(5), e001925 (2021).
Zhu, M. et al. Combination therapy with iRGD-antiCD3 and PD-1 blockade enhances antitumor potency of cord blood-Derived T Cells. OncoTargets Ther. 14, 835–844 (2021).
Zhang, W. et al. A bispecific peptide-polymer conjugate bridging target-effector cells to enhance immunotherapy. Adv. Healthc. Mater. 12(18), 2202977 (2023).
Yang, D. S. et al. A redox-triggered bispecific supramolecular nanomedicine based on peptide self-assembly for high-efficacy and low-toxic cancer therapy. Adv. Funct. Mater. 30(4), 1904969 (2019).
Dal Corso, A. et al. A non-internalizing antibody-drug conjugate based on an anthracycline payload displays potent therapeutic activity in vivo. J. Control. Release 264, 211–218 (2017).
Munn, D.H., et al., Inhibition of T cell proliferation by macrophage tryptophan catabolism. J. Experimental Medicine. 189(9), 1363–1372 (1999).
Li, G. et al. Dual-blockade immune checkpoint for breast cancer treatment based on a tumor-penetrating peptide assembling nanoparticle. ACS Appl. Mater. Interfaces 11(43), 39513–39524 (2019).
Chen, Y. et al. Prodrug-like, PEGylated protein toxin trichosanthin for reversal of chemoresistance. Mol. Pharm. 14(5), 1429–1438 (2017).
Met, Ö. et al. Principles of adoptive T cell therapy in cancer. Semin. Immunopathol. 41(1), 49–58 (2018).
Dong, Y. et al. iRGD-modified memory-like NK cells exhibit potent responses to hepatocellular carcinoma. J. Transl. Med. 21(1), 205 (2023).
Cogdill, A. P. et al. Targeting the MAGE A3 antigen in pancreatic cancer. Surgery 152(3), S13–S18 (2012).
Derouazi, M. et al. Novel cell-penetrating peptide-based vaccine induces robust CD4+ and CD8+ T cell-mediated antitumor immunity. Cancer Res. 75(15), 3020–3031 (2015).
Lim, S., Koo, J.-H. & Choi, J.-M. Use of cell-penetrating peptides in dendritic cell-based vaccination. Immune Netw. 16(1), 33 (2016).
Pouniotis, D. et al. Vaccine delivery by penetratin: Mechanism of antigen presentation by dendritic cells. Immunol. Res. 64(4), 887–900 (2016).
Backlund, C.M., et al., Cell-penetrating peptides enhance peptide vaccine accumulation and persistence in lymph nodes to drive immunogenicity. Proceedings of the National Academy of Sciences of the United States of America. 119(32) (2022).
Fu, X. et al. Staphylococcal enterotoxin C2 mutant drives T lymphocyte activation through PI3K/mTOR and NF-ĸB signaling pathways. Toxicol. Appl. Pharmacol. 333, 51–59 (2017).
Song, Y. et al. An iRGD peptide fused superantigen mutant induced tumor-targeting and T lymphocyte infiltrating in cancer immunotherapy. Int. J. Pharm. 586, 119498 (2020).
Wu, H. et al. Cell-penetrating peptide enhanced antigen presentation for cancer immunotherapy. Bioconjugate Chem. 30(8), 2115–2126 (2019).
Pouniotis, D. S. et al. Whole protein and defined CD8+ and CD4+ peptides linked to penetratin targets both MHC class I and II antigen presentation pathways. Immunol. Cell Biol. 89(8), 904–913 (2011).
Brooks, N. et al. Immunogenicity of a tripartite cell penetrating peptide containing a MUC1 variable number of tandem repeat (VNTR) and a T helper epitope. Molecules 23(9), 2233 (2018).
Jiang, J. Cell-penetrating peptide-mediated nanovaccine delivery. Curr. Drug Targets 22(8), 896–912 (2021).
Liu, X. et al. A cell-penetrating peptide-assisted nanovaccine promotes antigen cross-presentation and anti-tumor immune response. Biomater. Sci. 7(12), 5516–5527 (2019).
Tang, J. et al. A novel self-assembled nanoparticle vaccine with HIV-1 Tat49-57/HPV16 E749–57 fusion peptide and GM-CSF DNA elicits potent and prolonged CD8+ T cell-dependent anti-tumor immunity in mice. Vaccine 30(6), 1071–1082 (2012).
Staff, C. et al. Telomerase (GV1001) vaccination together with gemcitabine in advanced pancreatic cancer patients. Int. J. Oncol. 45(3), 1293–1303 (2014).
Lee, S.-A. et al. Heat shock protein-mediated cell penetration and cytosolic delivery of macromolecules by a telomerase-derived peptide vaccine. Biomaterials 34(30), 7495–7505 (2013).
Kim, H. et al. The telomerase-derived anticancer peptide vaccine GV1001 as an extracellular heat shock protein-mediated cell-penetrating peptide. Int. J. Mol. Sci. 17(12), 2054 (2016).
Yang, J. et al. Cell-penetrating Peptides: Efficient vectors for vaccine delivery. Curr. Drug Deliv. 16(5), 430–443 (2019).
Zhou, M. et al. The role of cell-penetrating peptides in potential anti-cancer therapy. Clin. Transl. Med. 12(5), e822 (2022).
Wang, K. et al. Cell penetrating peptide-based redox-sensitive vaccine delivery system for subcutaneous vaccination. Mol. Pharm. 15(3), 975–984 (2018).
Zhang, T. T. et al. LAH4 enhances CD8+ T cell immunity of protein/peptide-based vaccines. Vaccine 30(4), 784–793 (2012).
Saleh, T. et al. MPG-based nanoparticle: An efficient delivery system for enhancing the potency of DNA vaccine expressing HPV16E7. Vaccine 33(28), 3164–3170 (2015).
Granadillo, M. et al. LALF32-51-E7 therapeutic vaccine induces antitumor immunity against human papillomavirus type 16 E7-expressing murine tumor metastases in the lungs. Clin. Exp. Metastasis 34(3–4), 241–249 (2017).
Fisher, P. B. Is mda-7/IL-24 a “Magic Bullet” for Cancer?. Cancer Res. 65(22), 10128–10138 (2005).
Menezes, M. E. et al. MDA-7/IL-24: Multifunctional Cancer Killing Cytokine. In Anticancer Genes (ed. Grimm, S.) 127–153 (Springer London, London, 2014).
Singh, A. P. et al. IL-24 inhibits lung cancer cell migration and invasion by disrupting the SDF-1/CXCR4 signaling axis. Plos One 10(3), e0122439 (2015).
Yang, J. et al. Modification of IL-24 by tumor penetrating peptide iRGD enhanced its antitumor efficacy against non-small cell lung cancer. Int. Immunopharmacol. 70, 125–134 (2019).
Dharanipragada, R., New modalities in conformationally constrained peptides for potency, selectivity and cell permeation. Future Medicinal Chemistry. 5(7), 831–849 (2013).
Koppelhus, U., et al., Improved cellular activity of antisense peptide nucleic acids by conjugation to a cationic peptide-lipid (CatLip) domain. Bioconjugate Chemistry. 19(8), 1526–1534 (2008).
Shi, N.-Q. et al. A survey on “Trojan Horse” peptides: Opportunities, issues and controlled entry to “Troy”. J. Control. Release 194, 53–70 (2014).
Derakhshankhah, H. & Jafari, S. Cell penetrating peptides: A concise review with emphasis on biomedical applications. Biomed. Pharmacother. 108, 1090–1096 (2018).
Zakeri-Milani, P., et al., Cytotoxicity and Immunogenicity Evaluation of Synthetic Cell-penetrating Peptides for Methotrexate Delivery. Iran J Pharm Res. 20(3), 506–515 (2021).
Palm, C. et al. Peptide degradation is a critical determinant for cell-penetrating peptide uptake. Biochim. Biophys. Acta 1768(7), 1769–1776 (2007).
Ruoslahti, E. Tumor penetrating peptides for improved drug delivery. Adv. Drug Deliv. Rev. 110–111, 3–12 (2017).
Gustafson, H. H. et al. Nanoparticle uptake: The phagocyte problem. Nano Today 10(4), 487–510 (2015).
Patel, A., K. Cholkar, and A.K. Mitra, Recent developments in protein and peptide parenteral delivery approaches. Therapeutic delivery. 5(3), 337–65 (2014).
Di, L. Strategic approaches to optimizing peptide ADME properties. AAPS J. 17(1), 134–143 (2014).
Lee, J. S. & Tung, C.-H. Lipo-oligoarginines as effective delivery vectors to promote cellular uptake. Mol. BioSyst. 6(10), 2049 (2010).
Lu, J. et al. D- and unnatural amino acid substituted antimicrobial peptides with improved proteolytic resistance and their proteolytic degradation characteristics. Front. Microbiol. 11, 563030 (2020).
Nischan, N. et al. Stabilization of peptides for intracellular applications by phosphoramidate-linked polyethylene glycol chains. Angew. Chem. Int. Ed. 52(45), 11920–11924 (2013).
the polyethyleneglycol dilemma adva source biol pharm bull so 2013 36 6 892 9.pdf.
Kumar, S. et al. PEGylation and cell-penetrating peptides: Glimpse into the past and prospects in the future. Curr. Topics Med. Chem. 20(5), 337–348 (2020).
Qian, Z. et al. Intracellular delivery of peptidyl ligands by reversible cyclization: Discovery of a PDZ domain inhibitor that rescues CFTR activity. Angew. Chem. Int. Ed. 54(20), 5874–5878 (2015).
Qian, Z. et al. Discovery and mechanism of highly efficient cyclic cell-penetrating peptides. Biochem. 55(18), 2601–2612 (2016).
Lättig-Tünnemann, G. et al. Backbone rigidity and static presentation of guanidinium groups increases cellular uptake of arginine-rich cell-penetrating peptides. Nat. Commun. 2(1), 453 (2011).
Guarracino, D. A. et al. Macrocyclic control in helix mimetics. Chem. Rev. 119(17), 9915–9949 (2019).
Nieberler, M. et al. Exploring the role of RGD-recognizing integrins in cancer. Cancers 9(12), 116 (2017).
Zhu, L. et al. Research progress of radiolabeled asn-gly-arg (NGR) peptides for imaging and therapy. Mol. Imaging 19, 1536012120934957 (2020).
Säälik, P. et al. Peptide-guided nanoparticles for glioblastoma targeting. J. Control. Release 308, 109–118 (2019).
de Jong, H., Bonger, K. M. & Löwik, D. W. P. M. Activatable cell-penetrating peptides: 15 years of research. RSC Chem. Biol. 1(4), 192–203 (2020).
Schach, D. K. et al. Reversible activation of a cell-penetrating peptide in a membrane environment. J. Am. Chem. Soc. 137(38), 12199–12202 (2015).
Varkouhi, A. K. et al. Endosomal escape pathways for delivery of biologicals. J. Control. Release 151(3), 220–228 (2011).
Futaki, S., et al., Stearylated arginine-rich peptides: A new class of transfection systems. Bioconjugate Chemistry. 12(6), 1005–1011 (2001).
Pham, W. et al. Enhancing membrane permeability by fatty acylation of oligoarginine peptides. ChemBioChem 5(8), 1148–1151 (2004).
Lingasamy, P. et al. Tumor-penetrating peptide for systemic targeting of tenascin-C. Sci. Rep. 10(1), 5809 (2020).
Acknowledgements
Funding for this study was provided by the National Natural Science Foundation of China (82272811), Jiangsu Province Key Research and Development Program (BE2023654), Nanjing Jiangbei New Area Key Research and Development Program and Fundings for Clinical Trials from the Affiliated Drum Tower Hospital, Medical School of Nanjing University (2023-LCYJ-PY-29), Jiangsu Association for Science & Technology Youth Science & Technology Talents Lifting Project, The Third Level of the Jiangsu Province 333 High-Level Talent Training Project.
Author information
Authors and Affiliations
Contributions
D.Y. is responsible for collecting documents and writing the article. B.L. handles article editing, and H.S. is responsible for project design and article revisions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, D., Liu, B. & Sha, H. Advances and prospects of cell-penetrating peptides in tumor immunotherapy. Sci Rep 15, 3392 (2025). https://doi.org/10.1038/s41598-025-86130-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-86130-8
Keywords
This article is cited by
-
Efficient Transfection of siRNA with a Transcription Factor Oct-6-Derived Peptide
Journal of Pharmaceutical Innovation (2026)
-
Innovative gene engineering and drug delivery systems for dendritic cells in cancer immunotherapy
Journal of Biomedical Science (2025)
-
Harnessing the potential of HSV glycoproteins as cell penetrating peptides through in-silico methods
Network Modeling Analysis in Health Informatics and Bioinformatics (2025)