Abstract
The ABCB4 gene encodes multidrug resistance protein 3(MDR3), which is a phosphatidylcholine(PC) transfer enzyme that transfers lecithin from the inner part of the phospholipid bilayer to the extracellular bile. The occurrence of intrahepatic cholestasis of pregnancy(ICP) is closely related to ABCB4 variants, but there is limited research on this topic in southern Anhui, China. We sequenced ABCB4 in pregnant women with ICP and healthy pregnant women to explore the relationship. A total of 30 patients diagnosed with ICP were selected as the study objects and 90 healthy pregnant women were selected as the control group. DNA was extracted from peripheral blood of ICP patients and healthy pregnant women, 27 exons were sequencing by Sanger sequencing. Polymerase chain reaction (PCR) was used to amplify those exons. PolyPhen2, Mutation Taster, Provean, SIFT and Mutpred2 were used to predict protein structure, and Pymol software was used to predict the impact of missense variant c.1954 A > G(p.Arg652Gly) on proteins. Four exonic variants of ABCB4 gene were detected in ICP patients and healthy pregnant women, including synonymous variants c.175 C > T, c.504 C > T,c.711 A > T and missense variant c.1954 A > G(p.Arg652Gly). The incidence of the missense variant c.1954 A > G(p.Arg652Gly) was 6/90 in healthy pregnant womenand 8/30 in ICP patients.In healthy pregnant women with the missense variant c.1954 A > G(p.Arg652Gly), no other exonic variants were found. In ICP patients with missense variant c.1954 A > G(p.Arg652Gly), other exonic variants were found. PolyPhen2, Mutation Taster, Provean, SIFT and Mutpred2 were used to predict that the four exonic variants were benign, while Pymol was used to showed that the missense variant was located in the linker region of MDR3 and had a slight impact on protein function. Among ICP patients with missense variant c.1954 A > G(p.Arg652Gly), patients with three exonic variants(c.504 C > T, c.711 A > T, c.1954 A > G) had higher γ-GT, TBA, ALT and AST than those with two exonic variants. ABCB4 missense variant c.1954 A > G(p.Arg652Gly) requires the combination of other variants(c.175 C > T, c.504 C > T,c.711 A > T) to cause ICP symptoms, and when combined with other variants, it has a superimposed effect.
Similar content being viewed by others
Introduction
Intrahepatic cholestasis of pregnancy (ICP) is a common gestational liver disease in the world, which is a disease with unclear etiology. It is more common in the middle and late stages of pregnancy and rapidly subsides after delivery. In most women, ICP will recur in subsequent pregnancies. It is reported that the incidence rate of ICP varies from 0.2 to 15.6% according to race and geographical location, with the highest incidence in Chile1,2,3,4. The typical symptoms of ICP are skin itching, mainly occurring in the palms and soles of the feet, accompanied by elevated serum total bile acids (TBA) and abnormal liver function indicators. Its greatest harm is spontaneous premature birth, amniotic fluid fecal staining, neonatal depression, fetal respiratory distress syndrome, and high risk of death2,5,6,7.
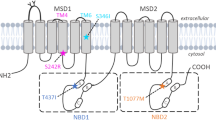
The ABCB4 gene is located on chromosome 7q21.1 and consists of 27 exons, with a total length of approximately 74kb8,9. The multidrug resistant protein3 (MDR3) encoded by ABCB4 is responsible for transporting phosphatidylcholine (PC) on the hepatic tubular membrane from the inner lobule to the outer lobule. When mixed with bile acids, it can protect the bile duct epithelium from bile salt damage. ABCB4 mutations can cause protein abnormalities, leading to bile duct damage and bile stasis10,11,12,13. Progressive familial intrahepatic cholestasis type 3 (PFIC3) that occurs during infancy or late adolescence, as well as adult onset syndromes such as low phospholipid associated gallstones (LPAC) or intrahepatic cholestasis of pregnancy (ICP), are all associated with ABCB4 mutations14.
The pathogenesis of ICP is multifactorial, with the interaction of genetic, hormonal, immune, and environmental factors5. Among genetic factors, mutations in ABCB4 are believed to play a major role in the pathogenesis of ICP15, with approximately 16% of ICP patients having ABCB4 mutations1. Most studies on ICP are focused on European and American populations, with little research on the Chinese population. This article aims to clarify the relationship between ABCB4 and genetic susceptibility to ICP by analyzing some unrelated populations in southern Anhui, China.
Materials and methods
Patients
30 ICP patients from Yijishan Hospital in Wuhu were selected as the experimental group, with an average age of (26.93 ± 3.25) and an average gestational age of (35.53 ± 2.42), and 90 healthy pregnant women were selected as the control group, with an average age of (27.19 ± 2.47) and an average gestational age of (36.62 ± 1.16). ICP patients met the ICP diagnostic criteria in the Guidelines for Diagnosis and Treatment of Intrahepatic Cholestasis of Pregnancy (2015)16. Inclusion criteria: All patients had no history of diabetes, hypertension, liver and kidney diseases and other complications. Blood was collected from all patients before delivery and stored in a -20 ℃ freezer.
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Yijishan Hospital of Wannan Medical College. All patients provided written informed consent.
DNA extraction
DNA was extracted from peripheral blood of ICP patients and healthy pregnant women, and 27 exons of ABCB4 were amplified by polymerase chain reaction (PCR) and sequenced17.
Polymerase chain reaction (PCR)
We designed primers for 27 exons of ABCB4, the NCBI version number of ABCB4 is NM_000443.4. The designed upstream and downstream primers were BLAST validated in NCBI, synthesized by Shanghai Jierui Biotechnology Co., Ltd., and used for PCR amplification (Additional Table 1).
PCR reaction conditions and reaction system: PCR reaction was performed according to 50 µl system. The concentration was 5 µg/µl template DNA 2 µl, Taq enzyme 2.5 U, 10×Buffer(including Mg2+) 5 µl, including 25 mM Tris-HCl pH 8.5, 50 mM KCl and 2 mM MgCl₂. dNTP is 0.2mmol/L, and the upstream and downstream primers with a concentration of 10µM are 0.5 µl each. Add ddH2O to 50 µl. Pre denature at 98 ℃ for 5 min, denature at 94℃ for 30 s, and add Taq enzyme (Promega company product) at 20 s; Refolding at 65℃ for 45 s, extending at 72℃ for 1 min, a total of 33 cycles, and finally extending at 72℃ for 4 min. The agarose gel electrophoresis at a concentration of 2% was used to verify that the PCR product had a unique specific band with the correct amplicon size.
DNA sequencing
PCR products were purified and recovered using the DNA Gel Extraction Kit (AXYGEN). The purified PCR products and upstream and downstream primers were sent to Beijing Nosai Genomics Research Center Co., LTD for sequencing.
Predict protein structure
Polyphen2 (http://genetics.bwh.harvard.edu/pph/)18, MutationTaster(https://www.mutationtaster.org/)19, Provean, SIFT (http://sift.jcvi.org/)20 and Mutpred2 (http://mutpred.mutdb.org/)21 were used to predict protein damage. Pymol software was used to predict structural missense variation of p.Arg652Gly protein.
Statistical analysis
GraphPad Prism8.0 software was used to perform statistical analysis on the data. Measurement data in mean ± standard deviation(\(\:\stackrel{-}{x\:}\)± s) indicates that ANOVA analysis of variance is used for comparison between multiple groups, and P < 0.05 indicates a statistically significant difference.
Results
DNA sequencing results
We detected four exonic variants in ABCB4 in ICP patients and healthy pregnant women, with a missense variant c.1954 A > G, and 3 synonymous variants c.175 C > T, c.504 C > T, c.711 A > T (Table 1) (Additional Fig. 1).Missense variant c.1954 A > G was found in 8 ICP patients and 6 healthy pregnant women. In 6 healthy pregnant women who carried the missense variant c.1954 A > G, no other exonic variants were found. But in 8 ICP patients with missense variant c.1954 A > G, other exonic variants were found (Table 2). Among the 8 ICP patients with c.1954 A > G variant, there were 6 ICP patients with 2 variants, including 1 patient with c.175 C > T variant, 2 patients with c.504 C > T variant, and 3 patients with c.711 A > T variant. And there were 2 ICP patients with 3 variants, all of whom carried the c.504 C > T and c.711 A > T variants (Table 3).
Protein structure prediction
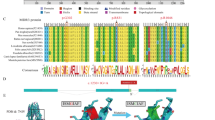
Polyphen2, MutationTaster, Provean, SIFT and Mutpred2 suggest that exonic variants may be nonpathogenic (Table 4) and missense variant c.1954 A > G are highly unconserved in species(Additional Table 2), including P.toglytes, M.mulatta, M.musculus, G.gallus, T.rubripes, D.rerio, D.melanogaster and C.elegans. Pymol software was used to show that the missense variant c.1954 A > G(p.Arg652Gly) was located in the linker region of the protein (Fig. 1).
c.1954 A > G variant(p.Arg652Gly)
The missense variant c.1954 A > G may need to be combined with other exonic variants to cause the symptoms of ICP and has a superimposed effect (Table 5).
Discussion
The incidence rate of ICP varies according to different geographical locations. It is most common in South America. In Europe, North America and Australia, about 1–2% of pregnant women suffer from ICP1,22. In southwest China, the incidence rate of ICP reached 2.8%23. Racial and regional differences are important factors in studying ICP and ABCB4 mutations. Since Jacquemin first discovered a T deletion at the 1712nd base of the 571st codon of ABCB4 in a proband of a cholestasis family, a mutation was subsequently discovered in four patients of this family15. Subsequent studies detected a wide range of ABCB4 mutations in ICP patients, but ABCB4 mutations have different outcomes in different racial populations. Wasmuth found that c.175 C > T and c.1954 A > G are genetic influencing factors for the onset of severe ICP in Swedes, while c.504T > C did not show any association with the disease24. Bacq believes that it is meaningful for Caucasian ICP patients with c.504T > C, while c.175 C > T and c.1954 A > G did not show any association with the disease25.
The pathogenesis of ICP is still unclear, and clinical and epidemiological studies suggest that ICP is related to genetics, hormones, and environment26,27,28,29. A recent study suggests that the phenotype of ICP is influenced by multiple environmental factors such as PM2.5, O3 and potential causes of various pregnancy complications. The ICP with elevated serum total bile acid level (TBA ≥ 10µmol/L) but no elevated serum total bile acid aminotransferase level or pruritus was defined as “monosymptomatic ICP” (ICPs). An ICP with elevated serum total bile acid levels and at least one symptom is defined as “multisymptomatic ICP” (ICPm).The occurrence of single symptom ICP is more attributed to endogenous risk factors, such as the expression of certain genes such as SLC10A1, while the occurrence of multi symptom ICP is more susceptible to external factors, such as environmental exposure30. In familial cluster analysis of pedigree studies, the higher incidence of ICP in mothers and sisters of ICP patients may suggest a genetic predisposition to the disease31,32.
The results of this study show that there is little association between ABCB4 synonymous variants c.175 C > T, c.504 C > T, and c.711 A > T with the incidence of ICP in ICP patients in southern Anhui. The missense variant c.1954 A > G is highly non conserved in other species. Through three-dimensional structural analysis, the missense variant is located in the linker region of MDR3 protein, and bioinformatics analysis shows that it has little effect on protein function. Previous studies abroad have shown that ABCB4 missense variant c.1954 A > G may not lead to the occurrence of idiopathic gallstones in children33. The missense variant c.1954 A > G found in patients with progressive familial intrahepatic cholestasis had the same variant frequency as the control group, but skin itching and cholestasis occurred during pregnancy in a mother carrying this mutation. This indicates that the missense variant c.1954 A > G itself may not cause clinical symptoms and may require specific circumstances, such as pregnancy or combination with another variant, to cause clinical symptoms34.
ABCB4 associated cholestasis is characterized by elevated levels of γ-GT, which may be due to a reduced concentration of phospholipids in bile, leading to impaired formation of mixed micelles, an increased relative concentration of bile salts, and subsequent toxic effects on the bile ducts9,34,35,36. According to Piatek K, genotype coexistence analysis suggests that mutant variants of genetic polymorphisms may have a superimposed effect on the development of ICP37. In our study, we found that the missense variant c.1954 A > G was found in both ICP patients and healthy pregnant women. The incidence of missense variant c.1954 A > G in ICP patients was slightly higher than that in healthy pregnant women. But in ICP patients with the missense variant c.1954 A > G, we found variants in other exons(c.175 C > T, c.504 C > T,c.711 A > T) that are not found in healthy pregnant women. Among ICP patients carrying the missense variant c.1954 A > G, ICP patients carrying three variants had higher γ-GT, TBA, ALT and AST than ICP patients carrying two variants, indicating an aggravation of ICP. This may suggest that the c.1954 A > G missense variant needs to combine with other variants(c.175 C > T, c.504 C > T,c.711 A > T) to cause clinical symptoms of ICP, and c.1954 A > G missense variant have superimposed effects when combined with other variants.
Data availability
The datasets generated during the current study are available in the [Clinvar] repositoryc.175 C> Thttps://www.ncbi.nlm.nih.gov/clinvar/variation/256158/?oq=rs2302387&m=NM_000443.4(ABCB4):c.175 C%3ET%20(p.Leu59=)c.504 C>Thttps://www.ncbi.nlm.nih.gov/clinvar/variation/198082/?oq=rs1202283&m=NM_000443.4(ABCB4):c.504 C%3ET%20(p.Asn168=)c.711 A>Thttps://www.ncbi.nlm.nih.gov/clinvar/variation/256166/?oq=rs2109505&m=NM_000443.4(ABCB4):c.711 A%3ET%20(p.Ile237=)c.1954 A>Ghttps://www.ncbi.nlm.nih.gov/clinvar/variation/256160/?oq=rs2230028&m=NM_000443.4(ABCB4):c.1954 A%3EG%20(p.Arg652Gly).
References
Piechota, J. & Jelski, W. Intrahepatic cholestasis in pregnancy: review of the literature. J. Clin. Med. 9, (2020).
Liu, X. et al. Whole-exome sequencing expands the roles of novel variants of organic anion transporting polypeptide, ATP-binding cassette transporter, and receptor genes in intrahepatic cholestasis of pregnancy. Front. Genet. 13, (2022).
Smith, D. D. R. K. Intrahepatic cholestasis of pregnancy. Clin. Obstet. Gynecol. 63, 134–151 (2020).
Stulic, M. et al. Intrahepatic cholestasis of pregnancy: a case study of the rare onset in the first trimester. Medicina 55, (2019).
Xiao, J. et al. Molecular pathogenesis of intrahepatic cholestasis of pregnancy. Can. J. Gastroenterol. Hepatol. 2021, 1–10 (2021).
Li, X. et al. Profiles and integration of the gut microbiome and fecal metabolites in severe intrahepatic cholestasis of pregnancy. BMC Microbiol. 23, (2023).
Wood, A. M. L. E., Hughes, B. L. & Kuller, J. A. A review of diagnosis and management. Obstet. Gynecol. Surv. 73, 103–109 (2018).
Avena, A. et al. ABCB4 variants in adult patients with cholestatic disease are frequent and underdiagnosed. Dig. Liver Dis. 53, 329–344 (2021).
Dröge, C. et al. Sequencing of FIC1, BSEP and MDR3 in a large cohort of patients with cholestasis revealed a high number of different genetic variants. J. Hepatol. 67, 1253–1264 (2017).
Stättermayer, A. F. et al. Variants in ABCB4 (MDR3) across the spectrum of cholestatic liver diseases in adults. J. Hepatol. 73, 651–663 (2020).
Pasmant, E. et al. First description of ABCB4 gene deletions in familial low phospholipid-associated cholelithiasis and oral contraceptives-induced cholestasis. Eur. J. Hum. Genet. 20, 277–282 (2011).
Khattak, M. F. Recurrent early onset severe obstetric cholestasis in a patient with two variants in the ABCB4 gene: A case report. Hepatol. Forum 2023, 142–144 (2023).
Nosol, K. et al. Structures of ABCB4 provide insight into phosphatidylcholine translocation. Proc. Natl Acad. Sci. USA 118, (2021).
Carbone, M. & Cardinale, V. ABCB4-alteration screening in adult-onset cholestasis. Dig. Liver Dis. 53, 261–262 (2021).
Jacquemin, E. et al. Heterozygous non-sense mutation of the MDR3 gene in familial intrahepatic cholestasis of pregnancy. Lancet 353, 210–211 (1999).
Obstetrics, Subgroup & Chinese Society of Obstetrics and Gynecology, Chinese Medical Association. Guidelines for the management of intrahepatic cholestasis of pregnancy (2015). J. Clin. Hepatol. 31, 1575–1578 (2015).
Di Pietro, F. et al. Genomic DNA extraction from whole blood stored from 15- to 30-years at -20 degrees C by rapid phenol-chloroform protocol: a useful tool for genetic epidemiology studies. Mol. Cell. Probes. 25, 44–48 (2011).
Adzhubei, I. A. et al. A method and server for predicting damaging missense mutations. Nat. Methods. 7, 248–249 (2010).
Schwarz, J. M. et al. MutationTaster2: mutation prediction for the deep-sequencing age. Nat. Methods. 11, 361–362 (2014).
Kumar, P., Henikoff, S. & Ng, P. C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 4, 1073–1081 (2009).
Pejaver, V. et al. Inferring the molecular and phenotypic impact of amino acid variants with MutPred2. Nat. Commun. 11, 5918 (2020).
Geenes, V. & Williamson, C. Intrahepatic cholestasis of pregnancy. World J. Gastroenterol. 15, 2049–2066 (2009).
Huang, L. et al. Effect of intrahepatic cholestasis of pregnancy on infantile food allergy: a retrospective longitudinal study cohort in Southwest China. Eur. J. Obstet. Gynecol. Reprod. Biol. 272, 110–115 (2022).
Wasmuth, H. E. et al. Intrahepatic cholestasis of pregnancy: the severe form is associated with common variants of the hepatobiliary phospholipid transporter ABCB4 gene. Gut 56, 265–270 (2007).
Bacq, Y. et al. ABCB4 gene variants and single-nucleotide polymorphisms in women with intrahepatic cholestasis of pregnancy. J. Med. Genet. 46, 711–715 (2009).
Williamson, C. & Geenes, V. Intrahepatic cholestasis of pregnancy. Obstet. Gynecol. 124, 120–133 (2014).
Beard, M. P. & Millington, G. W. M. Recent developments in the specific dermatoses of pregnancy. Clin. Exp. Dermatol. 37, 1–5 (2012).
Baliutavičienė, D., Zubruvienė, N. & Žalinkevičius, R. Pregnancy outcome in cases of intrahepatic cholestasis of pregnancy. Int. J. Gynecol. Obstet. 112, 250–251 (2011).
Bacq, Y. Liver diseases unique to pregnancy: a 2010 update. Clin. Res. Hepatol. Gastroenterol. 35, 182–193 (2011).
Sun, H. Z. et al. A Chinese longitudinal maternity cohort study (2013–2021) on intrahepatic cholestasis phenotypes: risk associations from environmental exposure to adverse pregnancy outcomes. J. Hazard. Mater. 463, (2024).
Hirvioja, M. L. & Kivinen, S. Inheritance of intrahepatic cholestasis of pregnancy in one kindred. Clin. Genet. 43, 315–317 (1993).
Eloranta, M. L. et al. Risk of obstetric cholestasis in sisters of index patients. Clin. Genet. 60, 42–45 (2001).
Bronský, J. et al. Role of common canalicular transporter gene variations in aetiology of idiopathic gallstones in childhood. Folia Biol. (Praha). 56, 9–13 (2010).
Jacquemin, E. et al. The wide spectrum of multidrug resistance 3 deficiency: from neonatal cholestasis to cirrhosis of adulthood. Gastroenterology 120, 1448–1458 (2001).
De Vree, J. M. J. et al. Variants in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc. Natl Acad. Sci. USA. 95, 282–287 (1998).
Fang, L. J. et al. Chinese children with chronic intrahepatic cholestasis and high γ-glutamyl transpeptidase. J. Pediatr. Gastroenterol. Nutr. 55, 150–156 (2012).
Piątek, K. et al. The role of ABC transporters’ gene polymorphism in the etiology of intrahepatic cholestasis of pregnancy. Ginekol. Pol. 89, 393–397 (2018).
Author information
Authors and Affiliations
Contributions
Dekun Zhang, Tiechen Li and Qing Sun contributed to the conception or design of the study and drafted the manuscript. Yuhong Li, Shufeng He, Jing Shu and Qing Sun collected clinical data. Dekun Zhang analyzed data. Tiechen Li and Qing Sun reviewed and revised the manuscript. All authors reviewed and approved the final manuscript.
Disclaimer
Due to the long period of sample acquisition, we only received two samples of ICP in the past few months, and the conclusion of this study should be interpreted with caution. Further research is needed with a larger, more representative population sample and a more comprehensive statistical analysis to validate the results of this study and clarify any uncertainties.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, D., Li, Y., He, S. et al. Association between ABCB4 variants and intrahepatic cholestasis of pregnancy. Sci Rep 15, 3300 (2025). https://doi.org/10.1038/s41598-025-87909-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-87909-5