Abstract
Heavy metal ions pollution in environmental waters has an increasing impact on human health. As two common metal ions, copper ions (Cu2+) and ferric ions (Fe3+) widely exist in nature and play a vital role in life process. Therefore, it is significant to design sensitive and simple detection approaches for Cu2+ and Fe3+. In our work, the ratiometric fluorescence analysis method (denoted as N-CDs/OPD) was established for Cu2+ and Fe3+ detection. The N-CDs exhibited a Cu2+ and Fe3+ fluorescence quenching response properties. The o-phenylenediamine (OPD) may be oxidized to 2,3-diaminophenazine (DAP) by Cu2+ and Fe3+. With addition of Cu2+ or Fe3+, the fluorescence of N-CDs (436 nm) was quenched and a new peak at 556 nm (DAP) appeared, which realized fluorescent ratiometric detection of Cu2+ and Fe3+. The Cu2+ concentration shows a good linear correlation versus fluorescence ratio (F436/F556) in the range of 10 to 30 µM (R2 = 0.9981) with detection limit (LOD) of 0.86 µM. In addition, a good linear relationship between fluorescence ratio (F436/F556) and Fe3+ concentration in the range of 20 to 80 µM (R2 = 0.9880) with LOD of 7.12 µM. This nanoprobe realizes the detection of authentic samples successfully, which is expected to serve as a testing kit for analysis in water samples.
Similar content being viewed by others
Introduction
With the development of modern industry, heavy metal ions pollution in environmental waters has an increasing impact on animals, plants and human health. As two common metal ions, copper ions (Cu2+) and ferric ions (Fe3+) widely exist in nature and play a significant role in life process1,2,3. The formation of intracellular oxidoreductase in the body requires sufficient amounts of copper4. However, excessive Cu2+ intake has been linked to several physical disorders, including Wilson’s disease and Alzheimer’s disease5,6. The irreversible liver and kidney damage may be caused by long-term accumulation of copper7. Unfortunately, due to widespread use of Cu2+ in industrial production and daily life, which has been enrolled in the EPA’s pollutant list of toxic metal species8. Fe3+ plays critical roles in cellular metabolism, electron transfer, enzyme catalysis, oxygen hemoglobin production9. Both of its deficiency or excess can cause Huntington10 or Parkinson’s disease11. Therefore, analysis of heavy metal ions, especially Cu2+ and Fe3+, is of great significance for environmental protection and clinical research.
Different analytical methods have been proposed for heavy metal ions detection, including atomic absorption spectrometry12, electrochemical analysis13, inductively coupled plasma mass spectroscopy14, colorimetry15 and fluorometry16,17. Among those ways, fluorescent probes show benefits of simplicity, high sensitivity, low cost and background signals18,19. Traditional fluorescence methods are based on intensity of individual emission peak to quantitatively analyze ions, which is susceptible to environmental interferences and results in inaccurate detection20,21,22. To ensure the accurate detection, ratiometric fluorescence probes have self-calibration capabilities. Two emission bands can act as internal reference, removing a majority of interfering factors, including probe concentration, environmental conditions photobleaching and instrumental efficiency23,24. However, the largest number of ratiometric fluorescent probes usually focus on detecting a single heavy metal ion specifically. The increased and complex detection requirements of multiple heavy metal ions are urgently needed.
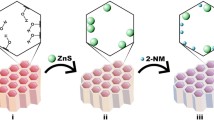
Among fluorescent probes, various fluorescent nanomaterials attract extensive interest, such as metal nanoclusters, quantum dots (QDs) and carbon dots (CDs), etc25. The CDs are new carbon-based nanomaterials, the size of which less than 10 nm with spherical particles26. Compared with QDs, CDs have better performance, including low toxicity, low cost, simple synthesis, photo stability, adjustable optical properties, good biocompatibility and water solubility27. Herein, we innovatively developed a N-doped carbon dots (N-CDs)-based ratiometric fluorescent nanoprobe (N-CDs/OPD) for detection of Cu2+ and Fe3+ in aqueous solution (Fig. 1). The N-CDs were synthesized via one-step solvothermal treatment of methionine and formamide. Due to inter filter effect (IFE) between metal ion and carbon dots, the N-CDs exhibited a Cu2+ and Fe3+ fluorescence quenching response properties. After Cu2+ and Fe3+ mixed with this probe, the fluorescence of N-CDs (436 nm) was quenched and a new peak at 556 nm (DAP) appeared. Cu2+/Fe3+ would quench CDs’ fluorescence and oxidize o-phenylenediamine (OPD) to 2,3-diaminophenazine (DAP). This strategy realized the fluorescence ratiometric detection of Cu2+ and Fe3+ in authentic samples successfully, which broadens the potential application of carbon dots.
Experimental section
Materials and apparatus
o-phenylenediamine (OPD), methionine and formamide and were obtained from Shanghai Aladdin Biochemical Techonology Co., Ltd. Phosphoric acid (H3PO4) and acetic acid (HAc) were purchased from Sinopharm Chemical Reagent Co. Cu2+ standard solution, Fe3+ standard solution, boric acid (H3BO3), NaOH, cobalt chloride hexahydrate (CoCl2·6H2O), ferrous sulfate heptahydrate (FeSO4·7H2O), nickel (II) sulfate hexahydrate (NiSO4·6H2O), barium chloride dihydrate (BaCl2·2H2O), chromium (III) nitrate nonahydrate (Cr(NO3)3·2H2O), KCl, CaCl2, NaCl, MgCl2, MnCl2 and ZnCl2 were acquired from Shanghai Macklin Biochemical Technology Co., Ltd. The section of “apparatus” was in “supplementary information”.
Synthesis of N-doped carbon dots
N-doped carbon dots (N-CDs) were synthesized by solvothermal synthesis approach with precursors of methionine and formamide. 0.2093 g of methionine was dissolved in 12.5 mL of formamide with ultrasound for 30 min. This mixed solution was added into a Teflon-lined stainless steel autoclave (50 mL) for 1 h with 180 °C. After the system cooling to room temperature, the light brown supernatant was filtered by filter membrane of 0.22 μm to remove large particles. Then, the solution was purified with dialysis bag (molecular weight cut-off of 1000 Da) for 8 h. And finally, the purified product stored in a refrigerator at 4 °C for further use.
Fluorescent detection of Cu2+ and Fe3+
The stock solution concentrations of Cu2+ and Fe3+ standard solution was 10 mM, respectively. 150 µL of Britton-Robinson buffer [pH 5.3(Cu2+) 5.8(Fe3+), 40 mM], ultrapure water, 150 µL of o-phenylenediamine solution (1 mM), various concentrations of Cu2+/Fe3+ solution were blended and diluted to 1.4 mL. After 60 min/10 min still standing at room temperature, 100 µL of N-doped carbon dots was added to above system. Fluorescence measurements were carried out under the excitation wavelength at 360 nm. It was used as the ordinate that the ratio of fluorescence intensity at 436 nm and 556 nm wavelengths (F436/F556). Concentrations of Cu2+/Fe3+ were regarded as horizontal ordinate and drawing standard curve for Cu2+ and Fe3+ detection.
In order to realize the Cu2+ and Fe3+ detection in tap water, samples were spiked with various concentrations of Cu2+ (20 µM, 30 µM) and Fe3+ (40 µM, 60 µM). After that, the samples were measured by this probe using the same experimental procedures as above. These fluorescent spectra were measured and concentrations of spiked samples were calculated by corresponding standard curve.
Selectivity of the proposed nanoprobe
150 µL of Britton-Robison buffer (pH 5.3, 40 mM), 150 µL of o-phenylenediamine solution (1 mM), the same concentration of Cu2+/Fe3+ standard solution, or pure water sample as blank sample, various metal interference ions (K+, Na+, Ca2+, Mg2+, Ba2+, Zn2+, Co2+, Mn2+, Fe2+, Ni2+ and Cr3+) mixed well and diluted to 1.4 mL. 100 µL of N-CDs were added to the above mixture after standing for 60 min. These fluorescent spectra were collected under 360 nm excitation wavelength.
Results and discussion
Characterization and optical properties of N-doped carbon dots
The N-doped carbon dots (N-CDs) were synthesized by solvothermal method with a simple procedure. As depicted in Fig. 2A, the TEM image indicates N-CDs have a monodisperse spherical structure with average diameter of 2 nm. In order to certify element components of N-CDs, XPS measurement was performed. The XPS full spectrum of N-CDs reflects five characteristic peaks, which represent C 1s, N 1s, O 1s, S 2s and S 2p, respectively (Fig. 2B). Atomic percentages of C, N, O and S were 52.23%, 8.06%, 31.01% and 8.71% by calculation, which showing the major components of N-CDs and doped by S and N. The high-resolution XPS spectra of N-CDs (C 1s, N 1s, O 1s and S 2p) are displayed in Fig. 2C–F. As shown in Fig. 2C, the high-resolution XPS spectrum of C 1s was deconvolved into two characteristic peaks, which were attributed to C = O (288.5 eV) and C-O/C = C (285.6 eV) bonds. High-resolution spectra of N 1s (Fig. 2D) displayed three kinds of N: pyrrole N (401.1 eV), amino N (400.3 eV) and pyridine N (399.3 eV)28,29. The high-resolution XPS spectra of O 1s (Fig. 2E) locates peaks at 532.1 eV and 533.0 eV, which associate with C-O and C = O bonds. As demonstrated in Fig. 1F, the S 2p spectrum illustrates two major peaks of 164.8 eV and 163.7 eV, which correspond to S 2p1/2 and S 2p3/2 bonding30.
The FT-IR spectrum also shows the surface functional groups of N-CDs (Fig. 3A). A broad absorption bands at 3200–3600 cm− 1 is attributed to stretching vibrations of O–H/N–H31. The peak located around 2876 cm− 1 and 1301 cm− 1 are regarded as stretching vibration of C–H32,33. Moreover, these specific peaks located at 1049 cm− 1, 1387 cm− 1, 1602 cm− 1 and 1665 cm− 1 represent the stretching vibration of C–O, C–N, C = C and C = O groups, respectively29,34,35,36. These results demonstrate the surface of carbon dots is decorated by oxygen containing and amino groups, and CDs doping with N are in accordance with FT-IR data. As demonstrated in Fig. 3B, these fluorescence emission spectra of N-CDs with different excitation wavelengths were measured. The emission peaks of N-CDs exhibit a slight red shift from 388 nm to 444 nm with the excitation wavelength from 310 to 370 nm, indicating the excitation-dependent emission of N-CDs. The property of excitation-dependence can be caused by surface heterogeneity of functional groups37.
(A) FT-IR spectrum of N-CDs, (B) Fluorescence emission spectra of N-CDs with different excitation wavelengths in range of 310 nm to 370 nm. (C) Fluorescence excitation (a) and emission (b) spectra of N-CDs, UV-vis spectra of Cu2+(c) and Fe3+(d). (D) Fluorescence emission spectra of N-CDs mixed with different metal ions (from top to bottom: Zn2+, Mn2+, Fe2+, Mg2+, Na+, Ca2+, K+, blank, Co2+, Fe3+, Cu2+).
When N-CDs were excited at 360 nm, a maximum fluorescence emission intensity was acquired at 436 nm (Fig. 3C). As depicted in inset photograph of Fig. 3C, the as-prepared N-CDs appear pale brown color under daylight and emit bright blue fluorescence with 365 nm UV light. The N-CDs may be acted as a fluorescence probe for Cu2+ and Fe3+ detection due to their optical property. To examine the selectivity of N-CDs for Cu2+ and Fe3+, different interfering metal ions were measured with N-CDs (Fig. 3D). The fluorescent intensity of N-CDs with relevant metal ions had little changes, while fluorescence quenching occurred with Cu2+ and Fe3+. These comparison data of fluorescence spectra demonstrate the excellent specificity of N-CDs.
Feasibility analysis and condition optimization
To testify the feasibility of this probe, the sensing mechanism need to be studied. The as-prepared N-CDs can be exploited as a probe to detect cupric ion and ferric ion. We speculated that the fluorescence quenching mechanism of N-CDs was static quenching, which can be strongly determined by fluorescence lifetime measurement. Static quenching usually does not change the fluorescence lifetime38. As demonstrated in Fig. 4A, compared with lifetime of N-CDs in the presence of Cu2+ and Fe3+, the three lifetime curves almost overlap, which illustrates the static quenching effect. In addition, the absorption peak of Cu2+ and Fe3+ (Fig. 3C) and excitation peak of N-CDs overlap significantly. Based on the almost unaffected lifetime curve of N-CDs by metal ion, it is certified that the existence of inter filter effect (IFE) between metal ion and carbon dots.
(A) Fluorescence lifetime curves of N-CDs in absence and presence of Cu2+ and Fe3+. (B) Fluorescence spectra of a: N-CDs, b: N-CDs + Cu2+, c: N-CDs + OPD + Cu2+, d: N-CDs + Fe3+, e: N-CDs + OPD + Fe3+. (C) UV–Vis absorption spectra of a: N-CDs, b: N-CDs + Cu2+, c: N-CDs + OPD + Cu2+, d: N-CDs + Fe3+, e: N-CDs + OPD + Fe3+.
To realize the ratiometric fluorescent detection of Cu2+ and Fe3+, we investigated the response mechanisms by the UV-vis absorption and fluorescence spectra. As shown in Fig. 4B, after Cu2+ and Fe3+ mixed with N-CDs/OPD system, the fluorescence of N-CDs (436 nm) was quenched and a new peak at 556 nm (DAP) appeared. Because Cu2+/Fe3+ would quench the fluorescence of N-CDs and oxidize OPD to DAP. In Fig. 4C, N-CDs exhibits an obvious UV-vis absorption peak at 319 nm. The N-CDs/OPD appeared new broad absorption peaks attributed to DAP in the presence of Cu2+ and Fe3+, which overlaps significantly with the excitation spectrum of N-CDs. These phenomena may be relative to Cu2+/Fe3+ react with OPD, causing the generation of new substance. These results can explain the further fluorescence quenching occurs for N-CDs induced by Cu2+ and Fe3+ after the addition of OPD in Fig. 4B. In terms of theoretical mechanisms, Cu2+ and Fe3+ both have a strong oxidation in acidic conditions, which could oxidize OPD to DAP in this sensing system. The relevant reaction process is shown in Fig. 1. Cu2+ oxidized OPD to DAP and it was reduced to Cu+/ Cu0. Fe3+ oxidized OPD to DAP and it was reduced to Fe2+.
Optimized experimental conditions (pH values, concentration of OPD and reaction time) were employed to obtain optimal response for Cu2+ and Fe3+ detection. As illustrated in Fig. 5A and C, as pH values increase, F436/F556 (the fluorescent peak of N-CDs at 436 nm and DAP at 556 nm) reduces and attains a stable condition. Therefore, pH 5.3 and pH 5.8 are selected as proper pH values for Cu2+ and Fe3+ detection respectively. To investigate whether pH has an effect on the fluorescence of N-CDs, the fluorescence response of N-CDs under different time with pH 7.0 and pH 5.3 were measured (Fig. S1). The N-CDs have an excellent fluorescent stability in weak acidic and neutral environment. Meanwhile, the OPD concentrations of Cu2+ and Fe3+ measurement have similar change trend (Figs. S2, S3). Thus, 100 µM of OPD was chosen for next test. Furthermore, the reaction time of Cu2+ and Fe3+ are shown in Fig. 5B and D. These values of F436/F556 for Cu2+ and Fe3+ remain steady after 60 min and 10 min, respectively. Hence, 60 min and 10 min are selected as optimal reaction time for Cu2+ and Fe3+ detection.
Fluorescent ratiometric detection of copper ions and ferric ions
The analytical performance of this probe for Cu2+ and Fe3+ was evaluated under optimized experimental conditions. As depicted in Fig. 6A, keeping the concentrations of N-CDs and OPD constant, the fluorescence emission peak at 436 nm (N-CDs) reduces whereas 556 nm (DAP) increases with enhancive amount of Cu2+ (0–100 µM). The dependence of Cu2+ concentration and fluorescence ratio (F436/F556) is shown in Fig. S4. When the Cu2+concentration enhanced, F436/F556 declined and achieved equilibrium. As displayed in Fig. 6B, the nice linear correlation is obtained between F436/F556 and Cu2+ concentration in range of 10 to 30 µM with R2 = 0.9981. The limit of detection (LOD) was determined by 3Sd/K, where Sd represents standard deviation of blank samples and K represents slope of calibration line. The limit detection of Cu2+ was calculated to be 0.86 µM. Similarly, as shown in Fig. 6C, the fluorescent ratiometric response of this probe for Fe3+ shows same variation trend with rising Fe3+ concentrations (0–120 µM) with optimal experimental conditions. A steady reduction in fluorescence ratio is recorded with Fe3+ concentration increasing (Fig. S5). In Fig. 6D, a good linear relationship (R2 = 0.9880) is obtained between fluorescence ratio (F436/F556) and Fe3+ concentration in range of 20 to 80 µM with LOD = 7.12 µM.
(A) Changes in fluorescence intensity of nanoprobe (N-CDs/OPD) in different concentrations (0, 10, 15, 20, 25, 30, 40, 70 and 100 µM) of Cu2+. (B) The linear plot of F436/F556 against Cu2+ concentration (10, 15, 20, 25 and 30 µM). (C) Changes in fluorescence intensity of nanoprobe (N-CDs/OPD) in different concentrations (0, 10, 20, 40, 50, 60, 80, 100 and 120 µM) of Fe3+. (D) The linear plot of F436/F556 against concentration of Fe3+ (20, 40, 50, 60 and 80 µM).
In Fig. 7, to access the selectivity of Cu2+ and Fe3+ detection, we performed fluorescent response of N-CDs/OPD toward various potentially metal ions, including K+, Na+, Ca2+, Mg2+, Ba2+, Zn2+, Co2+, Mn2+, Fe2+, Ni2+ and Cr3+. Figure 6 shows that only Cu2+ and Fe3+ could lead to significant increase of fluorescence intensity ratio and the influence of other metal ions is negligible. To examine the precision and accuracy of this nanoprobe, tap water samples were spiked with Cu2+ and Fe3+ ions and analyzed with this method in triple replicates with same conditions. These relative standard deviations (RSD) and recoveries results for the spiked samples are listed in Table 1. These results indicate a good consistency between added and found concentrations of Cu2+ and Fe3+. These recoveries in tab water samples are between 98.2% and 109.3%, and the RSD values are less than 1.40%. In order to strengthen scientific significance of this probe, standard addition detection of Cu2+ and Fe3+ in domestic wastewater were performed in Table S1, which acquired relatively good results. Therefore, these results demonstrate the probe has a good accuracy and precision for the analysis of tracking Fe3+ and Cu2+ in real water. Compared with other analytical methods in Table S2, the detection performance of current method was superior or comparable to previously reported probes for Cu2+ and Fe3+ detection. The above results indicate that the simple and sensitive fluorescent ratiometric system has potential for monitoring the level of copper ions and ferric ions in water.
Conclusion
In conclusion, a cost-effective N-doped carbon dots-based ratiometric fluorescent nanoprobe (N-CDs/OPD) is designed for the detection of copper ions and ferric ions. The OPD could be oxidized to DAP by Cu2+ and Fe3+. The N-CDs have fluorescence quenching response characteristics of Cu2+ and Fe3+ because of inner filter effect. After addition of Cu2+ and Fe3+, the fluorescence of N-CDs (436 nm) is quenched and a new peak at 556 nm (DAP) appears, which realizes fluorescence ratiometric change. Therefore, this strategy enables the fluorescence ratiometric determination of Cu2+ and Fe3+ in authentic samples successfully, which has potential application for point-of-care testing.
Data availability
The datasets used and analysed during the current study available from the corresponding author, Y.C. on reasonable request.
References
Liu, W. et al. Efficient and selective sensing of Cu2+ and UO22+ by a europium metal-organic framework. Talanta 196, 515–522. https://doi.org/10.1016/j.talanta.2018.12.088 (2019).
Mi, X. et al. Tunable light emission and multiresponsive luminescent sensitivities in aqueous solutions of two series of lanthanide metal-organic frameworks based on structurally related ligands. ACS Appl. Mater. Interface. 11, 7914–7926. https://doi.org/10.1021/acsami.8b18320 (2019).
Yu, Y. E. et al. Multiresponsive luminescent sensitivities of a 3D Cd-CP with visual turn-on and ratiometric sensing toward Al3+ and Cr3+ as well as turn-off sensing toward Fe3+. Inorg. Chem. 59, 3828–3837. https://doi.org/10.1021/acs.inorgchem.9b03496 (2020).
Sakaguchi, T., Okunaga, R., Irie, S., Urushisaki, M. & Hashimoto, T. Carbon dioxide-permselective polymer membranes composed of poly(vinyl ether)-based, ABA-type triblock copolymers with pendant oxyethylene chains. Polym. Bull. 74, 2017–2031. https://doi.org/10.1007/s00289-016-1820-2 (2017).
Patel, R. & Aschner, M. Commonalities between copper neurotoxicity and Alzheimer’s disease. Toxics 9, 1 (2021).
Russell, K., Gillanders, L. K., Orr, D. W. & Plank, L. D. Dietary copper restriction in Wilson’s disease. Eur. J. Clin. Nutr. 72, 326–331. https://doi.org/10.1038/s41430-017-0002-0 (2018).
Bost, M. et al. Dietary copper and human health: current evidence and unresolved issues. J. Trace Elem. Med. Biol. 35, 107–115. https://doi.org/10.1016/j.jtemb.2016.02.006 (2016).
Kleyer, H., Tecon, R. & Or, D. Resolving species level changes in a representative soil bacterial community using microfluidic quantitative PCR. Front. Microbiol. 8, 17. https://doi.org/10.3389/fmicb.2017.02017 (2017).
Li, S. et al. A novel solvent-dependently bifunctional NIR absorptive and fluorescent ratiometric probe for detecting Fe3+/Cu2+ and its application in bioimaging. Sens. Actuators B Chem. 224, 661–667. https://doi.org/10.1016/j.snb.2015.10.086 (2016).
Crabtree, R. H. Principles of bioinorganic chemistry. Science 266, 1591 (1994).
Burdo, J. R. & Connor, J. R. Brain iron uptake and homeostatic mechanisms: An overview. BioMetals 16, 63–75. https://doi.org/10.1023/A:1020718718550 (2003).
Pourjavid, M. R., Arabieh, M., Yousefi, S. R. & Akbari Sehat, A. Interference free and fast determination of manganese(II), iron(III) and copper(II) ions in different real samples by flame atomic absorption spectroscopy after column graphene oxide-based solid phase extraction. Microchem J. 129, 259–267. https://doi.org/10.1016/j.microc.2016.07.008 (2016).
Xu, W., Zhu, L., Shao, X., Huang, K. & Luo, Y. An electrochemical biosensor based on nucleic acids enzyme and nanochannels for detecting copper (II) ion. Biosens. Bioelectron. 120, 168–174. https://doi.org/10.1016/j.bios.2018.08.033 (2018).
Arslan, Z., Oymak, T. & White, J. Triethylamine-assisted mg(OH)2 coprecipitation/preconcentration for determination of trace metals and rare earth elements in seawater by inductively coupled plasma mass spectrometry (ICP-MS). Anal. Chim. Acta. 1008, 18–28. https://doi.org/10.1016/j.aca.2018.01.017 (2018).
Zhang, Y., Ren, T., Tian, H., Jin, B. & He, J. Hydrogel-encapsulated enzyme facilitates colorimetric acute toxicity assessment of heavy metal ions. ACS Appl. Mater. Interface. 10, 26705–26712. https://doi.org/10.1021/acsami.8b08949 (2018).
Geng, R. et al. Bimetallic Ag/Zn-ZIF-8: an efficient and sensitive probe for Fe3+ and Cu2+ detection. Colloids Surf. Physicochem Eng. Aspects. 632. https://doi.org/10.1016/j.colsurfa.2021.127755 (2022).
Kaushik, R. et al. Multianalytes sensing probe: fluorescent moisture detection, smartphone assisted colorimetric phosgene recognition and colorimetric discrimination of Cu2+ and Fe3+ ions. Sens. Actuators B Chem. 328. https://doi.org/10.1016/j.snb.2020.129026 (2021).
Mahata, P., Mondal, S. K., Singha, D. K. & Majee, P. Luminescent rare-earth-based MOFs as optical sensors. Dalton Trans. 46, 301–328. https://doi.org/10.1039/C6DT03419E (2017).
Zhao, S. N., Wang, G., Poelman, D. & Voort, P. V. Luminescent lanthanide MOFs: a unique platform for chemical sensing. Materials 11, 1 (2018).
Li, N. N. et al. A novel dimer-induced AIE material as a nano-sensor for colormetric and ratiometric sensing of Erythromycin and metal ions (Zn2+, Cd2+ and Cu2+) with different dissociation and re-aggregation processes and cellular imaging applications. Dyes Pigm. 184, 108872. https://doi.org/10.1016/j.dyepig.2020.108872 (2021).
Bhati, A. et al. Self-doped nontoxic red-emitting Mg–N-embedded carbon dots for imaging, Cu(ii) sensing and fluorescent ink. New. J. Chem. 42, 19548–19556. https://doi.org/10.1039/C8NJ04754E (2018).
Wang, C., Bi, X., Wang, M., Zhao, X. & Lin, Y. Dual-channel online optical detection platform integrated with a visible light absorption approach for continuous and simultaneous in vivo monitoring of ascorbic acid and copper(II) ions in a living rat brain. Anal. Chem. 91, 16010–16016. https://doi.org/10.1021/acs.analchem.9b04783 (2019).
Chuong, T. T. et al. Dual-reporter SERS-based biomolecular assay with reduced false-positive signals. PNAS 114, 9056–9061. https://doi.org/10.1073/pnas.1700317114 (2017).
Qiu, Z., Shu, J., Liu, J. & Tang, D. Dual-channel photoelectrochemical ratiometric aptasensor with up-converting nanocrystals using spatial-resolved technique on homemade 3D printed device. Anal. Chem. 91, 1260–1268. https://doi.org/10.1021/acs.analchem.8b05455 (2019).
Wang, Y. F. et al. One-pot synthesis of boron and nitrogen co-doped silicon-carbon dots for fluorescence enhancement and on-site colorimetric detection of dopamine with high selectivity. Appl. Surf. Sci. 573, 151457. https://doi.org/10.1016/j.apsusc.2021.151457 (2022).
Li, D. et al. Supra-(carbon nanodots) with a strong visible to near-infrared absorption band and efficient photothermal conversion. Light Sci. Appl. 5, e16120. https://doi.org/10.1038/lsa.2016.120 (2016).
Yue, J. et al. One-step synthesis of acriflavine-based carbon dots for adenine detection and a theoretical study on the detection mechanism. Microchem J. 148, 73–78. https://doi.org/10.1016/j.microc.2019.04.041 (2019).
Wang, X. et al. Bridging environmental and biological monitoring: constructing platform for hexavalent chromium detection and cancer-cells screening based on red fluorescent carbonized polymer dots. Chem. Eng. J. 451, 138524. https://doi.org/10.1016/j.cej.2022.138524 (2023).
Qin, J. et al. Carbon nanodot-based humidity sensor for self-powered respiratory monitoring. Nano Energy. 101, 107549. https://doi.org/10.1016/j.nanoen.2022.107549 (2022).
Wang, L. et al. Mesoporous nitrogen, sulfur co-doped carbon dots/CoS hybrid as an efficient electrocatalyst for hydrogen evolution. J. Mater. Chem. A. 5, 2717–2723. https://doi.org/10.1039/C6TA09580A (2017).
Tang, M., Ren, G. & Chai, F. A facile synthesis of magnetic fluorescence Fe3O4-carbon dots for the detection and removal of Hg2+. New. J. Chem. 44, 6635–6642. https://doi.org/10.1039/D0NJ00275E (2020).
Mondal, S., Vinod, C. P. & Gautam, U. K. Autophagy’ and unique aerial oxygen harvesting properties exhibited by highly photocatalytic carbon quantum dots. Carbon 181, 16–27. https://doi.org/10.1016/j.carbon.2021.04.054 (2021).
Tao, Y., Ju, E., Ren, J. & Qu, X. Polypyrrole nanoparticles as promising enzyme mimics for sensitive hydrogen peroxide detection. Chem. Commun. 50, 3030–3032. https://doi.org/10.1039/C4CC00328D (2014).
Yan, F. et al. Color emission carbon dots with quench-resixastant solid-state fluorescence for light-emitting diodes. ACS Sustain. Chem. Eng. 9, 3901–3908. https://doi.org/10.1021/acssuschemeng.0c09133 (2021).
Wang, J. et al. Tunable full-color solid-state fluorescent carbon dots for light emitting diodes. Carbon 190, 22–31. https://doi.org/10.1016/j.carbon.2022.01.001 (2022).
Tang, X. et al. Nitrogen-doped fluorescence carbon dots as multi-mechanism detection for iodide and curcumin in biological and food samples. Bioact Mater. 6, 1541–1554. https://doi.org/10.1016/j.bioactmat.2020.11.006 (2021).
Tang, X. et al. Exploration of nitrogen-doped grape peels carbon dots for baicalin detection. Mater. Today Phys. 22. https://doi.org/10.1016/j.mtphys.2021.100576 (2022).
Sun, P. et al. The fabrication of N-doped carbon dots by methionine and their utility in sensing Cu2+ in real water. Anal. Methods. 15, 1631–1638. https://doi.org/10.1039/D3AY00056G (2023).
Acknowledgements
This research was financially supported by Natural Science Foundation of Shandong Province (No. ZR2023QC284), Agricultural Science and Technology Innovation Project of Shandong Academy of Agricultural Sciences (No. CXGC2024F09).
Author information
Authors and Affiliations
Contributions
Yang Chunlei: Conceptualization, Methodology, Formal analysis, Investigation, Writing - Original Draft, Funding acquisition. Xu Guiju: Validation, Writing - Review & Editing. Hou Chenghao: Supervision. Zhang Hongwei: Project administration.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, C., Xu, G., Hou, C. et al. Ratiometric fluorescence nanoprobe based on nitrogen-doped carbon dots for Cu2+ and Fe3+ detection. Sci Rep 15, 6261 (2025). https://doi.org/10.1038/s41598-025-89327-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-89327-z