Abstract
This study introduces an innovative master-slave cardiac ablation catheter robot system that employs magnetorheological fluids. The system incorporates magnetorheological fluid to enable collision detection through haptic feedback, thereby enhancing the operator’s situational awareness. A modular clamping and propulsion mechanism has been engineered for the ablation catheter, facilitating omnidirectional operation and force feedback within the cardiac cavity. To evaluate the proposed system, an in vitro experiment was performed. Results from the experiment indicate that the system demonstrates high motion transmission accuracy. Furthermore, the system effectively alerts operators to potential collisions, enabling swift catheter position adjustments, minimizing the risk of vascular perforation, and ultimately enhancing the overall safety and efficiency of the procedure.
Similar content being viewed by others
Introduction
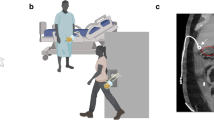
Atrial fibrillation (AF) is the most prevalent sustained cardiac arrhythmia encountered in clinical practice, affecting approximately 33 million individuals worldwide1. The deleterious effects of atrial fibrillation extend beyond the cardiovascular system. It is frequently associated with severe complications such as stroke, thromboembolism, and heart failure, which significantly elevate patient mortality and morbidity rates. Furthermore, atrial fibrillation is strongly correlated with longterm health sequelae including cognitive decline, dementia, and renal function impairment, which substantially diminish patients’ quality of life and longterm health outcomes2. Since the commencement of the 21st century, catheter ablation has progressively emerged as the preferred therapeutic modality for atrial fibrillation, offering advantages such as reduced hospital stay duration, minimal invasiveness, and expedited recovery3,4. The traditional cardiac ablation procedure is illustrated in Fig. 1. Despite its widespread application, this approach continues to exhibit numerous limitations. Primarily, the surgical process is heavily reliant on the physician’s manual dexterity, rendering it susceptible to operational fatigue and variations in technical proficiency, thereby increasing the potential for procedural errors and associated surgical risks5. Additionally, the absence of force feedback and tactile information during the intervention impedes the physician’s ability to accurately assess the contact status between the catheter and the myocardial wall, consequently affecting the precision and safety of the ablation procedure.
In recent years, the application of medical robot technology in minimally invasive interventional surgery has become a significant area of research. Investigators worldwide have conducted extensive studies on active flexible catheter configurations, force feedback technology, and robotic master-slave systems. Several research institutions and companies are dedicated to developing robotic systems for vascular interventional surgeries. For instance, the CorPath 200 system, launched by Corindus, utilizes standard catheters and guidewires to seamlessly integrate with mainstream X-ray imaging systems and demonstrates high cost-effectiveness6. Hanson Medical’s Sensei X system incorporates 3D catheter technology and 3D visualization technology, which substantially enhances the stability and accuracy of surgical procedures7. The subsequent Magellan surgical robot system represented an improvement on the Sensei platform and focused on remote guidance of interventional catheter systems. The Amigo system, developed by Catheter Robotics in the United States, is compatible with various commercial catheters and emulates manual surgical operations8. The GRX system, developed by Corindus Vascular Robotics, employs an innovative manipulator equipped with a force feedback mechanism to enhance the safety of catheter operation9.
Furthermore, researchers have developed numerous devices that enhance endovascular surgical techniques. For instance, Tavallaei et al. developed a remote catheter system that mitigates the operator’s physical stress and radiation exposure while maintaining compatibility with MRI10. Additionally, they designed a three-degree-of-freedom remote-controlled robotic catheter system capable of flexibly guiding the catheter for precise operation, and validated the system’s safety through in vitro experiments11. Moreover, a recently proposed low-cost catheter driving system with 4 degrees of freedom can substantially reduce radiation exposure during surgery and improve the stability of catheter operation12.
While these robotic systems have led to numerous advancements, challenges persist in advancing catheters through the complex vascular structures of the human body. Vessel tortuosity remains one of the primary causes of interventional surgery failure. Due to the lack of depth perception and the low resolution of fluoroscopic images, it becomes challenging to determine whether vessel collision has occurred13. To address this issue, some researchers have introduced force measuring devices into robotic catheter systems to provide more intuitive force feedback and assist physicians in avoiding blood vessel collisions14. Guo et al. proposed a master-slave robotic catheter system that combines force feedback and visual feedback to train novices in interventional surgery15. Bao et al. developed a remote control system based on an electromagnetic clutch to realize force feedback of the catheter16, and verified its efficacy through human experiments17. Furthermore, Guo et al. utilized closed-loop force feedback in a surgical robot system to enhance the accuracy of operations during catheterization18,19. In an effort to mitigate collision damage during catheterization, Yin et al. designed a tactile interface to detect the collision between the catheter tip and the blood vessel wall20.
Although existing systems have made significant progress in capturing tool-tissue interaction forces, surgeons still need to monitor force changes in real time, which inevitably increases the operational burden. The operational input of most systems is accomplished through multi-degree-of-freedom joysticks or tactile interfaces, lacking the natural operational feel found in traditional catheterization techniques. Furthermore, it is challenging for physicians to accurately control the force applied to the catheter/guidewire, which increases the risk of vascular damage. Therefore, it is crucial to develop more ergonomic, remotely operated robotic catheter systems that leverage the physician’s natural operational skills while providing tactile cues through force feedback. This approach can assist physicians in determining whether the vascular safety threshold has been exceeded during the procedure.
This study proposes a master-slave ablation catheter robotic system that utilizes magnetorheological (MR) fluids and high-precision load cell to facilitate collision detection based on tactile feedback. Through the development of a modular ablation catheter clamping and propulsion mechanism, the system achieves comprehensive operation and force feedback functionality of the catheter within the cardiac chamber.
Robotic system description
The robotic system for controlling the ablation catheter is depicted in Fig. 2. The surgeon operates the master manipulator, which captures the surgeon’s actions and transmits them via a hardware control system to the slave manipulator. The motion control system transmits control commands to the stepper motor actuator of the slave manipulator, enabling it to replicate the surgeon’s movements to perform the procedure. Concurrently, force information is fed back to the master manipulator, forming a closed-loop system. In addition to force feedback, precise catheter positioning is essential for successful vascular catheterization. Consequently, the motion transmission unit not only transmits movement signals but also feeds back position data to a PC screen, enabling the operator to accurately determine the catheter’s insertion distance.
Master manipulator
The master manipulator, developed and evaluated by our research group (as shown in Fig. 3)21,22, comprises a guidewire control unit, a magnetic field generator, a calibration unit, and a catheter control unit. The magnetic field generator is responsible for producing a magnetic field that acts on magnetorheological (MR) fluid, enabling tactile force feedback in the system23. It is developed based on electromagnetic principles, wherein the magnetic field and the state of the MR fluid can be altered by modifying the input current. The calibration unit is utilized to calibrate the system’s force feedback, specifically the relationship between the tactile force applied to the surgeon’s hand and the input current24. Additionally, the catheter and guidewire control units are equipped with four rotary encoders to capture the motion of the control levers. Surgeons can perform three types of operations using the levers: independent guidewire control, independent catheter control, and coordinated control of both guidewire and catheter. The master manipulator designed by our group accommodates the surgeon’s established operating techniques, allowing them to utilize their expertise and dexterity through cylindrical controls that emulate the real-life operation of guidewires and catheters.
Slave manipulator
The nonfixed modular ablation catheter operating mechanism is depicted in Fig. 4. This mechanism is utilized to manipulate the ablation catheter handle, with primary functionalities encompassing push-pull control, rotational control, and tip bending control, facilitating real-time measurement and feedback of the catheter’s advancement distance, rotational angle, and tip bending angle. For tip bending control, a control mapping is established to ensure that the tangential vector of the catheter tip is optimally perpendicular to the normal vector of the ablation target. A modular design approach is employed to facilitate the separation of the robotic body from the surgical instrument, enabling more efficient installation and disassembly during surgical procedures and allowing for future expansion of the system’s capabilities. The catheter operator comprises multiple modular units, including motion control, displacement measurement, angle measurement, and auxiliary support25.
The principle underlying the nonfixed modular ablation catheter clamping and advancing mechanism is illustrated in Fig. 5. Component (a) represents the catheter tip, the curvature of which is regulated by the bending drive mechanism in (b). Component (c) depicts the clamping device with an integrated load cell, while (d) represents the slave manipulator’s drive system, which is responsible for the rotation, bending, and insertion of the catheter. During the catheter’s advancement phase within the blood vessel, the catheter tip’s curvature drive mechanism (b) adjusts the catheter’s direction in real-time according to the blood vessel’s morphology, while its position is controlled by the slave manipulator drive system (d). When the ablation catheter is performing contact ablation within the heart, the tip’s curvature drive mechanism (b) remains stationary, and the slave manipulator drive system (d) executes active compliance control based on the feedback force.
The system employs an adaptive clamping mechanism (as illustrated in Fig. 5c) to ensure stable clamping of the catheter handle, preventing excessive clamping that could damage the catheter handle or insufficient clamping that could result in errors during catheter advancement. Research indicates that maintaining the contact force between the catheter tip and the atrial wall within the range of 0.2 ± 0.1 N achieves optimal therapeutic outcomes26. Excessive contact force risks perforating the heart wall, while insufficient force renders the treatment ineffective. During the ablation procedure, the catheter tip undergoes three stages: free motion, transient contact, and steady-state contact. During the transient contact stage, unavoidable overshoot in the contact force can occur. Therefore, to ensure precise and stable contact force tracking during the steady-state phase while maintaining the overshoot within a safe threshold during the transient phase, an impedance control strategy is proposed. This necessitates the development of an adaptive dynamic force control method within the proposed ablation catheter driving mechanism to establish and maintain precise and stable contact between the catheter tip and the atrial wall.
Safety mechanism
The cardiac ablation catheter robot safety interaction system comprises four components: serial port initialization setting, force visualization warning area, surgical catheter status information display unit, and surgical catheter motion control display unit, as illustrated in Fig. 6. Table 1 presents the system function description of the cardiac ablation catheter robot safety interaction system.
An S-type weighing sensor with a sensitivity of 1.0 ~ 2.0 ± 0.1 mV/V was utilized. The data measured by the sensor is transmitted to the PC through the RS232 serial port. As illustrated in Fig. 7, when the contact force (a) of the surgical catheter is below the vascular safety threshold, the system’s safety light remains unilluminated. When the contact force of the surgical catheter is equal to or exceeds the vascular safety threshold, the system’s warning light illuminates (red). In this scenario, the physician should exercise caution and cease the current operation (When the threshold is reached, the feedback resistance increases rapidly and it is impossible to move forward). The safety warning system’s primary advantage is its ability to prevent the surgical catheter from perforating the blood vessel, thereby avoiding unnecessary patient harm.
To evaluate the effectiveness of the master-side feedback tactile prompt collision detection, a dynamic force evaluation experiment was conducted to measure the magnitude of the feedback tactile force. The experimental setup is illustrated in Fig. 8. During the experiment, a rigid rod connected to a load cell (A single-axis force sensor) (TEAC, TU-UJ, Japan) was consistently driven by a slide to generate an insertion motion in the tactile interface. The PC recorded and displayed the force information generated by the slave-side acquisition and the master side feedback. To avoid affecting the single-axis contact force, the input speed was set to 5 mm/s22. To maximize the detection effect, the resistance provided by the hand at the slave side was utilized for feedback. The MR fluid transitions rapidly from fluid to solid27.
The experimental results are summarized in Fig. 9. The data indicate that upon application of resistance, the dynamic force increases rapidly from its initial value. Furthermore, the change in tactile force reaches approximately 6000 mN, which significantly exceeds the 19 mN human finger detection resolution (i.e., perceptible difference)28. Consequently, this substantial tactile change can swiftly elicit an operator’s stress response and achieve the intended purpose of collision detection even for novice users.
Experiments and results
Experimental setup
An in vitro test was conducted to verify the feasibility of this project. The experiment encompassed the advancement and withdrawal of the catheter, torsion, extension and bending of the catheter tip, and a force feedback test. A rotary encoder with a pinion and rack served as the input and feedback device for the axial motion (see Fig. 10a). An additional rotary encoder functions as the input and feedback device for radial motion (see Fig. 10a). The positions of the two encoders on the master side were decoded and transmitted to the two motion controllers, enabling the slave robot to follow the axial and radial positions of the master side. The Arduino Due development board (Smart Projects, Strambino, Italy) incorporated a 32-bit ARM architecture microcontroller for real-time orthogonal decoding and transmitting position data to the control unit. The control unit communicates with the master motion transmission unit to obtain the required reference position and simultaneously measures the position of the slave-side catheter through a laser displacement sensor (PANASONIC, HG-C1200, Japan) and a hollow rotary encoder (MUTOH, UN-2000, Japan) affixed to the drive shaft (see Fig. 10b).
Evaluation of bilateral surgical results
A bilateral manipulation experiment for the axial motion was conducted. Axial motion detection device: The axial motion input and feedback are achieved through a rotary encoder with a pinion and rack on the master end. This encoder captured the advancement and withdrawal distance of the catheter in real time to ensure the accuracy of its displacement signal. Slave side position measurement: On the slave side, a laser displacement sensor (PANASONIC HG-C1200) and a hollow rotary encoder (MUTOH UN-2000) are used to measure the position of the catheter in collaboration to ensure data redundancy and accuracy.As illustrated in Fig. 11, the translation error ranges between ± 0.98 mm. This result demonstrates that the slave robot can accurately replicate the trajectory of the master motion input unit and fulfill the requirements of the surgical procedure. Fifteen bilateral manipulation experiments were performed under identical conditions to assess the stability of the axial motion.
Figure 12 shows a summary of the average absolute errors for each trial. The results indicate that the maximum error does not exceed 1 mm. However, the substantial standard deviation depicted in the figure suggests considerable variability in the error distribution. This variability may be attributed to minor vibrations generated by the sliding table, potentially causing deviations in laser detection.
A bilateral manipulation experiment was conducted to evaluate the radial motion. The radial motion transmission performance and errors are shown in Fig. 13. The results indicate that the rotation error falls within the range of ± 1.8 degrees. Following 15 trials, Fig. 14 illustrates the average absolute error of each trial. The results demonstrate notable discreteness in the standard deviation relative to the mean. However, this is deemed acceptable as the absolute error within 2 degrees of the system does not significantly impact the direction adjustment of the catheter/guidewire tip.
Bilateral manipulation experiments were conducted to assess tip bending motion. The tip bending motion transmission performance and error are illustrated in Fig. 15. The results indicate that the bending error ranges between ± 1.9 degrees. Following 15 trials, the mean absolute error of each test is summarized in Fig. 16. The findings demonstrate that the standard deviation exhibits notable discreteness relative to its mean value, and the absolute error remains within 2 degrees, which is not expected to significantly impact the directional adjustment of the catheter tip.
Force measuring device performance evaluation
To enhance the sense of presence and improve task performance, the robot-assisted catheter system has incorporated force feedback. The critical component of force feedback is the precise measurement of force information. To mitigate the influence of gravity on force measurement, the mechanism is positioned horizontally, ensuring that the axis of the load cell is parallel to the ground. This configuration is then combined with an omnidirectional bearing as a support, resulting in a zero gravity component of the device acting on the axis of the load cell.
Evaluation from end force measurement device
The accuracy of the force measuring device is a critical factor that necessitates prioritization in the force measurement process. Its accuracy and response speed directly influence the safety of the operation, particularly when delicate blood vessels are involved. To evaluate the accuracy of the force measuring device, a step response experiment was conducted.
The experimental setup is illustrated in Fig. 17. A steel rod was secured by the slave manipulator, with the opposite end firmly connected to the load cell. Under conditions restricting the movement of the slave manipulator, the force applied to the rod was incrementally increased through micro-feeding via the sliding table. The loading force was gradually increased from 0 N to 2.5 N, with increments of 0.1 N. The real-time data of the applied force was collected by the main control board and displayed synchronously on the PC.
For each loading force condition, the experiment was replicated 10 times, and the mean value was calculated as the final measurement value, as depicted in Fig. 18. The experimental results indicate that the force measuring device exhibited minimal hysteresis throughout the entire load range, with an average hysteresis of 9.67 mN. The experiment demonstrates that the force measuring device possesses high accuracy and rapid response capability, with hysteresis characteristics that are deemed acceptable for precision surgery applications.
In vitro evaluation
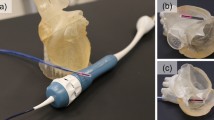
An experimental investigation was conducted to evaluate the collision detection capability of the proposed system. The experimental model utilized was a heart representation, as illustrated in Fig. 19. The specific task to be performed by the participant (non-medical personnel) involved maneuvering the catheter from the initial position A to the target position B without causing damage to the blood vessel. The experimental protocol incorporated two distinct modes. Mode 1: Tactile feedback (TF), which employed collision detection using tactile cues and visual feedback (HIKVISION, DS-E12, CHINA); Mode 2: No tactile feedback (NTF), which relied solely on visual feedback for collision detection.
The experimental setup is depicted in Fig. 9. The tip was positioned in proximity to the heart model. Based on the research team’s previous investigations29,30 and empirical tests, the maximum measured force exerted by the catheter from the initial position to the target position within the heart model, without significant tip collision, is approximately 1 N; consequently, the safety threshold was established at 1 N. The safety threshold for human blood vessels in clinical settings necessitates comprehensive evaluation by experienced surgeons, taking into account the specific vascular conditions and blood viscosity of individual patients. In mode 1, when the measured force exceeds the predetermined safety threshold, the tactile interface generates shear force on the catheter, thereby increasing resistance and effectively facilitating collision detection. By amplifying the feedback force, physicians can rapidly perceive the risk of collision. Tactile feedback, as an intuitive signal, is more direct than visual prompts and can reduce the cognitive burden of physicians, particularly in complex operational scenarios. During surgery, the collision between the catheter and the blood vessel wall may cause tissue damage, especially in narrow or fragile blood vessels. By increasing the feedback force, the system can compel physicians to decelerate the operation, reduce the risk of further collision, and contribute to ensuring patient safety. For physician training, moderate force feedback amplification aids in increasing physicians’ sensitivity to collision risks and familiarizing them with the safe operating range. This method is particularly suitable for the learning and practice of novice physicians.
The experimental results are presented in Fig. 20. In mode 2, the maximum force measured by the system (tip collision) significantly exceeds the set threshold, with the excess value surpassing 0.12 N, indicating a potential risk of perforation31. In mode 1, the maximum force measured by the system is substantially lower than that of mode 2. Although it also exceeds the set threshold, the excess value remains below 0.12 N, thereby enhancing safety and extending the operation time.
The rationale for this phenomenon is the inherent delay between the occurrence of a tip collision and the subsequent adjustment of the catheter position. During this brief interval, even a minimal forward movement of the catheter can exacerbate the collision. However, when the collision is communicated to the operator via tactile feedback, the operator can respond with maximal celerity. Furthermore, the tactile device in this system not only alerts the operator to potential collisions but also generates an instantaneous viscous resistance to the main control side operating rod, thereby impeding the catheter’s forward motion. This mechanism serves to mitigate the continuous increase in collision force and enhance the operational safety margin.
To further evaluate the efficacy of the proposed system in tactile prompt collision detection, 10 novel operators with no prior experience in either setting (mode 1 and mode 2) were subjected to testing under identical experimental conditions. The experimental tasks remained consistent with those previously conducted. For statistical validity, each participant completed 10 trials for each mode. This investigation primarily focused on two key performance indicators: (1) the mean maximum force exerted and (2) the average duration required for task completion.
According to the results summarized in Fig. 21, for each subject, the average maximum force of the task with collision detection was lower than the average maximum force of the task without collision detection. Consequently, the tactile cue collision detection of the robotic catheter system demonstrates a significant protective function. Figure 22 illustrates the average time required for all participating subjects to complete the task. The statistical analysis indicates that the average time required for mode 1 is shorter than that for mode 2. The reduction in surgical duration can decrease the patient’s exposure time to radiation, thereby mitigating the potential harmful effects of radiation on the human body.
The proposed collision detection robotic catheter system demonstrates potential for reducing surgical duration and promptly alerting surgeons to potential collisions. This functionality enables surgical personnel to respond and adjust the catheter expeditiously, thereby mitigating the risk of collision induced vascular perforation and enhancing surgical safety.
Conclusions
This paper proposes a master-slave ablation catheter robot system, which utilizes magnetorheological fluid and high-precision load cell to implement collision detection based on tactile feedback, as well as achieves comprehensive catheter operation and force feedback functions within the heart cavity through the development of a modular ablation catheter clamping and propulsion mechanism. Furthermore, axial and radial motion control experiments, force feedback experiments, and in vitro experiments were conducted to validate the proposed system. The experimental results demonstrate that the proposed master-slave ablation catheter robot system can effectively mitigate excessive collision force between the surgical catheter and the vascular wall, thereby preventing vascular puncture. In the axial and radial bilateral operation experiments, the system accurately follows the input of the main operation unit, with an axial error of less than ± 0.98 mm and a radial error controlled within ± 1.8 degrees, indicating that the system possesses good motion transmission accuracy. These findings suggest that the error is within the acceptable range and meets the design requirements. The experiment also indicates that tactile feedback can reduce task completion time and decrease patient exposure to radiation, thus minimizing harm to the human body. In conclusion, the proposed system can enhance surgical safety. Future work will involve further development and refinement of the robotic system, as well as conducting in vivo experiments using the developed catheter robotic system.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Change history
28 April 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41598-025-99040-6
References
Chugh, S. et al. Worldwide epidemiology of atrial fibrillation: A global burden of disease 2010 Study. Circulation 129 (8), 837–847 (2014).
Huang, C., Zhang, S. & Huang, D. Atrial fibrillation: Current understanding and treatment recommendations-2018. Chin. J. Cardiac Pacing Electrophysiol. 32, 315–368 (2018).
Yamane, T. Catheter ablation of atrial fibrillation: Current status and near future. J. Cardiol. 80 (1), 22–27 (2022).
Tanev, T. K. Minimally-invasive-surgery parallel robot with non-identical limbs. In Proceedings of the IEEE/ASME 10th International Conference on Mechatronic and Embedded Systems and Applications, Senigallia, Italy. 10–12 September 2014 1–6.
Hasanzadeh, S. & Janabi-Sharifi, F. Model-based force Estimation for intracardiac catheters. IEEE/ASME Trans. Mechatron. 21 (1), 154–162 (2015).
Carrozza, J. Robotic-assisted percutaneous coronary interventionfilling an unmet need. J. Cardiovasc. Transl. Res. 5 (1), 62–66 (2012).
Beasley, R. Medical robots: Current systems and research directions. J. Rob. 2012, 1–14 (2012).
Khan, E. et al. First experience with a novel robotic remote catheter system: Amigo™ mapping trial. J. Interventi. Cardiac Electrophysiol. 37 (2), 121–129 (2013).
Cercenelli, L., Bortolani, B. & Marcelli, E. CathROB: A highly compact and versatile remote catheter navigation system. Appl. Bion. Biomech. 2017 (2017).
Tavallaei, M., Thakur, Y., Haider, S. & Drangova, M. A. Magnetic-resonance-imaging-compatible remoto catheter navigation system. IEEE Trans. Bio-Med Eng. 60, 899–905 (2013).
Tavallaei, M. et al. Design, development and evaluation of a compact telerobotic catheter navigation system. Int. J. Med. Robot Comp. 12, 442–452 (2015).
Cha, H., Yi, B. & Won, J. An assembly-type master-slave catheter and guidewire driving system for vascular intervention. Part. H J. Eng. Med. 231, 69–79 (2017).
Payne, C. J., Rafii-Tari, H. & Yang, G. A. Force feedback system for endovascular catheterisation. In Proceedings of the 2012 IEEE/RSJ International Conference on Intelligent Robots and Systems, Vilamoura-Algarve, Portugal, 7–12 1298–1304. (2012).
Fu, Y., Gao, A., Liu, H. & Guo, S. The master-slave catheterization system for positioning the steerable catheter. Int. J. Mechatron. Autom. 1, 143–152 (2011).
Guo, J. et al. A novel robotic catheter system with force and visual feedback for vascular interventional surgery. Int. J. Mechatron. Autom. 2, 15–24 (2012).
Bao, X. et al. A Cooperation of catheters and guidewires-based novel remote-controlled vascular interventional robot. Biomed. Microdevices. 20, 20 (2018).
Bao, X. et al. Operation evaluation in-human of a novel remote-controlled vascular interventional robot. Biomed. Microdevices. 20, 34 (2018).
Guo, J., Jin, X. & Guo, S. Study of the operational safety of a vascular interventional surgical robotic system. Micromachines 9, 119 (2018).
Zhang, L., Guo, S., Yu, H. & Song, Y. Performance evaluation of a strain-gauge force sensor for a haptic robot-assisted catheter operating system. Microsyst. Technol. 23, 5041–5050 (2017).
Yin, X., Guo, S., Hirata, H. & Ishihara, H. Design and experimental evaluation of teleoperated haptic robot-assisted catheter operating system. J. Intell. Mater. Syst. Struct. 27, 3–16 (2016).
Yin, X., Guo, S., Hirata, H. & Ishihara, H. Design and experimental evaluation of a teleoperated haptic robot–assisted catheter operating system. J. Intell. Mater. Syst. Struct. 27 (1), 3–16 (2016).
Zhang, L. et al. Design and performance evaluation of collision protection-based safety operation for a haptic robot-assisted catheter operating system. Biomed. Microdevices. 20 (2), 22 (2018).
Yin, X., Guo, S., Xiao, N. & Tamiya, T. Safety operation consciousness realization of a MR fluids-based novel haptic interface for teleoperated catheter minimally invasive neurosurgery. IEEE/ASME Trans. Mechatron. 21 (2), 1043–1054 (2015).
Guo, S. et al. A novel robot-assisted endovascular catheterization system with haptic force feedback. IEEE Trans. Robot. 35 (3), 685–696 (2019).
Ma, X., Guo, S., Xiao, N., Yoshida, S. & Tamiya, T. Evaluating performance of a novel developed robotic catheter manipulating system. J. Micro-Bio Rob. 8, 133–143 (2013).
Faddis, M. et al. Novel, magnetically guided catheter for endocardial mapping and radiofrequency catheter ablation. Circulation 106 (23), 2980–2985 (2002).
Park, B., Fang, F. & Choi, H. Magnetorheology: Materials and application. Soft Matter. 6 (21), 5246–5253 (2010).
Jones, L. & Tan, H. Application of psychophysical techniques to haptic research. IEEE Trans. Haptics. 6 (3), 268–284 (2012).
Song, Y. et al. Performance evaluation of a robot-assisted catheter operating system with haptic feedback. Biomed. Microdevices. 20, 50 (2018).
Bao, X., Guo, S., Xiao, N., Li, Y. & Shi, L. Compensatory force measurement and multimodal force feedback for remote-controlled vascular interventional robot. Biomed. Microdevices. 20, 74 (2018).
Okumura, Y. et al. A systematical analysis of in vivo contact forces on virtual catheter tip/tissue surface contact during cardiac mapping and intervention. J. Cardiovasc. Electr. 19, 632–640 (2008).
Acknowledgements
This research was partly supported by the National Natural Science Foundation of China under Grant 62203071, the Sichuan Science and Technology Planning Project under Grant 2023YFG0178, and the College Students’ Innovation and Entrepreneurship Training Project under Grant 202310633027.
Author information
Authors and Affiliations
Contributions
L.Z. and J.Z. conceived and designed the advanced robotic system for precision cardiac ablation procedures, performed data collection, and drafted the sections of the manuscript. K.W., S.G. and Y.Z. assisted with the design of the mechanical system. L.X. and T.J. guided the evaluation of the synchronous evaluation experiments. All the authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained an error in the Conclusions section. Full information regarding the corrections made can be found in the correction for this Article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, L., Zuo, J., Wang, K. et al. An advanced robotic system incorporating haptic feedback for precision cardiac ablation procedures. Sci Rep 15, 6839 (2025). https://doi.org/10.1038/s41598-025-91342-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-91342-z