Abstract
Feeding intolerance (FI) is a common clinical problem in very preterm infants (VPIs) and it increases the risk for adverse outcomes. The value of regional tissue oxygen saturation in predicting FI remains unclear. A total of 57 VPIs were involved in this study, and the regional splanchnic and cerebral tissue oxygen saturation during minimal enteral feeding in the first 3 days after birth was monitored and analyzed. Compared with the feeding tolerance (FT) group, the FI group had a smaller gestational age, lower birth weight, and higher rate of maternal hypertensive disorders in pregnancy. Even more, the FI group had lower regional splanchnic tissue oxygen saturation and lower splanchnic-cerebral oxygenation ratio (SCOR) at the 1st hour and 2nd hour after feeding on the 3rd postnatal day than the FT group (P < 0.05). Multivariate logistic regression analysis showed that increased gestational age and elevated SCOR at the 2nd hour after feeding on the 3rd postnatal day reduced the risk for FI (P < 0.05). The areas under the curve (AUCs) of gestational age, SCOR at the 2nd hour after feeding on the 3rd postnatal day and their combination in predicting FT were 0.745 (95%CI 0.616 ~ 0.874), 0.756 (95%CI 0.628 ~ 0.883) and 0.820 (95%CI 0.710 ~ 0.929), respectively.
Similar content being viewed by others
Introduction
Feeding intolerance (FI) is a common clinical problem in preterm infants and may be a physical phenomenon caused by structural and functional immaturity of the gastrointestinal tract, or a pathological manifestation of necrotizing enterocolitis (NEC), sepsis or other serious complications. FI leads to enteral feeding interruptions, delayed full feeding, an increased hospitalization duration, and extrauterine growth restriction. Early identification of infants at risk for adverse gastrointestinal outcomes and the provision of refined management can improve clinical outcomes.
Near-infrared spectroscopy (NIRS) is a noninvasive monitoring tool for measuring the difference between oxyhemoglobin and deoxyhemoglobin that is expressed as regional tissue oxygen saturation, which can reflect tissue perfusion and oxygenation1. It was found that regional splanchnic tissue oxygen saturation (rsSO2) monitored by NIRS in the early postnatal period could predict the onset of NEC in preterm infants, and those who developed NEC had significantly lower rsSO2 values at one week after birth2,3. However, there are few studies on the use of rsSO2 to predict the occurrence of FI in preterm infants, and the results are inconsistent4,5. RsSO2 can be monitored alone or simultaneously with regional cerebral tissue oxygen saturation (rcSO2) to calculate the splanchnic-cerebral oxygenation ratio (SCOR). A recent meta-analysis showed that a low SCOR value was a biomarker of intestinal ischemia6. Since intestinal blood flow is more variable across pathophysiological states, whereas cerebral blood flow remains relatively stable through autoregulation, the SCOR makes changes in intestinal oxygenation more interpretable and comparable between individuals. In this study, we investigated the value of rsSO2, rcSO2 and SCOR during minimal enteral feeding in the early postnatal period in predicting the subsequent feeding tolerance (FT) of very preterm infants (VPIs).
Results
Characteristics of the preterm infants
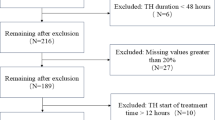
There were 90 VPIs included at admission, and 33 were excluded during hospitalization. Ultimately, 57 VPIs were eligible for analysis, with 27 included in the FT group and 30 included in the FI group (Fig. 1). There were 26 male and 31 female infants. The mean gestational age was 30.13 ± 1.16 weeks, and the mean birth weight was 1365 ± 322 g. All the infants were fed with preterm formula during minimal enteral feeding, because the transportation of mother’s own milk was interrupted by the prevention and control policies during the COVID-19 epidemic, and donor breast milk was not available in our center.
The median age at diagnosis of FI was 5 (4, 7.75) days. Among the 30 infants with FI, the main manifestations were abdominal distention (86.67%; 26 of 30), gastric residuals (83.33%; 25 of 30), vomiting (63.33%; 19 of 30), dark green residuals (46.67%; 14 of 30), and bloody stools (36.67%; 11 of 30). A total of three infants with symptom progression were diagnosed with NEC.
Comparison of clinical data between the FT group and the FI group
The summary and comparison of clinical data between the FT group and the FI group are detailed in Table 1. In the comparisons of maternal and prenatal factors, the FI group had a higher rate of hypertensive disorders in pregnancy than the FT group (P < 0.05). There was no statistically significant difference between the two groups in antenatal corticoid use, prenatal antibiotic use, cesarean section delivery, hyperglycemia in pregnancy, a duration of premature rupture of membranes > 18 h prior to delivery, multiple births, intrauterine distress or abnormal fetal umbilical blood flow (P > 0.05).
In the comparisons of neonatal factors, the infants in the FI group had a smaller gestational age and lower birth weight than those in the FT group (P < 0.05). No significant difference between the two groups was found in gender, small for gestational age, 1- and 5-minute Apgar scores, Hgb and HCT levels at admission, or pH, lactate, and BE values determined by blood gas analysis immediately after birth (P > 0.05). The postnatal treatments were similar between the two groups, including invasive ventilation, umbilical artery catheterization, umbilical vein catheterization, expansion treatment, the use of vasoactive drugs, caffeine and antibiotic treatment > 48 h after birth (P > 0.05).
Comparison of rsSO2, rcSO2 and SCOR values between the FT group and the FI group
The rsSO2, rcSO2 and SCOR values are summarized and demonstrated in Tables 2, 3 and 4, respectively. On the 1st and 2nd postnatal days, the FT group and the FI group showed similar rsSO2, rcSO2 and SCOR values. On the 3rd postnatal day, the rsSO2 and SCOR values in the FI group were lower than those in the FT group at the 1st hour and 2nd hour after feeding (P < 0.05). When the first 3 days served as a total, the mean rsSO2 value in the FI group was lower than that in the FT group at the 2nd hour after feeding (P < 0.05), and the SCOR value in the FI group was lower than that in the FT group at the 1st and 2nd hours after feeding (P < 0.05).
Multivariate logistic regression analysis of the factors related to FI
With feeding tolerance as the dependent variable (FI = 1, FT = 0), the independent variables shown in Tables 1, 2, 3 and 4 were included in the binary logistic regression analysis model, and forward logistic regression was performed. The results showed that an increased gestational age at birth and an elevated SCOR value at the 2nd hour after feeding on the 3rd postnatal day were protective factors against FI (P < 0.05) (Table 5).
The predictive value analyzed with ROC curves
ROC analysis showed that the areas under the curve (AUCs) for gestational age at birth, SCOR values at the 2nd hour after feeding on the 3rd postnatal day, and the combination of both in the prediction of subsequent FT were 0.745 (95%CI 0.616 ~ 0.874), 0.756 (95%CI 0.628 ~ 0.883) and 0.820 (95%CI 0.710 ~ 0.929), respectively. These results are demonstrated in Table 6; Fig. 2.
ROC curves for predicting FT, Blue lines represent gestational age (AUC = 0.745, 95%CI 0.616 ~ 0.874); Red lines represent SCOR at the 2nd hour after feeding on the 3rd postnatal day (AUC = 0.756, 95%CI 0.628 ~ 0.883); Green lines represent the combination of gestational age and SCOR at the 2nd hour after feeding on the 3rd postnatal day (AUC = 0.820, 95%CI 0.710 ~ 0.929). ROC, receiver operating characteristic; FT, feeding tolerance; SCOR, splanchnic-cerebral oxygenation ratio; AUC, area under the curve.
Discussion
Adequate nutrition is very important for the growth, development and health of preterm infants. Early enteral feeding may reduce mortality, the risk of sepsis and the length of hospital stay among preterm infants7. Nutritional status, such as weight gain and linear and head circumference growth, is even independently associated with long-term neurodevelopmental outcomes8. However, FI in preterm infants is extremely common in clinical practice and affects the implementation of enteral nutrition programs. The results of this study showed that the overall prevalence of FI among VPIs was 52.63% (30 of 57), the gestational age was significantly lower in the FI group than in the FT group, and increased gestational age was a protective factor against the occurrence of FI. This suggests that the smaller an infant’s gestational age is at birth, the more likely they are to develop FI, which relies on the maturation of gastrointestinal tract9. Mid to late gestation is a critical period for fetal gastrointestinal development. If premature birth occurs, it poses a challenge to nutritional intake due to immature gastrointestinal motility and imperfect digestion and absorption, and leads to extrauterine growth restriction and gastrointestinal complications (e.g., FI, NEC) in preterm infants. At the same time, the immaturity of the gastrointestinal tract adversely affects gut microbial colonization patterns, which in turn further alters the function and immunity of the gastrointestinal tract10; this abnormal colonization is closely related to the development of FI and NEC11.
Early identification of infants at high risk of developing FI through noninvasive screening methods can optimize feeding management protocols for preterm infants. Previously, visceral flow Doppler ultrasound was applied to assess the intestinal hemodynamic status of neonates. The results of a prospective cohort study by Bora et al.12showed that preterm infants who developed FI had impaired superior mesenteric artery blood flow perfusion after early postnatal feeding. However, Doppler examination has some limitations: it requires a specially trained sonographer and is prone to operator-dependent bias; it does not allow for continuous assessment; and does not allow direct estimation of regional tissue perfusion because it only measures blood flow velocity within large vessels. In contrast, NIRS monitoring provides a noninvasive, continuous, bedside method of monitoring regional tissue oxygenation, reflecting the balance between local microcirculatory oxygen supply and oxygen consumption, and is therefore more sensitive to the early detection of regional hypoxia and hypoperfusion13. Both animal and clinical trials have demonstrated that abdominal NIRS can be reliably used to measure splanchnic oxygenation changes and gastrointestinal perfusion in neonates14,15.
The results of this study showed that the infants who developed FI had lower rsSO2 and SCOR values at the 1st hour and 2nd hour after feeding on 3rd postnatal day, and a lower mean rsSO2 value at the 2nd hour after feeding on the first 3 days of life and a lower mean SCOR value at the 1st hour and 2nd hour after feeding than infants with FT; multivariate logistic regression analysis showed that increased SCOR values at the 2nd hour after feeding on the 3rd postnatal day were a protective factor against the occurrence of FI, with good predictive value for FT (AUC = 0.756). Dave et al.16 concluded that in healthy preterm infants, postprandial rsSO2 was increased, while rcSO2 was relatively stable; hence, the SCOR values increased. Al-Hamad et al.17 speculated that this altered oxygenation was physiologically significant because the body diverted blood flow and thus delivered more oxygen to the intestine during digestion; conversely, decreased rsSO2 and SCOR values could reflect FI. Corvaglia et al.4 performed continuous NIRS monitoring of cerebral and splanchnic regional oxygenation from 30 min before to 3 h after the first feeding in 61 preterm infants whose gestational age was ≤ 33 weeks and clinical conditions were stable. Enteral feeding was started within 48 to 72 h after birth. The results showed that the infants who developed FI had significantly lower rsSO2 and SCOR values at baseline, in the first 35 min after feeding and from 90 to 180 min after feeding compared to those with FT, and multivariate model analysis noted that lower mean 3-hour rsSO2 and SCOR values after feeding were risk factors for the development of FI. Martini et al.18 performed continuous mesenteric tissue NIRS monitoring from 30 min before to 3 h after the first introduction of enteral feeding in 20 preterm infants born at a gestational age ≤ 34 weeks for whom prenatal Doppler ultrasound suggested absent or reversed end-diastolic flow, starting enteral feeding within 48 h after birth. The results showed that the rsSO2 value was significantly lower at all periods within 3 h after feeding in infants with subsequent gastrointestinal complications compared with the control infants, and 8 infants had FI. However, some studies have suggested that early postnatal intestinal tissue oxygenation is not associated with the development of FI in preterm infants. Dani et al.5 performed NIRS monitoring during continuous feeding from 24 to 72 h after birth in 28 preterm infants born at a gestational age of 25 to 31+ 6 weeks and showed that the rsSO2 value was not significantly different between the FI group and the FT group. The inconsistent results of the current series may be related to the different feeding patterns of the study population and the inconsistent definition of FI. There is no widely accepted definition of FI19. Bolus and continuous feeding modes had different effects on splanchnic oxygenation20,21. There is one caveat that SCOR values do not reflect only changes in intestinal oxygenation when cerebral autoregulation has failed, moreover the predictive value of SCOR will be less reliable6.
Additionally, some studies found rsSO2 and SCOR were associated with anemia and RBC transfusion. Braski et al.22 noted that very low birth weight infants with a HCT ≤ 28% had a decrease in rsSO2 and SCOR with enteral feedings. Martini et al.23 observed pre-transfusion rsSO2 and SCOR increasing significantly in response to feeding; while decreasing significantly of rsSO2 and SCOR post-prandial after transfusion. In our study, there was no significant difference in Hgb and HCT values at birth between the two groups. Hgb values on the day of rsSO2 measurement would be more comparable, but to avoid iatrogenic anemia, we didn’t collect blood sample to make the test daily in the first week when the VPI’s condition was stable. As a result, none of the infants in our study received RBC transfusion within 7 days after birth. Anemia and transfusion were not important influencing factors to our study result.
In conclusion, NIRS monitoring of regional splanchnic and cerebral tissue during early postnatal minimal enteral feeding in VPIs has some predictive value for subsequent FT. However, this was a single-center study with a small sample size and failed to stratify the relevant influencing factors, and changes in intestinal oxygenation with the development of FI and the recovery process were not observed. Further studies with larger sample sizes are needed in the future.
Materials and methods
Study population
This was a prospective, observational cohort study. Preterm infants born at 28–31 weeks of gestation and transferred to the Neonatology Department of the Third Affiliated Hospital of Guangzhou Medical University within 24 h after birth from July 2022 to December 2022 were included as the study subjects. The exclusion criteria were as follows: (1) infants with severe congenital anomalies, including abdominal wall defects, cyanotic congenital heart disease, and gastrointestinal anomalies, that could affect the placement of the NIRS probe or NIRS assessment; (2) infants with severe conditions who could not tolerate NIRS monitoring; (3) infants who fasted in the first 3 days after birth; and (4) infants with incomplete or missing data. Eligible infants were divided into the FT group and the FI group according to subsequent feeding procedures.
Sample size Estimation
Previous study showed that the AUCs of rsSO2 and SCOR in predicting intestinal ischemia in newborns were 0.80 and 0.89 respectively6. We used software of PASS version 11.0 for sample size estimation. Setting the test level α at 0.05 and the power at 0.9, the required sample size was calculated to be 34 cases, and the estimated missing data rate was 10%, therefore, the total sample size was at least 38 cases.
Feeding schedule
The included VPIs were given minimal enteral feeding within 24 h after birth. Mother’s own milk was the preferred choice. Because we didn’t have breast milk bank in our center, donor breast milk was not available. Thus, preterm formula was used when mother’s own milk was not available. Feeding started at a volume of 15–20 ml/(kg.d), which was continued for 3 days with no increase. Then, the feeding volume was increased by 15–20 ml/(kg.d). The feeding interval was 3 h.
NIRS monitoring
RsSO2 and rcSO2 were measured using a near-infrared tissue oxygen nondestructive monitor (EGOS-600 series, Suzhou Aegean Biomedical Electronics Co., China). Before measurement, the sensors were disinfected with alcohol swabs, and the local skin was cleaned with a gauze block. RsSO2 was monitored with an A-type sensor placed below the umbilicus; and rcSO2 was monitored with a B-type sensor placed on the frontoparietal side of the head (random placement on the left or right side). The sensors were fitted to the skin to avoid light leakage, and then fixed with dressing. Patients were placed in a supine position and kept calm, and daily care or therapy was minimized during the monitoring period. NIRS monitoring was performed once per day in the afternoon from the first to the third day after birth, with continuous monitoring from 30 min before feeding to 2 h after feeding. The instrument was set to record data every 2 s, and started to record when the monitoring baseline was stable. Other vital signs and peripheral oxygen saturation were measured simultaneously during NIRS monitoring. When apnea, bradycardia or tachycardia occurred, the recording was suspended and the sensor position remained the same when rerecording. For patients receiving respiratory support therapy, the target oxygen saturation was set at 90–95%. Measurements were performed by trained investigators and were kept confidential to ensure that the bedside clinician was unaware of the data. Data View software was used to convert the NIRS monitoring data before statistical analysis. The SCOR value was calculated as follows: SCOR = rsSO2/rcSO2.
Clinical data collection
The following clinical data were collected: (1) maternal and prenatal factors, including whether antenatal corticosteroids were administered, whether prenatal treatment with antibiotics was provided, the type of delivery, pregnancy comorbidities and complications; (2) neonatal factors, including gestational age, birth weight, sex, Apgar scores, blood concentrations of hemoglobin (Hgb) and hematocrit (HCT) at admission, and pH, lactate and base excess (BE) values determined by blood gas analysis immediately after birth; (3) the enteral feeding process and whether manifestations of FI and NEC were present during hospitalization; and (4) treatment including the use of umbilical artery or/and vein catheters, the use of antibiotics, vasoactive drugs and caffeine, expansion treatment, and respiratory support.
Definitions and criteria
FI was defined as meeting one of the following conditions24: (1) a gastric residual volume exceeding 50% of the previous feeding volume with vomiting and/or abdominal distention; and (2) feeding program failure, including enteral feeding reductions, delays, or interruptions. Gastric residuals were not routinely checked during minimal enteral feeding, pre-feed gastric residual volume was checked only after a minimum feed volume (per feed) was attained25. Time to full enteral feeding was defined as the time to enteral feeding of a volume of at least 150 ml/(kg.d). Hypertensive disorders in pregnancy included gestational hypertension, preeclampsia-eclampsia, pregnancy combined with chronic hypertension, and chronic hypertension with preeclampsia26. Hyperglycemia in pregnancy included pregestational diabetes mellitus and gestational diabetes mellitus27.
Statistical analysis
Statistical analysis was performed using IBM SPSS version 22.0. Count data are expressed as the number of cases and percentages [n (%)], and comparisons between groups were made using the chi-square test or Fisher’s exact probability test. Normally distributed measures are expressed as the‾x ± s, and independent samples t-tests were used for comparisons between two groups; nonnormally distributed measurement data are expressed as the median and interquartile range [M (IQR)], and the Mann-Whitney U test was applied for comparisons between two groups. Multivariate analysis was performed using binary logistic regression analysis. Predictive value was analyzed by receiver operating characteristic (ROC) curves. P < 0.05 was considered to indicate a statistically significant difference.
Ethics declarations
The study was approved by the Clinical Research and Application Ethics Committee of the Third Affiliated Hospital of Guangzhou Medical University. All methods were carried out in accordance with relevant guidelines and regulations. Written informed consent was obtained from the parents of eligible infants at the time of admission.
Data availability
All the data used in this study are available from the corresponding author upon reasonable request.
References
Seager, E., Longley, C., Aladangady, N. & Banerjee, J. Measurement of gut oxygenation in the neonatal population using Near-Infrared spectroscopy: A clinical tool?? Arch. Dis. Child-Fetal Neonatal Ed. 105, 76–86 (2020).
Palleri, E. et al. Clinical usefulness of splanchnic oxygenation in predicting necrotizing Enterocolitis in extremely preterm infants: A cohort study. Bmc Pediatr. 23, 336 (2023).
Patel, A. K. et al. Abdominal Near-Infrared spectroscopy measurements are lower in preterm infants at risk for necrotizing Enterocolitis. Pediatr. Crit. Care Med. 15, 735–741 (2014).
Corvaglia, L. et al. Splanchnic oxygenation at first enteral feeding in preterm infants: correlation with feeding intolerance. J. Pediatr. Gastroenterol. Nutr. 64, 550–554 (2017).
Dani, C. et al. Splanchnic tissue oxygenation for predicting feeding tolerance in preterm infants. Jpen J. Parenter. Enter. Nutr. 39, 935–940 (2015).
Metcalfe, K., Stienstra, R. & McHoney, M. Nirs as a biomarker of bowel ischaemia & surgical pathology: A Meta-Analysis of studies in newborns. Early Hum. Dev. 161, 105437 (2021).
Chitale, R. et al. Early enteral feeding for preterm or low birth weight infants: A systematic review and Meta-Analysis. Pediatrics 150, e2022057092E (2022).
Skinner, A. M. & Narchi, H. Preterm nutrition and neurodevelopmental outcomes. World J. Methodol. 11, 278–293 (2021).
Fanaro, S. Feeding intolerance in the preterm infant. Early Hum. Dev. 89 (Suppl 2), 13–S20 (2013).
Indrio, F. et al. Development of the Gastrointestinal tract in newborns as a challenge for an appropriate nutrition: A narrative review. Nutrients 14, 1405 (2022).
Baldassarre, M. E. et al. Dysbiosis and Prematurity: Is there a Role for Probiotics? Nutrients. 11, 1273 (2019).
Bora, R., Mukhopadhyay, K., Saxena, A. K., Jain, V. & Narang, A. Prediction of feed intolerance and necrotizing Enterocolitis in neonates with absent end diastolic flow in umbilical artery and the correlation of feed intolerance with postnatal superior mesenteric artery flow. J. Matern-Fetal Neonatal Med. 22, 1092–1096 (2009).
Martini, S. & Corvaglia, L. Splanchnic Nirs monitoring in neonatal care: rationale, current applications and future perspectives. J. Perinatol. 38, 431–443 (2018).
Chen, M. W. et al. Abdominal Near-Infrared spectroscopy in a piglet model of Gastrointestinal hypoxia produced by graded hypoxia or superior mesenteric artery ligation. Pediatr. Res. 83, 1172–1181 (2018).
Thomas, R. A., Ballard, M. R., Aladangady, N. & Banerjee, J. Abdominal near infrared spectroscopy can be reliably used to measure splanchnic oxygenation changes in preterm infants. J. Perinatol. 43, 716–721 (2023).
Dave, V. et al. Splanchnic tissue oxygenation, but not brain tissue oxygenation, increases after feeds in stable preterm neonates tolerating full bolus orogastric feeding. J. Perinatol. 29, 213–218 (2009).
Al-Hamad, S. et al. Contrast-Enhanced ultrasound and Near-Infrared spectroscopy of the neonatal bowel: novel, bedside, noninvasive, and Radiation-Free imaging for early detection of necrotizing Enterocolitis. Am. J. Perinatol. 35, 1358–1365 (2018).
Martini, S., Aceti, A., Beghetti, I., Faldella, G. & Corvaglia, L. Feed-Related splanchnic oxygenation in preterm infants with abnormal antenatal doppler developing gut complications. J. Pediatr. Gastroenterol. Nutr. 66, 755–759 (2018).
Weeks, C. L., Marino, L. V. & Johnson, M. J. A systematic review of the definitions and prevalence of feeding intolerance in preterm infants. Clin. Nutr. 40, 5576–5586 (2021).
Corvaglia, L. et al. Bolus vs. Continuous feeding: effects on splanchnic and cerebral tissue oxygenation in healthy preterm infants. Pediatr. Res. 76, 81–85 (2014).
Sirota, G. L. et al. Regional Splanchnic Oxygenation During Continuous Versus Bolus Feeding Among Stable Preterm Infants. Children-Basel. 9, (2022).
Braski, K. et al. Splanchnic-Cerebral oxygenation ratio decreases during enteral feedings in anemic preterm infants: observations under Near-Infrared spectroscopy. Neonatology 113, 75–80 (2018).
Martini, S. et al. Red blood cell transfusions alter splanchnic oxygenation response to enteral feeding in preterm infants: an observational pilot study. Transfusion 60, 1669–1675 (2020).
Group, E. B. M., Society, N. & Chinese Medical Doctor Association. [Clinical guidelines for the diagnosis and treatment of feeding intolerance in preterm infants (2020)]. Zhongguo Dang Dai Er Ke Za Zhi. 22, 1047–1055 (2020).
Dutta, S. et al. Guidelines for feeding very low birth weight infants. Nutrients 7, 423–442 (2015).
Magee, L. A. et al. The 2021 international society for the study of hypertension in pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 27, 148–169 (2022).
American Diabetes Association Professional Practice Committee.15. Management of diabetes in pregnancy: standards of medical care in diabetes-2022. Diabetes Care. 45, S232–S243 (2022).
Acknowledgements
We thank all the included infants and their parents or guardians in the study.
Funding
This study was supported by Natural Science Foundation of Guangdong Province (No. 2021A1515011225); Basic Research Project of Guangzhou Science and Technology Bureau (No. 202102010080); Key Laboratory Construction Project of Guangzhou Science and Technology Bureau (No. 2023A03J0381).
Author information
Authors and Affiliations
Contributions
J. C. and X. F. conceptualized and designed the study, data collection and initial analysis, drafted the initial manuscript, and approved the final manuscript as submitted. S. H., Z. L. and Z. S. participated in data collection and sorting, and reviewed and revised the manuscript. F. W. conceptualized and designed the study, conducted data analysis, critically reviewed the manuscript and approved the final manuscript as submitted. All the authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, J., Fan, X., He, S. et al. Regional tissue oxygen saturation during minimal enteral feeding is associated with the subsequent feeding intolerance in very preterm infants. Sci Rep 15, 8558 (2025). https://doi.org/10.1038/s41598-025-92185-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-92185-4