Abstract
Wire guided localization is widely used as the standard method of pre-operative localization of breast lesions. The aim was to assess outcomes following the introduction of a novel non-wire guided, magnetic surgical marker navigation system. A prospective study between May 2022 and June 2023 established a data base of the first 200 procedures performed using the Sirius Pintuition GPS Detect magnetic marker. The primary outcome measures were the successful excision of the target lesion and retrieval of the magnetic marker. The primary lesion was excised and the magnetic marker was retrieved in all 200 procedures. In 17 procedures (8.5% of the total sample), the magnetic marker was dislodged during surgery; however, the primary lesion was still effectively excised with clear margins without the need for an additional procedure or radiologic assistance. The re-excision rate to achieve margin clearance was 9%. Insertion of the marker was classified as “easy” and “in contact with the target” by the radiologist in all cases (100%). This study has shown that surgical marker navigation reliably localizes lesions and is associated with low re-excision rates. We also perceived improvement in theater planning.
Similar content being viewed by others
Introduction
Breast cancer is the most diagnosed cancer in women in the European Union, with approximatively 400,000 new breast cancers cases diagnosed each year1. In developed countries, with breast screening programs, the estimated rates of non-palpable breast cancer diagnosed are from 30 to 50%2. These non-palpable cancers require appropriate pre-operative localization to guide the surgeon.
Wire-guided localization was first described by Dood et al., in 1965. The technique was then modified with the addition of a hooked tip to the wires to limit the risk of their displacement prior to surgery3. Wire guided localization evolved further during the late 1980’s, in the absence of better alternatives, to become the standard of care in non-palpable breast cancer.
This technique, although safe and accurate, is not without shortfalls; such as discomfort, hematomas, the need for bandages, pre- or intra-operative migration, and planning constraints. Wire insertion is usually carried out on the day of surgery, which can cause delays in the operating theater. Moreover, there must be good coordination between the radiologist and the operating room to facilitate this, which can cause organizational problems. Some have even described accidents of exposure to blood linked to the use of the wire.
In recent years, there has been a drive to develop alternative localization techniques which may optimize theater planning; including the use of radar techniques, radiofrequency based techniques, and radioactive seed localization4. Concerning radioactive seed localization, although this technique is discussed favorably in the literature, the regulatory barriers and the necessity for a nuclear medicine department on site circumvent this technique from being widely adopted5.
Non-wire, probe-guided technologies using the power of magnetism have been developed recently as an interesting alternative6. One of the advantages of using magnetic power is the removal of the need for radioisotopes. Moreover, the magnetic signal does not decay over time. Magnetic-type markers are non-radioactive inert metallic objects, which are detected intraoperatively using a hand-held probe. In Europe, the Sirius Pintuition is one such marker. The Sirius Pintuition uses a permanent magnet which always has a magnetic field allowing for detection from any direction, even in fluids (https://www.sirius-medical.com/pintuition-marker). The Sirius Pintuition works by generating a magnetic field that is detected by the probe provided with the system. Furthermore, it can be inserted up to 180 days before the surgery (long-term placement is allowed in the USA). Post insertion control of the correct placement is performed using mammography or ultrasound detection.
The aim of this study was to assess outcomes following the introduction of such a magnetic surgical marker navigation system for pre-operative localization of non-palpable breast cancer lesions at our specialized French breast cancer center.
Methods
Patient selection
This was a monocentric, prospective study which included the first 200 patients who underwent magnetic surgical marker navigation in a specialized French breast cancer center between the 5th of May 2022 and the 28th of June 2023.
Only one of the radiologists was entitled to use the magnetic marker during the study. The others still used traditional wire guided localization. In contrast, patients could be operated by any of the nine surgeons of the breast unit.
Patient eligibility for pre-operative localization of breast lesion was decided upon by the surgeon at the pre-operative consultation. The patient was then scheduled with the radiologist who confirmed the patient’s eligibility for surgery with the surgical marker navigation.
We used only non-palpable masses visible on ultrasound.
Patient inclusion criteria were: the presence of a nodular unifocal non-palpable confirmed diagnosis of invasive breast cancer, carcinoma in situ (DCIS or LCIS) or other high-risk features (atypical ductal hyperplasia, atypical lobular, hyperplasia, radial scar etc.), location of the lesion less than 50 mm under the skin (50 mm probe detection range according to the manufacturer), and visibility of the nodule on ultrasound for pre-operative identification under ultrasound guidance.
Patient exclusion criteria were: absence of visibility on ultrasound, i.e., calcifications alone, palpable lesion, bi or multifocality, location of the lesion more than 50 mm under the skin, absence of confirmed diagnosis of invasive breast cancer, carcinoma in situ or other high-risk features (atypical ductal hyperplasia, atypical lobular, hyperplasia, radial scar etc.). We did not include patients who had a marker placed during the biopsy for micro calcifications or distortions alone.
We excluded calcifications and distortions in order to have a homogeneous cohort in terms of means of localization, with markers placed only under ultrasound guidance.
Patient eligibility for breast conservative surgery with pre-operative localization of breast lesion was decided upon by the surgeon at the pre-operative consultation. Concerning non-palpable lesions, the patients selected were all from the french breast cancer screening program.
The patient was then scheduled with one of the departments radiologists. In the majority of cases, the patient was scheduled with our one full time radiologist dedicated to breast imaging, biopsy and pre-operative localization who performs the majority of the pre-operative localization at our unit. The radiologist then confirmed the patient’s eligibility for surgery with the surgical marker navigation.
Technique
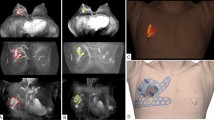
The magnetic surgical marker navigation system used in this study was the Sirius Pintuition GPS Detect™ (Sirius Medical Systems B.V., Eindhoven, The Netherlands). The magnetic marker is an inert metallic marker 5 × 1.6 mm in size. The marker surface is made of titanium. The marker insertion was performed by the radiologist. It was performed under local anesthesia, using ultrasound guidance, in every case. The marker was placed using the marker insertion device including a 14G pre-loaded needle with an ultrasound-enhanced tip. It was placed within the lesion, or just behind it if it was close to the skin (< 5 mm). The radiologist specified in the report for the surgeon the size of the lesion (length, width and height), its localization in relation to the nipple (radius, distance in centimeters) and the depth in relation to the skin. If the surgery was carried out the same day, we carried out a skin marking. Once in place, all the placement of the permanent magnetic markers were confirmed in the correct position using 2-axis mammography (Fig. 1). The unilateral post-procedure control mammogram was performed on 2 orthogonal views in order to check the correct positioning of the marker and it was considered as correct if less than 1 centimeter between the lesion and the marker, as shown in Fig. 1.
Intraoperative localization of the magnetic marker was performed using the surgical marker navigation probe. This specific probe allows detection of the magnetic marker with millimeter precision transmitted to the surgical marker navigation base unit. The base unit provides the surgeon with visual feedback showing the distance in millimeters between the magnetic marker and the detector and also includes sound feedback, using an audible tone, which increases in pitch as the probe gets closer to the marker.
All surgeries were performed under general anesthesia.
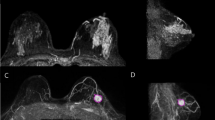
During the procedure, the marker location was confirmed repeatedly by regular probe guidance. The magnetic marker and the tumor excision were verified in two steps. Firstly, the location of the marker was confirmed within the excised specimen using the probe, and secondly, a specimen X-ray was performed immediately in the radiology unit (Fig. 2). Shaving of the cavity margins was decided upon by the individual surgeon, following review of the specimen X-ray.
Margins were considered as “clean” if greater than 2 mm for ductal carcinoma in situ (DCIS) alone, and if there was no ink on the tumor in cases of invasive cancer alone or those associated with DCIS, according to the National Comprehensive Cancer Network (NCCN) guidelines breast cancer version 4.20247, Society of Surgical Oncology-American Society for Radiation Oncology-American Society of Clinical Oncology Consensus Guidelines8,9, and actual French standards10.
Data collection
Data was collected from a prospective database. The results of this clinical trial are registered according to French standards with the CNIL. The patient characteristics recorded were age, body mass index (BMI) and personal history of cancer. Data collection on the history of care included was: time of the first biopsy, time of insertion of the magnetic marker, number of magnetic markers inserted, time of the surgery, and details of the neoadjuvant chemotherapy (if performed). Pre-operatively, we recorded: localization, radiological size, MRI (if performed), ease of insertion, quality of the insertion (distance from the tumor), patient acceptance to pain and tolerance of the marker’s insertion. Peri-operatively, we recorded: the pre-operative histological diagnosis, the type of surgery (standard, oncoplastic), axillary lymph node surgery (if performed), length of surgery, ease of the operative gesture, dislodgement of the marker during the procedure, dimensions of the lumpectomy, number of intra operative recut. Post-operatively, we recorded: margin status, the need for surgical revision, pathological size, receptor status, lymph node status, Ki67, and HER2 status.
According to French regulations and in line with the principles of the Declaration of Helsinki, all individual participants included in the study were informed of the research to be performed, and written informed consent was obtained. Approval was granted by the Ethics Committee of the Reims Unicancer Institute (Registration Number 19086206, date of registration 18/07/2024).
Outcome measures
The primary outcome measures were successful excision of the tumor and retrieval of the magnetic marker. The secondary outcomes analyzed were difficulty of insertion, localization accuracy, patient’s tolerance/pain, and breast margin re-excision rate.
Statistical analysis
Quantitative parameters were described by their mean and standard deviation (SD), qualitative parameters by their frequency and percentage. Continuous variables were compared using Student’s t-test. Categorical variables were compared using the chi-squared test. Statistical tests were performed at a significance level of 0.05. Analyses were performed using the R Development Core Team (2020) software11.
Results
Patient characteristics
During the study period, 194 surgeries were performed on patients using the surgical marker navigation system. This accounted for 200 procedures, as 6 surgeries were bilateral. The patient characteristics are detailed in Table 1. The mean age was 60.7 ± 12.4; mean BMI was 26.9 ± 5 kg/m2, 11% had a preexisting history of cancer, 37% underwent MRI evaluation before surgery, 19.7% of patients underwent neoadjuvant chemotherapy.
Tumor pre-operative characteristics
Table 2 summarizes the following tumor pre-operative characteristics as determined via ultra sound: localization of the tumor, size of the tumor (long axis, mean 12.6 mm +/- 8.3 standard deviation) and the depth in relation to the skin (mean 10.1 mm +/- 8.3 standard deviation); and histological type on the biopsy.
T1c (78 patients, 39%) and T2 (2 patients, 1%) lesions were non-palpable in relation to the breast volume, the deep localization and/or the histological type (DCIS, lobular).
Most patients underwent surgery for a pre-therapeutic diagnosis of invasive breast cancer with or without in situ carcinoma (181 patients, 90.5%). Twelve patients (6%) had DCIS alone, or high-risk lesions (7 patients, 3.5%).
Marker placement
All insertions of the magnetic markers were considered easy by the radiologist; which were cases that were without difficulty in locating the lesion, procedure duration of 10 to 15 minutes, and a simple insertion of the marker, without complications.” In all cases no complications were reported during the insertion procedure. The position of the magnetic markers in non-palpable breast lesions was considered as optimal (< 1 cm from the tumor) in all procedures. There was no need in any procedure in this study to place a 2nd marker due to incorrect positioning of the first marker and no migration of the marker.
The patients reported no pain or a low intensity of pain (1 or 2 on a pain scale from 0 to 10) in 193 cases (93%). One hundred and twenty-one magnetic markers (60.5%) were placed on the day of surgery or the day before surgery. Seventy-nine magnetic markers (39.5%) were placed between 2 and 29 days before surgery, including 19% at least one week earlier (n = 38).
Peri operative data
The primary tumor and the markers were retrieved in all cases.
Standard lumpectomy was performed in 114 procedures (57%) (Table 3). In 85 cases (42.5%), an oncoplastic procedure was performed (reduction mastoplasty, external oncoplastic incision, Round Block mastopexy, Thorek reduction mastoplasty). In 1 case (0.5%), an immediate breast reconstruction with implant was performed. The mean duration of tumor removal was 16.1 min (time from incision to removal of the tumor).
Detection of the magnetic marker was considered as “easy” in almost all procedures (98.5%). In 17 procedures (8.5%), the marker was dislodged during the surgery and was found in 13 cases (76% of the dislodged markers) in contact with a metallic surgical tool, and free in contact with tissues in 4 cases (24% of the dislodged markers). There was no need for re–excision in these patients as the primary lesion was still effectively removed.
Dislodged markers were significantly more superficial (7.9 vs. 10.3 mm, p = 0.02). A peri-areolar incision was significantly associated with dislodged markers compared with a direct radiary incision (p < 0.001). Lumpectomy with oncoplasty was significantly less associated with dislodged markers (p < 0.001). There was a significant trend towards dislodged markers with higher BMI (24.5 vs. 27.1 kg/m2, p = 0.05). The mean number of days between insertion and surgery (2.6 vs. 3.1 days, p = 0.72), as well as the size of the lesion (7.9 vs. 9.5 mm, p = 0.2), had no influence on dislodged markers.
Post-operative data
The overall rate of second procedure for positive margins was 7%, 9% for invasive cancer without neo-adjuvant chemotherapy (13 re-excision out of 145 procedures), 0% for invasive cancer after neo-adjuvant chemotherapy (0 re-excision out of 36 procedures), 8.3% for DCIS alone (1 re-excision out of 12 procedures).
The re-excision rate if surgery was the first treatment for invasive cancer was 8.2% for invasive cancer alone and 9.7% if invasive cancer was associated with DCIS. The re-excision rate when the marker was inserted the day of the surgery or the day before was 9.1%. The re-excision rate when the marker was inserted at least 2 days ahead of the surgery was 7.6% (p = 0.71).
The mean diameter of the surgical piece removed was 69.2 mm (Table 4); with a mean volume of 155 cm3 (range 12.5–3669); and its mean weight was 57.4 g (range 5-1485).
The mean pathological size was 12.6 mm for invasive carcinoma (with ou without DCIS) and 15.6 mm for DCIS alone. The majority of tumors were ductal invasive carcinoma (74.5%) and luminal histological subtype (73.0%). DCIS was associated with invasive carcinoma in less than half of all cases (45.4%). 10 cases were DCIS alone. 17.8% of lymph node investigations were positive.
Discussion
In this series of 200 surgical marker navigation procedures performed in a French cancer center, a 100% of the tumors and the magnetic markers were removed, with a low re excision rate (9%). A recent multicenter prospective study evaluated 946 Magseed versus 1170 wire-guided localizations of impalpable breast lesions in a population of 2116 patients12. The Magseed is a stainless steel paramagnetic marker that is detected using a magnetic probe to detect the reflected signal (https://www.endomag.com/products/magseed/). Using the device the authors showed that there was no difference in the median closest margin (p = 0.342), or re-excision rate (p = 0.574). The authors subsequently concluded that Magseed demonstrated similar safety and efficacy to wire guided localization.
In this study, 183 permanent magnetic markers (91.5%) were retrieved within the tumor and dislodged in 17 cases (8.5%). Concerning the 17 procedures with dislodgment of the markers, these were found in 13 cases in contact with one of the metallic surgical tools, and in 4 cases the marker was dislodged during the handling of the surgical piece. The tumors were, regardless of the dislodged markers, well excised without further necessity for re-excision.
Placing a stitch using a large needle, when getting close to the marker, in order to fix it, is an option that is recommended.
In our study, marker dislodgment was significantly higher if the lesion was more superficial (7.9 vs. 10.3 mm, p = 0.02) and if the incision was not direct (p < 0.001). The placement of the marker by the radiologist is important to reduce this risk. If the lesion is close to the skin, it is advised to place the magnetic marker a little behind it. This way, the risk of marker dislodgment when the surgeon dissects under the skin is greatly reduced, especially for non-direct incisions.
The timing of marker deployment might be important as well: we suggest that the earlier the better, as the tissue would have time to heal and encapsulate the marker, if the marker is placed earlier. We suppose it might reduce the risk of marker dislodgement during surgery, linked to surgical manipulations allowing movement of the marker in the insertion pathway. On the other hand, there might be some degree of marker migration during the process of healing. It may be the subject of a future work to evaluate the optimal timing of the marker placement.
Although the surgeons and radiologists involved in the surgical marker navigation procedure found it very user friendly and easy to implement, there was a tendency towards more marker dislodgement in the first hundred cases (10 dislodgements) than in the second one hundred cases (7 dislodgements) (p = 0.48). Although not statistically significant, we feel there is possibly a learning curve for this technique of detecting sub-clinical lesions, however simple, straightforward, and more efficient it might seem.
Other studies reported a 100% retrieval rate of the Magseed within the specimen of their studies13,14,15,16. These reassuring rates of tumor removal and marker retrieval confirm the validity of the procedure as an alternative to wire guided excision. Similarly, there were no cases of marker migration (i.e., clinically significant movement of the marker from the original placement position before surgery) in the present study, as in the literature13,14,15,16, regardless of the time of preoperative placement.
Theater planning was significantly easier, since the magnetic marker could be inserted a few days (at least 2) or weeks before the surgical procedure, if necessary. The re-excision rate for these procedures (7.6%) was not different to the rate of the procedures with the marker inserted the day of the surgery or the day before (9.1%). Although, it wasn’t the main end point of this study, it seems safe to place the marker a few days or even weeks before the surgery, with apparently a low risk of migration of the marker.
According to the manufacturer, the magnetic marker can be inserted up to 180 days before surgery and could be inserted as soon as neoadjuvant chemotherapy is indicated. However, the use of MRI (Magnetic Resonance Imaging) for monitoring might be a contraindication, as it can lead to artifacts and misinterpretation of the response to treatment17. In any case, it is recommended to use a magnetic marker after MRI is used for initial staging of prior to surgical treatment following neoadjuvant treatment18.
In this series, in cases of neo-adjuvant chemotherapy, standard radiological markers were usually used before the medical treatment. The magnetic marker was placed when neo-adjuvant chemotherapy and the MRI monitoring are complete, if conservative surgical treatment for non-palpable lesions is confirmed. An alternative option would be to use Contrast Enhance Mammography instead of MRI.
The re-operation rate using the Sirius Pintuition magnetic marker in our study (8.3% for invasive cancer alone and 9.7% if invasive cancer associated with DCIS) was quite low in comparison with published re-operation rates using standard wire-guided localization: 14.9 to 20.8% in literature review with pooled analysis4 and meta analysis of randomized controlled trials19,20. As a reminder, we only included patients with masses visible on ultrasound (diagnosis of invasive breast cancer, carcinoma in situ (DCIS or LCIS) or other high risk features), excluding micro calcifications or distortions alone, whether or not a marker from the previous biopsy was placed.
Our re-operation rate is however similar to other non-wire localization methods including radioactive seed localization, 6.8 to 10.3%4,20, Magseed, 11.25 to 13.44%20,21,22, radar techniques, 5.3 to 8.6% (Savi Scout Surgical guidance System)20 and radiofrequency based techniques, 13.9% (LOCalizer)23; each with its specific advantages and limitations24.
Finally, the lowest re-operation rates seem to have been obtained using intraoperative ultrasound localization (IOUS), 4.8 to 7%4,25. The other advantage of IOUS is the smaller surgical specimen volume necessary to achieve oncologic result26,27. The limitations of this technique are non-visible ultrasound lesions, operator dependency, necessity of specific training, and availability of an ultrasound machine24. Intra operative ultrasound detected marker is, however, a method that could be used in cases of non-visible ultrasound lesions24.
There was only one complication in the series. In that case, occult bleeding occurred during insertion of the marker, leading to the formation of a hematoma that was not immediately visible. As the marker was inserted the day of the surgery, the hematoma was discovered during the surgery. The magnetic marker was free in the hematoma. Fortunately, the lesion was in contact with the hematoma, allowing the surgeon to find it and remove it safely. There has been no re–excision in this case.
There are limitations to the study. It is a single site, non-randomized study with only one radiologist entitled to insert the magnetic markers. On the other hand, nine surgeons were involved in the surgical procedures, showing the ease to adapt to this procedure for the surgeons. Furthermore, we excluded calcifications in order to have a homogeneous cohort in terms of means of localization, with markers placed only under ultrasound guidance.
There was a lot of additional benefits using this magnetic marker for guided breast surgery of non-palpable lesions, which have not been evaluated, such as: more comfort for the patient, more freedom for the surgeon to decide the best incision, the localization of the magnetic marker was easier than with the end of the wire, and there was a possibility to check the margin with the probe (i.e., distance between the margin and the marker). Restrictions on the initiation of this technique in the department have led to the management of unifocal lesions only. Two markers can be placed in the same breast without interference if they are at least 2 cm apart. They can also be placed in the positive lymph node identified prior to neo-adjuvant chemotherapy for targeted axillary dissection28. These additional analyses may be the subject of future work as well as a direct comparison of magnetic markers versus wire guided localization.
Conclusion
The Sirius Pintuition magnetic surgical marker navigation system for the removal of non–palpable breast lesions has been easily implemented, with significant improvement in theater planning, and low complication and re-excision for margin clearance rates. The procedure is very safe and can be used as an alternative to wire guided excision.
Further prospective and comparative analyses could be the subject of future studies.
Data availability
The data generated during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- DCIS:
-
Ductal Carcinoma in situ
- LCIS:
-
Lobular Carcinoma in situ
- MRI:
-
Magnetic Resonance Imaging
- SD:
-
Standard Deviation
- BMI:
-
Body Mass Index
- HER 2:
-
Human Epidermal Growth Factor Receptor 2
- Ki67:
-
Prognostic biomarker in invasive breast cancer
- TNBC:
-
Triple Negative Breast Cancer
- IOUS:
-
intra operative ultrasound localization
References
Dafni, U., Tsourti, Z. & Alatsathianos, I. Breast cancer statistics in the European Union: Incidence and Survival across European countries. Breast Care (Basel) 14 (6), 344–353. https://doi.org/10.1159/000503219 (2019).
Ahmed, M., Rubio, I. T., Klaase, J. M. & Douek, M. Surgical treatment of nonpalpable primary invasive and in situ breast cancer. Nat. Rev. Clin. Oncol. 12 (11), 645–663. https://doi.org/10.1038/nrclinonc.2015.161 (nov 2015).
Frank, H. A., Hall, F. M. & Steer, M. L. Preoperative localization of nonpalpable breast lesions demonstrated by mammography. N. Engl. J. Med. 295 (5), 259–260. https://doi.org/10.1056/NEJM197607292950506 (1976).
Davey, M. G. et al. Optimal localization strategies for non-palpable breast cancers: A network meta-analysis of randomized controlled trials. Breast 62, 103–113. https://doi.org/10.1016/j.breast.2022.02.004 (2022).
Ahmed, M. & Douek, M. Radioactive seed localisation (RSL) in the treatment of non-palpable breast cancers: Systematic review and meta-analysis. Breast 22 (4), 383–388. https://doi.org/10.1016/j.breast.2013.04.016 (2013).
Schermers, B. et al. Feasibility of magnetic marker localisation for non-palpable breast cancer. Breast 33, 50–56. https://doi.org/10.1016/j.breast.2017.03.003 (2017).
National Comprehensive Cancer Network.. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1419 (2024).
Moran, M. S. et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. J. Clin. Oncol. 32, 1507–1515 (2014).
Morrow, M. et al. Society of Surgical Oncology-American Society for Radiation Oncology-American Society of Clinical Oncology Consensus Guideline on margins for breast-conserving surgery with whole-breast irradiation in Ductal Carcinoma in situ. J. Clin. Oncol. 34, 4040–4046 (2016).
Oncologik Référentiel inter - régional de prise en charge du cancer du sein. http://oncologik.fr/referentiels/dsrc/sein-principes-de-prise-en-charge (2019).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. Austria (2021). https://www.R-project.org
Dave, R. V. et al. Wire- and magnetic-seed-guided localization of impalpable breast lesions: iBRA-NET localisation study. Br. J. Surg. 109 (3), 274–282. https://doi.org/10.1093/bjs/znab443 (2022).
Murphy, E. et al. Initial experience of magnetic seed localization for impalpable breast lesion excision: First 100 cases performed in a single Irish tertiary referral centre. Surgeon. 20 (3), e36–e42. (2022). https://doi.org/10.1016/j.surge.2021.02.010
Thekkinkattil, D. et al. déc. A prospective, single-arm, multicentre clinical evaluation of a new localisation technique using non-radioactive Magseeds for surgery of clinically occult breast lesions. Clin. Radiol. 74 (12), 974.e7–974.e11. https://doi.org/10.1016/j.crad.2019.08.018 (2019).
Price, E. R., Khoury, A. L., Esserman, L. J., Joe, B. N. & Alvarado, M. D. Initial clinical experience with an inducible magnetic seed system for preoperative breast lesion localization. AJR Am. J. Roentgenol. 210 (4):913–917. https://doi.org/10.2214/AJR.17.18345 (2018).
Zacharioudakis, K. et al. Is the future magnetic? Magseed localisation for non palpable breast cancer. A multi-centre non randomised control study. Eur. J. Surg. Oncol. 45 (11), 2016–2021. https://doi.org/10.1016/j.ejso.2019.06.035 (2019).
Hayes, M. K. Update on preoperative breast localization. Radiol. Clin. N. Am. 55 (3), 591–603. https://doi.org/10.1016/j.rcl.2016.12.012 (2017).
Miller, M. E. et al. Hospital system adoption of magnetic seeds for Wireless breast and lymph node localization. Ann. Surg. Oncol. 28 (6), 3223–3229. https://doi.org/10.1245/s10434-020-09311-x (2021).
Jamaris, S. et al. Re-excision rates in breast-conserving surgery for invasive breast cancer after neoadjuvant chemotherapy with and without the use of a radiopaque tissue transfer and X-ray system. Breast Care (Basel). 14 (5), 302–307. https://doi.org/10.1159/000493017 (2019).
Shirazi, S. et al. Comparison of wire and non-wire localisation techniques in breast Cancer surgery: A review of the literature with pooled analysis. Medicina (Kaunas) 5 (2023).
Gera, R. et al. Evolving role of Magseed in Wireless localization of breast lesions: Systematic review and pooled analysis of 1,559 procedures. Anticancer Res. 40, 1809–1815 (2020).
Depretto, C. et al. Magnetic localization of breast lesions: A large-scale European evaluation in a National Cancer Institute. Clin. Breast Cancer. 23, e4 (2023).
Tayeh, S. et al. Reflector-guided localization of non-palpable breast lesions: The first reported European evaluation of the SAVI SCOUT® System. Anticancer Res.40, 3 (2020).
Cheung, B. H. H., Co, M., Lui, T. T. N. & Kwong, A. Evolution of localization methods for non-palpable breast lesions: A literature review from a translational medicine perspective. Transl. Breast Cancer Res. 5 https://doi.org/10.21037/tbcr-23-49 (2024).
Banys-Paluchowski, M. et al. Localization techniques for non-palpable breast lesions: Current status, knowledge gaps, and rationale for the MELODY study (EUBREAST-4/iBRA-NET, NCT 05559411). Cancers (Basel). 15, 1173 (2023).
Rahusen, F. D. et al. Ultrasound-guided lumpectomy of nonpalpable breast cancer versus wire-guided resection: A randomized clinical trial. Ann. Surg. Oncol. 9, 994–998 (2002).
Colakovic, N. et al. Intraoperative ultrasound in breast cancer surgery-from localization of non-palpable tumors to objectively measurable excision. World J. Surg. Oncol. 16, 184 (2018).
Alvarado, M. D. et al. (oct 2019) SentimagIC: A non-inferiority trial comparing superparamagnetic iron oxide versus technetium-99m and blue dye in the detection of axillary sentinel nodes in patients with early-stage breast cancer. Ann. Surg. Oncol. 26 (11), 3510–3516. https://doi.org/10.1245/s10434-019-07577-4
Funding
No funds, grants, or other support was received.
Author information
Authors and Affiliations
Contributions
Conceptualization: VC, LW, FR, JH; Acquisition of data: VC, LW, SM, TG, NG, LD, LP, EG, FR, BG, JH; Analysis of data: VC, BG, JH; Writing original draft: VC; Writing review and editing: CV, JH; Supervision: FR.Vivien Ceccato, Lauren Wallaert, Fabien Reyal and Judicaël Hotton contributed to the study conception and design. Material preparation and data collection were performed by Vivien Ceccato, Lauren Wallaert, Sophie Michel, Thomas Gaillard, Noémie Girard, Lauren Darrigues, Léa Pauly, Elodie Gauroy, Fabien Reyal, Beatriz Grandal and Judicaël Hotton.Analysis were performed by Vivien Ceccato, Beatriz Grandal and Judicaël Hotton.The first draft of the manuscript was written by Vivien Ceccato. Judicaël Hotton and Fabien REYAL commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Godinot Institute, Reims (registration number 19086206, date of registration 18/07/2024).
Informed consent
Written consent from included patients.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ceccato, V., Wallaert, L., Michel, S. et al. Magnetic surgical marker navigation for excision of non-palpable ultrasound visible breast lesions: first 200 cases in a French cancer center. Sci Rep 15, 5002 (2025). https://doi.org/10.1038/s41598-025-88430-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-88430-5