Abstract
Seabuckthorn (Hippophae rhamnoides L.) is known for its medicinal properties in treating various diseases, including neurological conditions. However, the therapeutic effect of inhaled seabuckthorn seed oil (SSO) on Alzheimer’s disease (AD) remains not fully understood. This study explores the effects of nebulized inhalation of SSO in 9-month-old APP/PS1 mice over 21 days. The results showed that nebulized SSO improved memory and cognition. Using 7.0T MRI to monitor blood oxygenation level dependent (BOLD) signals revealed that SSO altered the Amplitude of Low Frequency Fluctuations (ALFF) and Regional Homogeneity (ReHo) signaling such as in the amygdala and substantia innominate, and hippocampus. Enzyme-linked immuno sorbent assay (ELISA) and pathological analyses indicated reduced neuroinflammation in plasma and brain, decreased neuronal necrosis, lower β-amyloid (Aβ) protein levels, reduced amyloid deposition, and increased tyrosine hydroxylase activity. Additionally, SSO promoted gut microbiota remodeling by increasing alpha diversity and boosting levels of probiotics such as Verrucomicrobia, Bifidobacterium, Prevotella, and Akkermansia, without adverse effects on lung tissue. Nebulized inhalation of SSO may slow AD progression by modulating inflammation and amyloid deposition. Nebulized inhalation offered a potential method for enhancing drug delivery across the blood-brain barrier with reduced systemic side effects.
Similar content being viewed by others
Introduction
Cognitive dysfunction due to Alzheimer’s disease has become an important issue affecting the quality of life of elderly people1. It is characterized by the intraneuronal deposition of extracellular Aβ plaques and neuroprogenitor fiber tangles (NFTs)2. In particular, Aβ is generated from amyloid precursor protein (APP) by the action of β and γ secretases to produce Aβ40/42, and Aβ42 forms dense fibrillar neuroinflammatory plaques characteristic of AD, which drive inflammatory cytokines and microglia to migrate to the lesion to activate phagocytosis, ultimately leading to massive neuronal death3,4,5.
It was found that APP could influence AChE expression, as overexpression of APP695 in neuronal cells downregulated acetylcholinesterase (AChE) expression and activity, while knockdown of APP upregulated AChE expression6. Another aspect is that in AD patients, glutamate transporter protein capacity and protein expression are decreased, and vesicular glutamate transporter protein is selectively lost7. And, toxic Aβ may allow more glutamate availability by impairing glutamate uptake/recycling mechanisms8. This enhanced glutamate supply may lead to AD-associated excitotoxicity and neurodegeneration. Thus, the main therapeutic options for AD include anticholinesterase inhibitors and N-methyl-D-aspartate (NMDA) receptor modulators nowadays, both of which are administered orally9,10. Due to intestinal, hepatic, and renal first-pass elimination effects and the presence of the blood-brain barrier, the amount of medicine reaching the lesion is greatly reduced, making it difficult to reverse extensive neuronal and synaptic necrosis in brain tissue11,12,13,14. Increasing the oral dose to improve efficacy can lead to hepatotoxicity and unacceptable gastrointestinal and systemic adverse effects such as hypotension, severe vomiting, irregular respiration, and confusion15,16,17. Hamid and Zahid18 reported that using probiotics and donepezil to treat an AlCl3-induced AD mice model improved memory function and increased AChE levels, but caused anxiety symptoms in the mice. Farlow et al.19 compared the use of 23 mg/d versus 10 mg/d donepezil for treating patients with moderate to severe AD and found a greater percentage of discontinuation due to adverse events in the 23 mg/d group (12.1% versus 1.9%).
Seabuckthorn, a plant in the Hoodiaceae family, has received worldwide attention in recent years for its potential medicinal and nutritional value. Its nutritional and bioactive substances mainly include flavonoids, β-carotene, essential amino acids and so on, which have antioxidant, immunomodulatory, hypoglycemic, and anticancer activities20. Flavonoids contained in seabuckthorn have been shown to prevent common neurodegenerative diseases by stimulating neural-related signaling pathways21. Zhu et al.22 subcutaneously injected arginine-rich peptides obtained from seabuckthorn seed protein into D-galactose-induced memory-impaired mice, which increased hippocampal endothelial nitric oxide (NO) synthase and lowered serum inflammatory cytokine levels. Additionally, supercritical CO2 extracted SSO has significant anti-atherogenic and cardioprotective activity23.
In recent years, nasal drug delivery, especially nose-to-brain delivery, has been studied intensively24. Nasal inhalation drug delivery has emerged as a noninvasive and direct route for treating central nervous system disorders by bypassing the blood-brain barrier due to its fast absorption rate, painlessness, and good tolerability16,25. Studies show that nasal inhalation of non-controlled analgesics, such as ketoprofen and tramadol, in phospholipid nanovesicles achieves 2–5 times higher Cmax than oral administration26. Inhaling chitosan nanoparticles loaded with hesperidin enhances brain permeability and cognitive function in dementia mice27. Additionally, nasal delivery of a triple-drug therapy in APP/PS1 mice improves memory, reduces Aβ peptide levels, and lowers BACE-1 expression28. Vegetable glycerol (VG) and Propylene glycol (PG), as lipophilic carriers and permeation enhancers, can decompose and disrupt lipid arrangements, reducing solution viscosity, lowering mucosal ciliary clearance and enzyme activity by improving membrane fluidity. In this study, we combined heated nebulization of SSO with VG and PG to preliminarily investigate the effects of inhalation therapy on behavioral and inflammatory markers, neuroimaging, lung and brain histopathology, and intestinal flora in AD mice.
Results
Physicochemical properties, atomized concentration and compositional assay of SSO
SSO appeared as a brownish-yellow to brownish-red transparent oily liquid at room temperature, with a density of 0.85 g/mL, a greasy flavor, and white smoke upon heat atomization, producing liquid oily particles with diameters of 3–5 μm. High-performance liquid chromatography determined the contents as follows: 0.42% rutin, 0.63% quercetin, 0.51% isorhamnetin, 0.43% gallic acid, and 0.61% catechin (refer to the Supplementary File 1 for detailed assay methods). Each group of mice was placed in a 28 cm × 21 cm × 17 cm perforated container. Seabuckthorn oil was heated to 220 °C using a custom heating device (Qilu University of Technology) and then pumped through a custom electronic smoking machine (Qilu University of Technology) (Fig. 1). Smoke was extracted at 4-minute intervals, with 50 ml of smoke extracted each time. Over 30 min, the oil was pumped 7 times, totaling 100 µL, achieving an effective concentration of approximately 0.85 µg/L.
Effect of SSO in behavioral deficits and cognitive function
Figure 2 shows that over 4 consecutive days of training, group AD + NS had a longer avoidance latency compared to group WT + NS (P < 0.05), indicating it took them more time to find the platform. Compared to group AD + NS, group AD + SSO had a shorter avoidance latency (P < 0.05). On day 5, during the spatial exploration experiment, the number of crossings, dwell time, and total distance traveled by AD mice in the target quadrant were lower, suggesting impairments in spatial learning and memory. However, after inhalation of nebulized SSO, compared with group AD + NS, there was a significant improvement in escape latency, residence time (20.3 vs. 34.5s, P = 0.042), and total distance traveled (3123 vs. 6013 mm, P < 0.01). All data could be found in Supplementary File 2.
Effect of SSO in behavioral deficits and cognitive function. (A) Experimental design and protocol; (B) Time spent finding the platform hidden under the water surface during 4 consecutive days of water maze training; (C) Total time spent in the target quadrant during the spatial exploration trial on day 5; (D) Number of times crossing the original platform position in the target quadrant during the spatial exploration trial on day 5; (E) Total distance traveled in the target quadrant during the spatial exploration trial on day 5; (F) Mice trajectories during space exploration trials. *P < 0.05, **P < 0.01.
Effect of SSO in BOLD
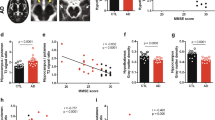
To assess the impact of nebulized inhalation of SSO on neuroimaging, we conducted 7.0T MRI scans and BOLD analyses in mice. Tables 1, 2, 3 and 4 and fMRI data revealed that SSO inhalation triggered significant BOLD signal changes. ALFF results showed decreased BOLD activation in several brain regions including the amygdala, substantia innominata, thalamus, caudate putamen, bed nucleus of the stria termina, globus pallidus, substantia nigra, piriform cortex, and hippocampus in AD mice compared to C57 mice. However, activation increased in the amygdala, caudate putamen, hypothalamus, piriform cortex, substantia innominata, and hippocampus after SSO treatment (Fig. 3). ReHo results similarly indicated decreased BOLD activation in the amygdala, caudate putamen, hypothalamus, substantia nigra, substantia innominata and so on in AD mice but increased activation in treated mice in various regions including the bed nucleus of the stria termina, ventral medial nucleus of the thalamus, cerebellum and so on.
Statistical analysis of ALFF activity in BOLD signals in the AD + NS group versus the AD + SSO group. The voxel-level height threshold was P < 0.005 (uncorrected). The cluster range threshold was 20 voxels. Detailed statistical information for these regions is provided in Table 2.
Effect of SSO in Neuropathology
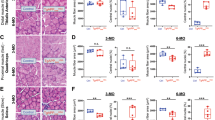
H&E and Nissl staining (Fig. 4) showed that the hippocampal cells in group (WT + NS) and group (AD + Donepezil-Memantine) were structurally intact, regularly arranged, and uniformly stained. In contrast, group (AD + NS) and group (AD + Solvent) displayed reduced hippocampal neurons, vacuolation, and disorganized cell arrangements. SSO inhalation partially restored hippocampal morphology. Congo red staining (Fig. 4) revealed no amyloid deposition in the hippocampus of C57 mice, whereas significant amyloid deposition was observed in AD mice. SSO inhalation markedly reduced this deposition (1.07 vs. 0.36%, P < 0.0001). Aβ protein deposition (Fig. 4) was observed in AD mice but not in C57 mice. SSO inhalation resulted in lighter and smaller Aβ plaques (9.04 vs. 1.83%, P < 0.0001). The average fluorescence intensity of tyrosine hydroxylase in the hippocampus of AD mice (Figs. 4 and 5) was lower than that in C57 mice. SSO inhalation significantly increased this intensity (29.34 vs. 47.65%, P = 0.018), unlike the group (AD + Solvent). All stained images including raw data could be found in Supplementary File 3.
Inflammation levels in serum and brain homogenate
Inflammatory factors in serum and brain homogenates (Fig. 6A and B) showed increased levels of lnterleukin-6 (IL-6), IL-17, tumor necrosis factor-α (TNF-α), IL-1β and decreased level of acetylcholine (ACh) in AD mice. SSO inhalation significantly reduced levels of IL-6, IL-17, TNF-α, IL-1β (P < 0.05) and increased ACh levels (P < 0.05), and effectiveness was comparable to traditional medication.
Safety of SSO in the lung tissue
To verify the safety of the nebulized inhalation of SSO on lung tissues, we performed H&E staining and IL-6 and TNF-α level evaluation in the group (AD + NS), group (AD + Solvent) and group (AD + SSO). The results (Fig. 7) confirmed the safety of inhalation of SSO on lung tissues. AD mice had intact lung structures, while solvent groups showed damage. SSO inhalation improved pathological damage and decreased IL-6 concentration (0.17 vs. 0.06pg/mg, P = 0.03).
Effect of SSO in the intestinal flora
Alpha diversity
Alpha diversity analysis mainly reflected the species diversity of the gut microbiota. The Chao1 index reflects the number of species, the Shannon index and Simpson index reflect the homogeneity and diversity of the gut flora community, and higher values indicate richer species diversity. The results (Fig. 8A) demonstrated that the intestinal microbiota of AD mice was damaged, and the species diversity decreased. After SSO inhalation, the Chao1 index (340.9 vs. 445.1, P = 0.05), and the Shannon index (5.3 vs. 6.0, P = 0.04) and Simpson index (0.91 vs. 0.94, P = 0.04) increased significantly, indicating that SSO could improve the diversity and species evenness in the fecal samples of APP/PS1 mice.
Effect of SSO in the intestinal flora. (A) Alpha-diversity analysis (Chao 1 index, Shannon index and Simpson index). (B) Phylum-level sample analysis. (C) Genus-level sample analysis. Relative abundances of (D) Phylum. (E) Genus in the relative abundance of various gut microbes among five groups. a: WT + NS; b: AD + NS; c: AD + Donepezil-Memantine; d: AD + Solvent; e: AD + SSO.
Analysis of differential species
Phylum-level sample analysis (Fig. 8B, D) revealed that the groups with higher abundances were Bacteroidetes, Firmicutes, Verrucomicrobia, Proteobacteria, and Actinobacteria. Proteobacteria and Verrucomicrobia were more abundant in AD mice than in C57 mice. After SSO inhalation, compared with group (AD + NS), the abundance of Actinobacteria (0.9 vs. 0.5%, P < 0.01) and Proteobacteria (24.7 vs. 4.0%, P < 0.0001) decreased, while Verrucomicrobia (6.0 vs. 17.8%, P < 0.01) increased.
Genus-level sample analysis (Fig. 8C, E) showed that the most abundant genera in each group were Akkermansia, Allobaculum, Prevotella, Oscillospira, Coprococcus, Bifidobacterium, and Lactobacillus. Compared to C57 mice, AD mice had relatively lower abundances of Bifidobacterium, Prevotella, and Akkermansia, and a higher abundance of Oscillospira. After SSO inhalation, compared with group (AD + NS), the abundance of Bifidobacterium (0.3 vs. 1.0%, P = 0.024), Prevotella(2.2 vs. 6.1%, P = 0.001), and Akkermansia (6.5 vs. 21.1%, P = 0.038) increased, while Oscillospira (6.2 vs. 1.4%, P = 0.01) decreased. The abundance of Lactobacillus also increased slightly, although these differences were not significant.
Discussion
Seabuckthorn has been used for centuries as a medicinal and nutritional supplement in Asia and Europe. Seabuckthorn oil contains a variety of biologically active components such as polyphenols (antioxidant), flavonoids (antihypertensive, promote wound healing), sterols (reduce blood cholesterol level), carotenoids (antioxidant and anticancer) and vitamins and so on29. Li et al. found that seabuckthorn polysaccharide gavage treatment in AD mice could improve learning and memory ability and reduce pathological damage by inhibiting the TLR4/MyD88 signaling pathway, and could positively regulate the interaction between inflammatory factors and oxidative stress by activating the Keap1/Nrf2 signaling pathway, reduce the accumulation of Aβ in the brain, and improve the hippocampal tissue pathological morphology30. Tsim et al. extracted flavonoids, known as isorhamnetin, from seabuckthorn fruits and used it to feed mice with high-fat and high-fructose-induced obesity. Isorhamnetin was found to inhibit microglia overactivation and downregulate inflammation levels in serum and brain by inhibiting MAPK and NFκB signaling pathways31. Similarly, Liu et al.32treated high-fat and high-fructose diet (HFFD)-induced obese mice with seabuckthorn flavonoids for 14 weeks and found that seabuckthorn flavonoids inhibited HFFD-induced salient dysfunction and neuronal damage by decreasing insulin resistance as well as increasing protein expression of PSD-95. In addition, seabuckthorn flavonoids activated ERK/CREB/BDNF and IRS-1/AKT pathways and inactivated NF-κB signaling and its downstream inflammatory mediator expression. However, the therapeutic effect of nebulized inhalation of seabuckthorn seed oil in AD has not been studied. Our study demonstrated similar efficacy in improving cognitive impairment and reducing neuroinflammation compared to conventional administration. While the nebulized inhalation of SSO significantly improved impaired intestinal flora, and the biosafety was confirmed. Additionally, we verified the impact of this treatment on brain function through neuroimaging.
AD is a neurodegenerative disease characterized by Aβ plaque deposition and intracellular hyperphosphorylated tau protein aggregates in NFTs33. Aβ aggregates lead to microglial dysfunction, hindering Aβ phagocytosis and promoting proinflammatory factors like IL-6, IL-1β, and TNF-α, further enhancing Aβ production and deposition, thus creating a cycle of Aβ deposition and microglial barrier disruption34. Tyrosine hydroxylase, a precursor for catecholamine synthesis, reflects norepinephrine levels, and its positive neuronal degeneration in APP/PS1 mice is associated with β-amyloid deposition, directly damaging neurons35.
The BOLD signal reflects changes in cerebral blood flow and volume, is used to study the dynamics of brain activity. It can be used as a marker of brain disease states36. ALFF is used to measure the amplitude of low-frequency oscillations in resting-state fMRI, defined as the total power in the frequency range of 0.01 to 0.1 Hz37. A study on ALFF white matter changes in AD patients showed that in AD patients, the right anterior thalamic radiation, left frontal anterior tract, left middle longitudinal fasciculus, right anterior thalamic radiation and left forceps minor had reduced ALFF values38. ReHo measures the similarity of voxels in the resting time series between a given voxel and its neighboring voxels39,40. Studies have demonstrated that, compared to cognitively normal individuals, patients with AD have diminished ReHo signals in the precuneus and posterior cingulate cortex and increased ReHo signals in the occipital and temporal lobes41,42, especially structural changes in the medial temporal lobe and hippocampus, which have been identified as typical MRI markers of AD43. In the present study, nebulized inhalation of SSO induced altered BOLD signaling in some brain regions, which we speculated was related to changes in Aβ deposition. Most of the existing studies suggest that the Aβ protein causes changes in BOLD signaling; specifically, early Aβ deposition causes metabolic hyperactivity, which may be related to tau protein accumulation or Aβ and tau protein interactions in the brain44. Or it may be related to the initial accumulation leading to neurological disruption, and the brain compensates for this damage by increasing neuronal activity45. When Aβ deposition exceeds the brain’s volume threshold, neuronal damage increases, cerebrovascular dysfunction occurs, and metabolic decompensation occurs46, with consequent changes in BOLD signaling throughout the process. For amyloidosis mice models (TG2576 and PDAPP mice), supersynchronous BOLD functional connectivity in the hippocampus has been observed prior to the observation of Aβ plaque deposition but when soluble Aβ was detected, and early treatment of TG2576 mice with anti-Aβ antibodies prevented BOLD supersynchronization47.
Vegetable glycerol and propylene glycol are used as mediating solvents in e-cigarettes, and the U.S. Food and Drug Administration considers these compounds safe for use as food additives. However, there is still no consensus on the safety of VG and PG. Yoon et al.48 administered a continuous two-week intratracheal drip of PG to mice, finding increased inflammatory cells and papery-rich macrophages in bronchoalveolar lavage fluid (BALF) and decreased ventilation during expiration. However, these changes resulted from PG doses 47–137 times greater than daily e-cigarette user exposure. In a simulated real smoking environment, mice exposed for 4 months to air, conventional tobacco smoke, an e-cigarettes carrier (containing only PG/VG), and carrier-nicotine, results showed no lung inflammation or emphysema in the e-cigarettes vapor group49. Interestingly, SSO exhibits anti-inflammatory activity that can treat lung inflammation. Seabuckthorn Wuwei Pulvis significantly improved lung function, reduced TNF-α, IL-8, IL-6, and IL-17 expression, and modulated intestinal microbiota dysregulation in rats with chronic obstructive pulmonary disease (COPD)50. Treatment with isorhamnetin significantly ameliorated inflammatory cell infiltration, proinflammatory cytokine overproduction, emphysema, and collagen deposition in the lungs of mice with cigarette smoke-induced COPD and inhibited the increase in peripheral blood T lymphocytes51. In this study, IL-6 levels in the alveolar lavage fluid of the AD + Solvent group were slightly higher than in the AD group, but IL-6 levels decreased to baseline after nebulized inhalation of SSO, possibly related to airway hyperresponsiveness in aged mice. TNF-α expression increased, but the difference was not statistically significant. According to lung histology, SSO significantly reduced inflammatory cell infiltration in small airways and alveoli and thinned airway walls, suggesting that SSO containing solvents is safe for mice. Our future research will focus on optimizing the solvent composition to minimize inflammatory responses while maintaining effective drug delivery. Additionally, we will also explore the therapeutic and preventive role of SSO for other neurological disorders.
As aging and AD progress, the gut mucosa is progressively disrupted, and the composition and diversity of the intestinal microbiota undergo dramatic changes52. These changes can promote the accumulation of Aβ proteins in the intestinal tract, which in turn shapes the intestinal and peripheral neurogenic inflammatory response, leading to inflammatory and degenerative changes in the central nervous system53,54. At six months of age, APP/PS1 mice showed a reduced abundance of intestinal flora and decreased relative levels of probiotics, which further deteriorated at eight months of age. For example, the increased abundance of Proteobacteria, Actinobacteria, Enterobacteriaceae, and Lactobacillus, as well as the decreased abundance of Firmicutes, Bacteroidetes, Akkermansia, and Prevotella was observed55,56,57, which is generally consistent with the present study. Notably, compared to the AD + NS group, the diversity and abundance of intestinal flora were increased, but not significantly different, in the AD + Donepezil-Memantine group, which may be related to constipation caused by memantine58. Lan et al.59 found that HRPI consumption in mice with high-fat diet-induced cognitive dysfunction effectively alleviated behavioral deficits, suppressed neuroinflammation, and ameliorated synaptic dysfunction through inhibition of the NF-κB pathway and upregulation of the CREB/BDNF/TrkB pathway. It also attenuated intestinal barrier damage, inflammatory response, and reversed dysbiosis. Flavonoids in sea buckthorn also have the ability to regulate the microbiota. Quercetin, as a prebiotic, can repair the damaged microbe-brain-gut axis in a repeated mild traumatic brain injury mice model60. In this study, the intestinal flora diversity of AD mice was damaged, and the diversity after nebulized inhalation of seabuckthorn oil tended to recover towards that of normal C57 mice. This indicates that nebulized inhalation of seabuckthorn oil is safer for the intestinal microbial environment and can improve the impaired diversity of the intestinal flora and reshape its composition.
In conclusion, our study demonstrated that APP/PS1 mice treated with nebulized SSO exhibited improved behavioral and cognitive abilities. There were significant decreases in the levels of inflammatory factors in the serum and brain homogenate, improvements in the pathological damage of brain tissues, and reductions in Aβ protein levels, amyloid deposition, and tyrosine hydroxylase. And we confirmed by fMRI that BOLD signal was altered in some brain regions after SSO treatment. Additionally, there was a restoration of the diversity of the intestinal flora and an increase in the relative content of probiotic bacteria, with no significant changes in inflammatory levels in the lung tissue. Overall, this study is the first to validate the effectiveness and feasibility of nebulized inhalation of SSO for the treatment of AD in mice.
Materials and methods
Materials and chemicals
SSO was provided by Jinan Quanding Biotechnology Co., Ltd. (Jinan, China). It is a brownish-yellow to brownish-red transparent oily liquid obtained from seabuckthorn seeds through supercritical extraction or subcritical low-temperature extraction (refer to Supplementary File 1 for detailed extraction methods and compositional analysis). VG and PG were provided by Dingguo Biotechnology Co., Ltd. (Jinan, China). Donepezil and memantine hydrochloride were supplied by Yuanye Biotechnology Co., Ltd. (Shanghai, China). IL-6, IL-17, IL-1β, and TNF-α detection kits were from BOSTER (Wuhan, China). The bicinchoninic acid (BCA) protein kit was from Abbkine Biotechnology Co., Ltd. (Wuhan, China). The H&E and nissl staining solution were from Beyotime (Shanghai, China). The Congo red staining solution was from Solarbio (Beijing, China). The Aβ protein and tyrosine hydroxylase antibody were from abcam (Shanghai, China).
Animals
Six-week-old C57BL/6 N male mice and APP/PS1 male mice were provided by Beijing Vital River Laboratory Animal Technology Co., Ltd. The mice were housed in the Laboratory Animal Center of Shandong University (5 mice per cage; size: 320 × 215 × 170 mm; light-dark cycle, 12 h; temperature: 24 ± 2 °C; humidity: 50–55%; and free access to feed and water) and were maintained until they reached 9 months of age. All the experimental procedures and animal care were approved by the Animal Ethics Committee of Shandong University (Ethical Approval No. DWLL-2024-091) and followed the National Institutes of Health Guidelines for the Care and Use of Animals.
20 APP/PS1 mice were randomly divided into four groups. (1) AD saline group (AD + NS): 5 APP/PS1 mice were gavaged with saline (0.2 ml per mice), (2) 1.5 mg/kg donepezil-memantine gavage group (AD + Donepezil-Memantine): 5 APP/PS1 mice were gavaged with donepezil (1.5 mg/kg) and memantine hydrochloride (1.5 mg/kg) in saline (0.2 ml per mice), (3) Nebulized solvent of VG: PG = 4:6 with heated at 220 °C group (AD + Solvent): 5 APP/PS1 mice were subjected to heated nebulization with solvent (VG: PG = 4:6) at 220 °C via inhalation every 4 min for 30 min, of 2 times one day, for a total of 14 times, for a total dose of 200 µl. (4) Nebulized SSO group (AD + SSO): 5 APP/PS1 mice inhaled SSO: solvent = 50%:50%, total dose of 400 µl; the method used was the same as that used for group (3). (5) Additionally, 5 C57BL/6 mice were given orally with saline and named the control group (WT + NS). After 21 days of exposure, three mice in each group were randomly selected for subsequent experiments.
Behavioral testing
All mice underwent daytime memory behavior tests in a quiet environment. The Morris water maze (ZH-Morris, Anhui Zhenghua Bio-Instrument and Equipment Co., Ltd., Huaibei, China) consisted of a black circular pool (r = 75 cm, h = 50 cm) divided into four quadrants, with a hidden platform in the center of the fourth quadrant. The pool was filled with 20 ± 2 °C water to a depth of 45 cm, and the platform was submerged 1.0 cm underwater. The experiment lasted 5 days, comprising an escape latency test and a spatial exploration test. During the 4-day escape latency test, each mouse underwent 3 trials per day with 60-second intervals. Mice were randomly placed in a quadrant and given 120 s to find and stay on the platform for 10 s. Mice failing to locate the platform were gently placed on it for 10 s, and their latency was recorded. On day 5, the platform was removed for the spatial exploration test. The software recorded the number of times each mouse passed the original platform position and the time spent in the target quadrant within 120 s.
Magnetic resonance image scanning
After behavioral testing, we selected group WT + NS, group AD + NS and group AD + SSO for BOLD imaging. To ensure the stability of the mice during the experiments to reduce movement artifacts, mice were anesthetized in an environment of isoflurane at a concentration of 1.5% until the end of the scanning, and their body temperatures were continuously monitored with a rectal probe and maintained at 37.0 ± 0.5 °C. The DASYLab software (National Instruments, Austin, TX, USA) was used to continuously monitor the respiratory rate, which was maintained at 110–130 breaths/minute. Functional magnetic resonance imaging (fMRI) imaging was performed on a 7.0T animal MRI scanner running ParaVision 6.0.1 (70/20 USR, Bruker Biospin MRI GmbH, Germany). BOLD data were acquired using echo planar imaging (EPI) sequence: Repetition time (TR) = 2000 ms, Echo time (TE) = 18.603 ms, Field of view (FOV) = 18 × 18 mm, 16 slices of 0.7 mm thickness, data matrix = 80 × 64, flip angle = 90°, repititions = 180, acquisition duration = 6 min.
Magnetic resonance imaging preprocessing
Data from the MRI scanner were exported as DICOM files and converted to NIFTI format for image data preprocessing using MATLAB 2014a and the SPM12 toolkit. The preprocessing steps included removing the first 20 time points, slice timing correction, head motion correction, image segmentation, spatial normalization, and spatial smoothing. After preprocessing, the time series of each voxel were filtered (0.01–0.1 Hz) to remove low-frequency drift and high-frequency noise. Whole-brain ALFF and ReHo for each mouse were then calculated using the DPABI package (DPABI_V6.0_210501). ALFF and ReHo were calculated using the spmIHEP toolkit61 for independent sample t-tests. Brain regions with significant ALFF and ReHo differences were identified based on voxel-level thresholds of P < 0.005 (uncorrected) and a cluster-extent threshold of 20 voxels.
Tissue sampling
After BOLD scanning, blood samples were collected into 1.5 ml centrifuge tubes immediately, allowed to coagulate at 25 ± 2 °C for 20 min, and centrifuged at 4 °C and 2000 r/min for 20 min. Brain tissues were bisected along the midline, with one portion washed in PBS and homogenized at a 1:9 ratio (tissue: PBS) on ice. The homogenate was centrifuged at 4 °C and 12,000 r/min for 20 min, and the supernatant was collected. The remaining brain tissue was fixed in 4% paraformaldehyde, dehydrated, and paraffin-embedded. The left lung was ligated, and the right lung lavaged with 1.0 ml of PBS via tracheal tube to collect BALF, achieving over 80% recovery. Fecal samples were collected in 2 ml tubes for colony analysis.
Biochemical analysis
ELISA kits were utilized to detect IL-6, IL-17, IL-1β, TNF-α, ACh, and AChE in both the blood supernatant and brain homogenate. The total protein content in the brain homogenate was determined using a BCA protein kit. The concentration in the blood supernatant was expressed as pg/ml, while that in the brain homogenate was expressed as pg/mg protein.
Staining of brain tissue sections
Paraffin-embedded brain tissues were sectioned to a thickness of 6 μm and prepared in a 42 °C spreader with a 60 °C roaster. Sections were stained with H&E, Nissl, Congo red, Aβ protein, and tyrosine hydroxylase (Supplementary File 1). Stained tissues were observed under a microscope, and images were captured. Using ImageJ (v1.52a, NIH, USA), we quantified the percentage of Congo red and Aβ-positive areas and the average fluorescence intensity of tyrosine hydroxylase.
Staining of lung tissue sections and BALF biochemical analysis
BALF was centrifuged (1500 × g for 10 min at 4 °C), and the supernatant was analyzed for IL-6, TNF-α, and total protein concentration. Lungs were perfused with 4% paraformaldehyde at 20 cm H2O via endotracheal intubation, dehydrated with ethanol, and paraffin-embedded. Sections of 5 μm were cut for H&E staining to observe histopathological changes under a microscope.
Detection of fecal flora
DNA was extracted using CTAB, and 16 S rRNA genes (V3-V4 regions) were amplified with specific primers (341 F and 806R) and sequenced on the Illumina NovaSeq PE250 platform. Sequences were processed using the DADA2 algorithm for length filtering and denoising, yielding ASV sequences and abundance tables. Alpha diversity of gut microbiota was analyzed, and species were annotated using the Greengenes database with a confidence threshold of 0.7.
Statistical analysis
All analyses were conducted using GraphPad Prism 10.0.2 (San Diego, CA, USA). Data are presented as the mean ± standard deviation. Normality of the data was assessed using the Shapiro-Wilk test. For the latency data across multiple days in the Morris water maze, repeated measures ANOVA was performed to account for within-subject variability over time, followed by Tukey’s post hoc tests for pairwise comparisons. The other data were analyzed using one-way ANOVA followed by Tukey’s post hoc test to detect inter-group differences. For comparisons between two groups, independent-sample t-tests were used for normally distributed data, and the Mann-Whitney U test was employed for non-normally distributed data. Statistical significance was defined as P < 0.05. For voxel-level analysis, the threshold was set at P < 0.005 (uncorrected) with a cluster-extent threshold of 20 voxels.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Files).
References
Supraja, P. et al. Towards point-of-care diagnosis of Alzheimer’s disease: multi-analyte based portable chemiresistive platform for simultaneous detection of β-amyloid (1–40) and (1–42) in plasma. Biosens. Bioelectron. 186, 113294. https://doi.org/10.1016/j.bios.2021.113294 (2021).
De-Paula, V. J., Radanovic, M., Diniz, B. S. & Forlenza, O. V. Alzheimer’s disease. Subcell. Biochem. 65, 329–352. https://doi.org/10.1007/978-94-007-5416-4_14 (2012).
Calsolaro, V. & Edison, P. Neuroinflammation in Alzheimer’s disease: current evidence and future directions. Alzheimers Dement. 12, 719–732. https://doi.org/10.1016/j.jalz.2016.02.010 (2016).
Simard, A. R., Soulet, D., Gowing, G., Julien, J. P. & Rivest, S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron 49, 489–502. https://doi.org/10.1016/j.neuron.2006.01.022 (2006).
Baik, S. H., Kang, S., Son, S. M. & Mook-Jung, I. Microglia contributes to plaque growth by cell death due to uptake of amyloid β in the brain of Alzheimer’s disease mouse model. Glia 64, 2274–2290. https://doi.org/10.1002/glia.23074 (2016).
Ramos-Rodriguez, J. J. et al. Rapid β-Amyloid deposition and cognitive impairment after cholinergic denervation in APP/PS1 mice. J. Neuropathol. Exp. Neurol. 72, 272–285. https://doi.org/10.1097/NEN.0b013e318288a8dd (2013).
Kirvell, S. L., Esiri, M. & Francis, P. T. Down-regulation of vesicular glutamate transporters precedes cell loss and pathology in Alzheimer’s disease. J. Neurochem. 98, 939–950. https://doi.org/10.1111/j.1471-4159.2006.03935.x (2006).
Fernández-Tomé, P., Brera, B., Arévalo, M. A. & de Ceballos, M. L. Beta-amyloid25-35 inhibits glutamate uptake in cultured neurons and astrocytes: modulation of uptake as a survival mechanism. Neurobiol. Dis. 15, 580–589. https://doi.org/10.1016/j.nbd.2003.12.006 (2004).
Passeri, E. et al. Alzheimer’s disease: treatment strategies and their limitations. Int. J. Mol. Sci. 23, 89. https://doi.org/10.3390/ijms232213954 (2022).
Briggs, R. & Kennelly, S. P. O’Neill, D. Drug treatments in Alzheimer’s disease. Clin. Med. (Lond). 16, 247–253. https://doi.org/10.7861/clinmedicine.16-3-247 (2016).
Ruan, S., Zhou, Y., Jiang, X., Gao, H. & Rethinking, C. R. I. T. I. D. Procedure of Brain Targeting Drug Delivery: circulation, blood brain barrier Recognition, Intracellular Transport, diseased cell targeting, internalization, and drug release. Adv. Sci. (Weinh) 8, 2004025. https://doi.org/10.1002/advs.202004025 (2021).
Dong, X. Current strategies for brain drug delivery. Theranostics 8, 1481–1493. https://doi.org/10.7150/thno.21254 (2018).
Sweeney, M. D., Kisler, K., Montagne, A., Toga, A. W. & Zlokovic, B. V. The role of brain vasculature in neurodegenerative disorders. Nat. Neurosci. 21, 1318–1331. https://doi.org/10.1038/s41593-018-0234-x (2018).
Pardridge, W. M. Drug transport across the blood-brain barrier. J. Cereb. Blood Flow. Metab. 32, 1959–1972. https://doi.org/10.1038/jcbfm.2012.126 (2012).
Ali, T. B., Schleret, T. R., Reilly, B. M., Chen, W. Y. & Abagyan, R. Adverse effects of Cholinesterase inhibitors in Dementia, according to the Pharmacovigilance databases of the United-States and Canada. PLoS One 10, e0144337. https://doi.org/10.1371/journal.pone.0144337 (2015).
Djupesland, P. G., Messina, J. C. & Mahmoud, R. A. The nasal approach to delivering treatment for brain diseases: an anatomic, physiologic, and delivery technology overview. Ther. Deliv. 5, 709–733. https://doi.org/10.4155/tde.14.41 (2014).
Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics (review). Mol. Med. Rep. 20, 1479–1487. https://doi.org/10.3892/mmr.2019.10374 (2019).
Hamid, M. & Zahid, S. Ameliorative effects of probiotics in AlCl(3)-induced mouse model of Alzheimer’s disease. Appl. Microbiol. Biotechnol. 107, 5803–5812. https://doi.org/10.1007/s00253-023-12686-y (2023).
Farlow, M. et al. Safety and tolerability of donepezil 23 mg in moderate to severe Alzheimer’s disease. BMC Neurol. 11, 57. https://doi.org/10.1186/1471-2377-11-57 (2011).
Suryakumar, G. & Gupta, A. Medicinal and therapeutic potential of Sea Buckthorn (Hippophae rhamnoides L). J. Ethnopharmacol. 138, 268–278. https://doi.org/10.1016/j.jep.2011.09.024 (2011).
Numakawa, T. Possible protective action of neurotrophic factors and natural compounds against common neurodegenerative diseases. Neural Regen Res. 9, 1506–1508. https://doi.org/10.4103/1673-5374.139474 (2014).
Zhu, X. et al. Effect of seabuckthorn seed protein and its arginine-enriched peptides on combating memory impairment in mice. Int. J. Biol. Macromol. 232, 123409. https://doi.org/10.1016/j.ijbiomac.2023.123409 (2023).
Basu, M. et al. Anti-atherogenic effects of seabuckthorn (Hippophaea rhamnoides) seed oil. Phytomedicine 14, 770–777. https://doi.org/10.1016/j.phymed.2007.03.018 (2007).
Keller, L. A., Merkel, O. & Popp, A. Intranasal drug delivery: opportunities and toxicologic challenges during drug development. Drug Delivery Translational Res. 12, 735–757. https://doi.org/10.1007/s13346-020-00891-5 (2022).
Mittal, D. et al. Insights into direct nose to brain delivery: current status and future perspective. Drug Deliv. 21, 75–86. https://doi.org/10.3109/10717544.2013.838713 (2014).
Touitou, E., Natsheh, H., Boukeileh, S. & Awad, R. Short onset and enhanced analgesia following nasal administration of non-controlled drugs in nanovesicular systems. Pharmaceutics 13, 89. https://doi.org/10.3390/pharmaceutics13070978 (2021).
Chakraborty, S. et al. Chitosan nanoparticle-mediated nose-to-brain delivery of naringenin: attenuating memory decline in experimental animals via behavioural assessment and modulation of biochemical parameters. Int. J. Biol. Macromol. 286, 138336. https://doi.org/10.1016/j.ijbiomac.2024.138336 (2025).
Lee, D., Shen, A. M., Shah, M., Garbuzenko, O. B. & Minko, T. Donepezil, memantine, and BACE-1 siRNA for Alzheimer’s disease therapy. Int. J. Mol. Sci. 25, 85. https://doi.org/10.3390/ijms251910357 (2024). Vivo Evaluation of Nose-to-Brain Delivery of Liposomal.
Zielińska, A. & Nowak, I. Abundance of active ingredients in sea-buckthorn oil. Lipids Health Dis. 16, 95. https://doi.org/10.1186/s12944-017-0469-7 (2017).
Zhao, H. et al. Polysaccharides from sea buckthorn (Hippophae rhamnoides L.) berries ameliorate cognitive dysfunction in AD mice induced by a combination of d-gal and AlCl(3) by suppressing oxidative stress and inflammation reaction. J. Sci. Food Agric. 103, 6005–6016. https://doi.org/10.1002/jsfa.12673 (2023).
Mulati, A. et al. Isorhamnetin attenuates high-fat and high-fructose diet induced cognitive impairments and neuroinflammation by mediating MAPK and NFκB signaling pathways. Food Funct. 12, 9261–9272. https://doi.org/10.1039/d0fo03165h (2021).
Mulati, A. et al. Sea-Buckthorn flavonoids Alleviate High-Fat and High-Fructose Diet-Induced Cognitive impairment by inhibiting insulin resistance and neuroinflammation. J. Agric. Food Chem. 68, 5835–5846. https://doi.org/10.1021/acs.jafc.0c00876 (2020).
Hyman, B. T. et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 8, 1–13. https://doi.org/10.1016/j.jalz.2011.10.007 (2012).
Chen, Y. & Colonna, M. Two-faced behavior of microglia in Alzheimer’s disease. Nat. Neurosci. 25, 3–4. https://doi.org/10.1038/s41593-021-00963-w (2022).
Liu, L. et al. Degenerative alterations in noradrenergic neurons of the locus coeruleus in Alzheimer’s disease. Neural Regen Res. 8, 2249–2255. https://doi.org/10.3969/j.issn.1673-5374.2013.24.004 (2013).
Wang, Z. et al. Spatial patterns of intrinsic brain activity in mild cognitive impairment and Alzheimer’s disease: a resting-state functional MRI study. Hum. Brain Mapp. 32, 1720–1740. https://doi.org/10.1002/hbm.21140 (2011).
Fox, M. D. & Raichle, M. E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 8, 700–711. https://doi.org/10.1038/nrn2201 (2007).
Yan, Y. et al. White Matter Changes as an independent predictor of Alzheimer’s Disease. J. Alzheimers Dis. 93, 1443–1455. https://doi.org/10.3233/jad-221037 (2023).
Yue, J. et al. Functional brain activity in patients with amnestic mild cognitive impairment: an rs-fMRI study. Front. Neurol. 14, 1244696. https://doi.org/10.3389/fneur.2023.1244696 (2023).
Yuan, X. et al. Regional homogeneity changes in amnestic mild cognitive impairment patients. Neurosci. Lett. 629, 1–8. https://doi.org/10.1016/j.neulet.2016.06.047 (2016).
Zhang, Z. et al. Altered spontaneous activity in Alzheimer’s disease and mild cognitive impairment revealed by Regional Homogeneity. Neuroimage 59, 1429–1440. https://doi.org/10.1016/j.neuroimage.2011.08.049 (2012).
He, Y. et al. Regional coherence changes in the early stages of Alzheimer’s disease: a combined structural and resting-state functional MRI study. NeuroImage 35, 488–500. https://doi.org/10.1016/j.neuroimage.2006.11.042 (2007).
Scheltens, P. et al. Alzheimer’s disease. Lancet 388, 505–517. https://doi.org/10.1016/s0140-6736(15)01124-1 (2016).
Bakhtiari, A. et al. Early cerebral amyloid-β accumulation and hypermetabolism are associated with subtle cognitive deficits before accelerated cerebral atrophy. Geroscience 46, 769–782. https://doi.org/10.1007/s11357-023-01031-w (2024).
Cohen, A. D. et al. Basal cerebral metabolism may modulate the cognitive effects of Abeta in mild cognitive impairment: an example of brain reserve. J. Neurosci. 29, 14770–14778. https://doi.org/10.1523/jneurosci.3669-09.2009 (2009).
Smith, E. E. & Greenberg, S. M. Beta-amyloid, blood vessels, and brain function. Stroke 40, 2601–2606. https://doi.org/10.1161/strokeaha.108.536839 (2009).
Shah, D. et al. Early pathologic amyloid induces hypersynchrony of BOLD resting-state networks in transgenic mice and provides an early therapeutic window before amyloid plaque deposition. Alzheimers Dement. 12, 964–976. https://doi.org/10.1016/j.jalz.2016.03.010 (2016).
Yoon, S. H. et al. Comparative study of lung toxicity of E-cigarette ingredients to investigate E-cigarette or vaping product associated lung injury. J. Hazard. Mater. 445, 130454. https://doi.org/10.1016/j.jhazmat.2022.130454 (2023).
Madison, M. C. et al. Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine. J. Clin. Invest. 129, 4290–4304. https://doi.org/10.1172/jci128531 (2019).
Wang, J., Ren, C., Jin, L. & Batu, W. Seabuckthorn Wuwei Pulvis attenuates chronic obstructive pulmonary disease in rat through gut microbiota-short chain fatty acids axis. J. Ethnopharmacol. 314, 116591. https://doi.org/10.1016/j.jep.2023.116591 (2023).
Xu, Y. et al. Isorhamnetin alleviates Airway inflammation by regulating the Nrf2/Keap1 pathway in a mouse model of COPD. Front. Pharmacol. 13, 860362. https://doi.org/10.3389/fphar.2022.860362 (2022).
Huo, J. et al. Protective effects of Natural polysaccharides on Intestinal Barrier Injury: a review. J. Agric. Food Chem. 70, 711–735. https://doi.org/10.1021/acs.jafc.1c05966 (2022).
Mancuso, C. & Santangelo, R. Alzheimer’s disease and gut microbiota modifications: the long way between preclinical studies and clinical evidence. Pharmacol. Res. 129, 329–336. https://doi.org/10.1016/j.phrs.2017.12.009 (2018).
Cattaneo, A. et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging. 49, 60–68. https://doi.org/10.1016/j.neurobiolaging.2016.08.019 (2017).
Tang, W. et al. Roles of gut microbiota in the regulation of hippocampal plasticity, inflammation, and Hippocampus-Dependent behaviors. Front. Cell. Infect. Microbiol. 10, 611014. https://doi.org/10.3389/fcimb.2020.611014 (2020).
Shen, L., Liu, L. & Ji, H. F. Alzheimer’s disease histological and behavioral manifestations in transgenic mice correlate with specific gut Microbiome State. J. Alzheimers Dis. 56, 385–390. https://doi.org/10.3233/jad-160884 (2017).
Beilharz, J. E., Kaakoush, N. O., Maniam, J. & Morris, M. J. The effect of short-term exposure to energy-matched diets enriched in fat or sugar on memory, gut microbiota and markers of brain inflammation and plasticity. Brain Behav. Immun. 57, 304–313. https://doi.org/10.1016/j.bbi.2016.07.151 (2016).
Nguyen, V. T. T., Sallbach, J., Dos Santos Guilherme, M. & Endres, K. Influence of acetylcholine esterase inhibitors and memantine, clinically approved for alzheimer’s dementia treatment, on intestinal properties of the mouse. Int. J. Mol. Sci. 22, 89. https://doi.org/10.3390/ijms22031015 (2021).
Lan, Y. et al. Sea Buckthorn polysaccharide ameliorates high-fat diet induced mice neuroinflammation and synaptic dysfunction via regulating gut dysbiosis. Int. J. Biol. Macromol. 236, 123797. https://doi.org/10.1016/j.ijbiomac.2023.123797 (2023).
Balasubramanian, R. et al. Involvement of microbiome gut-brain axis in neuroprotective effect of quercetin in mouse model of repeated mild traumatic brain injury. Neuromol. Med. 25, 242–254. https://doi.org/10.1007/s12017-022-08732-z (2023).
Nie, B. et al. A stereotaxic MRI template set of mouse brain with fine sub-anatomical delineations: application to MEMRI studies of 5XFAD mice. Magn. Reson. Imaging. 57, 83–94. https://doi.org/10.1016/j.mri.2018.10.014 (2019).
Acknowledgements
We thank Yingkun Zhou, MS (Department of general surgery, The First Affiliated Hospital of Soochow University, Soochow, China) and Xiaonan Yang, MS (Department of Radiology, The Affiliated Hospital of Qingdao University, Qingdao, China), who provided support for this research.
Funding
This study has received funding by the Shandong Provincial Natural Science Foundation (ZR2023MH284).
Author information
Authors and Affiliations
Author notes
These authors contributed equally: Ruichen Ren and Gaorui Zhang.
Contributions
Ruichen Ren: Data curation, Formal Analysis, Writing – original draft. Gaorui Zhang: Formal Analysis, Writing – review & editing. Junqing Ma: Data curation, Validation. Yongze Zheng: Data curation, Validation. Yuxuan Zhao: Data curation, Supervision, Validation. Yang Zhang: Investigation, Funding, Writing – review & editing. Lin Zhao: Investigation, Methodology, Validation. All authors reviewed the manuscript. All data were generated in-house, and no paper mill was used. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study adhered to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines, ensuring ethical treatment of the mice involved. The euthanasia procedures complied with these guidelines, and the study received approval from the Ethics Committee of Shandong University (Ethical Approval No. DWLL-2024-091). Mice were anesthetized using inhalation of isoflurane. All methods employed in this study were conducted in strict accordance with relevant guidelines and regulations.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ren, R., Zhang, G., Ma, J. et al. Nebulized seabuckthorn seed oil inhalation attenuates Alzheimer’s disease progression in APP/PS1 mice. Sci Rep 15, 6368 (2025). https://doi.org/10.1038/s41598-025-89747-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-89747-x
Keywords
This article is cited by
-
The long-term neuroprotective effect of MIND and Mediterranean diet on patients with Alzheimer’s disease
Scientific Reports (2025)