Abstract
Mirabegron and tamsulosin have recently been prescribed to men with overactive bladder for the treatment of benign prostatic hypertrophy. An efficient and environmentally friendly HPLC method was developed to accurately measure the levels of mirabegron and tamsulosin in both their pure form and in medication formulations. Full separation was achieved on an X-Bridge C18 column using a gradient elution of (The A mobile phase was a buffer solution containing 1 mL of trifluoroacetic acid and 3 mL of triethylamine in 1,000 milliliters of water, the pH of the solution was then adjusted to 3 using triethylamine and the B-mobile phase was acetonitrile). The chromatographic peaks were obtained at a wavelength of 220 nm. Mirabegron and tamsulosin were identified with retention time values of 2.4 min and 8.9 min, respectively. In the concentration ranges of 2.5–55 µg/mL for mirabegron and 5–110 µg/mL for tamsulosin, remarkable linearity was seen. The limits of detection for the two analytes were 0.28 and 0.55 µg/mL, respectively, and their R2 values were 0.9999. The new HPLC method was evaluated for its environmental friendliness using the Analytical GREEness (AGREE) metric. Furthermore, the suggested technique was considered practicable based on the evaluation conducted using the Blue Applicability Grade Index (BAGI) assessment. Both evaluation methods were quite successful, yielding scores of 0.52 and 80, respectively. Compared to the TLC-reported method, HPLC is the preferred choice for the separation of the two analytes due to its sensitivity.
Similar content being viewed by others
Introduction
Overactive bladder syndrome (OAB) is a type of storage lower urinary tract symptoms (LUTS) that includes urgent need to go to the bathroom, with or without incontinence, often happening at night1. Overactive bladder syndrome is a persistent medical problem that affects individuals of both genders, resulting in a significant decline in their overall quality of life2,3. Although the occurrence of this ailment is more common among those aged 40 and beyond, it may affect both children and young people4. According to research conducted in 2011 and using a sample of 10,000 people from Europe, it was shown that about 36% of males and 43% of females aged 40 and above had symptoms indicative of OAB5. According to the findings of the National Overactive Bladder Evaluation (NOBLE) program, the prevalence of approximately 33 million persons with overactive bladder (OAB) in the United States population was observed. The OAB has a substantial impact on the economy. For example, a study conducted in the United States in 2005 found that the yearly costs incurred by individuals with OAB surpassed 12 billion USD. These expenditures include indirect costs, such as the temporary impairment of work performance6. The dominant pharmaceutical treatment for LUTS in males with benign prostatic hyperplasia (BPH) is the alpha-1-adrenoceptor blocker (A1B). Nevertheless, even receiving A1B therapy, signs of OAB may continue to exist7. The guideline established by the European Urologic Association in 2015 suggests that antimuscarinics or beta-3-adrenoceptor agonists (B3A) should be used in men with benign prostatic hyperplasia (BPH) for the treatment of moderate-to-severe lower urinary tract symptoms characterized by predominant bladder storage symptoms. In cases where both monotherapies prove inadequate in alleviating symptoms, a combination therapy is recommended8.
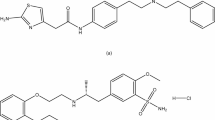
Mirabegron (MIR) is a recently approved medicine by the FDA for the treatment of idiopathic overactive bladder9. Mirabegron functions as a β3 specific receptor agonist, exerting its effects through the relaxation of the detrusor muscle. Regarding the chemical composition of the compound, MIR can be described as (N-[4-[2-[[(2R)−2-hydroxy-2-phenylethyl] amino] ethyl] phenyl] acetamide)10(Fig. 1a). Tamsulosin (TAM), is an α1-adrenoceptor antagonist with the chemical formula 5-[(2R)−2-[2-(2-ethoxyphenoxy)ethylamino]propyl]l.2-methoxybenzene-1-sulfonamide11 that is employed in the management of lower urinary tract symptoms linked to benign prostatic hyperplasia (BPH) (Fig. 1b).
Multiple trials have demonstrated the efficacy and safety of MIR as an add-on therapy to TAM as a treatment for symptoms of obstruction of the bladder (OAB) caused by benign prostatic hyperplasia (BPH) in males with a minimal incidence of adverse effects12. Mirabegron and tamsulosin combination treatment for OAB has shown benefits in symptom relief without producing extra adverse effects in recent clinical studies. The combined treatment of tamsulosin and mirabegron is thus anticipated to be actively investigated as a substitute for traditional medications in individuals who are unable to tolerate the inadequacies of current drug regimens or adverse drug reactions13. Even though there have been a number of chromatographic studies done on MIR detection14,15,16,17,18,19,20and TAM determination21,22,23,24,25,26,27,28,29,30, the literature review revealed that there was just one single HPTLC technique documented for the quantification of MIR and TAM together31.
Liquid chromatography (LC) is the most often used and applied technique in the pharmaceutical industry. Furthermore, it is the most efficient and versatile method for the concurrent separation and quantitative measurement of different components found in a mixture32. To reduce the amount of hazardous organic solvents produced every day worldwide or to replace them with safer alternatives, it is crucial to apply the principles of green analytical chemistry (GAC) while continuing to routinely analyze pharmaceuticals using liquid chromatography (LC) for quality control (QC) without compromising performance33. The current research used the Analytical GREENNESS metric methodology (AGREE) to evaluate the environmental greenness of the suggested strategy34. The findings demonstrate that the proposed method has outstanding environmental sustainability. In addition, the newly implemented Blue Applicability Grade Index (BAGI) tool was used to evaluate the blueness of the technique35. The main goal of the proposed study is to use the GAC principle to make a simple, low organic consumption, sensitive and cost-effective high-performance liquid chromatography (HPLC) method for evaluating the mixture.
Experimental
Materials and reagents
Chemicals and reagents
The chemicals used in this study were of HPLC quality, including trifluoroacetic acid, triethylamine, methanol, and acetonitrile. Sigma Aldrich supplied these for use in the mobile phase and diluent production. The MIR and TAM reference standards were purchased from Sigma–Aldrich. The medications Betmiga 50 mg® and Tamsul 0.4 mg® were obtained from a local market. The study was carried out with Milli-Q water, which was produced internally.
Instruments and software details
An EAB 224e Adam equinox semi-microbalance is used to measure the weight of all samples, compounds, and standards. The mobile phase buffer with a pH of 3.0 was created and then assessed using a JENWAY 3510 pH meter. The whole study was carried out using an Agilent HPLC type Alliance 1260, equipped with 2,998 Photodiode-Array Detector (PDA) detectors and 2,489 UV detectors. The development of the chromatographic technique included the use of an X-Bridge C18 column (4.6 × 150 mm, 3.5 μm) obtained from Waters.
Stock standard solutions
Stock standard solutions were made with concentrations of 100 µg/mL MIR and 200 µg/mL TAM, respectively, by measuring and transferring 5 mg of MIR and 10 mg of TAM into 50 mL clean and dry volumetric flasks. After that, 10 ml of methanol was added. The flask was filled to the mark with distilled water. The resulting stock solution was filtered using a 0.22 μm nylon filter.
General procedures
Chromatographic conditions
A buffer solution containing 1 mL of trifluoroacetic acid and 3 mL of triethylamine in 1,000 milliliters of water was used to create Phase A of the mobile phase. The pH of the solution was then adjusted to 3 using triethylamine. The B-mobile phase was acetonitrile. The process of separation was accomplished using a linear gradient procedure in the following manner: The developed gradient program started with a high proportion of the aqueous phase (80:20, A: B) for a duration of 5 min, followed by a transition to (60:40, A: B) over a period of 4 min. After a duration of 4 min, the gradient program was reverted back to its original conditions. The rate of flow was one milliliter per minute. The injection volume was 10 µl. The total duration of the experiment was 13 min, with a wavelength of 220 nm.
Linearity
The linearity of the samples was assessed by systematically diluting each analyte from the respective stock standard solutions individually. This process resulted in solutions with concentrations ranging from 2.5 to 55.0 µg/mL of MIR and 5.0 to 110.0 µg/mL of TAM. The mobile phase was used as the solvent for this dilution. Injecting the solutions in triplicate allowed us to obtain chromatograms under the chromatographic conditions mentioned previously. We plotted the average peak area against the relevant concentrations on both the integrated and calibration plots.
Analysis of dosage forms
The Betmiga® dosage form had ten tablets that were accurately weighed, crushed, and mixed. An amount of powder equal to one tablet was transferred to a 100 mL volumetric flask. Twenty Tamslu® capsules were properly measured, and their average weights were calculated. The capsule contents were well mixed before being transferred to a 25 mL volumetric flask at a specific weight comparable to 5 mg. The volume of each flask was then adjusted to 30 mL and 15 mL respectively with methanol. An ultrasonic shaker was used to stir the flasks for 15 min. After filtering using a 0.45-µm membrane filter, the contents of the flasks were further diluted. To create a solution including MIR and TAM, aliquots of Betmiga® and Tamsul® filtrates were placed in a 25-mL volumetric flask and diluted with the mobile phase.
Results and discussion
Optimization of chromatographic conditions
It is not practicable to simultaneously determine MIR and TAM using zero-order simple UV spectrophotometry. A novel technique was devised to analyze mixes of MIR-TAM using a gradient liquid chromatographic method combined with diode array detection (Table 1). This method offers a viable approach for conducting regular quality control examinations. The primary objective in the development of LC methods is to attain adequate resolution with satisfactory peak symmetry within a tolerable analytical timeframe. This objective was accomplished by performing many experiments aimed at optimizing the stationary and mobile phases. In order to optimize the stationary phase, two columns were tested: the X-Bridge C18 column with dimensions of 4.6 × 150 mm, 3.5 μm and the X-Bridge C18 column with dimensions of 4.6 × 250 mm, 5 μm. The X-Bridge C18 column, size 4.6 × 150 mm x 3.5 μm, was determined to be optimal due to its ability to achieve the best retention period for the two medicines. Different volumes of various compositions of mobile phases were subjected to testing. A mixture of 0.001% trifluoroacetic acid solution, triethylamine and acetonitrile was found to be the most effective mobile phase. An experiment was conducted using orthophosphoric acid, which led to the occurrence of a tailing in the peaks. Additionally, octansulphonate and heptansulphonte were used, resulting in the occurrence of peaks overlap. In addition, the use of a mobile phase containing a significant amount of acetonitrile in an isocratic manner led to a complication in the MIR peak. Also, there was a lack of retention and clear co-elution between the two peaks. In order to solve these issues and achieve a perfect resolution between MIR and TAM, a gradient elution technique was used, initiating with a low amount of acetonitrile.
Method validation
The process of validating the proposed approach was conducted in accordance with the principles set out by the ICH36.
System suitability
Table 2 displays the computed system suitability characteristics, namely retention time, theoretical plates, tailing factor, resolution, and selectivity.
The method selectivity
The comparison of chromatograms between the raw analytes and those obtained from dosage forms (Fig. 2) indicated the absence of any interference caused by commonly used excipients in tablets and capsules.
Linearity and range
The correlation between peak area and concentration was shown to be linear for MIR and TAM within the concentration ranges of 2.5–55 and 5–110 µg/mL, respectively. The results are presented in Table 2.
LOQ and LOD
The slope of the relative standard calibration curves and standard deviations of the intercept were used to calculate the limits of detection (LOD) and quantitation (LOQ) of both medications (Table 2).
Accuracy and precision
The accuracy of the measurements was effectively determined by calculating the percentage of recovered substance in the three replicates of the three distinct concentrations falling within the linear range for MIR and TAM, as shown in Table 2. The different three concentration levels were 5, 25, and 50 µg/mL, 10, 50, and 100 µg/mL for MIR and TAM, respectively. According to Table 3, the suggested approach is precise. The study aimed to assess the repeatability precision by administering standard solutions at three different concentration levels, three times daily. The process was replicated on three separate days to evaluate the intermediate level of precision. The different three concentration levels for repeatability and intermediate precision were 5, 25, and 50 µg/mL, 10, 50, and 100 µg/mL for MIR and TAM, respectively.
Chromatographic method robustness
In order to demonstrate the durability of the suggested approach, minor modifications were made to the chromatographic components, and the impact on certain chromatographic parameters was noted (Table 4). The findings of the study suggest that the approach exhibits robustness.
Application to pharmaceutical formulations
The application of the suggested research was used for different dosage forms MIR in tablets and TAM in capsules. Regardless, there is a newly published HPTLC technique for determining the binary mixture31. The suggested research exhibits many advantages in various aspects. The proposed research exhibits greater sensitivity for MIR and TAM. Also the analysis conducted on MIR in tablets and TAM in capsules yielded recovery rates of 100.11% and 99.53%, respectively (Table 5).
The statistical comparison between the proposed approach and the published one was carried out using Student’s t-test and F-test. According to Table 6, there was no discernible difference between the two approaches. Additionally, the interval plot test was used. The vertical lines representing confidence intervals on plots imply that the interval mean is located at their midpoint. According to these graphs, the group intervals are not significantly different from one another (Fig. 3). The Boxplot is another useful tool for data visualization since it depicts the distribution of data across multiple categories. It displays boxplots of the proposed methods. The interquartile range is shown by the middle box, which includes a line showing the median, upper lines representing higher values, and whiskers signifying lower values. The boxplot depicts the distribution of data within each group. (Fig. 3)
Assessment of ecological effect and comparison to the previously documented analytical technique
While developing analytical techniques, it became crucial to evaluate their ecological effect to provide a clear and objective evaluation that could be used to compare proposed and reported approaches in the future31. AGREE offers a clock-shaped graph that is split into 12 pieces, representing the twelve fundamentals of Green Analytical Chemistry37,38,39. The suggested and reported techniques31 were evaluated using the AGREE tool. Table 6presents a comprehensive comparison between the suggested methodology and previously published methods that included real data. The two approaches indicate the presence of a red zone (3) on the AGREE scale. A red zone has emerged as a result of the offline sampling and transportation to quality control (QC) labs, which is essential owing to the separation between pharmaceutical manufacturing and QC locations. The suggested LC method demonstrated advantages over the other documented chromatographic methodology in terms of the number of stages, analysis duration, and the usage of significant amounts of organic solvents (Table 6). The proposal introduces a new measure called the Blue Applicability Grade Index (BAGI) to evaluate the practicability of an analytical method40,41,42. When used in conjunction with the well-known green metrics, the BAGI framework shines a light on the practical aspects of White Analytical Chemistry. The color gradient of the pictogram indicates the extent to which the method conforms to the defined criteria. If the method wants to be called “practical,” it has to get 60 or more points Table 6.
Conclusion
A novel and cost-efficient technique was shown for the concurrent HPLC analysis of mirabegron and tamsulosin in pharmaceutical formulations, ensuring accuracy and precision. Moreover, a comparative analysis was conducted between the suggested methodology and previously documented approaches, which revealed no discernible difference between the two approaches. The evaluation of the greenness using the AGREE metric tool included a comparison with the previously published method. The assessment of the Blue Applicability Grade Index methodology further shows that the recently proposed method is practicable. The proposed technique was validated using the rules set by the International Council for Harmonization.
Data availability
All data generated or analysed during this study are included in this published article.
Change history
24 April 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41598-025-98289-1
References
Alexandre, E. C. et al. Mirabegron relaxes urethral smooth muscle by a dual mechanism involving β3-adrenoceptor activation and α1‐adrenoceptor blockade. Br. J. Pharmacol. 173, 415–428 (2016).
Leron, E., Weintraub, A. Y., Mastrolia, S. A. & Schwarzman, P. Overactive bladder syndrome: evaluation and management. Curr. Urol. 11, 117–125 (2018).
Abrams, P., Kelleher, C. J., Kerr, L. A. & Rogers, R. G. Overactive bladder significantly affects quality of life. Am. J. Manag Care. 6, S580–S590 (2000).
Milsom, I. et al. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int. 87, 760–766 (2001).
Stewart, W. F. et al. Prevalence and burden of overactive bladder in the United States. World J. Urol. 20, 327–336 (2003).
Scarneciu, I. et al. Overactive bladder: a review and update. Exp. Ther. Med. 22, 1–8 (2021).
Abrams, P. et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Eur. Urol. 61, 37–49 (2003).
Gratzke, C. et al. EAU guidelines on the assessment of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur. Urol. 67, 1099–1109 (2015).
El Helou, E. et al. The use of mirabegron in neurogenic bladder: a systematic review. World J. Urol. 38, 2435–2442 (2020).
Bahgat, E. A., Saleh, H., Hendawy, H. A. M. & Darwish, I. M. Challenging electroanalytical method for the determination of mirabegron in biological samples and pharmaceutical formulations. Microchem J. 200, 110258 (2024).
Kamel, E. B. Two green chromatographic methods for the quantification of tamsulosin and solifenacin along with four of their impurities. J. Sep. Sci. 45, 1305–1316 (2022).
Kang, T. W. & Chung, H. C. Add-on treatment with mirabegron may improve quality of life in patients with benign prostatic hyperplasia complaining of persistent storage symptoms after tamsulosin monotherapy. Ther. Adv. Urol. 12, 1–7 (2020).
Shin, W., Yang, A. Y., Yoo, H. & Kim, A. Drug–drug interactions between tamsulosin and mirabegron in healthy individuals do not affect pharmacokinetics and hemodynamic parameters significantly. Pharmaceuticals 16, 1457 (2023).
Mishra, S., Surekha, N. & Chauhan, A. Development and validation of stability indicating RP-HPLC method for simultaneous estimation of silodosin and mirabegron in synthetic mixture. in Ann. Pharm. Fr. 82 243–262 (Elsevier, 2024).
Lou, Y. et al. Simultaneous quantification of mirabegron and vibegron in human plasma by HPLC-MS/MS and its application in the clinical determination in patients with tumors associated with overactive bladder. J. Pharm. Biomed. Anal. 240, 115937 (2024).
Kadam, M. M. M., Singh, R. P. & Charde, M. S. Development and validation of stability indicating UHPLC method for the quantitative estimation of mirabegron and solifenacin succinate in pharmaceutical dosage form. J. Pharm. Negat. Results. 6727–6737. https://doi.org/10.47750/pnr.2022.13.S07.815 (2023).
Andhale, G., Shankarpure, M., Kadam, J., Shelar, M. & Singh, S. RP-HPLC method development and validation for the simultaneous estimation of Mirabegron and Solifenacin succinate in pharmaceutical dosage form. NeuroQuantology 20, 2652 (2022).
Rezaei, M. & Ramazani, A. RP-HPLC method development and validation for the quantitative estimation of mirabegron in extended-release tablets. J. Med. Chem. Sci. 1, 36–40 (2018).
Yehia, A. M., Sami, I., Riad, S. M. & El-Saharty, Y. S. Comparison of two stability-indicating chromatographic methods for the determination of mirabegron in presence of its degradation product. Chromatographia 80, 99–107 (2017).
Van Teijlingen, R. et al. Development and validation of LC–MS/MS methods for the determination of mirabegron and its metabolites in human plasma and their application to a clinical pharmacokinetic study. J. Chromatogr. B. 887, 102–111 (2012).
Sangshetti, J. N., Chivte, D. K., Imran, M. & Mahaparale, P. Development of Hplc method for determination of tamsulosin using quality by design (QBD) approach. Eur. Chem. Bull. 8, 409–414 (2019).
Afshari, A., Qomi, M. & R. & A novel application of three phase hollow fiber based liquid phase microextraction (HF-LPME) for the HPLC determination of tamsulosin from biological fluids. Curr. Pharm. Anal. 12, 258–265 (2016).
Ganthi, H. K. R. et al. Stability indicating HPLC method for quantification of solifenacin succinate & tamsulosin hydrochloride along with its impurities in tablet dosage form. Am. J. Anal. Chem. 7, 840–862 (2016).
Nasare, M. K. et al. Simultaneous determination of finasteride and tamsulosin in combined dosage form by using RP-HPLC method. J. Liq Chromatogr. Relat. Technol. 37, 1176–1186 (2014).
Ishaq, B. M., Prakash, K. V. & Mohan, G. K. Simultaneous determination of dutasteride and tamsulosin in pharmaceutical dosage forms by RP-HPLC. in International Conference on Harmonization (ICH) analytical method validation guidelines vol. 15 16 (2014).
Kumar, G. S. & Kumar, B. S. P. Stability-indicating RP-HPLC method for determination of tamsulosin hcl in pharmaceutical dosage form. J. Basic. Clin. Pharm. 3, 255 (2012).
Mhamunkar, S. M., Vyavaharkar, R. Y., Bhoir, S. & I. RP-HPLC method development and validation for the simultaneous estimation of tamsulosin HCl and tolterodine tartrate in pharmaceutical dosage form. Int. J. Pharm. Pharm. Sci. 4, 319–322 (2012).
Choi, C. I. et al. Determination of tamsulosin in human plasma by liquid chromatography/tandem mass spectrometry and its application to a pharmacokinetic study. J. Chromatogr. B. 909, 65–69 (2012).
Thimmaraju, M. K., Rao, V. & Gurrala, S. RP HPLC method for the determination of finasteride and tamsulosin in bulk and pharmaceutical formulations. Der Pharm. Lett. 3, 79–86 (2011).
Kumari, R., Dash, P. P., Lal, V. K., Mishra, A. & Murthy, P. N. RP–HPLC method for the estimation of tamsulosin hydrochloride in tablet dosage form. Indian J. Pharm. Sci. 72, 785 (2010).
Abou El-Alamin, M. M., Toubar, S. S., Mohamed, D. A. & Helmy, M. I. Development of Green HPTLC method for simultaneous determination of a promising combination Tamsulosin and Mirabegron: stability-indicating assay was examined. BMC Chem. 17, 1–18 (2023).
El-Sayed, H. M., Abdellatef, H. E., Hendawy, H. A. M., El-Abassy, O. M. & Ibrahim, H. DoE-enhanced development and validation of eco-friendly RP-HPLC method for analysis of safinamide and its precursor impurity: QbD approach. Microchem J. 190, 108730 (2023).
Hafez, H. M. et al. Micellar Organic-solvent free HPLC design of experiment for the determination of Ertapenem and meropenem; assessment using GAPI, AGREE and analytical eco-scale models. Microchem J. 185, 108262 (2023).
Pena-Pereira, F., Wojnowski, W. & Tobiszewski, M. AGREE - Analytical GREEnness metric approach and software. Anal. Chem. 92, 10076–10082 (2020).
Manousi, N., Wojnowski, W., Płotka-Wasylka, J. & Samanidou, V. Blue applicability grade index (BAGI) and software: a new tool for the evaluation of method practicality. Green. Chem. 25, 7598–7604 (2023).
ICH Q2 (R2). Validation of Analytical Procedures: Text and Methodology (November, 2023).
Magdy, G., Aboelkassim, E., El-Domany, R. A. & Belal, F. Ultrafast one-pot microwave-assisted green synthesis of silver nanoparticles from Piper longum fruit extract as a sensitive fluorescent nanoprobe for carbamazepine and risperidone in dosage forms and human plasma. Microchem J. 197, 109755 (2024).
El-Sayed, H. M. et al. Safinamide detection based on prussian blue analogue modified solid-contact potentiometric sensor. Microchem J. 191, 108829 (2023).
Alossaimi, M. A., Altamimi, A. S. A., Elmansi, H. & Magdy, G. A green sustainable ultrasensitive micelle-sensitized spectrofluorimetric approach for the estimation of tafamidis in different matrices at picogram levels. Sustain. Chem. Pharm. 36, 101321 (2023).
El-Abassy, O. M., Maged, K., El-Henawee, M. M. & Abd El-Hay, S. S. Development of eco-friendly spectrophotometric methods for analysis of metformin hydrochloride and linagliptin in presence of metformin toxic impurity in their pure and dosage forms: validation, practicality and greenness studies. Spectrochim Acta Part. Mol. Biomol. Spectrosc. 309, 123844 (2024).
Halim, M. K., Badran, O. M. & Abbas, A. E. F. Sustainable chemometric methods boosted by latin hypercube technique for quantifying the recently FDA-approved combination of bupivacaine and meloxicam in the presence of bupivacaine carcinogenic impurity: Comprehensive greenness, blueness, and whiteness. Microchem J. 110276 https://doi.org/10.1016/j.microc.2024.110276 (2024).
Ntorkou, M., Kabir, A., Furton, K. G., Tzanavaras, P. D. & Zacharis, C. K. Sol-gel Carbowax 20 M-zwitterionic ionic liquid composite sorbent-based capsule phase microextraction device combined with HPLC/post-column derivatization for the determination of lanreotide, a human somatostatin analogue in urine. J. Chromatogr. A. 1717, 464674 (2024).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Eman A. Bahgat: Writing – review & editing, Writing – original draft, Validation, Supervision, Software, Resources, Investigation, Formal analysis, Data curation, Conceptualization. Hanaa Saleh: Writing – review & editing, Writing – original draft, Supervision, Methodology, Conceptualization. Islam M. Darwish: Writing – original Software, Validation, Formal analysis, Investigation, Resources, Writing – original draft. Omar M El-Abassy: Writing – review & editing, Supervision, Project administration, Methodology, Investigation, Data curation, Conceptualization.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: In the original version of this Article authors Islam M. Darwish and Omar M El-Abassy were incorrectly affiliated. The correct affiliations are listed in the correction notice.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bahgat, E.A., Saleh, H., Darwish, I.M. et al. Green HPLC technique development for the simultaneous determination of the potential combination of Mirabegron and Tamsulosin. Sci Rep 15, 7005 (2025). https://doi.org/10.1038/s41598-025-89216-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-89216-5
Keywords
This article is cited by
-
Eco-friendly spectrophotometric quantification of the potential combination of mirabegron and tamsulosin
Scientific Reports (2025)
-
Development and validation of a green/blue UHPLC-MS/MS method for trace pharmaceutical monitoring
Scientific Reports (2025)