Abstract
Research the expression of USP4 in lung adenocarcinoma and its correlation with clinicopathological features and prognosis analysis, to explore the invasion and metastasis mechanism of USP4 in lung adenocarcinoma, and to clarify the mechanism of USP4’s involvement in the occurrence and development of lung adenocarcinoma. The expressions of USP4, VEGF, MMP2 and Ki67 in lung adenocarcinoma and adjacent tissues of 139 patients with lung adenocarcinoma were detected by immunohistochemical method, and the correlation between expression and clinicopathological features and survival curve were analyzed by statistical method. The expression of USP4 was interfered by LIP-2000 cell transfection technology, and the expression of USP4 and its related factors in protein level was detected by Western Blot, and their correlation was analyzed. After silencing USP4 expression, the effects of USP4 on proliferation, invasion and migration of lung adenocarcinoma cells were detected by cell scratches assay, MTT assay, Transwell assay and tumorigenesis assay in nude mice. The expression of USP4 in lung adenocarcinoma tissues was higher than that in normal adjacent tissues, and the high expression of USP4 was significantly correlated with the differentiation degree of lung adenocarcinoma, clinical stage and pathological grade lymph node metastasis. After silencing USP4 expression, the expression of cyclin apoptosis protein invasion related proteins and phosphorylation factors were affected, and then cell migration and the proliferation ability decreased, the number of invasion and metastasis decreased, and the tumor volume decreased in nude mice. USP4 may play a certain role in the invasion and metastasis of lung adenocarcinoma by regulating the expression of tumor-related factors and affecting the prognosis of patients with lung adenocarcinoma. USP4 can be used as a potential therapeutic target for clinical diagnosis of lung adenocarcinoma and provide a new opportunity for clinical research on lung adenocarcinoma.
Similar content being viewed by others
Introduction
Lung cancer is one of the most common malignant tumors worldwide, and it is the malignant tumor that causes the highest morbidity and mortality in the world. Lung adenocarcinoma is the major histopathological type of lung cancer1. Despite continuous improvements in medical technology and the development of immunotherapy in recent years, the survival rate for patients with lung adenocarcinoma is still very low. Therefore, it is important to further study the pathogenic mechanism of lung adenocarcinoma to find new therapeutic targets.
Ubiquitination and deubiquitination are highly dynamic processes that regulate protein stability and degradation, and their abnormal regulation is closely related to the occurrence and development of cancer2,3. Ubiquitination enzymes add ubiquitin to substrate proteins, and ubiquitinated target proteins are transported to proteasomes for degradation; in contrast, deubiquitinating enzymes deubiquitinate ubiquitinated substrate proteins, thus blocking protein degradation4. Ubiquitin-specific protease (USP) is the most diverse subfamily of deubiquitinating enzymes. Currently, a total of 56 USP family member have been identified, including USP4, USP7, USP11 and USP225,6,7,8. USP22 is a tumor stem cell marker that plays an important role in tumor genesis, development and cell cycle progression. It is highly expressed in colorectal cancer, hepatocellular carcinoma and oral cancer, and is considered as an indicator of poor prognosis by activating signaling pathways to promote tumor cell proliferation, invasion and metastasis through changing cell cycle activity USP11 mediates cell proliferation and participates in cell signal transfer pathway. Studies have shown that USP11 can both inhibit tumor (glioma) and promote tumor (breast cancer). USP11 is highly expressed in non-small cell lung cancer and promotes cell proliferation. USP4 is an important deubiquitination enzyme in the family of ubiquitin specific modification enzymes (UBPs), which changes its characteristics by recognizing specific target proteins and making them deubiquitinated, and plays an important role in regulating related proteins such as cell cycle oncogenes and tumor suppressor genes. Studies have shown that USP4 plays an important role in rectal cancer bladder cancer melanoma The high expression of USP4 in liver cancer and cervical squamous cell carcinoma implies that USP4 may be a potential oncogene, and it has been confirmed that the abnormal expression of USP4 promotes the occurrence, development, invasion and metastasis of rectal cancer9,10. In addition, USP4 promotes the proliferation, migration, and invasion of esophageal squamous cell carcinoma by targeting TAK1. The expression of USP4 is low in breast cancer, and the high expression of USP4 inhibits the proliferation of breast cancer cells and the occurrence and development of breast cancer, thus USP4 may be a potential tumor suppressor gene. Meanwhile, USP4 is an important p53 regulatory molecule, and its deubiquitination can reduce the level of p53 and inhibit p53-related apoptosis5,11,12.
USP4 is a relatively new factor in tumor research and has rarely been reported in tumor research, and its mechanism of action is still unclear. However, it has not been reported in lung adenocarcinoma that USP4, as an important deubiquitination enzyme, plays an important role in the ubiquitin-proteasome system and regulates protein expression Therefore, the regulation of USP4 protein signaling pathway is expected to become a target for tumor therapy, bringing a new chapter for tumor therapy.
In this study, we evaluated the expression of USP4 in human lung adenocarcinoma and its correlation with malignancy, and further explored the mechanism of USP4 in lung adenocarcinoma in vitro and in vivo.
Materials and methods
Patients and tissue samples
The human lung adenocarcinoma tissue samples were acquired from the Department of Pathology, Affiliated Hospital of Guilin Medical College with informed consent of the patients, which was approved by the Joint Ethics Committee of the Ethics Committee of Affiliated Hospital of Guilin Medical College. The patients underwent radical surgery at the Affiliated Hospital of Guilin Medical University without other treatment from 2009 to 2012. And 4 μm paraffin sections were prepared according to manufacturing process for HE and immunohistochemical staining. Fresh lung adenocarcinoma tissue samples protein were extracted for Western Blot assay.
Immunohistochemistry
All specimens were soaked and fixed in neutral formalin, embedded in paraffin, and serially sectioned (4-µm thick). The sections were deparaffinized and hydrated according to the routine procedure, boiled in EDTA for 2 min for antigen retrieval, naturally cooled to room temperature, and treated with endogenous peroxidase blockers for 15 min. The samples were incubated with primary antibodies against USP4 (ab236987, Abcam), vascular endothelial growth factor (VEGF, ab135505, Abcam), matrix metalloproteinase-2 (MMP2, ab86607, Abcam), or Ki67 overnight at 4 °C. Then, the samples were incubated with biotin-labeled secondary antibody for 20 min at room temperature and incubated with avidin-labeled horseradish peroxidase (HRP) at room temperature for 30 min, followed by chromogenic reaction with DAB, counterstaining with hematoxylin, and differentiation and dehydration with hydrochloric acid-alcohol, after which the samples became transparent. The samples were sealed with neutral resin and observed under a microscope. After staining, all sections were viewed and evaluated for histopathological diagnoses using a double-blind method. Based on the staining intensity, light yellow staining received 1 point, yellow staining received 2 points, and brown yellow staining received 3 points. Based on the percentage of positive cells, < 25% positive cells received 1 point, 25 -50% received 2 points, 51 -75% received 3 points, and > 75% received 4 points. Then, the 2 scores for intensity and percentage were multiplied to obtain the total score; if the total score was 0–3, the sample was defined as -; a score of 4–6 was defined as +; a score of 7–9 was defined as ++, and a score of 10–12 was defined as +++.
Cell culture
Human lung adenocarcinoma cell lines, NCI-H1975 and A549, were purchased from the Cell Bank of the Type Culture Collection of the Chinese Academy of Sciences. The cells were cultured in 1640 medium containing 10% fetal bovine serum and 1% penicillin and streptomycin and cultured in a 5% CO2 incubator at 37 °C.
Small interfering RNA (siRNA) interference
Human lung adenocarcinoma cells were seeded in 6-cm Petri dishes at a confluency of 50–60%. After the cells adhered to the dishes, negative control siRNA (si-NC) or USP7 siRNA (si-USP7) (GenePharma) was dissolved in Opti-MEM serum-free medium and mixed with lipofectamine 2000 (lip-2000). After standing for 20 min, the mixed solution was added to dishes for cell transfection. Four to six hours after transfection, the culture medium was replaced with medium containing 10% serum.
Immunofluorescence
Cells were seeded on glass slides. The cells were transfected when they reached a confluency of 50–60%. Forty-eight hours after transfection, the cells were fixed in paraformaldehyde for 10 min at room temperature and permeabilized with 0.1% Triton-X100 for 20 min. The cells were incubated with primary antibody against tubulin (1:1000) at room temperature for 1 h and then incubated with FITC-labeled secondary antibody at room temperature for 30 min. The nuclei were stained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI, 1: 1000) for 3 min. The cells were mounted with anti-fluorescent quenching sealing agent and observed under a fluorescence microscope.
Western blot
Tissues or cells were sonicated in lysis buffer. The supernatant was collected by centrifugation, and the protein concentration was quantified using the bicinchoninic acid (BCA) assay (Bio-Rad Laboratories). Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels were prepared, and 30 µg of protein was loaded, electrophoresed, and transferred to membranes. The membranes were blocked with 5% nonfat milk for 2 h and then incubated with primary antibody overnight at 4 °C. After incubation with a secondary antibody at room temperature for 1 h, the membrane was incubated in HRP chemiluminescent substrate, and protein bands were visualized on a Bio-Rad gel imaging system.
Western blot length gels image settings and processing manipulations
I used the image lab software to open length gels, and then used the screenshot tool to take a screenshot and save the picture. Then used the photoshop software to crop and process the image.
Wound healing assay
Twelve hours after transfection, cells were seeded into 6-well plates at seeding density of 1 × 10^6 cells per well and cultured in a 5% CO2 incubator at 37 °C. When the cells were confluent, a 100-µl pipette tip and ruler were used to draw a vertical line. The culture medium was discarded. After rinsing with PBS twice, 2 ml of serum-free medium was added. Photographs were taken at 0 h, 12 h, 24 h, and 48 h after scratching. Cell healing ability was observed and compared.
Cell proliferation assay (MTT)
Forty-eight hours after transfection, cells were seeded into 96-well plates at a seeding density of 1500 cells per well and cultured in a 5% CO2 incubator at 37 °C. After the cells attached to the plate for 0 h, 24 h, 48 h, 72 h, and 96 h, 10 µl of 5 mg/ml MTT (protected from light) was added to each well, and the cells were cultured for 4 h. The medium was discarded, and 150 µl of DMSO was added to each well. The optical density (OD) value (wavelength, 490 nm) for each well was measured with a microplate reader, and a cell proliferation curve was plotted with the OD as the ordinate.
Cell migration and invasion assay
Cell migration and invasion assays were performed using 24-well transwell chambers with 8 μm pore size polycarbonate membrane (Corning Incorporated). Forty-eight hours after transfection, 5 * 104 cells were seeded in serum-free medium in the upper chamber of the membrane (without Matrigel for cell migration assay) or seeded on the top side of the membrane pre-coated with Matrigel for cell invasion ability assay (BD, USA). Culture medium containing 10% FBS was added to the lower chamber. The cells that passed through the membrane were fixed in 4% paraformaldehyde for 10 min and stained with hematoxylin for 10 min. The number of cells that passed through the membrane was counted under a microscope. Four fields were selected randomly for each group (× 100), and the average number was calculated and plotted by Image J software.
Tumorigenesis experiment in nude mice
Lung cancer cell line A549 was subcultured and logarithmic growth cells were collected. 10 mice were randomly divided into 3 groups: blank group and experimental group (lung cancer cells were transfected with USP4 siRNA), with 5 nude mice in each group. The lung cancer cells were injected intradermal into the armpit of nude mice, and the tumor formation was observed and recorded. After 2 months, all mice were sacrificed, the transplanted tumor was removed and weighed, and the weight of transplanted tumor and tumor inhibition rate of mice were recorded and calculated. Immunohistochemistry detects the expression of USP4 protein in tumor tissues. Protein was extracted from fresh tumor tissues, and the relative expression level of USP4 protein was detected by Western blot.
Statistical analysis
SPSS20.0 software was used for statistical analysis. The correlation between USP4, VEGF, MMP2 and clinicopathological factors was evaluated. Χ2 test was used for comparison between two groups, Spearman rank correlation analysis was used for correlation analysis, and P < 0.05 was considered statistically significant. Western Blot, MTT and Transwell results were performed by T test. P < 0.05 was considered statistically significant.
Ethical approval
All animal experiments in this study have been approved by the Laboratory Animal Ethics Committee of Affiliated Hospital of Guilin Medical College and all methods were performed in accordance with relevant regulations and guidelines including the ARRIVE guideline.
Results
USP4 is highly expressed in human lung adenocarcinoma and is associated with the prognosis of patients
Immunohistochemistry detects the expression of USP4, VEGF and MMP2 in lung adenocarcinoma: The positive staining sites of USP4 were in the nucleus and cytoplasm of tumor cells, while the positive staining sites of VEGF and MMP2 were mainly in the cytoplasm of tumor cells. The results showed that the expressions of USP4, VEGF and MMP2 in lung adenocarcinoma tissues were higher than those in adjacent normal lung tissues, with statistically significant differences (P < 0.05) (Fig. 1A; Table 1). The correlation between USP4 and VEGF and MMP2 expression: The growth, development, invasion and metastasis of tumor cannot be separated from the nutrition and support of tumor blood vessels. VEGF and MMP2 are closely related to the generation of blood vessels and tumor metastasis. Immunohistochemical results were analyzed and the expressions of USP4 and VEGF, USP4 and MMP2 in lung adenocarcinoma tissues were compared for statistical analysis, and the results showed statistically significant differences, USP4 is positively correlates to Ki67, MMP2, VEGF expression (P < 0.05) (Table 2). Correlation between USP4 expression and clinicopathological features in lung adenocarcinoma: Analyze the correlation between the expression of USP4 and age, sex, lymph node metastasis and pathological stage in lung adenocarcinoma. The results showed that the expression of USP4 in lung adenocarcinoma tissues was not correlated with the age and gender of patients, but was significantly correlated with the degree of differentiation, TNM stage and pathological grade (P < 0.05). And the USP4 expression became more prominent in the later tumor stage (III + IV) but less prominent in earlier tumor stage (I + II). This finding indicated that USP4 was associated with poor prognosis of lung adenocarcinoma patients (Table 3). Analysde the relationship between USP4 expression and prognosis of patients and made urvival curve: Kaplan-meier survival analysis and log-rank test were used to analyze the relationship between USP4 expression and prognosis of patients with lung adenocarcinoma. The results showed that high USP4 expression was associated with poor prognosis of patients with lung adenocarcinoma (Fig. 1B).
USP4 is highly expressed in lung adenocarcinoma tissues, interference the expression of USP4 inhibits the proliferation of lung adenocarcinoma cells
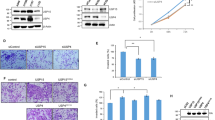
Collect 23 cases of fresh lung adenocarcinoma tissue and adjacent lung tissues, detect the expression of USP4 by WesternBlot. The results showed that the expression of USP4 in lung adenocarcinoma tissues was significantly higher than that in adjacent lung tissues (Fig. 2A & (a)). The expression of USP4 in lung adenocarcinoma cells was interfered, and the expression of USP4 was detected by Western Blot after 48 H protein extraction. The down-regulation trend was obvious for transfection agent USP4 siRNA1 (Fig. 2B) comparing the down-regulation effect of USP4 siRNA1 in NCI-H1975 and A549 cells. The results showed that the down-regulation trend was more obvious in A549 cell line (Fig. 2C). The interference effect of transfection agent siRNA1 on A549 cell line was further verified with a significant down-regulation trend, which could be used in subsequent experiments (Fig. 2D). Observed cell morphology at 0 H, 24 H and 48 H after USP4 transfection, and it was found that the cell morphology became round and the number decreased after interfering USP4 expression (Fig. 3A&B). Therefore, we guessed that USP4 could promote cell proliferation and inhibit apoptosis. MTT assay was used to detect the cell proliferation. SPSS statistical analysis showed that silencing USP4 expression inhibited the proliferation of lung adenocarcinoma cells, and the difference was statistically significant P < 0.05(Fig. 3C). To verify the effect of USP4 on promoting proliferation and inhibiting apoptosis: Western Blot was used to detect the expression of cell cycle proliferation and apoptosis-related factors. P21, P27, P53 is a cell cycle suppressor protein, both of which are negative regulators of cell cycle with anti-cancer effects and affect the regulation of cell proliferation and differentiation. CyclinA CyclinD1 is a cell cycle regulator gene, which is closely related to cell division and proliferation activities. Bcl-2 is a gene that inhibits apoptosis, while Bax is a gene that can promote apoptosis. Bax is a member of bcl-2 gene family, which can promote apoptosis by antagonizing Bcl-2 gene. Caspase family plays a key role in the process of apoptosis Caspase-3 is its core member, and its high expression can induce apoptosis, which plays a central role in the process of apoptosis. Caspase-3 is the last execution factor of apoptosis known at present. Studies have found that Cleaved caspase-3 high expression in normal tissues can induce apoptosis of abnormal hyperplasia cells, maintaining the cell number at a stable level. However, low expression in tumor tissue, abnormal cell proliferation accelerated, apoptosis decreased. Western Blot was used to detect the effects of USP4 silencing and expression on P21, P27, P53, CyclinA, CyclinD, Bax, Bcl2, caspase-3, Cleaved caspase-3 to study the proliferation and apoptosis of lung adenocarcinoma cells by USP4. The results showed that the expression of tumor-related tumor suppressor gene and cycle factor P21, P27, P53, increased and the expression of CyclinA, CyclinD decreased after USP4 expression was interfered. Apoptosis related protein Bax, Cleaved caspase-3 expression was increased, and Bcl2, caspase-3 expression was decreased (Fig. 3D), indicating that USP4 could promote cell apoptosis and inhibit cell proliferation.
Expression of USP7 in lung adenocarcinoma and adjacent tissues by western blot. (A) Expression of USP4 after interference with 3 siRNAs by western blot. (B) Western blot analysis of USP4 interference in A549 and NCI-1975 lung adenocarcinoma cells. (C) Western blot further confirmed the interference effect of USP4 in A549 lung adenocarcinoma cells (D).
The A549 cell morphology detection by microscope after interference with si-USP4. (A) The A549 cell morphology and number detection by fluorescence microscope after interference with si-USP4. (B) Cell viability was detected by MTT assay after A549 cells interference with si-USP4. (C) Cell cycle and apoptosis-related signal pathway were detected by western blot after interference with si-USP4 (D).
USP4 can promote the migration and invasion of lung adenocarcinoma cells
Research the effect of USP4 on the migration of lung adenocarcinoma cells, the cell migration ability was detected by cell scratch test and uncoated Transwell chamber method (the migration number of cells was observed at 0 H, 24 H and 48 H) The results showed that the healing rate of cell scar area was slowed down after silencing USP4 expression, and the number of cells passing through uncoated Transwell cell decreased (Fig. 4A,B). Research the invasion effect of USP4 on lung adenocarcinoma cells, Transwell gel chamber method was used to observe the number of cells passing through 0 H, 12 H, 24 H, 48 H and detect the invasion ability of lung adenocarcinoma cells. The results showed that after interfering USP4 expression, the number of cells passing through the gel chamber was reduced and the invasion ability was weakened (Fig. 4C). Western Blot was used to detect the expression of tumor invasion-related protein (MMP2 VEGF e-cadherin n-cadherin), further verifying the invasion ability of USP4 in lung adenocarcinoma. Results Showed that MMP2, VEGF after silencing USP4 expression The expression of e-cadherin was decreased, while the expression of N-cadherin was increased, indicating that downregulation of USP4 expression inhibited the invasion ability of cells (Fig. 4D). In addition, ERK, SRC, FAK, AKT and P38 pathways play an important role in tumor proliferation, migration and invasion. Western blot was used to detect the changes in phosphorylation levels of these proteins, and further explore the invasion and metastasis mechanism of USP4 in lung adenocarcinoma. The decreased levels of P-Src and P-ERK suggest that USP4 may affect the proliferation and metastasis of lung adenocarcinoma cells by regulating RSK, SRC and ERK signaling pathways (Fig. 5A). The mechanism of action is shown below (Fig. 6).
The migration ability of A549 cells were detected by wound healing assay after interference with si-USP4. (A) The migration ability of A549 cells were detected by transwell assay after interference with si-USP4. (B) The invasion ability of A549 cells were detected by transwell assay after interfering USP4. (C) EMT and metastasis signal pathway were detected by western blot (D).
In vivo experiment (tumorigenesis experiment in nude mice): The lung cancer cell line A549 was injected intradermal into the armpit of nude mice, and the tumorigenesis was observed. 2 months after successful tumorigenesis, the transplanted tumor was removed and weighed in 6 nude mice, and the weight and tumor inhibition rate of the transplanted tumor were recorded and calculated, and the differences were statistically significant (P<0.05)(Table 4). Immunohistochemical staining was performed to detect the expression of USP4 protein in tumor tissues. Protein was extracted from the fresh tumor tissues, and the relative expression level of USP4 protein was detected by Western blot to study the proliferation and invasion ability of USP4 on tumors. The results showed that the expression of USP4 in tumor tissues of transfected mice was decreased, and the volume of tumor formation was small The slow growth rate indicated that USP4 had tumor-promoting effect in vivo (Fig. 7A&B& C).
The expression of USP4 in tumor tissues was detected by immunohistochemistry. (A) Comparison of tumor size between blank group and transfected component in nude mouse tumor formation experiment. (B) Protein was extracted from the fresh tumor tissues, and the relative expression level of USP4 protein was detected by Western blot (C).
Discussion
By binding to ubiquitin, USP4 deubiquitinates and regulates the expression of a variety of proteins.Studies have shown that USP4 is highly expressed in colon cancer, pancreatic cancer and liver cancer, USP4 regulates the growth, invasion and metastasis of colorectal cancer. Knockdown of USP4 expression is significantly correlated with distant metastasis of tumor size differentiation and can inhibit the growth of colorectal cancer cells13,14,15. Our study found that USP4 was highly expressed in human lung adenocarcinoma and that the expression of USP4 was associated with the differentiation of lung adenocarcinoma, TNM stage, pathological grade and lymph node metastasis. Notably, the lung adenocarcinoma patients with high USP4 expression had shorter survival times. The results suggested that USP4, as a predictor of the prognosis of lung adenocarcinoma patients, plays an important role in the malignant progression of lung adenocarcinoma.
Furthermore, using a cell proliferation assay, we confirmed that the proliferation of lung adenocarcinoma cells decreased after USP4 expression interference, indicating that USP4 plays an important role in regulating the proliferation of lung adenocarcinoma cells. The cell cycle is regulated by both positive and negative factors. P21, P27, and P53 are cell cycle negative regulators with tumor suppressor functions, and they regulate cell proliferation and differentiation16,17,18. Human double minute 2 homolog (HDM2) is a p53-specific E3 ubiquitin-protein ligase that can negatively regulate p53 expression and inhibit p53 activity19. Studies have shown that USP7 deubiquitinates HDM2 and maintains the deubiquitination state, further mediating p53 ubiquitination and degradation and resulting in decreased intracellular p53 levels20. p21 and p27 are cyclin-dependent kinase inhibitors downstream of p53, regulate the G1/S phase transition together with p53 and negatively regulate the cell cycle21. Our study showed that the expression levels of p21, p27 and p53 increased after USP7 expression interference; this result might be due to the ubiquitination and degradation of HDM2 caused by the decreased expression of USP7, thereby inhibiting p53 ubiquitination, resulting in increased p53 expression and further causing an increase in the expression of downstream p21 and p27. Cyclin A and cyclin D1 are cell cycle regulatory genes that play important roles in cell division and proliferation22,23. Furthermore, they are regulated by p53. Therefore, USP4 may affect the expression of downstream proteins through the regulation of p53 expression, thereby affecting the cell cycle of lung adenocarcinoma cells. In addition, p53 is also an important factor that regulates cell apoptosis and the expression of Bcl-2 and Bax24. Bax translocates to the mitochondrial membrane to form homodimers or multimers and forms permeability transition (PT) pores on the mitochondrial membrane, allowing the release of pro-apoptotic factors, such as cytochrome c, which causes a caspase cascade reaction, exerting a pro-apoptotic function25. Bcl-2 can form a heterodimer with Bax and plays a role in inhibiting cell apoptosis26. Our study showed that after USP4 expression interference, Bax expression increased, Bcl-2 expression decreased, and caspase-3 activation increased, indicating that USP4 might regulate the apoptosis of lung adenocarcinoma cells through p53.
EMT is closely associated with tumor metastasis27. During the EMT process, the polarity and adhesion ability of epithelial cells are lost, thereby increasing the migration ability of mesenchymal cells, which facilitates tumor cell metastasis. In addition, the phenotype of the cells changes, and tumor cells lose the epithelial phenotype, e.g. E-cadherin is downregulated, and exhibit a mesenchymal phenotype, e.g., vimentin and N-cadherin are downregulated28. The downregulation of E-cadherin levels could result in decreased cell adhesion and facilitate cell migration and invasion29. Our study showed that after interfering with USP4 expression, lung adenocarcinoma cells changed from a long spindle shape to round, the migration and invasion abilities of cells decreased, N-cadherin expression decreased, and E-cadherin expression increased, indicating that USP4 promoted the invasion and migration of lung adenocarcinoma cells by promoting the EMT of cancer cells. MMP2 can degrade type IV collagen, the major structural component of basement membranes, and plays an important role in tumor cell invasion and metastasis30. MMP2 can also promote lymphangiogenesis and lymph node metastasis31,32. VEGF can induce lymphangiogenesis and promote lymph node metastasis33,34. Our study showed that in human lung adenocarcinoma, the expression of USP4 was positively correlated with the expression of VEGF and MMP2, and the probability of lymph node metastasis was higher in lung adenocarcinoma patients with high USP4 expression. In addition, USP4 expression interference in lung adenocarcinoma cells caused a decrease in MMP2 and VEGF expression, indicating that USP4 induced lymphangiogenesis and promoted lymph node metastasis of lung adenocarcinoma by upregulating VEGF and MMP2 expression35.
The MAPK/ ERK, SRC, FAK, AKT, P38 signaling pathway play important roles in tumor proliferation, migration and invasion. The MAPK/ERK signaling pathway regulates cell proliferation, differentiation and survival and plays an important role in tumor invasion and metastasis. Abnormal MAPK/ERK signaling pathway activation can promote malignant transformation and abnormal cell proliferation, ultimately leading to tumorigenesis and tumor cell proliferation36. Studies have shown that the inhibition of ERK phosphorylation inhibits the proliferation of and promotes the apoptosis of human gastric cancer cells37. Our study showed that after USP4 expression interference, the expression of p-ERK, p-RSC and p-RSK decreased, indicating that USP4 may affect the proliferation, apoptosis, migration and invasion of lung adenocarcinoma cells through the regulation of p-ERK, p-RSC and p-RSK. The results of tumorigenesis experiment in nude mice showed that USP4 could promote tumor proliferation and promote tumor in vivo.
In summary, we found that USP4 is highly expressed in lung adenocarcinoma. It may induce lymphangiogenesis and promote lymph node metastasis of lung adenocarcinoma by regulating the expression of VEGF and MMP2. USP4 regulates the cell cycle and apoptosis and promotes tumor cell proliferation by regulating the p53 and downstream protein expression. USP4 promotes the migration and invasion of lung adenocarcinoma cells by regulating cellular EMT processes. Therefore, our results suggest that USP4 may be a potential target for the treatment of lung adenocarcinoma.
Data availability
The original contributions presented in the study are included in the article/Supplementary material; further inquiries can be directed to the author, Yamin Wei: 13026592854@163.com.
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
Shiromizu, T. et al. Targeting E3 ubiquitin ligases and deubiquitinases in ciliopathy and cancer. Int. J. Mol. Sci. 21 (2020).
Chen, Y. et al. USP4 promotes the proliferation and glucose metabolism of gastric cancer cells by upregulating PKM2. PLoS One 18 (8), e0290688 (2023).
Yun, S. I. et al. Binding of USP4 to cortactin enhances cell migration in HCT116 human colon cancer cells. FASEB J. 37 (5), e22900 (2023).
Wang, Y. et al. USP4 function and multifaceted roles in cancer: a possible and potential therapeutic target. Cancer Cell. Int. 20, 298 (2020).
Yeasmin, K. F., Chen, F. Z. & Chen, H. C. Targeting ubiquitin specific protease 7 in cancer: a deubiquitinase with great prospects. Cell. Biochem. Funct. 36, 244–254 (2018).
Zhang, S. et al. Ubiquitin-specific protease 11 serves as a marker of poor prognosis and promotes metastasis in hepatocellular carcinoma. Lab. Invest. 98, 883–894 (2018).
McCann, J. J. et al. USP22 functions as an oncogenic driver in prostate cancer by regulating cell proliferation and DNA repair. Cancer Res. 80, 430–443 (2020).
Cao, W. H. et al. USP4 promotes invasion of breast cancer cells via Relaxin/TGF-β1/Smad2/MMP-9 signal. Eur. Rev. Med. Pharmacol. Sci. 20 (6), 1115–1122 (2016).
Guo, W. et al. Up-regulated deubiquitinase USP4 plays an oncogenic role in melanoma. J. Cell. Mol. Med. 22 (5), 2944–2954 (2018).
Li, Y., Jiang, D., Zhang, Q., Liu, X. & Cai, Z. Ubiquitin-specific protease 4 inhibits breast cancer cell growth through the upregulation of PDCD4. Int. J. Mol. Med. 38 (3), 803–811 (2016).
Zhang, H. et al. USP4 promotes the proliferation, migration, and invasion of esophageal squamous cell carcinoma by targeting TAK1. Cell. Death Dis. 14 (11), 730 (2023).
Zhong, M., Jiang, Q. & Jin, R. USP4 expression independently predicts favorable survival in lung adenocarcinoma. IUBMB Life (2018).
Xing, C. et al. Ubiquitin-specific protease 4-mediated deubiquitination and stabilization of PRL-3 is required for potentiating colorectal oncogenesis. Cancer Res. 76 (1), 83–95 (2016).
Sho, S., Court, C. M., Winograd, P., Russell, M. M. & Tomlinson, J. S. A prognostic mutation panel for predicting cancer recurrence in stages II and III colorectal cancer. J. Surg. Oncol. 116 (8), 996–1004 (2017).
Xu, J. et al. FBXO3 stabilizes USP4 and Twist1 to promote PI3K-mediated breast cancer metastasis. PLoS Biol. 21 (12), e3002446 (2023).
Engeland, K. Cell cycle arrest through indirect transcriptional repression by p53: I have a DREAM. Cell. Death Differ. 25, 114–132 (2018).
Jeannon, J. P., Soames, J. V., Aston, V., Stafford, F. W. & Wilson, J. A. Molecular markers in dysplasia of the larynx: expression of cyclin-dependent kinase inhibitors p21, p27 and p53 tumour suppressor gene in predicting cancer risk. Clin. Otolaryngol. Allied Sci. 29, 698–704 (2004).
Li, F. et al. The deubiquitinase USP4 stabilizes twist1 protein to promote lung cancer cell stemness. Cancers (Basel). 12 (6), 1582 (2020).
Fan, Y. H. et al. USP7 inhibitor P22077 inhibits neuroblastoma growth via inducing p53-mediated apoptosis. Cell. Death Dis. 4, e867 (2013).
Lee, J. T. et al. Targeting prostate cancer based on signal transduction and cell cycle pathways. Cell. Cycle. 7, 1745–1762 (2008).
Zhang, W. et al. PI3K and notch signal pathways coordinately regulate the activation and proliferation of T lymphocytes in asthma. Life Sci. 92, 890–895 (2013).
Liu, Z. et al. MCM family in HCC: MCM6 indicates adverse tumor features and poor outcomes and promotes S/G2 cell cycle progression. BMC Cancer. 18, 200 (2018).
Wang, X., Simpson, E. R. & Brown, K. A. p53: Protection against tumor growth beyond effects on cell cycle and apoptosis. Cancer Res. 75, 5001–5007 (2015).
Pena-Blanco, A. & Garcia-Saez, A. J. Bax, Bak and beyond - mitochondrial performance in apoptosis. FEBS J. 285, 416–431 (2018).
Correia, C. et al. Emerging understanding of Bcl-2 biology: implications for neoplastic progression and treatment. Biochim. Biophys. Acta. 1853, 1658–1671 (2015).
Saitoh, M. Involvement of partial EMT in cancer progression. J. Biochem. 164, 257–264 (2018).
Paolillo, M. & Schinelli, S. Extracellular matrix alterations in metastati processes. Int. J. Mol. Sci. 20 (2019).
Yang, M. et al. Ubiquitin-specific protease 22: a novel molecular biomarker in cervical cancer prognosis and therapeutics. Tumour Biol. 35, 929–934 (2014).
Tao, Y. & You, W. The deubiquitinating enzyme USP4 functions as an oncoprotein in gastric cancer and mediates NF-κB signaling by regulating PRL-3 expression. Front. Biosci. (Landmark Ed). 27 (10), 286 (2022).
Fu, Z. et al. The expression of tumor-derived and stromal-derived matrix metalloproteinase 2 predicted prognosis of ovarian cancer. Int. J. Gynecol. Cancer. 25, 356–362 (2015).
Wu, Z. Y., Li, J. H., Zhan, W. H. & He, Y. L. Lymph node micrometastasis and its correlation with MMP-2 expression in gastric carcinoma. World J. Gastroenterol. 12, 2941–2944 (2006).
Zheng, H. et al. Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinoma. Anticancer Res. 26, 3579–3583 (2006).
Scheiflinger, A. et al. High USP4 mRNA is associated with an HPV-positive status in head and neck squamous cell carcinoma patients. J. cancer Res. Clin. Oncol. vol. 149 (12), 10675–10683 (2023).
Dai, Y., Tong, R., Guo, H., Yu, T. & Wang, C. Association of CXCR4, CCR7, VEGF-C and VEGF-D expression with lymph node metastasis in patients with cervical cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 214, 178–183 (2017).
Kitamura, H. Ubiquitin-specific proteases (USPs) and metabolic disorders. Int. J. Mol. Sci. 24(4), 3219 (2023).
Yan, W., Chen, S., Zhao, Y. & Ye, X. Fisetin inhibits the proliferation of gastric cancer cells and induces apoptosis through suppression of ERK 1/2 activation. Oncol. Lett. 15, 8442–8446 (2018).
Acknowledgements
The study was in part supported by Guilin Scientific Research and Technology Development Program (2020011204-13);Guangxi Medical and Health Appropriate Technology Development and Application Project (S2022126);Guangxi Graduate Education Innovation Project 2022 (GYYK2022017);The project of Innovation and Entrepreneurship Training Program for college students of Autonomous Region in 2021 (202110601007).
Author information
Authors and Affiliations
Contributions
YAMIN WEI: Conceptualization, Methodology, Software, Investigation, Formal Analysis, Writing - Original Draft; SHANWANG WEI: Data Curation, Writing - Original Draft; ZHONGTENG LEI: Investigation; Icon making; YAN ZHANG:InvestigationJINXIAO WU: Resources, Supervision; JINLI HUANG: Software, Validation; LIJUAN FU: Writing - Review & Editing; GUIYING HUANG: Data processing; ZHIMENG LI: Image processing; YUANNA LIANG: Image processing; JINHUA ZHENG: Conceptualization, Funding Acquisition, Resources, Supervision, Writing - Review & Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Statement
(i) Identifying the institutional and/or licensing committee approving the experiments, including any relevant details; (ii) confirming that all experiments were performed in accordance with relevant guidelines and regulations.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wei, Y., Wei, S., Lei, Z. et al. USP4 promotes proliferation and metastasis in human lung adenocarcinoma. Sci Rep 15, 11096 (2025). https://doi.org/10.1038/s41598-025-89377-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-89377-3