Abstract
Nowadays, due to the high demand and cost of conventional ingredients like fishmeal, as well as the need to create nutritionally effective diets for shrimp, alternative ingredients are being actively explored. In this study, novel feed ingredients were selected, and an eight-week feeding trial was carried out to assess the impact of diets formulated with different inclusion levels of novel ingredient combination. The study evaluated the growth performance, digestibility, digestive enzyme activity, and IGF-I and IGF-II gene expression in Penaeus vannamei. Four isonitrogenous (crude protein, 36%), isolipidic (crude fat, 6%), and isoenergetic (gross energy, 16 MJ/Kg) diets were formulated. The ingredients, namely poultry byproduct meal (PBM), insect meal (IM), rapeseed meal (RM), peanut meal (PM), fish waste (FW) and single cell protein (SCP) were incorporated into the diets at 24.82% (Diet 1), 28.82% (Diet 2), 32.82% (Diet 3) and 36.82% (Diet 4). A total of 420 shrimps (average initial weight of 1 g) were distributed into 12 tanks (35 shrimp per tank). The experimental diets were fed to triplicate groups of P. vannamei three times a day until satiation for 60 days. Among the dietary groups, significantly (p < 0.05) higher weight gain, final weight, ADG and SGR were observed in shrimp fed with Diet 3 and 4 than other diets. FCR, PER and survival remain unaffected with no significant difference (p > 0.05). In hepatopancreas, digestive enzyme such as lipase shows significantly higher activity in shrimp fed with Diet 4, which was not different from Diet 3 (p < 0.05). In the intestine, significantly higher amylase activity was found in Diets 3 and 4, which was not different from Diet 1. Likewise, lipase activity was significantly higher in Diets 3 and 4 in the intestine (p < 0.05). No significant difference (p > 0.05) was found in whole-body proximate composition, gene expression activities and apparent digestibility coefficients of shrimp fed with varying inclusion levels of novel ingredient combination. The results of this study revealed that the growth performance and nutrient utilization of P. vannamei remain unaffected, even with the inclusion of novel ingredient combination at a level of 36.82%. Therefore, this research suggests that the potential of the novel ingredient combination as a sustainable and cost-effective alternative to conventional, increasingly expensive ingredients without adverse effects. Even at maximum inclusion levels, the combination proved beneficial for shrimp, making it a promising solution for future feed formulations.
Similar content being viewed by others
Introduction
The worldwide consumption of edible fish grew at an annual rate of 3.0% from 1961 to 2019, nearly twice the rate of global population growth1,2. Aquaculture has developed quickly as a result of this increase in demand, especially through intensified culture, which reached a historic high of 87.5 million tonnes in 20202. Intense aquaculture accounted for 49.2% of worldwide seafood output.
Penaeus vannameiis a widely cultured crustacean all over the world due to its wide endurance to temperature and salinity3. Fish oil (FO) and fish meal (FM) are important dietary components and nutrient inputs used by feed manufacturers worldwide to prepare aquafeeds4. Although more sources with high protein are available, fishmeal is still recommended because of its high nutritional content, more easily absorbed crude protein and good balanced amino acid composition5. However, the use of fishmeal in diets is clearly trending lower due to factors such as rising global demand, rising costs, supply fluctuations, as well as sustainability difficulties6,7. Therefore, the quest for a substitute for fishmeal in shrimp diets has been the focus of numerous studies8,9. The amount of fishmeal produced over the last ten years has stabilized at about five million tons, and advancements in FM processing methods have made it easier to produce and distribute FO and FM that are obtained from fish byproducts2. The constrained global availability as well as the elevated price of fishmeal have led to a boom in the development and use of alternative protein sources over the past two decades10,11. Fishmeal has been replaced in shrimp diet by a wide range of different sources of protein that have been investigated until now. These include sources of protein found in plants, fisheries and aquaculture byproducts; land animal products such as insects and food industry coproducts; microalgae; low trophic species such as krill; and microbial biomass such as bacteria and yeast. In the production of shrimp feed, this might decrease our dependence on FM12.
As the demand and cost of conventional feed ingredients like fishmeal continue to rise, there is an increasing need to search for alternative sources with nutritional values comparable to fishmeal. Novel feed ingredients refer to alternative ingredients used in animal feed that are obtained from non-conventional sources. These ingredients are incorporated into animal diets to supplement conventional feed ingredients, aiming to improve sustainability, reduce costs, and meet the nutritional requirements of shrimp. Research on novel feed ingredients is focused on enhancing the efficiency and environmental sustainability of animal production. However, limited research has been conducted in this area13,14,15. By identifying viable alternatives, we can reduce much dependence on conventional ingredients like fishmeal16. While unconventional feed research has a long history, current efforts are intensifying worldwide, driven by both researchers and industries. They are developing novel ingredients based on circular economy principles to maximize resource efficiency and achieve zero waste in the agro-food value chain while emphasizing cost-effectiveness. Instead of replacing fishmeal with a single ingredient, the emerging approach combines various novel ingredients to meet animal’s dietary needs. The nutritional content of shrimp may be better when different novel feed ingredients are combined than when they are used individually.
The novel feed ingredients chosen for this research include poultry byproduct meal (PBM), insect meal (IM), fish waste (FW) (by-products such as discarded fish fillets, trimmings, and offal), rapeseed meal (RM), peanut meal (PM), and single-cell protein (SCP). These ingredients were combined using a concept that integrates plant, animal, and microbial-based ingredients12,17,18 at different inclusion levels. The various inclusion levels of the novel ingredient combination in the experimental diets are as follows: Diet 1 contains 248.2 g, Diet 2 contains 288.2 g, Diet 3 contains 328.2 g, and Diet 4 contains 368.2 g of the novel ingredient combination per kg of experimental diet.
Insect meal is a sustainable and cost-effective alternative to fish meal and plant-based diets in shrimp farming. It requires minimal land, water, and energy and serves as a highly viable protein source, rich in essential amino acids, under controlled farming conditions19,20,21. Poultry by-product meal (PBM) is rich in protein and essential amino acids, except for lysine and methionine22,23,24. Peanut meal, known for its high protein and arginine content, offers a palatable and economical substitute for fish meal and soybean meal in aquafeeds25,26. Rapeseed meal is a promising alternative to other plant proteins in aquafeeds due to its balanced amino acid profile, global availability, and cost-effectiveness27. Single-cell proteins (SCPs), derived from microorganisms such as microalgae, yeast, and bacteria, help reduce environmental waste and offer an effective alternative to conventional protein sources in aquaculture28,29. Fish waste (discarded fish fillets, trimmings and offal) biomass is a rich source of bio functional compounds such as omega-3 oils, enzymes, polysaccharides, gelatin, and bioactive peptides30,31, although its biochemical composition, including protein, ash, and lipid levels, varies depending on the source species32.
Additional protein sources were used in place of FM in practical diets by combining novel feed ingredients at different inclusion levels that were developed specifically for this study. This helped to compensate for nutrient deficiencies in a single ingredient and also leads the better nutritional value of novel protein sources. It is important to maintain nutritional balance in FM-free diets, as demonstrated by this study on analysing the utilization of novel protein sources at different inclusion levels in shrimp feed. It highlights areas for further research on FM replacement and points out the limitations of the current studies. Therefore, this research was done to assess the effects of diets formulated with different inclusion levels of novel ingredient combination on growth performance, whole body proximate composition, apparent digestibility, digestive enzymes and gene expression of P. vannamei.
Materials and methods
Shrimp and experimental conditions
A 32 m³ nursery tank with continuous aeration was used to raise 1,000 post-larvae (PL 12) of Penaeus vannameito the juvenile stage (1 g). The larvae were obtained from Star Aqua Hatchery in Koovathur, Chengalpattu, Tamil Nadu, India. The shrimp were fed a commercial diet (Royal Dragon DT311, Sheng Long Biotech International Co., Ltd., India) containing 37% protein at four intervals a day (at 9:00, 10:00, 14:00, and 18:00 h) throughout their acclimatization. An eight-week feeding trial was carried out at the wet lab of the Institute of Fisheries Post Graduate Studies, TNJFU, Vaniyanchavadi, India. After proper acclimatization, the shrimp were gradually introduced into the experimental tanks, where shrimp in the treatment groups were fed the prepared diets till apparent satiation four times daily (09:00, 10:00, 14:00, and 18:00 h). Weekly checks were conducted on shrimp weight, survival, and health. Feed rations were adjusted based on weight to minimize uneaten feed. Brackish water from the wet lab (salinity: 15 ± 1 ppt) was used, with water exchanges performed every third day. Daily monitoring of water quality during the trial recorded the following values: salinity (15.01 ± 0.71 ppt) was measured with a refractometer, temperature (28.43 ± 0.50 °C) measured with a digital thermometer, dissolved oxygen (6.21 ± 0.81 ppm) measured with a dissolved oxygen (DO) meter, and pH (8.24 ± 0.43) measured with a pH meter. Total ammonia N (0.01 ± 0.01 ppm), nitrite-N (0.05 ± 0.01 ppm), and nitrate N (10 ± 0.1 ppm) were measured according to APHA33 standards.
Diet preparation
The formulation and the nutrient composition of the experimental diets are shown in Table 1. Four isonitrogenous (crude protein, 36%), isolipidic (crude fat, 6%), and isoenergetic (gross energy, 16 MJ/Kg) diets were formulated with ingredients, namely poultry byproduct meal (PBM), insect meal (IM), rapeseed meal (RM), peanut meal (PM), fish waste (FW), and single-cell protein (SCP), at 24.82% (Diet 1), 28.82% (Diet 2), 32.82% (Diet 3), and 36.82% (Diet 4). The dry feed components were all finely pulverized in a pulverizer, passed through a 180-micron mesh screen, and mixed well. Then, the ground ingredients were combined with the oil sources (fish oil, soy lecithin), all of the additives, and the necessary amount of water. The mixture was vigorously mixed for 15 min using an electric blender (Gaocheng-GC-MT1200, Mogli Labs, India Pvt. Ltd.) to achieve homogeneity. The feed included a chromic oxide marker so that the apparent digestibility coefficient (ADC) could be determined. The soft dough was properly mixed and then cooked for 15 min at 80 °C. After cooling, the soft dough was pelletized using a 1.6 mm die in a tabletop pelletizer. The pellets were then air-dried for 12 h at 45 °C to achieve the appropriate moisture content. At last, every dried feed was put into an airtight plastic container and kept at 4 °C until needed.
Growth parameters and sample collection
Shrimp were mass-weighed, counted, and anesthetized with MS-222 (Sigma-Aldrich Inc.) after the growth trial was over. Calculations were made for mean final body weight, weight gain, FCR, SGR, ADG, PER, and survival rate. For the purpose of gene expression analysis and digestive enzyme assays, nine shrimp per treatment (three per replicate) were chosen at random, and the remaining shrimp were utilized for evaluation of whole-body chemical composition. The growth parameters computed on the basis of the collected data were listed below:
Proximate analysis
Standard methods were used to evaluate the dry matter, crude protein, ether extract, and ash in the diets, whole body, and faeces, according to AOAC34. A final whole-body chemical composition assessment was conducted on 10 randomly selected shrimp from each tank (30 shrimp per treatment) at the termination of the growth trial.
Faeces collection
The control and experimental diets were hand-fed to the shrimp four times daily at 09:00, 10:00, 14:00, and 18:00 h, until the shrimp appeared satiated. After a one-week acclimation period, faecal samples were collected according to the method described by Lin et al.35. After each feeding, one hour later, unconsumed leftover feed and faeces were disposed of. Faecal samples were drawn twice a day at 12:00 and 16:00 h from every replicate. The samples were then combined according to treatment, oven-dried, and kept for chemical examination.
Determination of digestibility
Chromic oxide (Cr2O3), an inert marker, was used to calculate the ADC, or apparent digestibility coefficient, of the diets in the study at a dose of 5 g/kg of feed. An inductively coupled plasma atomic emission spectrometer was used to determine the amount of chromium present in diets and faecal samples. In accordance with method of Cho et al.36, the apparent digestibility coefficient (ADC) of diets’ dry matter, protein, and lipid was computed as follows:
Quantification of Digestive enzymes
After removing the hepatopancreas from three shrimp and midgut of intestine tissue samples from five shrimp, the materials were homogenized with a 0.25 M sucrose solution and centrifuged for 10 min at 6000 rpm. For the examination of digestive enzymes, such as lipase, amylase, and protease, the supernatant was maintained at −20°C. Lipase activity was measured as the amount of NaOH needed to neutralize released fatty acids per minute of incubation, following the method of Cherry and Crandall37. Protease activity was measured as µmol of tyrosine released per milligram of protein per minute, with casein serving as the substrate, according to Drapeau38. Amylase activity was measured using 1% starch and DNS according to Rick and Stegbauer39, quantified by a maltose standard curve, and expressed as µmol of maltose per minute per mg of protein. Total protein content was determined following the method of Bradford40.
Quantitative real time PCR (qRT-PCR)
Hepatopancreas tissue from three shrimp in each group was collected at the end of the experiment under cold, sterile conditions and preserved at −80 °C to analyse the gene expression of IGF-I and IGF-II. As directed by the manufacturer, total RNA was extracted using TRIzol (easy-RED). Samples were used for cDNA synthesis after RNA quality (A260/A280 ratio ≥ 1.8) was assessed using NanoDrop. DNase-treated RNA, reverse transcriptase, and a heat cycler were used in the PCR amplification process. Melting curve analysis (62–95 °C) was used for real-time PCR. Using the 2-ΔΔCt technique according to Livak and Schmittgen41, gene expression was measured and normalized to β-actin. Table 2 lists the primers that were used in the gene expression.
Statistical computation
To find significant differences between treatments, all findings were submitted to a one-way analysis of variance, which was followed by Duncan’s multiple range tests using SPSS version 20 (SPSS Inc., Chicago, IL, USA). When a P value was 0.05 or below, the treatment effects were deemed significant.
Ethical approval
The Institutional Animal Ethics Committee (IAEC) of Tamil Nadu Dr. J. Jayalalithaa Fisheries University in Nagapattinam has approved this research under the grant number 3/1128/IAEC/TNJFU/IFPGS.
Results
Growth performance and feed utilization
The growth performance of P. vannamei is summarized in Table 3. Shrimp fed diet 3 and 4 showed significantly higher final weight, weight gain, average daily growth, and specific growth rate (p < 0.05). However, no significant differences were observed in survival, PER, or FCR (p > 0.05) of P. vannamei fed diets formulated with different inclusion levels of novel ingredient combination.
Whole-body proximate composition
The whole-body proximate composition of P. vannamei is presented in Table 4. No statistically significant difference was found in the whole-body proximate composition, such as crude protein, crude lipid, moisture, and ash (p > 0.05) of P. vannamei fed diets formulated with different inclusion levels of novel ingredient combination.
Determination of digestibility
Apparent digestibility coefficient (ADC) of dry matter, crude protein, and crude lipid of Penaeus vannamei are shown in Table 5. The results indicate that there was no statistically significant difference in the digestibility of dry matter, crude protein, or crude lipid (p > 0.05) among shrimps fed diets formulated with different inclusion levels of novel ingredient combination.
Digestive enzyme activity
The activities of digestive enzymes, including amylase, protease, and lipase, in the hepatopancreas of Penaeus vannamei are shown in Table 6. The amylase and protease activities showed no statistically significant difference (p > 0.05) among shrimp fed diets formulated with varying inclusion levels of novel ingredient combination. In contrast, lipase activity was significantly higher in shrimp fed diet 4, which was not significantly different from diet 3 (p < 0.05).
The digestive enzyme activities, including amylase, protease, and lipase, in the intestine of Penaeus vannamei are shown in Table 7. The amylase activity was significantly increased in shrimp fed diet 3 and 4 than in shrimp fed with diet 1 (p < 0.05). Shrimp-fed diets 3 and 4 have shown significantly higher lipase activity compared to other diets, whereas protease activity was found with no significant difference in shrimp-fed diets formulated with varying inclusion levels of novel ingredient combination.
Growth gene expression
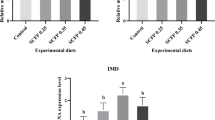
Growth genes such as IGF-I and IGF-II expression in Penaeus vannamei are illustrated in Figs. 1 and 2. IGF-I and IGF-II were neither upregulated nor downregulated (i.e.,) found with no significant difference (p < 0.05), in shrimp-fed diets formulated with different inclusion levels of novel ingredient combination.
Discussion
Several studies have examined the effects of incorporating individual ingredients with a nutritional profile nearly equivalent to fishmeal42,43, but there is limited research on the use of a combination of animal, plant, and microbial-based ingredients14,15. The purpose of this experiment was to investigate the impact of different inclusion levels of this novel ingredient combination. Supporting the results of better growth performance and nutrient utilization, shrimp fed Diet 4 exhibited significantly higher RBA and proPO activity (p> 0.05), indicating enhanced growth without compromising their health and immune status. The improved growth may also be attributed to the presence of balanced amino acids and fatty acids in Diet 4. Incorporating a single ingredient into a diet may sometimes lead to negative effects, so the concept of using a combination of ingredients helps mitigate these issues13,14. Studies have demonstrated that the use of ingredient combination, even at high inclusion levels44,15, can improve the growth and health of shrimp. In line with the results of previous studies and the findings of this study, the growth parameters observed suggest that Diet 4, formulated with a combination of novel feed ingredients (PBM, IM, FW, RM, PM, and SCP) at an inclusion level of 36.82%, effectively meets the nutritional requirements of shrimp without compromising their growth or nutrient utilization.
Whole-body carcass composition mostly depends on the nutrient composition of diets. The components of proximate, such as moisture, fat, protein, and ash, are crucial markers of an organism’s metabolic state. Higher protein and lipid content generally reflects greater energy density45. However, these components can differ widely depending on species, size, sex, feeding season, and physical activity46. In the present research, the whole-body proximate composition of P. vannameialigns with the findings of Manikandan and Felix18, who supplemented plant-based diets containing a corn gluten meal and soybean meal protein blend with dietary L-lysine and phytase to enhance the growth performance of P. vannamei and reported no significant differences in the shrimp’s proximate composition.
The breakdown and absorption of food and its nutrients depend critically on the activity of digestive enzymes47,48. According to earlier research49,50,51, diets formulated with varying inclusion levels of novel ingredient combination can either positively or negatively alter the activities of digestive enzymes. The present research revealed no significant alterations in the amylase and protease enzyme activities of the hepatopancreas (p > 0.05). In the same way, no statistically significant difference was noted in the intestinal protease activity (p > 0.05) of P. vannamei. On the other hand, the inclusion level of novel ingredient combination in diet 3 (32.82%) and diet 4 (36.82%) of P. vannamei was significantly higher in terms of hepatopancreas lipase activity (p < 0.05) and the same was found in intestinal amylase and lipase activities. These results can be correlated with the different digestibility, such as crude protein, crude lipid and dry matter digestibility, which has shown no significant difference (p > 0.05) in shrimp-fed diets formulated with different inclusion levels of novel ingredient combination in P. vannamei. The results of the digestive enzyme analysis in the present research showed a clear correlation with digestibility, indicating that the enzyme activity aligns closely with the observed digestibility findings. Therefore, the combination of ingredients, even at an inclusion level of 36.82%, did not adversely affect the nutrient digestion and absorption in P. vannamei, and all these were reflected in the results obtained in the present study.
Digestibility of feedstuffs is essential for assessing their utilization52. The amount of feed sample that is absorbed in an animal’s digestive tract is indicated by its digestibility35. In high-density culture conditions, where leftover that are not properly assimilated can lead to water pollution, higher water treatment costs, and increased susceptibility to shrimp illnesses and mortalities. Therefore, extremely digestible feedstuffs are particularly significant53. Determination of apparent digestibility of ingredients is crucial for evaluating the potential of novel protein sources54. Enhancing apparent digestibility coefficients (ADCs) through research and innovation is vital for the sustainable growth of aquaculture feed. In this study, no significant differences were found in the digestibility of crude protein, crude lipid, and dry matter (p> 0.05). This can be linked to the activity of digestive enzymes, such as amylase and protease in the hepatopancreas and protease in the intestine. These findings suggest that the digestive efficiency of shrimp fed diets with varying inclusion levels of novel ingredient combination was positively influenced by their nutritional composition, including the presence of balanced amino acids and fatty acids, which have beneficial effects55,56,57. Therefore, the observed trends in digestibility and the nutritional profile of the diets in the present study support the conclusion that the combination of novel feed ingredients, even at an inclusion level of 36.82%, did not negatively impact the digestibility capacity of P. vannamei.
Over the last decade, the inclusion levels of various feed ingredients have been researched according to growth statistics of animals used for research. The GH/IGF-Iaxis is a key signalling pathway for growth and nutrient distribution58 and plays roles in muscle differentiation, metabolism, behaviour, and immunity. In aquatic animals, dietary modifications can be modulated by the GH/IGF-I axis due to IGF-Imetabolic functions59. In this research, the IGF-I and IGF-II gene expression activities were not significantly (p > 0.05) affected by the diets formulated with different inclusion levels of novel ingredient combination in P. vannamei. These results could be attributed to better nutrient utilization, and required amino acids and fatty acids were met by the diet fed to shrimp. This result concluded that even at a 36.82% inclusion level of novel ingredient combination, the growth of P. vannamei was not negatively influenced.
Conclusion
The outcomes of the present research indicated that different inclusion levels of novel ingredient combination did not compromise the growth performance, whole-body composition, digestibility, digestive enzymes, and gene expression activities of P. vannamei. The 36.82% inclusion level of novel ingredient combination has improved the growth performance and nutrient utilization of P. vannamei without compromising growth and nutrient utilization. These findings suggest that alternative dietary formulations can effectively replace conventional ingredients, and furthermore, this study provides insights into the feasibility and sustainability of incorporating novel feed ingredient combination at varying inclusion levels in P. vannamei diets to optimize growth, nutrient utilization, and immune response, paving the way for improved shrimp feed formulations.
Data availability
All data of the research were provided within this manuscript.
References
FAO. Fishery and Aquaculture Statistics (2018).
FAO. The State of World Fisheries and Aquaculture (2022).
Landsman, A. et al. Impact of aquaculture practices on intestinal bacterial profiles of Pacific Whiteleg shrimp Litopenaeus vannamei. Microorganisms 7(4), 93. https://doi.org/10.3390/microorganisms7040093 (2019).
Hua, K. et al. The future of aquatic protein: Implications for protein sources in aquaculture diets. One Earth 1(3), 316–329 (2019).
Nunes, A. J., Sá, M. V., Browdy, C. L. & Vazquez-Anon, M. Practical supplementation of shrimp and fish feeds with crystalline amino acids. Aquaculture 431, 20–27. https://doi.org/10.1016/j.aquaculture.2014.04.003 (2014).
Aas, T. S., Ytrestøyl, T. & Åsgård, T. Utilization of feed resources in the production of Atlantic salmon (Salmo salar) in Norway: An update for 2016. Aquac Rep. 15, 100216. https://doi.org/10.1016/j.aqrep.2019.100216 (2019).
Tacon, A. G., Metian, M. & McNevin, A. A. Future feeds: Suggested guidelines for sustainable development. Rev. Fish. Sci. Aquac. 30(2), 135–142. https://doi.org/10.1080/23308249.2020.1860474 (2022).
McLean, E., Barrows, F. T., Craig, S. R., Alfrey, K. & Tran, L. Complete replacement of fishmeal by soybean and poultry meals in Pacific whiteleg shrimp feeds: Growth and tolerance to EMS/AHPND and WSSV challenge. Aquaculture 527, 735383. https://doi.org/10.1016/j.aquaculture.2020.735383 (2020).
Xie, J. J. et al. Fishmeal levels can be successfully reduced in white shrimp (Litopenaeus vannamei) if supplemented with DL-methionine (DL‐Met) or DL‐methionyl‐DL‐methionine (Met‐Met). Aquac. Nutr. 24(3), 1144–1152. https://doi.org/10.1111/anu.12653 (2018).
Sánchez-Muros, M. J. et al. Innovative protein sources in shrimp (Litopenaeus vannamei) feeding. Rev. Aquac. 12(1), 186–203. https://doi.org/10.1111/raq.12312 (2020).
Sookying, D., Davis, D. A. & Soller Dias da Silva, F. A review of the development and application of soybean-based diets for Pacific white shrimp Litopenaeus vannamei. Aquac Nutr. 19(4), 441–448. https://doi.org/10.1111/anu.12050 (2013).
Chen, Y. et al. Evaluation of the dietary black soldier fly larvae meal (Hermetia illucens) on growth performance, intestinal health, and disease resistance to Vibrio parahaemolyticus of the Pacific white shrimp (Litopenaeus vannamei). Front. Mar. Sci. 8, 706463. https://doi.org/10.3389/fmars.2021.706463 (2021).
Rajalakshmi, K. et al. Effects of diets formulated with different combinations of novel feed ingredients on growth performance, apparent digestibility, digestive enzymes and gene expression activities of Pacific white shrimp, Penaeus vannamei. Aquac Int. 33(1), 120 (2025).
Yang, P. et al. Evaluation of composite mixture of protein sources in replacing fishmeal for Pacific white shrimp (Litopenaeus vannamei): Based on the changing pattern of growth performance, nutrient metabolism and health status. Aquac. Rep. 21, 100914. https://doi.org/10.1016/j.aqrep.2021.100914 (2021).
Ye, J. D., Wang, K., Li, F. D., Sun, Y. Z. & Liu, X. H. Incorporation of a mixture of meat and bone meal, poultry by-product meal, blood meal and corn gluten meal as a replacement for fish meal in practical diets of Pacific white shrimp Litopenaeus vannamei at two dietary protein levels. Aquac Nutr. 17(2), 337–e347. https://doi.org/10.1111/j.1365-2095.2010.00768.x (2011).
Cai, Y. et al. Effects of fish meal replacement by three protein sources on physical pellet quality and growth performance of Pacific white shrimp (Litopenaeus vannamei). Aquac. Rep. 25101210. https://doi.org/10.1016/j.aqrep.2022.101210 (2022).
Eroldoğan, O. T. et al. From the sea to aquafeed: A perspective overview. Rev. Aquac. 15(3), 1028–1057. https://doi.org/10.1111/raq.12740 (2023).
Manikandan, K. & Felix, N. Evaluation of dietary supplementation of L-lysine and phytase in corn gluten meal-soybean meal-based diets for Pacific white shrimp Penaeus vannamei Boone, 1931). Indian J. Fish. 67(1), 1-91358. https://doi.org/10.21077/ijf.2019.67.1.91358-11 (2020).
Barroso, F. G. et al. The potential of various insect species for use as food for fish. Aquaculture 422, 193–201. https://doi.org/10.1016/j.aquaculture.2013.12.024 (2014).
Nogales-Mérida, S. et al. Insect meals in fish nutrition. Rev. Aquac. 11(4), 1080–1103. https://doi.org/10.1111/raq.12281 (2019).
Van Huis, A. Potential of insects as food and feed in assuring food security. Annu. Rev. Entomol. 58(1), 563–583 (2013).
González-Rodríguez, Á. et al. Evaluation of poultry by‐product meal as partial replacement of fish meal in practical diets for juvenile tench (Tinca tinca L). Aquac Res. 47(5), 1612–1621. https://doi.org/10.1111/are.12622 (2016).
Gupta, S. K. et al. Impact of varied combinatorial mixture of non-fishmeal ingredients on growth, metabolism, immunity and gut microbiota of Lates calcarifer (Bloch, 1790) fry. Sci. Rep. 10(1), 17091 (2020).
Zhou, Q. C., Zhao, J., Li, P., Wang, H. L. & Wang, L. G. Evaluation of poultry by-product meal in commercial diets for juvenile cobia (Rachycentron canadum). Aquaculture 322, 122–127. https://doi.org/10.1016/j.aquaculture.2011.09.042 (2011).
Batal, A., Dale, N. & Café, M. Nutrient composition of peanut meal. J. Appl. Poult. Res. 14(2), 254–257. https://doi.org/10.1093/japr/14.2.254 (2005).
NRC. Nutrition Requirements of Fish 114 (National Academy, 2013).
Friedt, W., Tu, J. & Fu, T. Academic and economic importance of Brassica napus rapeseed. The Brassica napus genome 1–20 (2018).
Barka, A. & Blecker, C. Microalgae as a potential source of single-cell proteins. A review. Base. https://doi.org/10.25518/1780-4507.13132 (2016).
Palmegiano, G. B. et al. Spirulina as a nutrient source in diets for growing sturgeon (Acipenser Baeri). Aquac Res. 36(2), 188–195. https://doi.org/10.1111/j.1365-2109.2005.01209.x (2005).
Messina, C. M., Renda, G., La Barbera, L. & Santulli, A. By-products of farmed European sea bass (Dicentrarchus labrax L.) as a potential source of n-3 PUFA. Biologia 68, 288–293 (2013).
Pangestuti, R. & Kim, S. K. Bioactive peptide of marine origin for the prevention and treatment of non-communicable diseases. Mar. Drugs 15(3), 67. https://doi.org/10.3390/md15030067 (2017).
Le Gouic, A. V., Harnedy, P. A. & FitzGerald, R. J. Bioactive peptides from fish protein by-products. Bioactive Molecules in Food 1–35 (2018).
APHA. Standard Methods for the Examination of Water and Waste Water 22nd edn (American Public Health Association, 2012).
AOAC. Association of Official Analytical Chemists–Official Methods of Analysis 18th edn (2010).
Lin, H. Z., Guo, Z., Yang, Y., Zheng, W. & Li, Z. J. Effect of dietary probiotics on apparent digestibility coefficients of nutrients of white shrimp Litopenaeus vannamei Boone. Aquac Res. 35(15), 1441–1447. https://doi.org/10.1111/j.1365-2109.2004.01169.x (2004).
Cho, C. Y., Slinger, S. J. & Bayley, H. S. Bioenergetics of salmonid fishes: Energy intake, expenditure and productivity. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 73(1), 25–41. https://doi.org/10.1016/0305-0491(82)90198-5 (1982).
Cherry, I. S. & Crandall Jr, L. A. The specificity of pancreatic lipase: Its appearance in the blood after pancreatic injury. Am. J. Physiol. Cell. Physiol. 100(2), 266–273. https://doi.org/10.1152/ajplegacy.1932.100.2.266 (1932).
Drapeau, G. R. [38] Protease from Staphyloccus aureus. In Methods in Enzymology vol. 45, 469–475 (Academic Press, 1976). https://doi.org/10.1016/S0076-6879(76)45041-3
Rick, W. & Stegbauer, H. P. α-Amylase measurement of reducing groups. In Methods of Enzymatic Analysis 885–890. Academic Press. https://doi.org/10.1016/B978-0-12-091302-2.50074-8 (1974).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72(1–2), 248–254. https://doi.org/10.1016/0003-2697(76)90527-3 (1976).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 – ∆∆CT method. Methods 25(4), 402–408. https://doi.org/10.1006/meth.2001.1262 (2001).
Cruz-Suárez, L. E. et al. Apparent dry matter, energy, protein and amino acid digestibility of four soybean ingredients in white shrimp Litopenaeus vannamei juveniles. Aquaculture 292(1–2), 87–94. https://doi.org/10.1016/j.aquaculture.2009.03.026 (2009).
Hernández, C. Partial replacement of fish meal by porcine meat meal in practical diets for Pacific white shrimp (Litopenaeus vannamei). Aquaculture 277(3–4), 244–250 (2008).
Guo, J., Wang, Y. & Bureau, D. P. Inclusion of rendered animal ingredients as fishmeal substitutes in practical diets for cuneate drum, Nibea miichthioides (Chu, Lo et Wu). Aquac. Nutr. 13(2), 81–87 (2007). https://doi.org/10.1111/j.1365-2095.2007.00456.x
Dempson, J. B., Schwarz, C. J., Shears, M. & Furey, G. Comparative proximate body composition of Atlantic salmon with emphasis on parr from fluvial and lacustrine habitats. J. Fish. Biol. 64(5), 1257–1271. https://doi.org/10.1111/j.0022-1112.2004.00389.x (2004).
Rosa, R. & Nunes, M. L. Biochemical composition of deep-sea decapod crustaceans with two different benthic life strategies off the Portuguese south coast. Deep Sea Res. Part. I Oceanogr. Res. Pap. 50(1), 119–130. https://doi.org/10.1016/S0967-0637(02)00147-4 (2003).
Deng, J. M. et al. Effects of replacing plant proteins with rubber seed meal on growth, nutrient utilization and blood biochemical parameters of tilapia (Oreochromis niloticus× O. Aureus). Aquac Nuttr. 23(1), 30–39. https://doi.org/10.1111/anu.12355 (2017).
Silva, F. C. et al. Influence of partial substitution of dietary fish meal on the activity of digestive enzymes in the intestinal brush border membrane of gilthead sea bream, Sparus aurata and goldfish, Carassius auratus. Aquaculture 306(1–4), 233–237. https://doi.org/10.1016/j.aquaculture.2010.05.018 (2010).
Feng, Q. F. et al. Effects of oxidized silkworm (Bombyx mori L.) pupae on growth performance, and intestine, liver and muscle histology and function of Gif Tilapia (Oreochromis niloticus). Aquac Res. 52(9), 4127–4137. https://doi.org/10.1111/are.15251 (2021).
Gangadhar, B., Umalatha, H., Ganesh, H., Saurabh, S. & Sridhar, N. Digestibility of dry matter and nutrients from three ingredients by the carps, Labeo fimbriatus (Bloch, 1795) and Cyprinus carpio Linnaeus, 1758 with a note on digestive enzyme activity. Indian J. Fish. 64(3), 75–84. https://doi.org/10.21077/ijf.2017.64.3.69091-11 (2017).
Hosseini Shekarabi, S. P., Shamsaie Mehrgan, M. & Banavreh, A. Feasibility of superworm, Zophobas Morio, meal as a partial fishmeal replacer in fingerling rainbow trout, Oncorhynchus mykiss, diet: Growth performance, amino acid profile, proteolytic enzymes activity and pigmentation. Aquac Nutr. 27(4), 1077–1088. https://doi.org/10.1111/anu.13249 (2021).
Akiyama, D. M., Coelho, S. R., Lawrence, A. L. & Robinson, E. H. Apparent digestibility of feedstuffs by the marine shrimp Penaeus vannamei BOONE. 日本水産学会誌 55(1), 91–98. https://doi.org/10.2331/suisan.55.91 (1989).
Lin, H. Z., Li, Z. J., Chen, Y. Q., Zheng, W. H. & Yang, K. Effect of dietary traditional Chinese medicines on apparent digestibility coefficients of nutrients for white shrimp Litopenaeus vannamei. Boone Aquaculture. 253(1–4), 495–501. https://doi.org/10.1016/j.aquaculture.2004.11.048 (2006).
Jannathulla, R., Dayal, J. S., Vasanthakumar, D., Ambasankar, K. & Muralidhar, M. Effect of fungal fermentation on apparent digestibility coefficient for dry matter, crude protein and amino acids of various plant protein sources in Penaeus vannamei. Aquac Nutr. 24(4), 1318–1329. https://doi.org/10.1111/anu.12669 (2018).
Li, X. et al. Evaluation of six novel protein sources on apparent digestibility in Pacific white shrimp, Litopenaeus vannamei. Aquac Nutr. 2022(1), 8225273. https://doi.org/10.1155/2022/8225273 (2022).
Oujifard, A., Seyfabadi, J., Kenari, A. A. & Rezaei, M. Growth and apparent digestibility of nutrients, fatty acids and amino acids in Pacific white shrimp, Litopenaeus vannamei, fed diets with rice protein concentrate as total and partial replacement of fish meal. Aquaculture 342, 56–61. https://doi.org/10.1016/j.aquaculture.2011.12.038 (2012).
Shin, J. & Lee, K. J. Digestibility of insect meals for Pacific white shrimp (Litopenaeus vannamei) and their performance for growth, feed utilization and immune responses. PloS One. 16(11), e0260305. https://doi.org/10.1371/journal.pone.0260305 (2021).
Beckman, B. R. & Dickhoff, W. W. Plasticity of smolting in spring chinook salmon: Relation to growth and insulin-like growth factor‐I. J. Fish. Biol. 53(4), 808–826. https://doi.org/10.1111/j.1095-8649.1998.tb01834.x (1998).
Sharawy, Z. Z. et al. Effects of dietary marine microalgae, Tetraselmis Suecica, on production, gene expression, protein markers and bacterial count of Pacific white shrimp Litopenaeus vannamei. Aquac Res. 51(6), 2216–2228. https://doi.org/10.1111/are.14566 (2020).
Acknowledgements
The authors sincerely thank the Dean, Institute of Fisheries Post Graduate Studies and the Director of Directorate of Incubation and Vocational training in Aquaculture, Tamil Nadu Dr. J. Jayalalithaa Fisheries University for extending facilities and support to carry out this research as a part of the doctoral thesis research work.
Funding
The study received no funding support; however, first author sincerely acknowledges the fellowship provided by the Tamil Nadu Dr. J. Jayalalithaa Fisheries University for completing the research.
Author information
Authors and Affiliations
Contributions
K.R. -Investigation, Formal analysis, Writing - original draft. N.F.- Supervision, Conceptualization, Methodology, Investigation, Resources, Writing - review & editing and Funding acquisition. A.R.- Supervision, Conceptualization, Methodology, Visualization, Writing - review & editing. G. S.- Sampling and Formal analysis. Arumugam Uma: Supervision and Visualization.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rajalakshmi, K., Felix, N., Ranjan, A. et al. Evaluation of different inclusion levels of a novel ingredient combination on growth performance, nutrient utilization and gene expression in Penaeus vannamei. Sci Rep 15, 13311 (2025). https://doi.org/10.1038/s41598-025-90208-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-90208-8