Abstract
Excessive cell proliferation and migration of vascular smooth muscle cells (VSMCs) exacerbate atherosclerotic plaque formation. Peroxisome proliferation-activated receptor gamma (PPARγ) has an atheroprotective effect by inhibiting foam cell formation in macrophages. However, the anti-proliferative mechanism of PPARγ has not been fully explained in VSMCs. Therefore, we investigated the effect of PPARγ by full or partial agonist on VSMC proliferation and migration to suppress atherosclerosis. We found that PPARγ activation by pioglitazone (a full agonist) decreased PDGF-BB-induced mTORC2 signaling pathway and cell proliferation. Pioglitazone treatment downregulated the expression of VSMC contractile markers, cell migration, and lipid accumulation. In contrast, a PPARγ partial agonist, telmisartan, did not influence mTORC2 signaling pathway, VSMC phenotype switching, and lipid droplet markers despite PPARγ activation. We identified differential expression of PPARγ between pioglitazone and telmisartan to regulate VSMC phenotype switching and atherosclerosis. The anti-proliferative effect of pioglitazone was attributed to inhibition of mTORC2 activation, leading to atheroprotective effect. Our findings show that PPARγ suppresses mTORC2 signaling pathway to mitigate VSMC proliferation and migration, which accelerates the atherosclerosis development. These results demonstrate that full and partial PPARγ agonists exert different effects through specific mechanisms, respectively, despite both agonists against PPARγ.
Similar content being viewed by others
Introduction
Atherosclerosis is one of major causes of death worldwide and is considered a chronic inflammation state1,2. Although it has been showed that lipid accumulation is a causative factor in all stage of atherosclerosis process3, numerous studies suggest that the proliferation of vascular smooth muscle cells (VSMCs) play an important role in atherosclerosis4. Abnormal VSMC proliferation and migration which are induced by cytokines and growth factors such as tumor necrosis factor-alpha (TNF-α), platelet-derived growth factor-BB (PDGF-BB), and angiotensin II (Ang II) lead to VSMC accumulation in blood vessel walls and aggravate the formation of atherosclerotic plaques5,6,7. Furthermore, vascular disease is associated with the VSMC phenotype switching from a contractile to a synthetic phenotype8. Increased levels of synthetic markers (collagen I, fibronectin, and matrix metalloproteinase (MMP)) are detected in vascular diseases such as atherosclerosis and neointima, accompanied by decreased expression of contractile markers (α-SMA, calponin, SM22α, and SM-MHC)8. Previous study has determined that a contractile marker is decreased, whereas a synthetic marker is increased in advanced atherosclerotic plaque of ApoE-deficient mice fed a Western diet9. Therefore, it means that the regulation of VSMC proliferation, migration, and phenotype switching is a key mediator in prevention of atherosclerosis development.
The mammalian target of rapamycin (mTOR) is a conserved serine/threonine kinase that plays a major role in cellular growth, metabolism, and survival10. mTOR is composed of two structurally and functionally distinct complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) which include mTOR kinase in common. Among several subunits composed each complex, Raptor and Rictor play a key regulatory role in mTORC1 and mTORC2, respectively11,12. mTORC1 is stimulated by stress, growth factors, amino acids, and energy and regulates protein/lipid synthesis and autophagy through various downstream targets10. Because mTORC1 activation by the PI3K-Akt signaling pathway induces cell proliferation and migration, the mTORC1 signaling pathway has been reported in several pathological and physiological states including atherosclerosis, cancer, and aging13. As a mTORC1 chemical inhibitor, immunosuppressive drug rapamycin suppresses atherosclerosis and is used to treat human atherosclerotic coronary artery disease via drug-eluting stents14. In contrast, mTORC2 is activated only by growth factors and insulin15, and few studies have been conducted to determine the effect of mTORC2 in atherosclerosis compared to those on mTORC1. Recent reports have focused on the novel effects of mTORC2 such as autophagy, diseases, and aging, suggesting the necessity for research on mTORC2 in various fields. We previously demonstrated that mTORC2 activation causes VSMC senescence by increasing the production of reactive oxygen species (ROS) and autophagy impairment16,17. Moreover, it has been identified that mTORC2 activation induces cell proliferation and survival of pulmonary arterial VSMCs and cancer cells18,19,20,21. Therefore, we hypothesized that mTORC2-induced cell proliferation and migration might trigger atherosclerosis in VSMCs and examined the potential regulatory mechanism.
Peroxisome proliferation-activated receptor gamma (PPARγ) is a member of the nuclear receptor subfamily and plays a role in transcription factor22. Although PPARγ is expressed at moderate levels in VSMCs compared to levels in adipose tissue, it is sufficient to mediate cardiovascular diseases, including atherosclerosis23. PPARγ deletion in vascular cells, including endothelial cells and VSMCs, induces pulmonary arterial hypertension24,25. Additionally, smooth muscle-specific PPARγ deletion in mice causes vascular dysfunction and exacerbates the pathogenesis of atherosclerosis26,27. In previous studies, thiazolidinediones (TZDs) such as pioglitazone and rosiglitazone (PPARγ full agonists) exerted protective effects on vessel walls and inhibited VSMC proliferation28,29,30. However, the anti-proliferative effect of PPARγ has been mainly associated with the regulation of mTORC130,31, and there is no known relationship between PPARγ and mTORC2 in VSMC proliferation and migration.
In addition to full agonists, PPARγ is activated by telmisartan which is an Ang II receptor blocker (ARB) and is widely used to treat hypertension by decreasing blood pressure32. Among several ARBs, only telmisartan increases PPARγ activation. However, telmisartan is considered as a PPARγ partial agonist because activation level of PPARγ by telmisartan corresponds to just 25–30% compared to full agonist33. Previous studies have identified that telmisartan ameliorates oxidative damage and inflammation via PPARγ activation in neuronal PC12 cells and BV2 microglial cells34,35. In particularly VSMCs, several studies have reported that telmisartan-induced PPARγ activation prevents mitochondrial dysfunction, cell proliferation, and lipid accumulation36,37,38. Therefore, we investigated the underlying mechanisms of telmisartan and compared the regulatory effects of pioglitazone and telmisartan on PPARγ and mTORC2 in VSMCs.
Results
PDGF-BB treatment for a long period induces mTORC2 activation and the pathogenesis of atherosclerosis in VSMCs
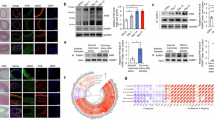
First, we treated VSMCs with PDGF-BB for a short period (15 min, 30 min, 1 h, and 2 h) to investigate PDGF-BB-induced mTORC2 activation and atherosclerotic phenotypes in VSMCs. The levels of phosphorylated mTOR (Ser2481; a marker of mTORC2 activation) and Akt (Ser473; downstream of mTORC2) were consistently activated by PDGF-BB (10 ng/ml) treatment for 2 h (Fig. 1A). However, PDGF-BB treatment for a short period did not change the expression of ADRP (a marker of lipid droplets) and BODIPY staining (a lipid staining dye) (Fig. 1B,C), whereas cholesterol (a positive control; 5 μg/ml for 24 h) induced BODIPY staining in VSMCs (Fig. S1A). Thus, we examined the effect of long-term exposure (24, 48, and 72 h) of VSMCs to PDGF-BB (10 ng/ml). As shown in Fig. 1D, treatment with PDGF-BB induced activation of the mTORC2 signaling pathway from 24 to 72 h. Furthermore, PDGF-BB increased the proliferation of VSMCs, and maximal proliferation was observed at 48 h (Fig. 1E). Analysis of lipid droplet accumulation showed that PDGF-BB activated ADRP expression and BODIPY staining which peaked at 48 h (Fig. 1F,G). Therefore, we selected PDGF-BB for 48 h as an appropriate period to induce the activation of mTORC2, cell proliferation, and atherosclerotic phenotypes in VSMCs for subsequent experiments. These results suggested that long-term exposure to PDGF-BB activates the mTORC2 signaling pathway and cell proliferation, and this process is related to the pathogenesis of atherosclerosis in VSMCs.
Effects of PDGF-BB on mTORC2 activation and the pathogenesis of atherosclerosis in VSMCs. (A–C) VSMCs were treated with PDGF-BB (10 ng/ml) for a short period (15 min, 30 min, 1 h, and 2 h) in DMEM supplemented with 2% FBS. (A) mTORC2 phosphorylation (Ser2481) and Akt phosphorylation (Ser473; downstream of mTORC2) were investigated via western blot analysis. (B) Expression of ADRP, a lipid droplet marker, was examined via western blot analysis. (C) VSMCs were stained with BODIPY (a lipid staining dye; green), and nuclei were stained with DAPI (blue). (D–G) VSMCs were incubated with PDGF-BB (10 ng/ml) for a prolonged time (24, 48, and 72 h) in DMEM supplemented with 2% FBS. (D) The mTORC2 signaling pathway was investigated via western blot analysis. (E) After drug treatment, cell proliferation was confirmed using MTT assay. (F) ADRP expression was investigated by western blot analysis. (G) Cells were stained with BODIPY (a lipid staining dye; green), and nuclei were stained with DAPI (blue). Scale bar = 50 μm. The results are representative of three independent experiments. *p < 0.05.
Pioglitazone-induced PPARγ expression inhibits mTORC2 activation and cell proliferation in VSMCs
To investigate the effects of PPARγ on the mTORC2 signaling pathway and cell proliferation, we used pioglitazone, a PPARγ full agonist, and PPARγ siRNA-transfected cells. VSMCs were pretreated with different concentrations of pioglitazone (10, 20, 50, and 100 μM) for 30 min and were then treated with PDGF-BB (10 ng/ml) for 48 h. As a result, 100 μM pioglitazone enhanced PPARγ expression in VSMCs and was selected for subsequent experiments (data not shown). In control siRNA-transfected cells, pretreatment with pioglitazone (100 μM) increased PPARγ expression compared with control and PDGF-BB-treated cells (Fig. 2A). Moreover, the mTORC2 signaling pathway was decreased by pioglitazone pretreatment. In contrast, knockdown by PPARγ siRNA induced the mTORC2 signaling pathway more than control siRNA. Pretreatment with pioglitazone did not inhibit the expression of p-mTOR (Ser2481) and p-Akt (Ser473). PDGF-BB and pioglitazone had same effect on Akt phosphorylation (Thr308; upstream of mTORC1). Also, the expression of p-Akt (Thr308) was not changed by PPARγ knockdown. Immunofluorescence analysis showed that p-mTOR (Ser2481) was consistently expressed in PPARγ siRNA-transfected cells despite pioglitazone pretreatment (Fig. 2B). Next, we confirmed cell proliferation using MTT assay and cell counting. Pretreatment with pioglitazone decreased PDGF-BB-induced cell proliferation (Fig. 2C,D). In PPARγ siRNA-transfected cells, cell proliferation was further increased. Pioglitazone pretreatment decreased cell proliferation, but there was no statistically significant difference between PDGF-BB and PDGF-BB + pioglitazone in PPARγ siRNA-transfected cells. Furthermore, overall proliferation in PPARγ-knockdown cells increased more than control siRNA. Taken together, these results showed that pioglitazone-induced PPARγ expression alleviates mTORC2 activation and proliferation in VSMCs.
Regulatory effects of PPARγ on mTORC2 activation and proliferation in VSMCs. VSMCs were transfected with control siRNA or PPARγ siRNA. Cells were pretreated with pioglitazone (100 μM) for 30 min and then treated with PDGF-BB (10 ng/ml) for 48 h. (A) Expression of PPARγ, p-mTORC2 (Ser2481), p-Akt (Ser473), and p-Akt (Thr308) was investigated via western blot analysis. (B) VSMCs were stained with anti-PPARγ antibody (green) and p-mTORC2 (Ser2481; red), and nuclei were stained with DAPI (blue). Cells were observed under a confocal microscope. (C, D) Cell proliferation was confirmed using MTT assay and cell counting. Scale bar = 50 μm. The results are representative of three independent experiments. *p < 0.05.
Pioglitazone-induced PPARγ expression mitigates VSMC phenotype switching and the pathogenesis of atherosclerosis
In VSMCs, growth factors such as PDGF-BB cause excessive cell proliferation and migration, leading to a switch in the VSMC phenotype from a contractile to a synthetic phenotype39,40. Moreover, it is well known that VSMC phenotype switching occurs in atherosclerotic plaques7. Thus, we investigated alterations in VSMC phenotype switching after treatment with PDGF-BB and pioglitazone. As shown in Fig. 3A, PDGF-BB (10 ng/ml) exposure for long-term exposure increased the levels of collagen I and fibronectin (markers of the synthetic phenotype) and decreased the expression of α-SMA, calponin, and SM22α (markers of contractile phenotypes) in control siRNA-transfected cells. Furthermore, pretreatment with pioglitazone (100 μM) inhibited the expression of synthetic phenotypes but did not change the contractile phenotypes. Compared to control siRNA, the knockdown of PPARγ aggravated the expression of collagen I and fibronectin and induced the failure of pioglitazone effects. Since there is a relationship between cell proliferation, migration, and VSMC phenotype switching, we examined cell migration using wound healing assay (Fig. 3B). These results indicated that PDGF-BB-induced cell migration was decreased by pioglitazone pretreatment in control siRNA-transfected cells. However, the effect of pioglitazone did not appear in VSMCs transfected with PPARγ siRNA. Finally, knockdown of PPARγ increased the expression of ADRP and eliminated the effects of pioglitazone (Fig. 3C). Additionally, BODIPY staining was consistently maintained by knockdown of PPARγ despite pioglitazone pretreatment (Fig. 3D). These results demonstrated that pioglitazone-induced PPARγ regulates VSMC phenotype switching, cell migration and atherosclerosis.
Inhibitory effects of pioglitazone-induced PPARγ on VSMC phenotype switching, migration, and atherosclerosis. VSMCs were transfected with control siRNA or PPARγ siRNA. Cells were pretreated with pioglitazone (100 μM) for 30 min and then treated with PDGF-BB (10 ng/ml) for 48 h. (A) VSMC synthetic markers (collagen I and fibronectin) and contractile markers (α-SMA, calponin, and SM22α) were confirmed by western blot analysis. (B) Cell migration was investigated using wound healing assay. (C) ADRP expression was examined using western blot analysis. (D) Cells were stained with BODIPY (a lipid staining dye; green), and nuclei were stained with DAPI (blue). Scale bar = 50 μm. The results are representative of three independent experiments. *p < 0.05.
Telmisartan does not affect mTORC2 activation, VSMC phenotype switching, and the pathogenesis of atherosclerosis in VSMCs
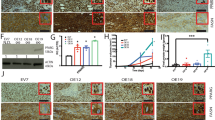
Since it has been reported that telmisartan functions as a PPARγ partial agonist33, we investigated whether telmisartan regulates the activation of PPARγ and mTORC2 to alleviate VSMC phenotype switching and atherosclerosis in PDGF-BB-treated VSMCs. VSMCs were cotreated with various concentrations of telmisartan (10, 20, 30, and 40 μM) and PDGF-BB for 48 h. As shown in Fig. 4A, telmisartan induced PPARγ expression but did not inhibit the mTORC2-Akt (Ser473) signaling pathway. Moreover, PDGF-BB-induced ADRP expression and BODIPY staining were not altered by telmisartan treatment (Fig. 4B,C). Next, we identified VSMC phenotype switching by treatment with PDGF-BB and telmisartan. As a result, PDGF-BB increased markers of the synthetic phenotype, collagen I and fibronectin, and decreased the contractile phenotype markers, α-SMA, calponin, and SM22α (Fig. 4D). However, none of the telmisartan concentrations altered the markers of VSMC phenotype switching. Moreover, telmisartan did not inhibit PDGF-BB-induced cell migration (Fig. 4E). Finally, we compared the capacity for PPARγ activation between full and partial agonists (pioglitazone and telmisartan). In PDGF-BB-treated cells, pioglitazone induced greater PPARγ expression than telmisartan (Fig. 4F). The phosphorylation of mTOR at Ser2481 was suppressed by pioglitazone alone. These findings indicated that differential expression of PPARγ by full and partial agonists results in disparity on regulation of VSMC phenotype switching and atherosclerosis.
Effects of telmisartan-induced PPARγ on mTORC2 activation, cell migration, and atherosclerosis in VSMCs. VSMCs were cotreated with telmisartan (10, 20, 30, and 40 μM) and PDGF-BB (10 ng/ml) for 48 h. (A) The activation of PPARγ and mTORC2 signaling pathway was investigated via western blot analysis. (B) Accumulation of lipid droplets was confirmed by western blot analysis using an anti-ADRP. (C) Cells were stained with BODIPY (a lipid staining dye; green), and nuclei were stained with DAPI (blue). (D) VSMC synthetic markers (collagen I and fibronectin) and contractile markers (α-SMA, calponin, and SM22α) were investigated by western blot analysis. (E) Cell migration was investigated using wound healing assay. (F) The differential expression of PPARγ and p-mTORC2 (Ser2481) was assessed via western blot analysis. Scale bar = 50 μm. The results are representative of three independent experiments. *p < 0.05.
PPARγ improves PDGF-BB-induced cell proliferation, migration, and pathogenesis of atherosclerosis by inhibiting mTORC2 activation
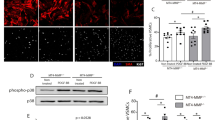
Next, we verified whether pioglitazone had an anti-proliferative effect through PPARγ-mTORC2 signaling pathway. Since there was no specific inhibitor for mTORC2, previous studies used the technique of transfection with Rictor (a regulatory subunit of mTORC2) siRNA41,42,43. Therefore, VSMCs were transfected with Rictor siRNA in the present study. Both pioglitazone pretreatment and knockdown of Rictor decreased the mTORC2-Akt (Ser473) signaling pathway (Fig. 5A). Thus, these findings indicate that PPARγ activation by pioglitazone inhibits the mTORC2 signaling pathway. Next, we confirmed the effect of PPARγ-mTORC2 signaling pathway on cell proliferation. Increased cell numbers after PDGF-BB were diminished by pioglitazone pretreatment or Rictor knockdown (Fig. 5B). As shown in Fig. 5C,D, the accumulation of lipid droplets was decreased in Rictor siRNA-transfected cells. Furthermore, pioglitazone pretreatment and Rictor knockdown inhibited the markers of synthetic phenotype (collagen I and fibronectin), whereas the markers of contractile phenotype (α-SMA, calponin, and SM22α) were unchanged (Fig. 5E). The results of wound healing assay indicated that cell migration was reversed by pioglitazone pretreatment and Rictor knockdown (Fig. 5F). These data showed that pioglitazone-induced PPARγ mitigates cell proliferation, migration, and VSMC phenotype switching by suppressing the mTORC2 signaling pathway and these effects are involved in the regulation of atherosclerotic pathogenesis.
Anti-proliferative effect of pioglitazone via PPARγ-mTORC2 signaling pathway in VSMCs. VSMCs were transfected with control siRNA or Rictor siRNA. Cells were pretreated with pioglitazone (100 μM) for 30 min and then treated with PDGF-BB (10 ng/ml) for 48 h. (A) The expression of Rictor, PPARγ, p-mTORC2 (Ser2481), and p-Akt (Ser473) was investigated by western blot analysis. (B) Cell proliferation was examined using cell counting. (C) Expression of ADRP, a lipid droplet marker, was confirmed via western blot analysis. (D) Cells were stained with BODIPY (a lipid staining dye; green), and nuclei were stained with DAPI (blue). (E) VSMC synthetic markers (collagen I and fibronectin) and contractile markers (α-SMA, calponin, and SM22α) were investigated by western blot analysis. (F) Cell migration was investigated using wound healing assay. Scale bar = 50 μm. The results are representative of three independent experiments. *p < 0.05.
Discussion
Growth factors induce cell proliferation and migration through mTOR signaling pathway and act as activators of mTORC213. Similarly, we verified that both PDGF-BB (10 ng/ml, 48 h) and insulin (100 nM, 45 min) activated the mTORC2 signaling pathway in VSMCs (Fig. S2A). In our study, short- and long-term exposure to PDGF-BB increased mTORC2 phosphorylation at Ser2481; however, short-term exposure did not induce lipid accumulation (Fig. 1). Accordingly, mTORC2 activation caused by long-term exposure promotes abnormal cell proliferation and migration, leading to an increase in atherosclerotic markers (ADRP and BODIPY staining) in VSMCs. Previous study has demonstrated that high cholesterol diet-induced VSMC proliferation co-localizes with atherosclerotic plaques in the thoracic aorta of ApoE knockout mice5. Because the mTOR signaling pathway plays a significant role in cell proliferation and migration which are key mediators of atherosclerosis10, mTOR inhibition is a potential strategy to alleviate atherosclerotic plaque formation. As mTOR kinase inhibitors, sirolimus and PP242 inhibit PDGF-BB-induced proliferation and migration in VSMCs5,18. Moreover, myeloid lineage-specific Rictor deletion decreases cell survival and atherosclerotic plaques by inhibiting mTORC2 signaling pathway in LDL receptor-knockout mice44. In the present study, we verify that mTORC2 activation is accompanied by VSMC proliferation and migration, and further it is associated with the development of atherosclerosis (Fig. 1).
Increasing evidence supports that PPARγ has vasoprotective effects in vascular cells23. PPARγ activation suppresses inflammation which is causative of VSMC proliferation and migration, by decreasing NF-κB and C/EBP-induced the inflammatory genes45,46. PPARγ also induces cell cycle arrest through inhibition of cyclin/cyclin-dependent kinase (CDK), leading to the blockade of cell growth in VSMCs47. These previous studies have described that PPARγ enhances the function of VSMC and further facilitates improvement in vascular diseases such as atherosclerosis and hypertension. Additionally, PPARγ activation delays aging in aged mouse models which is the cause of cardiovascular diseases48,49. In our previous studies, PPARγ activation by pioglitazone or fisetin exerted anti-aging effects by inhibiting mTORC2 signaling pathway in VSMCs16,17. Based on these findings, we considered that PPARγ-induced mTORC2 inhibition may be associated with VSMC proliferation and migration, leading to atherosclerosis. To verify the relation between PPARγ and mTORC2, we investigated the alterations of PPARγ and mTORC2 in PPARγ siRNA- or Rictor siRNA-transfected cells (Fig. S2B and S2C). In PPARγ knockdown cells, pretreatment with pioglitazone failed to suppress PDGF-BB- or insulin-induced mTORC2 signaling pathway. Also, mTORC2 inhibition by Rictor siRNA had same effect of pioglitazone, but the expression of PPARγ was not altered. These findings demonstrated that pioglitazone-induced PPARγ acted as an upstream of mTORC2. Additionally, PPARγ activation suppressed cell proliferation and migration by inhibiting mTORC2 signaling pathway in VSMCs (Fig. 5). Importantly, PPARγ expression was upregulated by not only PDGF-BB but also PDGF-BB + pioglitazone in VSMCs. These results are consistent with results of other studies that investigated pulmonary arterial and human aortic VSMCs30,50,51,52. Since it is an obvious fact that PDGF-BB represents a significant risk factor in cell proliferation and migration, we speculate that PDGF-BB-induced PPARγ activation is a negative feedback mechanism to block the effects of PDGF-BB. This opinion is supported by the observation that knockdown of PPARγ more potently increased mTORC2 signaling pathway, cell proliferation, and migration than control siRNA-transfected VSMCs. Finally, PPARγ activation by pioglitazone diminished markers of lipid accumulation in VSMCs (Fig. 3 and 5). Therefore, we suggested that the anti-proliferative effect of PPARγ is related to the mTORC2 signaling pathway, thereby preventing the pathogenesis of atherosclerosis.
The activation of Ang II type 1 receptor causes an increase in blood pressure and fibrous cap development by inducing vasoconstriction, cell proliferation, and migration and thus reducing blood pressure exerts favorable effects against atherosclerosis53. Previous studies have demonstrated that telmisartan partially activates PPARγ through interaction with the ligand-binding domain of PPARγ33,54. It has been reported that telmisartan-induced PPARγ activation inhibits lipid accumulation in macrophages and telmisartan induces target genes of PPARγ in adipocyte tissues33,55. Therefore, it might be predicted that PPARγ activation by telmisartan inhibits the pathogenesis of atherosclerosis. Interestingly, pioglitazone failed to inhibit cell proliferation in PPARγ-deficient NIH3T3 cells and VSMCs derived from SMC-specific PPARγ knockout mice, whereas telmisartan decreased cell proliferation regardless of PPARγ expression37. Similarly, in our study, telmisartan did not influence the mTORC2 signaling pathway, cell migration, and markers of lipid droplets despite PPARγ activation in VSMCs (Fig. 4). Although we could not clearly describe the effect of telmisartan on cell proliferation and atherosclerosis, telmisartan might regulate cell proliferation and migration via molecular mechanisms other than the mTORC2 signaling pathway or regardless of PPARγ activation in VSMCs. Other studies have proposed that the target gene expressions and effects by PPARγ partial agonists are different from those of full agonists56,57. Therefore, it is necessary to further investigate the effects of telmisartan on VSMC proliferation, migration, and atherosclerosis.
In conclusion, the PDGF-BB-induced mTORC2 signaling pathway increases cell proliferation, migration, and the pathogenesis of atherosclerosis, and these processes are inhibited by pioglitazone, a PPARγ full agonist in VSMCs (Fig. 6). However, telmisartan which is a PPARγ partial agonist has no anti-proliferative effects. Our results suggest that differential expression of PPARγ by pioglitazone and telmisartan might mediate cell proliferation and migration via specific mechanisms, respectively. In addition, PPARγ and mTORC2 may represent significant therapeutic targets for preventing cardiovascular diseases such as atherosclerosis.
Schematic representation of the anti-atherosclerotic mechanism of PPARγ in VSMCs. PDGF-BB induced mTORC2 signaling pathway with increased cell proliferation and VSMC switching, leading to pathogenesis of atherosclerosis. PPARγ activation by pioglitazone suppressed mTORC2 activation to mitigate VSMC proliferation and migration, which accelerated the atherosclerosis development.
Materials and methods
Reagents and antibodies
Pioglitazone, insulin, and PDGF-BB were purchased from Sigma-Aldrich (St. Louis, MO, USA), and telmisartan was purchased from Selleckchem (Houston, TX, USA). Antibodies against PPARγ, p-mTOR (Ser2481), mTOR, p-Akt (Ser473), and Akt were acquired from Cell Signaling Technology (Danvers, MA, USA), and antibodies against Actin, p-mTOR (Ser2481), ADRP, fibronectin, calponin, and SM22α were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against p-Akt (Thr308), collagen I and α-SMA were purchased from Abcam (Cambridge, UK).
Primary cell culture
Sprague–Dawley rats were supplied by Koatech (Gyeonggi-do, Korea) and euthanized with 95% CO2. Thoracic aortas were isolated and placed in serum free high-glucose Dulbecco’s Modified Eagle’s medium (DMEM; WELGENE, Gyeongsangbuk-do, Korea), and fatty tissues and adventitia were removed. The aortas were then cut longitudinally, and the lumens were scoured with cotton swabs to remove the intima and then cut into 3–5 mm long pieces. These pieces were then explanted lumen side down on new 10 cm dishes containing DMEM supplemented with 50% fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA, USA) and incubated in a 5% CO2/95% air incubator for 3 days at 37 °C. Sprouted VSMCs were collected and cultured in a growth media (DMEM supplemented with 10% FBS and 1% antibiotics). For experiments, 70–90% confluent primary VSMCs from passages 3 to 7 were used. All the experimental procedures of this study are in accordance with the ARRIVE guidelines and all procedures involving animals were approved beforehand by the Institutional Animal Care and Use Committee of Yeungnam University College of Medicine (YUMC-AEC2023-014). All methods were performed in accordance with the relevant guidelines and regulations.
Western blot analysis
Equal amounts of protein were mixed with 5 × SDS-PAGE loading buffer (iNtRON Biotechnology, Gyeonggi-do, Korea), heated for 5 min at 95 °C, and subjected to SDS-PAGE using 8–15% gels. Separated proteins were electrophoretically transferred to PVDF membranes, which were then blocked with 3% bovine serum albumin (BSA; GenDEPOT, Baker, TX, USA), incubated with primary antibodies (1:500–1:1000) overnight, and then in 5% skim milk (KisanBio, Seoul, Korea) containing anti-rabbit or anti-mouse IgG (GeneTex, Irvine, CA, USA). The membranes were developed using enhanced chemiluminescence (ECL) and ECL Plus Western blot analysis detection reagents (Millipore, Burlington, MA, USA).
Transfection of siRNA
VSMCs were transfected with siRNA using Lipofectamine 2000 reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions. VSMCs were transfected with 10 μM control siRNA, PPARγ siRNA, or Rictor siRNA (Santa Cruz) for 6 h in Opti-MEM reduced serum medium (Thermo Fisher Scientific). The media were replaced with growth media, cells were incubated for 24 h, and then used for experiments. All the experiments were repeated at least three times.
Cell proliferation assay
VSMCs were seeded on 12-well plates at 1 × 104 cells/well in growth media. After the replacement of the media with DMEM supplemented with 2% FBS, cells were pretreated with pioglitazone (100 μM) for 30 min and then treated with PDGF-BB (10 ng/ml) for 48 h. 50 μl of 1 mg/ml MTT solution (VWR, Radnor, PA, USA) was added to each well (0.1 mg/well) and incubated for 4 h at 37 °C. The supernatants were aspirated, and the formazan crystals in each well were solubilized with 200 μl dimethyl sulfoxide (DMSO). Aliquots (100 μl) were placed in 96-well plates. Cell viability was assessed by measuring optical absorbance at 570 nm using a microplate reader (Agilent BioTek, Santa Clara, CA, USA). All the experiments were repeated at least three times.
Cell numbers were determined using an automatic cell counter (NanoEntek, Seoul, Korea). Cells were seeded on 6-well plates at 4 × 104 cells/well in growth media. After drug treatment, cells were trypsinized and counted using trypan blue dye (Thermo Fisher Scientific). All experiments were repeated at least three times.
Wound healing assay
VSMCs were seeded on 6-well plates in growth media, which was replaced with DMEM supplemented with 2% FBS. Cells were pretreated with mitomycin C (1 μg/ml) for 1 h. Wounds were made with a sterile 200 μl pipet tip by drawing a line through the plated cells perpendicular to the line above. After the replacement of the media with fresh DMEM supplemented with 2% FBS, cells were pretreated with pioglitazone (100 μM) for 30 min and then treated with PDGF-BB (10 ng/ml) for 48 h or co-treated with telmisartan (10, 20, 30, and 40 μM) and PDGF-BB (10 ng/ml) for 48 h. Cells were fixed in 4% paraformaldehyde (Thermo Fisher Scientific) for 10 min and stained with 1% crystal violet (Sigma-Aldrich) for 15 min. The cells were subsequently washed five times with PBS and imaged under a phase-contrast microscope (× 40). The wound area was quantified by measuring the wound closure area versus that of the initial wound using ImageJ software (NIH, Bethesda, MD, USA). All the experiments were repeated at least three times.
Labeling with BODIPY dye
To assess lipid accumulation, VSMCs were stained with 2 μM BODIPY 493/503 (Thermo Fisher Scientific) which is fluorescent neutral lipid dye used to detect a wide range of lipids58 and then incubated for 15 min at 37 °C. After incubation, cells were washed three times with PBS and stained with DAPI (Thermo Fisher Scientific; 1:2500) for 10 min. Images were captured using a K1-Fluo confocal microscope (NANOSCOPE SYSTEMS, Daejeon, Korea). All the experiments were repeated at least three times.
Immunofluorescence analysis
VSMCs were fixed with 4% paraformaldehyde (Thermo Fisher Scientific) for 10 min, permeabilized with 0.2% Triton X-100 (Daejung, Gyeonggi-do, Korea) for 5 min, blocked with 5% normal goat serum (Vector Laboratories, Newark, CA, USA) for 1 h, and incubated with anti-PPARγ (1:250) and p-mTOR (Ser2481) (1:250) overnight at 4 °C. The cells were then treated with mouse or rabbit FITC secondary antibodies (Thermo Fisher Scientific) in 2% BSA at a 1:1000, and nuclei were stained with DAPI (1:2500) for 40 min at room temperature. After immunofluorescent staining, cells were imaged using a K1-Fluo confocal microscope. All the experiments were repeated at least three times.
Statistical analysis
Data acquisition and analysis were performed using Microsoft Excel (Microsoft, Redmond, WA, USA) and GraphPad Prism (GraphPad Software, San Diego, CA, USA). The significance of intergroup differences was determined using one- or two-way analysis of variance (ANOVA) or Bonferroni’s t-test. Results are expressed as means ± standard error of means (SEMs) of at least three independent experiments, and p values < 0.05 were considered statistically significant.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Ang II:
-
Angiotensin II
- ARB:
-
Angiotensin II receptor blocker
- PDGF-BB:
-
Platelet-derived growth factor-BB
- PPARγ:
-
Peroxisome proliferation-activated receptor gamma
- mTOR:
-
Mammalian target of rapamycin
- mTORC1:
-
MTOR complex 1
- mTORC2:
-
MTOR complex 2
- VSMC:
-
Vascular smooth muscle cell
References
Libby, P., Ridker, P. M. & Hansson, G. K. Progress and challenges in translating the biology of atherosclerosis. Nature 473, 317–325. https://doi.org/10.1038/nature10146 (2011).
Hansson, G. K. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 352, 1685–1695. https://doi.org/10.1056/NEJMra043430 (2005).
Gaggini, M., Gorini, F. & Vassalle, C. Lipids in atherosclerosis: Pathophysiology and the role of calculated lipid indices in assessing cardiovascular risk in patients with hyperlipidemia. Int. J. Mol. Sci. 24, 75. https://doi.org/10.3390/ijms24010075 (2022).
Bennett, M. R., Sinha, S. & Owens, G. K. Vascular smooth muscle cells in atherosclerosis. Circ. Res. 118, 692–702. https://doi.org/10.1161/circresaha.115.306361 (2016).
Chen, C. C. et al. Corylin inhibits vascular cell inflammation, proliferation and migration and reduces atherosclerosis in ApoE-deficient mice. Antioxidants (Basel) 9, 275. https://doi.org/10.3390/antiox9040275 (2020).
Chen, C. C., Liang, C. J., Leu, Y. L., Chen, Y. L. & Wang, S. H. Viscolin inhibits in vitro smooth muscle cell proliferation and migration and neointimal hyperplasia in vivo. PLoS ONE 11, e0168092. https://doi.org/10.1371/journal.pone.0168092 (2016).
Kansakar, U., Jankauskas, S. S., Gambardella, J. & Santulli, G. Targeting the phenotypic switch of vascular smooth muscle cells to tackle atherosclerosis. Atherosclerosis 324, 117–120. https://doi.org/10.1016/j.atherosclerosis.2021.03.034 (2021).
Cao, G. et al. How vascular smooth muscle cell phenotype switching contributes to vascular disease. Cell Commun. Signal 20, 180. https://doi.org/10.1186/s12964-022-00993-2 (2022).
Ding, Y. et al. AMP-activated protein kinase alpha 2 deletion induces VSMC phenotypic switching and reduces features of atherosclerotic plaque stability. Circ. Res. 119, 718–730. https://doi.org/10.1161/circresaha.116.308689 (2016).
Saxton, R. A. & Sabatini, D. M. mTOR signaling in growth, metabolism, and disease. Cell 168, 960–976. https://doi.org/10.1016/j.cell.2017.02.004 (2017).
Kim, D. H. et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110, 163–175. https://doi.org/10.1016/s0092-8674(02)00808-5 (2002).
Oh, W. J. & Jacinto, E. mTOR complex 2 signaling and functions. Cell Cycle 10, 2305–2316. https://doi.org/10.4161/cc.10.14.16586 (2011).
Das, A., Reis, F., Maejima, Y., Cai, Z. & Ren, J. mTOR signaling in cardiometabolic disease, cancer, and aging. Oxid. Med. Cell Longev. 2017, 6018675. https://doi.org/10.1155/2017/6018675 (2017).
Kurdi, A., De Meyer, G. R. & Martinet, W. Potential therapeutic effects of mTOR inhibition in atherosclerosis. Br. J. Clin. Pharmacol. 82, 1267–1279. https://doi.org/10.1111/bcp.12820 (2016).
Xie, J., Wang, X. & Proud, C. G. Who does TORC2 talk to?. Biochem. J. 475, 1721–1738. https://doi.org/10.1042/bcj20180130 (2018).
Kim, S. G., Sung, J. Y., Kang, Y. J. & Choi, H. C. Fisetin alleviates cellular senescence through PTEN mediated inhibition of PKCδ-NOX1 pathway in vascular smooth muscle cells. Arch. Gerontol. Geriatr. 108, 104927. https://doi.org/10.1016/j.archger.2023.104927 (2023).
Kim, S. G., Sung, J. Y., Kang, Y. J. & Choi, H. C. PPARγ activation by fisetin mitigates vascular smooth muscle cell senescence via the mTORC2-FoxO3a-autophagy signaling pathway. Biochem. Pharmacol. 218, 115892. https://doi.org/10.1016/j.bcp.2023.115892 (2023).
Goncharov, D. A. et al. Mammalian target of rapamycin complex 2 (mTORC2) coordinates pulmonary artery smooth muscle cell metabolism, proliferation, and survival in pulmonary arterial hypertension. Circulation 129, 864–874. https://doi.org/10.1161/circulationaha.113.004581 (2014).
Krymskaya, V. P. et al. mTOR is required for pulmonary arterial vascular smooth muscle cell proliferation under chronic hypoxia. FASEB J. 25, 1922–1933. https://doi.org/10.1096/fj.10-175018 (2011).
Evangelisti, C. et al. Targeted inhibition of mTORC1 and mTORC2 by active-site mTOR inhibitors has cytotoxic effects in T-cell acute lymphoblastic leukemia. Leukemia 25, 781–791. https://doi.org/10.1038/leu.2011.20 (2011).
Goncharova, E. A. et al. mTORC2 is required for proliferation and survival of TSC2-null cells. Mol. Cell Biol. 31, 2484–2498. https://doi.org/10.1128/mcb.01061-10 (2011).
Smirnov, A. N. Nuclear receptors: nomenclature, ligands, mechanisms of their effects on gene expression. Biochemistry (Mosc) 67, 957–977. https://doi.org/10.1023/a:1020545200302 (2002).
Duan, S. Z., Usher, M. G. & Mortensen, R. M. Peroxisome proliferator-activated receptor-gamma-mediated effects in the vasculature. Circ. Res. 102, 283–294. https://doi.org/10.1161/circresaha.107.164384 (2008).
Guignabert, C. et al. Tie2-mediated loss of peroxisome proliferator-activated receptor-gamma in mice causes PDGF receptor-beta-dependent pulmonary arterial muscularization. Am. J. Physiol. Lung Cell Mol. Physiol. 297, L1082-1090. https://doi.org/10.1152/ajplung.00199.2009 (2009).
Hansmann, G. et al. An antiproliferative BMP-2/PPARgamma/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J. Clin. Invest. 118, 1846–1857. https://doi.org/10.1172/jci32503 (2008).
Halabi, C. M. et al. Interference with PPAR gamma function in smooth muscle causes vascular dysfunction and hypertension. Cell Metab 7, 215–226. https://doi.org/10.1016/j.cmet.2007.12.008 (2008).
Subramanian, V., Golledge, J., Ijaz, T., Bruemmer, D. & Daugherty, A. Pioglitazone-induced reductions in atherosclerosis occur via smooth muscle cell-specific interaction with PPAR{gamma}. Circ. Res. 107, 953–958. https://doi.org/10.1161/circresaha.110.219089 (2010).
Law, R. E. et al. Expression and function of PPARgamma in rat and human vascular smooth muscle cells. Circulation 101, 1311–1318. https://doi.org/10.1161/01.cir.101.11.1311 (2000).
Phillips, J. W. et al. Rosiglitazone reduces the accelerated neointima formation after arterial injury in a mouse injury model of type 2 diabetes. Circulation 108, 1994–1999. https://doi.org/10.1161/01.Cir.0000092886.52404.50 (2003).
Osman, I. & Segar, L. Pioglitazone, a PPARγ agonist, attenuates PDGF-induced vascular smooth muscle cell proliferation through AMPK-dependent and AMPK-independent inhibition of mTOR/p70S6K and ERK signaling. Biochem. Pharmacol. 101, 54–70. https://doi.org/10.1016/j.bcp.2015.11.026 (2016).
Kim, J. S. et al. Anti-proliferative effect of rosiglitazone on angiotensin II-induced vascular smooth muscle cell proliferation is mediated by the mTOR pathway. Cell Biol. Int. 36, 305–310. https://doi.org/10.1042/cbi20100524 (2012).
Siragy, H. M. Angiotensin receptor blockers: how important is selectivity?. Am. J. Hypertens. 15, 1006–1014. https://doi.org/10.1016/s0895-7061(02)02280-x (2002).
Benson, S. C. et al. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activity. Hypertension 43, 993–1002. https://doi.org/10.1161/01.Hyp.0000123072.34629.57 (2004).
Eslami, H., Sharifi, A. M., Rahimi, H. & Rahati, M. Protective effect of telmisartan against oxidative damage induced by high glucose in neuronal PC12 cell. Neurosci. Lett. 558, 31–36. https://doi.org/10.1016/j.neulet.2013.10.057 (2014).
Wang, Z. F., Li, J., Ma, C., Huang, C. & Li, Z. Q. Telmisartan ameliorates Aβ oligomer-induced inflammation via PPARγ/PTEN pathway in BV2 microglial cells. Biochem. Pharmacol. 171, 113674. https://doi.org/10.1016/j.bcp.2019.113674 (2020).
Takeuchi, K. et al. Telmisartan modulates mitochondrial function in vascular smooth muscle cells. Hypertens. Res. 36, 433–439. https://doi.org/10.1038/hr.2012.199 (2013).
Yamamoto, K., Ohishi, M., Ho, C., Kurtz, T. W. & Rakugi, H. Telmisartan-induced inhibition of vascular cell proliferation beyond angiotensin receptor blockade and peroxisome proliferator-activated receptor-gamma activation. Hypertension 54, 1353–1359. https://doi.org/10.1161/hypertensionaha.109.138750 (2009).
Li, B. H. et al. Telmisartan-induced PPARγ activity attenuates lipid accumulation in VSMCs via induction of autophagy. Mol. Biol. Rep. 42, 179–186. https://doi.org/10.1007/s11033-014-3757-6 (2015).
Han, J. H. et al. Regulation of autophagy by controlling Erk1/2 and mTOR for platelet-derived growth factor-BB-mediated vascular smooth muscle cell phenotype shift. Life Sci 267, 118978. https://doi.org/10.1016/j.lfs.2020.118978 (2021).
Munshaw, S. et al. Thymosin β4 protects against aortic aneurysm via endocytic regulation of growth factor signaling. J Clin Invest 131, e127884. https://doi.org/10.1172/jci127884 (2021).
Guo, X., Yu, M., Kang, X. & Yin, H. mTOR complex 2 activation by reconstituted high-density lipoprotein prevents senescence in circulating angiogenic cells. Arterioscler. Thromb. Vasc. Biol. 31, 1421–1429. https://doi.org/10.1161/atvbaha.111.224089 (2011).
Xiong, Y. et al. ARG2 impairs endothelial autophagy through regulation of MTOR and PRKAA/AMPK signaling in advanced atherosclerosis. Autophagy 10, 2223–2238. https://doi.org/10.4161/15548627.2014.981789 (2014).
Yang, C. et al. Inhibitory effect of 14,15-EET on endothelial senescence through activation of mTOR complex 2/Akt signaling pathways. Int. J. Biochem. Cell Biol. 50, 93–100. https://doi.org/10.1016/j.biocel.2014.02.020 (2014).
Babaev, V. R. et al. Loss of rictor in monocyte/macrophages suppresses their proliferation and viability reducing atherosclerosis in LDLR null mice. Front Immunol. 9, 215. https://doi.org/10.3389/fimmu.2018.00215 (2018).
Park, K. G. et al. The ascochlorin derivative, AS-6, inhibits TNF-alpha-induced adhesion molecule and chemokine expression in rat vascular smooth muscle cells. Life Sci. 80, 120–126. https://doi.org/10.1016/j.lfs.2006.08.030 (2006).
Takata, Y. et al. Vascular inflammation is negatively autoregulated by interaction between CCAAT/enhancer-binding protein-delta and peroxisome proliferator-activated receptor-gamma. Circ. Res. 91, 427–433. https://doi.org/10.1161/01.res.0000031271.20771.4f (2002).
Wakino, S. et al. Peroxisome proliferator-activated receptor gamma ligands inhibit retinoblastoma phosphorylation and G1–> S transition in vascular smooth muscle cells. J. Biol. Chem. 275, 22435–22441. https://doi.org/10.1074/jbc.M910452199 (2000).
Werner, C. et al. Pioglitazone activates aortic telomerase and prevents stress-induced endothelial apoptosis. Atherosclerosis 216, 23–34. https://doi.org/10.1016/j.atherosclerosis.2011.02.011 (2011).
Xu, L. et al. PPARγ agonists delay age-associated metabolic disease and extend longevity. Aging Cell 19, e13267. https://doi.org/10.1111/acel.13267 (2020).
Fu, M. et al. Platelet-derived growth factor promotes the expression of peroxisome proliferator-activated receptor gamma in vascular smooth muscle cells by a phosphatidylinositol 3-kinase/Akt signaling pathway. Circ. Res. 89, 1058–1064. https://doi.org/10.1161/hh2301.099642 (2001).
Li, F. et al. Activation of PPARγ inhibits HDAC1-mediated pulmonary arterial smooth muscle cell proliferation and its potential mechanisms. Eur. J. Pharmacol. 814, 324–334. https://doi.org/10.1016/j.ejphar.2017.08.045 (2017).
Zhang, Q. et al. PPARγ activation inhibits PDGF-induced pulmonary artery smooth muscle cell proliferation and migration by modulating TERT. Biomed. Pharmacother. 152, 113233. https://doi.org/10.1016/j.biopha.2022.113233 (2022).
Duval, C., Chinetti, G., Trottein, F., Fruchart, J. C. & Staels, B. The role of PPARs in atherosclerosis. Trends Mol. Med. 8, 422–430. https://doi.org/10.1016/s1471-4914(02)02385-7 (2002).
Amano, Y. et al. Structural basis for telmisartan-mediated partial activation of PPAR gamma. Hypertens. Res. 35, 715–719. https://doi.org/10.1038/hr.2012.17 (2012).
Matsumura, T. et al. Telmisartan exerts antiatherosclerotic effects by activating peroxisome proliferator-activated receptor-γ in macrophages. Arterioscler Thromb. Vasc. Biol. 31, 1268–1275. https://doi.org/10.1161/atvbaha.110.222067 (2011).
Berger, J. P. et al. Distinct properties and advantages of a novel peroxisome proliferator-activated protein [gamma] selective modulator. Mol. Endocrinol. 17, 662–676. https://doi.org/10.1210/me.2002-0217 (2003).
Camp, H. S. et al. Differential activation of peroxisome proliferator-activated receptor-gamma by troglitazone and rosiglitazone. Diabetes 49, 539–547. https://doi.org/10.2337/diabetes.49.4.539 (2000).
Elle, I. C., Olsen, L. C., Pultz, D., Rødkaer, S. V. & Faergeman, N. J. Something worth dyeing for: molecular tools for the dissection of lipid metabolism in Caenorhabditis elegans. FEBS Lett. 584, 2183–2193. https://doi.org/10.1016/j.febslet.2010.03.046 (2010).
Acknowledgements
This work was supported by the Medical Research Center Program (2022R1A5A2018865) through the National Research Foundation of Korea (NRF) funded by the Korean government.
Author information
Authors and Affiliations
Contributions
S.G.K.: Investigation, Methodology, Validation, Formal analysis, Writing—original draft. J.Y.S.: Methodology, Validation, Formal analysis. S.-Y.P.: Supervision, Funding acquisition, Writing—review & editing. H.C.C.: Conceptualization, Supervision, Project administration, Funding acquisition, Writing—review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kim, S.G., Sung, J.Y., Park, SY. et al. Differential expression of PPARγ by pioglitazone and telmisartan causes effect of disparity on mTORC2-mediated cell proliferation and migration. Sci Rep 15, 22112 (2025). https://doi.org/10.1038/s41598-025-93320-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-93320-x