Abstract
Chromium (Cr) is a highly toxic heavy metal that can negatively impact crop yield and food quality by causing chlorosis and reduced root and shoot growth. To address this issue, rhizobacteria has emerged as a viable and safe technology. Additionally, gibberellins (GA3) can act as allied factors for regulating various physiological processes in plants, particularly cell division and elongation under Cr stress. That’s why the current study aimed to investigate the individual and combined effects of gibberellic acid (GA3) application and biofertilizer (Agrobacterium fabrum) in alleviating chromium toxicity in wheat. The treatments included two concentrations of chromium (Cr): 300Cr (300 mg Cr/kg soil) and 600Cr (600 mg Cr/kg soil), as well as the application of gibberellic acid (GA3 = 5 mg/L solution) with and without biofertilizer, i.e., A. fabrum. Results showed that the addition of GA3 + A. fabrum showed a significant increase in shoot fresh weight (~ 13%), shoot dry weight (~ 90%), root fresh weight (~ 76%), root dry weight (~ 88%), root length (~ 39%), shoot length (~ 18%) over control (no GA3 and No A. fabrum). In conclusion, GA3 + A. Farum is a better treatment for mitigating Cr toxicity in soil. More investigations are suggested at field levels under different cereal crops to declare GA3 + A. fabrum as the best treatment for alleviating Cr adverse effects on crops. Future research should focus on field-level investigations across cereal crops to validate GA3 + A. fabrum as the best treatment for alleviating Cr adverse effects on different crops and exploring its potential for integration into sustainable agricultural practices.
Similar content being viewed by others
Introduction
Chromium (Cr) is a toxic heavy metal that causes a decline in yield and deterioration of food quality1,2,3,4,5. High concentrations of Cr in the soil can accumulate in the wheat’s root system and be absorbed into the plant, leading to toxicity symptoms such as leaf chlorosis, yellowing of the stem and leaves, reduced root and shoot growth, and reduced grain yields. Cr toxicity can lead to plant death6. Its exposure can harm wheat, causing stunted growth and altering membrane permeability, generating reactive oxygen species (ROS). ROS accumulation causes electrolyte leakage7 and cell membrane disruption, leading to further damage8 production of green and dry mass of seedlings has been reduced due to changes in aerial parts and parts.
Additionally, the oxidation process of membrane proteins and lipids is commonly hindered, leading to cellular demise. Chromium toxicity triggers adverse impacts on plant development, such as leaf chlorosis, hindered root and shoot growth, and wilting. Once absorbed, chromium disrupts the lamellar system of plant tissues, as demonstrated by the inhibition of growth parameters reported9.
To address this challenge, microbial formulations, or biofertilizers, offer an economically feasible, safe alternative to toxic chemicals in sustainable wheat production systems10. Biofertilizers, which can colonize the cultivable habitat upon initial application, have been demonstrated to improve wheat yields through various mechanisms11,12,13. Wheat exhibits a high vulnerability to nitrogen deficiency, leading to symptoms such as leaf chlorosis resulting from compromised chlorophyll synthesis, decreased tillering, disruptions in cellular growth and division, and a decline in both the rate and extent of protein production14,15,16. Furthermore, gibberellins (GA3) constitute a significant class of plant hormones that are essential for regulating diverse physiological functions, notably in the kingdom of cell division and elongation17,18. Their multifaceted effects encompass nearly all phases of plant growth, encompassing pivotal events such as seed germination, stem and leaf development, floral induction, and fruit growth17,19. Notably, GA3 exerts its influence by synergizing with other phytohormones, specifically auxins and cytokinin’s, thereby orchestrating and promoting overall plant growth and development17,20.
That’s why the current study was conducted to explore the effectiveness of GA3 and rhizobacteria A. fabrum inoculation as a sole and combined amendment to mitigate Cr toxicity in wheat. While previous research has explored the individual roles of GA3 or microbial inoculants in mitigating different heavy metal stress, this study is novel in terms of integrated approach, combining the phytohormonal regulation potential of gibberellic acid (GA3) with the plant growth-promoting Agrobacterium fabrum (A. fabrum) against Cr toxicity in wheat. The study covers the knowledge gap regarding the use of GA3 and A. fabrum inoculation to alleviate Cr stress in wheat. It is hypothesized that the combined use of GA3 and A. fabrum inoculation might potentially alleviate the Cr toxicity in wheat.
Materials and methods
Experimental site
A current pot study was conducted on wheat (Triticum aestivum L.) in the Department of Botany, Islamia University Bahawalpur research area. Before starting the experiment, composite soil and irrigation samples were collected for pre-experimental soil characterization. The soil and irrigation water characteristics are provided in Table 1.
Treatment plan
The treatments included three concentrations of chromium (Cr): 0Cr (0 mg Cr/kg soil), 300Cr (300 mg Cr/kg soil), and 600Cr (600 mg Cr/kg soil)29. There were two levels of gibberellic acid (GA3 = 5 mg/L solution and no GA3).
GA3 application
A total of 2 sprays of GA3 were applied on the wheat, i.e., the first one after seven days of germination and the second one after 30 days of germination. Additionally, a control group was maintained without any GA3.
Agrobacterium fabrum
The rhizobacteria Agrobacterium fabrum (NR_074266.1) was previously isolated from rhizosphere of wheat as described by30. The bacteria was initially tested for its survival under Cr toxicity by growing on DF media31 (4.0 g KH2PO4, 0.2 g, MgSO4·7H2O, 6.0 g Na2HPO4, 2.0 g gluconic acid, 2.0 g glucose, 2.0 g citric acid, 1 mg FeSO4·7H2O, 11.19 mg MnSO4·H2O, 10 mg H3BO3, 78.22 mg CuSO4·5H2O, 124.6 mg ZnSO4·7H2O, 10 mg MoO3, pH = 7.2 and 0.5 M ACC as a sole nitrogen source) having 600 ug/L Cr introduced by using K2Cr2O7.
Pot dimensions and filing
Plastic bags with dimensions of 10-inch depth and 15-inch diameter were used as pots. In each pot, 10 kg of soil was filled.
Seed sterilization, inoculation and sowing
Before sowing, the seeds were sterilized using a sodium hypochlorite solution. The seeds were soaked in a dilute sodium hypochlorite solution for a specified period to eliminate surface pathogens and enhance seed health. Inoculating wheat seeds with A. fabrum was conducted using peat-based inoculation and 10% sugar solution. For 100 g seeds 10 ml of inoculum having optical density 0.5 at 600 nm wavelength was used. After inoculation, the seeds were allowed to dry for 1 h under controlled conditions to ensure proper adhesion of the inoculum to the seed surface. The characteristics of A. fabrum is provided in Table 2. The inoculated seeds of wheat (Akbar 2019) were manually sown in pots, with ten seeds per pot.
Fertilizer application and irrigation
During the experimental period, recommended fertilizers application rates (Nitrogen (N): 52 kg/ac [0.64 g/pot], Phosphorus (P): 46 kg/ac [0.57 g/pot], Potassium (K): 25 kg/ac [0.31 g/pot]) were applied to provide essential nutrients to the wheat plants. Irrigation was provided to maintain optimal soil moisture levels (65FC (w/w)) necessary for wheat growth throughout the experiment.
Experimental setup and growth duration
After seed sowing and treatment application, the pots were placed in a controlled environment chamber under standardized temperature (20 ± 3 °C), and humidity (50%) for wheat growth. The crop was allowed to grow for 50 days, ensuring sufficient time for plant development and the expression of treatment effects.
Crop harvesting
The wheat crop was harvested at the end of the 50-day growth period. The aboveground parts of the plants, including leaves and stems, were carefully cut at the base using sharp tools. The harvested crop material was then separated and processed for subsequent measurements and analysis.
Data collection and analysis
After a specified duration of treatment, various growth parameters were measured to evaluate the effects of the treatments. These parameters included germination percentage, shoot length, root length, seedling length, shoot fresh weight, shoot dry weight, root fresh weight, root dry weight, and vigor index. The measurements were taken for each replicate within a treatment group soon after harvesting.
Chlorophyll analysis
Fresh leaf samples of 0.5 g were taken, and 80% acetone (20 ml) was added to a pestle mortar. The grinding was done manually, and filtration was performed to obtain the filtrate. Absorbance measurements were taken at 663 nm and 645 nm wavelengths using a spectrophotometer. A blank solution containing the extraction solvent was also run as a reference32.
Gas exchange attributes
The gas exchange attributes were assessed using the CI-340 Photosynthesis system, manufactured by CID, Inc. USA, operating as an infrared gas analyzer. Data collection took place during a sunny period between 10:30 and 11:30 AM, coinciding with peak light intensity levels conducive to photosynthesis saturation33.
Antioxidants
Superoxide dismutase (SOD) activity measuring the reduction inhibition of nitro blue tetrazolium (NBT) at 560 nm wavelength34. The study evaluated catalase (CAT) activity by observing the breakdown of hydrogen peroxide and the decrease in absorbance at 240 nm35. Ascorbate peroxidase (APX) activity was calculated by observing ascorbate oxidation in the presence of H2O2 at 290 nm36.
Free proline determination
The study assessed free proline content using glacial acetic acid, ninhydrin solutions, and sulfosalicylic acid, and combined solution heating at 100 °C, adding 5 ml of toluene, and recording the absorbance at 520 nm37.
N, P, and K leaves
The study used a modified micro-Kjeldahl method for nitrogen content determination38, a flame photometer for potassium content analysis, and a spectrophotometer for phosphorus content quantification at 420 nm39.
Statistical analysis
The collected data was analyzed using Origin Software. The liner mixed model was used keeping chromium and GA3 as fixed factor while replication a random factor. The data was tested to ensure the assumption of models40. The significance of differences between the treatment groups was determined using analysis of variance (ANOVA), followed by Fisher’s LSD using OriginPro 202141.
Results
Germination, seedling length, shoot and root length
Adding GA3, A. fabrum, and GA3 + A. fabrum showed a significant ~ 8%, ~ 17%, and ~ 25% rise in germination rate under control condition. Under 300Cr stress, applying GA3, A. fabrum, and GA3 + A. fabrum resulted ~ 10%, ~ 22%, and ~ 35% increase in germination rate over the control in 300Cr stress. Adding GA3, A. fabrum, and GA3 + A. fabrum treatments in comprison to the control under 600Cr stress the germination rate increased by ~ 22%, ~ 57%, and ~ 81%, respectively (Fig. 1A).
The seedling length increased by ~ 2%, ~ 7%, and ~ 11% with GA3, A. fabrum, and GA3 and A. fabrum treatments under control. Adding GA3, A. fabrum, and GA3 and A. fabrum treatments resulted in a significant ~ 4%, ~ 8%, and ~ 11% increase in seedling length under 300Cr stress and 600Cr stress showed ~ 3%, ~ 7%, and ~ 11% increase in comparison to their respective controls (Fig. 1B).
Shoot length increased ~ 2%, ~ 6%, and ~ 10% by applying GA3, A. fabrum, and GA3 and A. fabrum under control. Under 300Cr stress, the shoot length increased by ~ 2%, ~ 6%, and ~ 9% with the application of GA3, A. fabrum, and GA3 and A. fabrum. Compared to the control adding GA3, A. fabrum, and GA3 and A. fabrum under 600Cr, a significant ~ 6%, ~ 11%, and ~ 18% increase in shoot length were recorded (Fig. 1C).
In control, applying GA3, A. fabrum, and GA3 and A. fabrum treatment showed a significant ~ 5%, ~ 10%, and ~ 15% rise in root length. In comparison to the control under 300Cr stress, root length increased by ~ 7%, ~ 19%, and ~ 30% with GA3, A. fabrum, and GA3 and A. fabrum, and under 600Cr stress showed ~ 15%, ~ 25%, and ~ 39% than their control (600Cr) (Fig. 1D).
Shoot/root fresh and dry weight
The application of GA3, A. fabrum, and GA3 and A. fabrum showed ~ 3%, ~ 6%, and ~ 8% increase in shoot fresh over the control under no stress. Under 300Cr stress, shoot fresh weight increased by ~ 3%, ~ 7%, and ~ 10% with GA3, A. fabrum, and GA3 and A. fabrum than the 300Cr stressed control. Compared to the control under 600Cr stress, shoot fresh weight increased by ~ 4%, ~ 8%, and ~ 13% than the 600Cr stressed control (Fig. 2A).
Under control, the shoot dry weight increased by ~ 6% with GA3, ~ 11% with A. fabrum, and ~ 20% with GA3 and A. fabrum. Under 300Cr stress, shoot dry weight increased by ~ 22% with GA3, ~ 22% with A. fabrum, and ~ 30% with GA3 and A. fabrum than the 300Cr stressed control. Under 600Cr, shoot dry weight increased by ~ 21% with GA3, ~ 65% with A. fabrum, and ~ 90% with GA3 and A. fabrum over the 600Cr stressed control (Fig. 2B).
The root fresh weight increased by ~ 13% with GA3, ~ 20% with A. fabrum, and ~ 29% with GA3 and A. fabrum than the control under no stress. Under 300Cr, root fresh weight showed a ~ 10% increase with GA3, ~ 20% with A. fabrum, and ~ 33% with GA3 and A. fabrum over the 300Cr stressed control. Under 600Cr, root fresh weight increased by ~ 2%, ~ 44%, and ~ 76% with GA3, A. fabrum, and GA3 and A. fabrum than the 600Cr stressed control (Fig. 2C).
The root dry weight increased by ~ 13%, ~ 26%, and ~ 48% with the application of GA3, A. fabrum, and GA3 and A. fabrum than the control. Under 300Cr stress, adding GA3, A. fabrum, and GA3 and A. fabrum treatments showed ~ 15%, ~ 38%, and ~ 56% rise in root dry weight than the 300Cr stressed control. Applying GA3, A. fabrum, and GA3 and A. fabrum treatments compared to the control under 600Cr stress showed ~ 28%, ~ 28%, and ~ 88% increase in root dry weight (Fig. 2D).
Vigor index and chlorophyll content
In control, adding GA3, A. fabrum, and GA3 and A. fabrum treatments showed a significant ~ 2%, ~ 4%, and ~ 9% increase in vigor index. Under 300Cr stress, the vigor index increased by ~ 2%, ~ 3%, and ~ 5% with GA3, A. fabrum, and GA3 and A. fabrum than the 300Cr stressed control. Compared to the control under 600Cr stress, the vigor index showed ~ 2%, ~ 6%, and ~ 10% rise with GA3, A. fabrum, and GA3 and A. fabrum than the 600Cr stressed control (Fig. 3A).
The chlorophyll a content showed ~ 6%, ~ 10%, and ~ 16% increase with the addition of GA3, A. fabrum, and GA3 and A. fabrum under control. Under 300Cr stress, the chlorophyll a showed ~ 17% increase with GA3, ~ 33% with A. fabrum, and ~ 45% with GA3 and A. fabrum over the 300Cr stressed control. Compared to the 600Cr stressed control, chlorophyll a showed ~ 26%, ~ 46%, and ~ 68% rise under 600Cr stress (Fig. 3B).
The chlorophyll b content showed ~ 4% with GA3, ~ 8% with A. fabrum, and ~ 13% with GA3 and A. fabrum than the control under no stress. Under 300Cr stress, chlorophyll b content showed a ~ 5% increase with GA3, ~ 8% with A. fabrum, and ~ 13% with GA3 and A. fabrum than the 300Cr stressed control. Compared to the 600Cr stressed control, chlorophyll b showed ~ 13% rise with GA3, ~ 32% with A. fabrum, and ~ 40% with GA3 and A. fabrum (Fig. 3C).
Adding GA3, A. fabrum, and GA3 + A. fabrum showed a significant ~ 5%, ~ 9%, and ~ 14% rise in total chlorophyll content under control conditions. Under 300Cr stress, applying GA3, A. fabrum, and GA3 + A. fabrum resulted ~ 10%, ~ 19%, and ~ 27% increase in total chlorophyll over the 300Cr stressed control. Adding GA3, A. fabrum, and GA3 + A. fabrum treatments in comprison to the control under 600Cr stress the total chlorophyll increased by ~ 40%, ~ 68%, and ~ 84%, respectively (Fig. 3D).
Gass exchange attributes
The photosynthetic rate under control showed a ~ 3% increase with GA3, ~ 4% with A. fabrum, and ~ 6% with GA3 + A. fabrum than the control. Compared to the control under 300Cr stress, the photosynthetic rate showed a ~ 6% increase with GA3, ~ 13% with A. fabrum, and ~ 19% with GA3 + A. fabrum over 300Cr stressed control. Under 600Cr, adding GA3 showed a ~ 5% rise in photosynthetic rate, A. fabrum ~ 11%, and GA3 + A. fabrum ~ 18% compared to 600Cr stressed control (Fig. 4A).
The transpiration rate showed ~ 1% with GA3, ~ 3% with A. fabrum and ~ 4% with GA3 + A. fabrum than the control under no stress. Under 300Cr stress, the transpiration rate showed a ~ 2% increase with GA3, ~ 3% with A. fabrum, and ~ 5% with GA3 + A. fabrum than the 300Cr stressed control. Compared to the 600Cr stressed control, the transpiration rate showed a ~ 3% rise with GA3, ~ 8% with A. fabrum, and ~ 11% with GA3 and A. fabrum (Fig. 4B).
Adding GA3, A. fabrum, and GA3 + A. fabrum showed a significant ~ 4%, ~ 7%, and ~ 10% rise in stomatal conductance under control condition. Under 300Cr stress, applying GA3, A. fabrum, and GA3 + A. fabrum resulted ~ 5%, ~ 7%, and ~ 11% increase in stomatal conductance over the control in 300Cr stress. Adding GA3, A. fabrum, and GA3 + A. fabrum treatments in comprison to the control under 600Cr stress the stomatal conductance increased by ~ 3%, ~ 8%, and ~ 13%, respectively (Fig. 4C).
Proline, SOD, CAT, and APx
In control, adding GA3, A. fabrum, and GA3 and A. fabrum treatments showed a significant ~ 6%, ~ 15%, and ~ 16% decrease in proline. Under 300Cr stress, proline level decreased by ~ 6%, ~ 14%, and ~ 22% with GA3, A. fabrum, and GA3 and A. fabrum than the 300Cr stressed control. Compared to the control under 600Cr stress, the proline level showed ~ 6%, ~ 11%, and ~ 17% decrease with GA3, A. fabrum, and GA3 and A. fabrum than the 600Cr stressed control (Fig. 5A).
The effect of treatments on proline (A), SOD (Superoxide dismutase) (B), CAT (Catalase) (C), and APx (Ascorbate peroxidase) of wheat cultivated with and without Cr stress. The Fisher LSD test measured significant differences at (p < 0.05); distinct letters on the bars are the mean of four replicates.
The SOD activity showed ~ 7% decrease with GA3, ~ 14% with A. fabrum, and ~ 18% with GA3 + A. fabrum than the control under no stress. Under 300Cr stress, SOD activity showed ~ 6% decrease with GA3, ~ 13% with A. fabrum, and ~ 24% with GA3 + A. fabrum than the 300Cr stressed control. Compared to the 600Cr stressed control, SOD activity showed ~ 3% decrease with GA3, ~ 7% with A. fabrum, and ~ 11% with GA3 and A. fabrum (Fig. 5B).
The CAT activity under control showed a ~ 3% decrease with GA3, ~ 5% with A. fabrum, and ~ 7% with GA3 + A. fabrum than the control. Compared to the control under 300Cr stress, CAT activity showed a ~ 2% decrease with GA3, ~ 3% with A. fabrum, and ~ 5% with GA3 + A. fabrum over 300Cr stressed control. Under 600Cr, adding GA3 showed a ~ 1% decrease in CAT activity, A. fabrum ~ 2%, and GA3 + A. fabrum ~ 3% compred to 600Cr stressed control (Fig. 5C).
Adding GA3, A. fabrum, and GA3 + A. fabrum showed a significant ~ 6%, ~ 10%, and ~ 22% decrease in APx activity under control condition. Under 300Cr stress, applying GA3, A. fabrum, and GA3 + A. fabrum resulted ~ 3%, ~ 7%, and ~ 12% decrease in APx activity over the control in 300Cr stress. Adding GA3, A. fabrum, and GA3 + A. fabrum treatments in comprison to the control under 600Cr stress the APx activity decreased by ~ 5%, ~ 8%, and ~ 13%, respectively (Fig. 5D).
Shoot N, P, and K
The shoot N under control showed a ~ 9% increase with GA3, ~ 16% with A. fabrum, and ~ 26% with GA3 + A. fabrum than the control. Compared to the control under 300Cr stress, shoot N showed a ~ 10% increase with GA3, ~ 23% with A. fabrum, and ~ 33% with GA3 + A. fabrum over 300Cr stressed control. Under 600Cr, adding GA3 showed a ~ 13% rise in shoot N, A. fabrum ~ 33%, and GA3 + A. fabrum ~ 52% compared to 600Cr stressed control (Fig. 6A).
The shoot P showed ~ 10% increase with GA3, ~ 14% with A. fabrum, and ~ 17% with GA3 + A. fabrum than the control under no stress. Under 300Cr stress, shoot P showed ~ 10% increase with GA3, ~ 19% with A. fabrum, and ~ 23% with GA3 + A. fabrum than the 300Cr stressed control. Compared to the 600Cr stressed control, shoot P showed ~ 3% rise with GA3, ~ 22% with A. fabrum, and ~ 37% with GA3 and A. fabrum (Fig. 6B).
Adding GA3, A. fabrum, and GA3 + A. fabrum showed a significant ~ 4%, ~ 9%, and ~ 14% rise in shoot K under control condition. Under 300Cr stress, applying GA3, A. fabrum, and GA3 + A. fabrum resulted ~ 12%, ~ 25%, and ~ 39% increase in shoot K over the control in 300Cr stress. Adding GA3, A. fabrum, and GA3 + A. fabrum treatments in comprison to the control under 600Cr stress the shoot K increased by ~ 20%, ~ 42%, and ~ 63%, respectively (Fig. 6C).
Cr in shoot and root
The Cr in the leaf showed ~ 15%, ~ 39%, and ~ 64% decrease with the addition of GA3, A. fabrum, and GA3 and A. fabrum under control. Under 300Cr stress, the Cr in the leaf showed a ~ 14% decrease with GA3, ~ 28% with A. fabrum, and ~ 45% with GA3 and A. fabrum over the 300Cr stressed control. Compared to the 600Cr stressed control, Cr in the leaf showed ~ 5%, ~ 9%, and ~ 19% decrease under 600Cr stress (Table 3).
The Cr in root showed ~ 27% with decreased GA3, ~ 71% with A. fabrum, and ~ 86% with GA3 and A. fabrum than the control under no stress. Under 300Cr stress, Cr in root showed a ~ 12% decrease with GA3, ~ 33% with A. fabrum, and ~ 61% with GA3 and A. fabrum than the 300Cr stressed control. Compared to the 600Cr stressed control, Cr in root showed ~ 7% decrease with GA3, ~ 16% with A. fabrum, and ~ 31% with GA3 and A. fabrum (Table 3).
Pearson correlation analysis
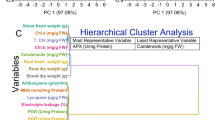
The analysis utilized correlation coefficients on a scale from − 1 to 1, where values nearer to 1 signify stronger positive correlations, those closer to − 1 denote stronger negative correlations, and those around 0 imply no significant correlation. Plant growth parameters including germination percentage, shoot length, root length, seedling length, shoot fresh weight, shoot dry weight, root fresh weight, and root dry weight demonstrated robust positive correlations, with coefficients ranging from approximately 0.99 to 1. These parameters exhibited a cohesive grouping, indicating their interconnectedness in influencing plant growth and development. Conversely, physiological parameters such as chlorophyll content, photosynthetic rate, transpiration rate, and stomatal conductance formed another distinct cluster, suggesting their mutual reliance in regulating plant physiological processes. The correlation coefficients within this cluster ranged from approximately 0.98 to 1, reflecting strong positive correlations. A separate cluster encompassed stress response and biochemical activity parameters, including proline concentration, superoxide dismutase (SOD) activity, catalase (CAT) activity, and ascorbate peroxidase (APX) activity. These variables displayed negative correlations, implying potential antagonistic interactions within stress response mechanisms. Additionally, parameters associated with nutrient levels such as shoot nitrogen (N), phosphorus (P), and potassium (K) percentages clustered together, indicating their collective impact on plant nutrient status and metabolism. Lastly, variables concerning heavy metal accumulation in plant tissues, such as chromium (Cr) concentration in leaf and root tissues, formed a distinct cluster due to their unique characteristics compared to other measured parameters in the study (Fig. 7).
Discussion
Chromium (Cr) contamination in soil and water is a significant environmental concern due to its widespread industrial use. Cr toxicity in plants depends on its valence state, with Cr(VI) being more toxic than Cr(III)42. Plants lack a specific transport system for Cr, which is taken up by carriers of essential ions like sulfate or iron43. Cr adversely affects plant growth, development, and physiological processes such as photosynthesis, water relations, and mineral nutrition44. It induces oxidative stress by generating reactive oxygen species, leading to lipid peroxidation and cellular damage45. Cr toxicity also impairs seed germination, chlorophyll biosynthesis, and enzymatic activities42. Plants have developed various defense mechanisms, including antioxidant enzymes, proline accumulation, and stress proteins, to cope with Cr toxicity42. Bioremediation and phytoremediation have gained interest as potential strategies for cleaning up Cr-contaminated areas44. Moreover, heightened generation of reactive oxygen species (ROS) as reported by46 could potentially diminish the photosynthetic efficiency of plants subjected to chromium stress. Chromium accumulation may have further decreased energy utilization, leading to a decrease in the activity of photosynthetic pigments47. The detrimental impact of chromium (Cr) appears to have hindered the functionality of enzymes involved in carbon fixation and the electron transport chain, leading to a notable decline in the photosynthetic rate (A) of plants, specifically in terms of CO2 fixation. Additionally, the diminished assimilation of CO2 may be correlated with a decrease in excitation capture efficiency6 and PS-II quantum yield. Chromium toxicity significantly impacted the transpiration rate (E) of plants, affecting water loss from their surfaces. This effect can be linked to diminished water potential and heightened diffusive resistance. The reduction in transpiration rate corresponds to the regulation of stomatal openings by guard cells, facilitating gas exchange but leading to increased water loss, which is influenced by the presence of chromium48.
The application of plant growth-promoting rhizobacteria A. fabrum49 has been demonstrated to confer numerous benefits to plants, such as disease suppression and improved nutrient availability and assimilation. Directly, A. fabrum has been shown to produce growth regulators, solubilize phosphate, and generate 1-aminocyclopropane-1-carboxylate (ACC) deaminase49. It can decrease the stress generating ethylene in plants which played an important role in degradation of chlorophyll by activation of chlase49,50. In addition to above, siderophores can reduce metal pollution impact and aid phytoremediation by binding trace element ions51. Metal resistance is crucial in heavy metal remediation. Variations in peroxidase, APX, and catalase activities vary across strains45. The minimal increase in antioxidant enzyme activities observed in some instances may result from the inoculated strains stimulation of plant defense mechanisms52. Better uptake of nutrients and water was another allied factor which played an important role in mitigation of Cr toxicity. Nutrients uptake strengthen the plants growth attributes. On the other hand, ample water uptake caused dilution effect against Cr toxicity. It also enhanced the fresh weight and other attributes of plants due to optimum uptake of nutrients which developed a nutrients balance in plant body53.
Gibberellic acid (GA3) acts as an adaptogen that helps the plant to overcome stressful conditions. Its application triggers the the antioxidant enzymatic activity to protect the plant from the damaging effects of heavy metal toxicity54,55,56. This increase in tolerance allows plants to withstand heavy metal-induced oxidative stress, thus improving growth and yield57. Low concentrations of GA3 increased cell number, protein content, and photosynthetic pigments under cadmium and lead stress58. Similarly, GA3 application improved growth and chlorophyll content in plants exposed to lead, while reducing lead uptake and regulating phenolic compounds56.
Conclusion
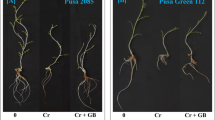
In conclusion, the combined application of GA3 and Agrobacterium fabrum has shown promising potential to improve wheat growth under chromium (Cr) toxicity. The study indicates that this treatment can significantly enhance chlorophyll content, as well as the fresh and dry weight of roots and shoots, in wheat cultivated in Cr-contaminated environments. These findings suggest that the application of GA3 and A. fabrum can mitigate the adverse effects of Cr toxicity, leading to healthier and more robust wheat plants. For growers, this combination presents a viable strategy to enhance wheat growth in areas affected by Cr contamination, potentially improving crop yields and contributing to food security in regions facing heavy metal pollution. The application of GA3 and A. fabrum can be particularly beneficial in areas where soil contamination poses a significant challenge to agricultural productivity. However, while these initial results are promising, further investigations at the field level are necessary to confirm the efficacy of GA3 and A. fabrum under diverse environmental conditions and to establish standardized application protocols. Such field-level studies would help in understanding the long-term effects, optimal dosages, and practical implementation of this treatment in different soil types and climatic conditions. Ultimately, these efforts could lead to the declaration of GA3 and A. fabrum as the best treatment for cultivating cereals in Cr-affected areas, thereby offering a sustainable solution to a critical agricultural problem.
Data availability
All data generated or analysed during this study are included in this published article.
References
Rizvi, A. et al. Heavy metal induced stress on wheat: Phytotoxicity and microbiological management. RSC Adv. 10, 38379–38403 (2020).
Haider, I., Arif Ali, M. & Sanaullah, M. Change in lettuce nutrients, soil enzymes, soil microbial biomass and activities under chromium toxicity. Pak. J. Bot. 55, 89–96 (2023).
Bedar Bekhat Naseem, M., Ali, Q., Ali, S., Rehan Khalid, M. & Nawaz, M. Selenium application reduces cadmium uptake in tomato (Lycopersicum esculentum Mill.) by modulating growth, nutrient uptake, gas exchange, root exudates and antioxidant profile. Pak. J. Bot. 55, 1633–1646 (2023).
Ghafoor, I. et al. Silicon regulates growth, yield, physiological responses, and tissue concentration of lead in Brassica campestris L. grown in lead contaminated soil. Pak. J. Bot. 55, 127–134 (2023).
Sheikh, L. et al. Evaluating the effects of cadmium under saline conditions on leafy vegetables by using acidified biochar. Pak. J. Bot. 55, 33–39 (2023).
Fahsi, N., Mahdi, I., Mesfioui, A., Biskri, L. & Allaoui, A. Plant growth-promoting rhizobacteria isolated from the Jujube (Ziziphus lotus) plant enhance wheat growth, Zn uptake, and heavy metal tolerance. Agriculture 11, 316 (2021).
Ilyas, N. et al. Efficacy of citric acid chelate and Bacillus sp. in amelioration of cadmium and chromium toxicity in wheat. Chemosphere 290, 133342 (2022).
Elnahal, A. S. M. et al. The use of microbial inoculants for biological control, plant growth promotion, and sustainable agriculture: A review. Eur. J. Plant Pathol. 162, 759–792 (2022).
Xu, T. et al. Deciphering soil amendments and actinomycetes for remediation of cadmium (Cd) contaminated farmland. Ecotoxicol. Environ. Saf. 249, 114388 (2023).
Jain, D. et al. Zinc tolerant plant growth promoting bacteria alleviates phytotoxic effects of zinc on maize through zinc immobilization. Sci. Rep. 10, 1–13 (2020).
Zafar-ul-Hye, M. et al. Rhizobacteria having ACC-deaminase and biogas slurry can mitigate salinity adverse effects in wheat. Pak. J. Bot. 54, 297–303 (2022).
Hussain, A. et al. Comparative effectiveness of plant growth promoting rhizobacteria and various organic carriers on wheat growth, physiology, antioxidative activities and rhizosphere properties. Pak. J. Bot. 54, 317–324 (2022).
Ali, F., Bano, A., Hassan, T. U., Nazir, M. & Khan, R. T. Plant growth promoting rhizobacteria induced modulation of physiological responses in rice under salt and drought stresses. Pak. J. Bot. 55, 447–452 (2023).
Hamid, B. et al. Bacterial plant biostimulants: A sustainable way towards improving growth, productivity, and health of crops. Sustainability 13, 2856 (2021).
Ullah, A., Bano, A. & Javed, H. PGPR assisted bioremediation of heavy metals and nutrient accumulation in Zea mays under saline sodic soil. Pak. J. Bot. 53, 31–38 (2021).
Khan, A., Asad, S., Bano, A., Khan, R. A. & Qadri, T. A. Evaluation of plant growth promoting rhizobacteria (PGPR) for the control of charcoal rot of Mung bean. Pak. J. Bot. 54, 285–289 (2022).
Morcillo, R. & Manzanera, M. The effects of plant-associated bacterial exopolysaccharides on plant abiotic stress tolerance. Metabolites 11, 337 (2021).
Long, W.-H. et al. Cloning and characterization of two gibberellin receptor genes in yam (Dioscorea opposita). Pak. J. Bot. 53, 1717–1725 (2021).
Iqbal, I. et al. Improvement of wheat (Triticum aestivum L.) productivity with the applications of plant growth regulators. Pak. J. Bot. 54, 1675–1684 (2022).
Salih, E. G. I. et al. Effects of seeds priming with plant growth regulators on germination and seedling growth of Hargel (Solenostemma argel (Del.) Hayne) under salinity stress. Pak. J. Bot. 54, 1579–1587 (2022).
McLean, E. O., Page, A. L., Miller, R. H., Keeny, D. R. & McLean, E. O. Soil pH and lime requirement. in Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties, 9.2.2/Agronomy Monographs (ed. Page, A. L.) vol. 9 199–224 (American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America, Madison, 1982).
Estefan, G., Sommer, R. & Ryan, J. Methods of Soil, Plant, and Water Analysis: A Manual for the West Asia and North Africa Region (International Center for Agricultural Research in the Dry Areas (ICARDA), 2013).
Rhoades, J. D. Salinity: Electrical conductivity and total dissolved solids. In Methods of Soil Analysis, Part 3, Chemical Methods Vol. 5 (eds Sparks, D. L. et al.) 417–435 (Soil Science Society of America, 1996).
Nelson, D. W. A. & Sommers, L. Total carbon, organic carbon, and organic matter. Methods Soil Anal. Part 2 Chem Microbiol. Prop. 9, 539–579 (1983).
Bremner, M. Nitrogen-total. In Methods of Soil Analysis Part 3. Chemical Methods-SSSA Book Series 5 (eds Sumner, D. L. et al.) 1085–1121 (Wiley, 1996).
Kuo, S. Phosphorus. In Methods of Soil Analysis Part 3: Chemical Methods (eds Sparks, D. L. et al.) 869–919 (Wiley, SSSA, 2018). https://doi.org/10.2136/sssabookser5.3.c32.
Pratt, P. F. Potassium. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties (ed. Norman, A. G.) 1022–1030 (Wiley, 2016). https://doi.org/10.2134/agronmonogr9.2.c20.
Gee, G. W. & Bauder, J. W. Particle-size Analysis. In Methods of Soil Analysis. Part 1. Physical and Mineralogical Methods (ed. Klute, A.) 383–411 (Wiley, 2018). https://doi.org/10.2136/sssabookser5.1.2ed.c15.
López-Luna, J., González-Chávez, M. C., Esparza-García, F. J. & Rodríguez-Vázquez, R. Toxicity assessment of soil amended with tannery sludge, trivalent chromium and hexavalent chromium, using wheat, oat and sorghum plants. J. Hazard Mater. 163, 829–834 (2009).
Danish, S. & Zafar-ul-Hye, M. Co-application of ACC-deaminase producing PGPR and timber-waste biochar improves pigments formation, growth and yield of wheat under drought stress. Sci. Rep. 9, 5999 (2019).
Dworkin, M. & Foster, J. W. Experiments with some microorganisms which utilize ethane and hydrogen. J. Bacteriol. 75, 592–603 (1958).
Arnon, D. I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant. Physiol. 24, 1–15 (1949).
Nazar, R., Khan, M. I. R., Iqbal, N., Masood, A. & Khan, N. A. Involvement of ethylene in reversal of salt-inhibited photosynthesis by sulfur in mustard. Physiol. Plant. 152, 331–344 (2014).
Dhindsa, R. S., Plumb-Dhindsa, P. L. & Reid, D. M. Leaf senescence and lipid peroxidation: Effects of some phytohormones, and scavengers of free radicals and singlet oxygen. Physiol. Plant 56, 453–457 (1982).
Aebi, H. Catalase in vitro. Methods Enzymol. 105, 121–126 (1984).
Nakano, Y. & Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22, 867–880 (1981).
Bates, L. S., Waldren, R. P. & Teare, I. D. Rapid determination of free proline for water-stress studies. Plant Soil 39, 205–207 (1973).
Steyermark, A. L. & McGee, B. E. Progress in elemental quantitative organic analysis: 1960. Microchem. J. 5, 389–410 (1961).
Olsen, S. R. & Sommers, L. E. Phosphorus. In Method of Soil Analysis, Agron. No. 9, part 2: Chemical and Microbiological Properties (ed. Page, A. L.) 403–430 (American Society of Agronomy, 1982).
Steel, R. G. D. & Torrie, J. H. Principles and Procedures of Statistics A Biometrical Approach 3rd edn, 334–381 (McGraw Hill Book Company Inc., 1996). https://doi.org/10.2307/2333165.
OriginLab Corporation. OriginPro (OriginLab, 2021).
Yıldız, M. Chromium toxicity and cellular responses in plants (2011).
Shanker, A. K., Cervantes, C., Loza-Tavera, H. & Avudainayagam, S. Chromium toxicity in plants. Environ. Int. 31, 739–753 (2005).
Bhalerao, S. A. & Sharma, A. S. Chromium: As an Environmental Pollutant. Int. J. Curr. Microbiol. Appl. Sci. 4, 732–746 (2015).
Sharma, A. et al. Chromium bioaccumulation and its impacts on plants: An overview. Plants 9, 100 (2020).
Khalid-Chaudhry, U. et al. Integration of biochar and chemical fertilizer to enhance quality of soil and wheat crop (Triticum aestivum L.). J. Biodivers. Environ. Sci. 9, 348–358 (2016).
Raklami, A., Meddich, A., Oufdou, K. & Baslam, M. Plants—microorganisms-based bioremediation for heavy metal cleanup: Recent developments, phytoremediation techniques, regulation mechanisms, and molecular responses. Int. J. Mol. Sci. 23, 5031 (2022).
Etesami, H. Bacterial mediated alleviation of heavy metal stress and decreased accumulation of metals in plant tissues: Mechanisms and future prospects. Ecotoxicol. Environ. Saf. 147, 175–191 (2018).
Khanna, K., Ohri, P., Bhardwaj, R. & Ahmad, P. Unsnarling plausible role of plant growth-promoting rhizobacteria for mitigating Cd-toxicity from plants: An environmental safety aspect. J. Plant Growth Regul. 41, 2514–2542 (2021).
Matile, P., Schellenberg, M. & Vicentini, F. Localization of chlorophyllase in the chloroplast envelope. Planta 201, 96–99 (1997).
Pandey, N. & Sharma, C. P. Chromium interference in iron nutrition and water relations of cabbage. Environ. Exp. Bot. 49, 195–200 (2003).
Choppala, G. K., Bolan, N. S., Megharaj, M., Chen, Z. & Naidu, R. The influence of biochar and black carbon on reduction and bioavailability of chromate in soils. J. Environ. Qual. 41, 1175–1184 (2012).
Danish, S., Hussain, G. S., Hussain, M. B., Elgorban, A. M. & Datta, R. Unveiling the potential of A. fabrum and γ-aminobutyric acid for mitigation of nickel toxicity in fenugreek. Sci. Rep. 14, 11042 (2024).
Danish, S. et al. Effect of methyl jasmonate and GA3 on canola (Brassica napus L.) growth, antioxidants activity, and nutrient concentration cultivated in salt-affected soils. BMC Plant Biol. 24, 363 (2024).
Khalid, A. & Aftab, F. Effect of exogenous application of IAA and GA3 on growth, protein content, and antioxidant enzymes of Solanum tuberosum L. grown in vitro under salt stress. In Vitro Cell. Dev. Biol. Plant 56, 377–389 (2020).
Anwar, T. et al. Alleviation of cadmium and drought stress in wheat by improving growth and chlorophyll contents amended with GA3 enriched deashed biochar. Sci. Rep. 13, 18503 (2023).
Siddikee, M. A., Chauhan, P. S., Anandham, R., Han, G. H. & Sa, T. Isolation, characterization, and use for plant growth promotion under salt stress, of ACC deaminase-producing halotolerant bacteria derived from coastal soil. J. Microbiol. Biotechnol. 20, 1577–1584 (2010).
Falkowska, M. et al. The effect of gibberelilic acid (GA3) on growth, metal biosorption and metabolism of the green algae Chlorella vulgaris (chlorophyceae) beijerinck exposed to cadmium and lead stress. Pol. J. Environ. Stud. 20, 53–59 (2011).
Acknowledgements
The authors extend their appreciation to the Researchers Supporting Project number (RSP2025R98), King Saud University, Riyadh, Saudi Arabia, for financial support.
Funding
The authors extend their appreciation to the Researchers Supporting Project number (RSP2025R98), King Saud University, Riyadh, Saudi Arabia, for financial support.
Author information
Authors and Affiliations
Contributions
Conceptualization; S.D.; G.S.; Conducted experiment; M.F.; Formal analysis; S.D.; M.F.; Methodology; S.D.; G.S.; Writing—original draft; M.J.A.; S.D.; S.A.A.; A.A.A.; Writing—review & editing; M.J.A.; G.S.; S.A.A.; A.A.A.; All authors have reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
We all declare that manuscript reporting studies do not involve any human participants, human data, or human tissue. So, it is not applicable. Study protocol must comply with relevant institutional, national, and international guidelines and legislation Our experiment follows the with relevant institutional, national, and international guidelines and legislation.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sarwar, G., Fatima, M., Danish, S. et al. Enhancing wheat growth under chromium toxicity using gibberellic acid and microbial inoculants as modulating agents. Sci Rep 15, 8356 (2025). https://doi.org/10.1038/s41598-025-92828-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-92828-6