Abstract
The rapid spread of COVID-19 have overwhelmed health systems, especially in the care of chronic disease such as tuberculosis and diabetes. The objective of the study was to analyze the magnitude and relevance of tuberculosis-diabetes and diabetes-COVID-19 comorbidities in spatial risk areas and their factors associated with unfavorable outcomes in the Brazilian population between 2020 and 2022. An ecological study was carried out in Brazilian municipalities. The population was composed by cases of tuberculosis-diabetes and diabetes-COVID-19 comorbidities, registered in the Influenza Epidemiological Surveillance Information System (SIVEP-GRIPE) and in DATASUS from 2020 to 2022. The Scan Statistics technique was used to identify spatial risk clusters. Binary logistic regression was then employed to understand the relationship between outcomes and comorbidities, considering clinical and sociodemographic variables. A total of 24,750 cases of tuberculosis-diabetes comorbidity were identified, which consisted of an incidence of 3.2 cases per 100,000 inhabitants. Risk clusters were identified in the Central-West and North regions. 303,210 cases of diabetes- COVID-19 comorbidity were identified, resulting in an incidence of 0.4 cases per 100,000 inhabitants. São Paulo-SP, Rio de Janeiro-RJ and Belo Horizonte-MG were the municipalities with the highest spatial risk of illness. The analysis of the spatial risk areas revealed distinct patterns in the geographic distribution of comorbidities. Based on the findings, it is concluded that comorbidities between tuberculosis and diabetes, as well as between COVID-19 and diabetes, represent significant challenges for public health in Brazil, deserving attention from health authorities and the scientific community.
Similar content being viewed by others
Introduction
The prevalence of diabetes mellitus (DM) has been increasing globally at an alarming rate, being considered one of the major public health issues of the twenty-first century. This chronic condition results in high mortality and disability rates worldwide, affecting around 422 million people in 2016 and leading to 5 million deaths, with approximately 75% of cases concentrated in developing countries1,2. In 2021, the number of adults with DM reached 537 million, with projections for 643 million by 20303. In Brazil, 13 million people live with the disease, representing 6.9% of the population, with a prevalence rate of 11.2%4.
The relationship between DM and COVID-19 has been widely justified in the literature due to the impact that diabetes has on COVID-19 severity and outcomes. Patients with DM show greater susceptibility and worse clinical progression when contracting COVID-19, with an increased risk of hospitalization, respiratory complications, and mortality5,6. However, the interaction between DM and Tuberculosis (TB), although less frequently discussed, also has important public health implications. People with DM have an increased risk of developing active TB and experience worse therapeutic outcomes, such as higher rates of recurrence and mortality, as DM impairs the host’s immune response against Mycobacterium tuberculosis7,8. Given the growing global prevalence of DM, particularly in countries endemic for TB, studying this comorbidity is essential for designing more effective control strategies for both diseases9.
The significant increase in DM is related to a combination of factors such as a sedentary lifestyle, rising obesity, and an aging population. People with DM often present with other chronic conditions, such as hypertension and cardiovascular diseases, as well as increased susceptibility to and severity of respiratory infections, including COVID-19 and TB. This poses a substantial burden on health systems, increasing the complexity of treatment and resulting in higher rates of hospitalization, healthcare service usage, and direct and indirect costs. According to the Pan American Health Organization (PAHO), diabetic patients are two to three times more likely to be hospitalized compared to those without DM10.
TB, an ancient disease, persists as a significant public health issue and, in 2015, surpassed HIV infection as the leading cause of death from infectious disease11. In 2023, it was estimated that there were 10.5 million new TB cases, the highest number since the WHO began global monitoring of TB in 1995. In Brazil, the incidence is 32 cases per 100,000 inhabitant12.
With the pandemic, in 2022, Brazil ranked 3rd globally for confirmed COVID-19 cases and 2nd for deaths13. TB remained the second leading cause of infectious disease-related deaths, with 1.3 million deaths, only behind COVID-1914. Given this interaction between diseases, the need for health policies and complementary actions that address these three conditions emerges.
Studies on syndemics—the interaction of two or more diseases causing greater harm than the sum of their parts15—highlight the importance of integrated approaches to tackle the complex interplay between DM, TB, and COVID-19, especially in high-risk areas. In areas with moderate to high TB risk, latent TB infection screening and preventive treatment for those testing positive are recommended16. Similarly, DM screening is recommended among TB patients due to its significant association with TB development and complications, including more frequent relapses and increased mortality.
With the pandemic, managing these diseases has been further complicated by impacts on socioeconomic inequalities and access to treatment. However, few studies in Brazil address this topic, revealing a significant knowledge gap. Therefore, the objective of this study was to analyze the magnitude and relevance of diabetes-tuberculosis (DM-TB) and diabetes-COVID-19 (DM-COVID-19) comorbidities in spatial risk areas and the factors associated with unfavorable outcomes in the Brazilian population between 2020 and 2022.
Methods
Study design and setting
This is a retrospective cohort study with data from Brazil.
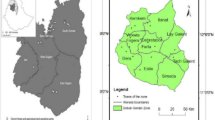
Regarding the study setting, Brazil is the largest country in South America by land area; it has 26 states and a Federal District (Brasília, the capital). The country is divided into five major regions: North, Northeast, South, Southeast, and Central-West, with a total of 5,570 municipalities (Fig. 1). In 2018, 64% of the population was covered by the Family Health Strategy, and 49% of Basic Health Units used electronic medical records, though this rate varied across regions, from 29% in the Northeast to 87% in the South17.
Created using ArcGIS 10.5 (https://www.esri.com/).
The country is located in South America. With an area of 8,510,417.771 km2, it is considered the fifth largest country in the world by territorial extension. In South America, it occupies nearly 50% of the total area, being the largest country in the region, as well as the third largest country in the American continent18. According to the Brazilian Institute of Geography and Statistics (IBGE), the Brazilian population reached 203,080,756 inhabitants on August 1, 202219. Brazil has a Human Development Index (HDI) of 0.760, considered high for the country, and a Gini Coefficient of 0.59, indicating significant social inequality, with a poverty rate of 31.6%20.
Study population and information sources
The study population included cases of DM diagnosed with COVID-19, recorded in the Influenza Epidemiological Surveillance Information System (SIVEP-GRIPE), and cases of DM diagnosed with TB, recorded in the Notification Diseases Information System (SINAN) of the Department of Informatics of the Unified Health System (DATASUS), in Brazil from 2020 to 2022. Both systems aim for epidemiological surveillance and monitoring of disease cases. These systems have retroactive data, allowing for the registration and consultation of information online throughout the patient’s treatment journey. The systems aim to maintain a unique record for each patient, where the entire treatment history is entered. For each patient, one or more treatments are opened, and follow-ups are recorded for each treatment21.
Statistical analysis
Spatial analysis
To detect spatial clusters of the comorbidities DM-TB and DM-COVID-19, the technique known as Scan Statistics or Scan Statistic was used. Developed by Kulldorff and Nagarwalla (1995)22, this technique creates circles that cover the entire study area centered on the centroids of each analyzed territorial unit. The radius of these circles can range from zero up to a limit determined by the researcher23.
The identification of spatial clusters occurs by calculating the number of events within each circle. If the observed number is significantly higher than expected in the region z defined by the circle, a cluster is identified. Otherwise, the radius of the circle is increased to test a new centroid, and this process is repeated until all centroids are evaluated. The hypotheses tested are as follows: H0 indicates the absence of clusters in the study region, while H1 suggests that region z is a cluster24.
The numerous generated circles are tested using Monte Carlo simulations based on the null hypothesis. During the centralization process, the Log Likelihood Ratio (LLR) of each possible cluster is calculated from the difference between the observed and expected incidence within and outside the circular window, where a p-value is assigned, according to the following formula25:
where "O" represents the observed cases, "E" the expected cases, "Oin" and "Ein" denote the observed and expected numbers within the circular window, respectively, and "Ein" is calculated by multiplying TB deaths by the population of the census sectors. The larger the LLR value, the lower the probability that the detected cluster occurred by chance. Additionally, the Spatial Relative Risk (SRR)26,27 is calculated for each statistically significant cluster, comparing the risk within the cluster to the risk outside of it28. The spatial scanning analysis was conducted using SaTScan 10.0.2 software (https://www.satscan.org/).
After the cluster is identified, the software calculates the SRR, obtained using the following formula29:
where “N” is the total number of cases, “NZ” is the number of cases in cluster Z, “EA” is the expected number of cases in the region under the null hypothesis, and “EZ” is the expected number of cases in area Z under the null hypothesis. The SRR value determines whether the cluster is of risk (SRR > 1) or protection (SRR < 1)30,31.
Additionally, thematic maps were created from the scanning analyses, displaying the risk clusters identified using ArcGIS 10.5 software (https://www.esri.com/).
Binary logistic regression
To investigate the main factors associated with the outcomes of the comorbidities DM-TB and DM-COVID-19, binary logistic regression analysis was conducted based on variables from the SIVEP-GRIPE and DATASUS databases. Two separate analyses were performed, one for TB-DM and another for DM-COVID-19, with the objective of identifying the factors associated with unfavorable outcomes for both comorbidities. The dependent variable was dichotomized as unfavorable outcome (1) and favorable outcome (0), as illustrated in Fig. 2.
For DM-TB, the following variables were considered: age group, gender, race/ethnicity, pregnancy status (yes/no), education level, type of entry (new case, TB discovered post-mortem, TB relapse, TB relapse after abandonment), histopathology (positive, suggestive of TB, not suggestive of TB), Directly Observed Treatment (DOT) (yes/no), life history (alcoholism, mental illness, incarceration, homelessness, immigrant status, drug use, smoking), diagnosis by rapid molecular test for TB, drug resistance (isoniazid resistance, isoniazid and rifampin resistance, resistance to other drugs), and HIV testing.
For DM-COVID-19, the following variables were considered: age group, gender, race/ethnicity, education level, diagnostic criterion (COVID-19), pregnancy (1st trimester, 2nd trimester, 3rd trimester), comorbidities (cardiopathy, asthma, Down syndrome, neurological disease, kidney disease, obesity), and COVID-19 vaccination status.
Categorization
The dichotomized dependent variable indicated whether there was a favorable or unfavorable outcome for TB (0 and 1, respectively). This variable was analyzed in conjunction with all independent variables, which were also dichotomized (0 and 1).
The independent variables considered for the DM-TB regression, along with the dichotomization process into 0 and 1 values, were as follows:
• Age: 1 to 5 years (1 = Yes; 0 = No); 6 to 19 years (1 = Yes; 0 = No); 40 to 49 years (1 = Yes; 0 = No); 50 to 59 years (1 = Yes; 0 = No); 60 to 69 years (1 = Yes; 0 = No); 70 to 79 years (1 = Yes; 0 = No); 80 to 89 years (1 = Yes; 0 = No); above 90 years (1 = Yes; 0 = No).
• Sex: (1 = Male; 0 = Female).
• Pregnancy: (1 = Yes; 0 = No).
• Race/Color: White (1 = Yes; 0 = No); Black (1 = Yes; 0 = No); Yellow (1 = Yes; 0 = No); Brown (1 = Yes; 0 = No); Indigenous (1 = Yes; 0 = No).
• Educational level: No schooling (0 = Yes; 1 = No); Complete high school (1 = Yes; 0 = No); Complete higher education (1 = Yes; 0 = No); Incomplete higher education (1 = Yes; 0 = No).
• Case classification: TB discovered post-mortem (1 = Yes; 0 = No); TB relapse (1 = Yes; 0 = No); TB relapse post-abandonment (1 = Yes; 0 = No).
• Directly Observed Treatment (DOT): (1 = Received; 0 = Do not received).
• Histopathology: (1 = Positive; 0 = Negative).
• Comorbidities and drug use: Alcoholism (1 = Yes; 0 = No); Mental illness (1 = Yes; 0 = No); HIV status (1 = Positive; 0 = Negative); Illicit drug use (1 = Yes; 0 = No);
• Smoking (0 = Yes; 1 = No).
• Vulnerable populations: Incarceration (1 = Yes; 0 = No); Homelessness (1 = Yes; 0 = No); Immigrant (1 = Yes; 0 = No).
• Government beneficiary: (1 = Yes; 0 = No).
• Diagnosis by Tuberculosis Rapid Molecular Test—TRM-TB: (1 = Yes; 0 = No).
• Drug resistance: Isoniazid resistance (1 = No; 0 = Yes); Isoniazid and rifampicin resistance (1 = No; 0 = Yes); Resistance to other drugs (1 = No; 0 = Yes).
The independent variables considered for DM-COVID-19 regression:
• ICU Admission: (1 = Yes; 0 = No).
• Sex: (1 = Male; 0 = Female).
• Diagnostic criterion: Laboratory (1 = Yes; 0 = No); Epidemiological (1 = Yes; 0 = No); Clinical (1 = Yes; 0 = No); Imaging (1 = Yes; 0 = No).
• Age: 1 to 5 years (1 = Yes; 0 = No); Age 6 to 19 years (1 = Yes; 0 = No); Age 30 to 49 years (1 = Yes; 0 = No); Age 50 to 59 years (1 = Yes; 0 = No); Age 60 to 69 years (1 = Yes; 0 = No); Age 70 to 79 years (1 = Yes; 0 = No); Age 80 to 89 years (1 = Yes; 0 = No); Age 90 years or older (1 = Yes; 0 = No).
• Gestational age: Pregnant—1st Trimester (0 = Yes; 1 = No); Pregnant—2nd Trimester (0 = Yes; 1 = No); Pregnant—3rd Trimester (0 = Yes; 1 = No).
• Race/Color: White (1 = Yes; 0 = No); Black (1 = Yes; 0 = No); Yellow (1 = Yes; 0 = No); Brown (1 = Yes; 0 = No); Indigenous (1 = Yes; 0 = No).
• Educational level: No schooling (1 = Yes; 0 = No); Incomplete Elementary School (1 = Yes; 0 = No); Complete Elementary School (1 = Yes; 0 = No); Complete High School (1 = Yes; 0 = No); Complete Higher Education (1 = Yes; 0 = No).
• Comorbidities: Cardiopathy (1 = Yes; 0 = No); Down Syndrome (1 = Yes; 0 = No); Asthma (1 = Yes; 0 = No); Neurological Disease (1 = Yes; 0 = No); renal disease (1 = Yes; 0 = No); Obesity (1 = Yes; 0 = No).
• COVID-19 Vaccination Scheme: (1 = Complete; 0 = Not completed).
Statistical analysis
Initially, exploratory analyses were conducted to check for collinearity among independent variables using the Variance Inflation Factor (VIF). Variables with a VIF greater than 10 were removed from the statistical modeling 32,33.
Modeling was performed using the Backward stepwise selection method, starting with a full model and removing one variable at a time based on the minimization of the Akaike Information Criterion (AIC) 34,35. The final model was chosen based on the lowest AIC value, and the Odds Ratio (OR) with 95% Confidence Intervals (CI) was calculated.
To validate the final model, Hosmer–Lemeshow, likelihood ratio, Cox-Snell, Nagelkerke, and McFadden tests were performed. The predictive ability and accuracy of the models were assessed by the area under the ROC (Receiver Operating Characteristic) curve and its corresponding CIs 36. All analyses and validation tests were conducted using RStudio software.
Ethical aspects
This study was approved by the Research Ethics Committee of the Ribeirão Preto School of Nursing, University of São Paulo, in accordance with the Guidelines and Regulatory Standards for Research with Human Subjects, Resolution No. 466/2012 of the National Health Council, under Certificate of Presentation for Ethical Appreciation No. 57933622.4.0000.5393, issued on June, 06th 2022. There was no consent required to participate in the study, as the study was conducted using secondary data from registered in the SINAN and SIVEP-GRIPE databases. The databases used for the study are anonymous, so it is not possible to identify the subjects included in the study. All methods in the study were performed in accordance with relevant guidelines and regulations.
Results
Risk clusters for comorbidities
The incidence rate of the DM-TB comorbidity was 3.2 cases per 100,000 inhabitants between 2020 and 2022. The Central-West and North regions presented major spatial risk clusters and were among the most affected areas of the country. In contrast, the South, Southeast, and Northeast regions exhibited minor clusters, composed of fewer municipalities, as evidenced by Fig. 3A.

Created using ArcGIS 10.5 (https://www.esri.com/).
- Spatial Risk Clusters (A) and incidence (B) for the DM-TB Comorbidity, Brazil (2020 to 2022).
The cluster in the Central West region showed 250 cases of DM-TB, an incidence of 5.3 cases per 100,000 inhabitants, and SRR = 1.64 (95% CI 1.4 – 2.3) (Fig. 3B). Meanwhile, the cluster located in the North region of the country has 2,562 cases, an incidence of 8.1 cases of DM-TB per 100,000 inhabitants, and SRR = 2.68 (95% CI 2.0 – 3.5). The main cluster located in the Southeast region has 42,481 cases, an incidence of 299.1 cases of DM-COVID-19 per 100,000 inhabitants, and SRR = 830.8 (95% CI 800.4 – 901.3).
Regarding the incidence of the DM-COVID-19 comorbidity, it was 39.7 cases per 100,000 inhabitants between 2020 and 2022. All regions presented high spatial clusters, though the South (44 clusters) and Southeast (61 clusters) regions concentrated most of the clusters (Fig. 4). The three highest clusters for DM-COVID-19 were located in the Southeast region with SRR = 830.8; 95% (CI 800.4 – 901.3), SRR = 301.24; 95% (CI 290.0 – 371.2), SRR = 187.07; 95% (CI 172.8 – 191.5). The North, Central-west and Northeast presented 12, 14 and 38 clusters (Fig. 4).
Created using ArcGIS 10.5 (https://www.esri.com/).
Factors associated with unfavorable outcomes
Through the Logistic regression analysis (Table 1) for the DM-TB comorbidity, it was identified several factors associated with a higher probability of unfavorable outcomes. These include male sex, Black race/color, TB relapse, HIV-positive status, alcoholism, illicit drug use, and smoking. Social determinants also played a significant role, with homelessness, immigrant status, Indigenous identity, and government assistance beneficiaries being associated with unfavorable outcomes.
The analysis of the completeness of the variables used in both logistic regressions can be found in the supplementary materials (see Supplementary Table S1 e S2).
To validate the model, the Hosmer–Lemeshow test (p = 0.41), likelihood ratio (p < 0.01), Cox-Snell (0.07), Nagelkerke (0.15), and McFadden (0.11) tests were performed. The model showed an ROC value of 0.73, as shown in Fig. 5.
In the context of DM-COVID-19 comorbidity, individual variables associated with unfavorable outcomes included male sex, educational attainment, and race/color categories such as Black, Brown, Yellow, and Indigenous. Additional factors were ICU admissions, diagnoses based on clinical-epidemiological, laboratory, and imaging criteria, and age 50 years or older. Other comorbidities, including cardiopathy, neurological disease, renal disease, and obesity, also contributed to worsening outcomes (Table 2).
To validate the model, the Hosmer–Lemeshow test (p = 2.45), likelihood ratio (p < 0.01), Cox-Snell (0.16), Nagelkerke (0.21), and McFadden (0.13) tests were performed. The model showed an ROC value of 0.73, as shown in Fig. 6.
Discussion
The study aimed to analyze the magnitude and significance of the comorbidities tuberculosis-diabetes and diabetes-COVID-19, spatial risk areas, and their factors associated with unfavorable outcomes. Key findings include the geo-spatial distribution of DM-TB and DM-COVID-19 comorbidities in Brazil, revealing important implications for public health. The results highlighted significant factors associated with unfavorable outcomes in DM-TB and DM-COVID-19 comorbidities, emphasizing both individual characteristics and social variables. This analysis is extremely important for guiding public health interventions, improving clinical outcomes for these vulnerable populations, and strengthening local health systems.
We found an incidence of 3.2 cases of DM-TB per 100,000 inhabitants in Brazil. Brazil shows an increasing trend of comorbidity, in addition to being a country with a high burden of TB13. The Brazilian Ministry of Health recommends screening for TB in individuals diagnosed with DM, as well as screening for DM in people with TB37.
The results also showed that the distribution of the comorbidity in the country is heterogeneous. The analyses revealed two prominent risk clusters: one in the Northeast region and another in the Central-Western region of Brazil. These regions have distinct profiles regarding TB control. Historically, the Central-West region has recorded lower incidence and mortality rates for the disease, while the Northern region bears a significant TB case burden38. However, concerning DM incidence, the values are reversed: the Central-West region has the highest national incidence, while the Northern region has the lowest. Thus, despite differences in TB and DM epidemiological profiles, it is plausible that these regions develop interaction patterns resulting in high co-occurrence of these two diseases.
Although both regions presented most of the clusters, the highest SRRs for DM-TB were located in the Southeast region, indicating a higher burden of comorbidity in this area. The Southeast region is characterized by a high population density, especially in its capital city, which is the most populous in the country39. This results in a significant influx of people and intense economic activity in the region. However, it also has high inequality rates, particularly in urban settings, creating socio-spatial environments with stark disparities in housing quality, public infrastructure, and access to health services40. This contributes to conditions that facilitate TB transmission in the region, which also has the highest prevalence of DM (8.5%)37, potentially explaining the high risk of comorbidity in this area.
The higher incidence in the Northeast can be attributed to adverse socioeconomic conditions, such as higher poverty rates and lower access to education and formal employment, and greater geographic dispersion, which hinder access to quality healthcare services41,42, which may impact the diagnosis of both TB and DM.
It is important to highlight that not only does TB have a heterogeneous distribution across the country, but DM also varies in prevalence between regions. The Southeast and South show the highest occurrences, with an estimated 8.5% and 7.9% of the population affected by diabetes, respectively43. Another significant factor influencing the distribution of this comorbidity is age, which also varies regionally43. Given that TB typically affects adults and young adults, while DM is more prevalent in older adults and the elderly, these demographic differences are likely to impact the distribution of the comorbidity and make it more challenging to interpret.
Conversely, the lower incidence in the Central-West region may be attributed to better socioeconomic and health indicators, including moderate population density and a more accessible healthcare network44, which may contribute to improved detection of the comorbidity. However, intra-regional inequalities persist affecting specific subgroups of the population. These findings underscore the need for targeted public policies considering regional specifics, with investments in healthcare infrastructure and social programs to reduce socioeconomic inequalities and improve the quality of life for the most vulnerable populations44.
The COVID-19 was initially recorded in Brazil in the state of São Paulo, where the first case was reported. The delayed implementation of social distancing measures in the state contributed to the rapid spread of the virus. Consequently, the Southeast region of the country became the epicenter of COVID-19-related hospitalizations45.
The incidence of DM-COVID-19 comorbidity was relatively high, at 39.7 cases per 100,000 inhabitants. The Southeast region showed the highest spatial risks, which may be correlated with high population density and significant urban mobility, which are factors facilitating virus spread46. As previously highlighted, the Southeast Region emerged as the epicenter of the COVID-19 pandemic in Brazil and has the highest percentage of people diagnosed with diabetes in the country, reinforcing the relevance of these factors in the concentration of cases45.
Given the regional disparities presented in the interaction of these diseases, it is essential to consider health systems through collaborative partnership networks. Integration between different government levels, non-governmental organizations, research institutions, and the private sector can promote the efficient sharing of resources, knowledge, and technologies. For instance, implementing integrated programs for controlling TB and DM can be optimized through partnerships that enhance healthcare infrastructure and ensure equity in access to care. Furthermore, public health strategies that consider regional specifics and invest in social programs are essential for mitigating socioeconomic inequalities and improving the quality of life for vulnerable populations47,48.
The factors associated with favorable and unfavorable outcomes in comorbidities highlighted the significant influence of individual and sociodemographic factors on unfavorable outcomes. In the case of DM-TB comorbidity, relevant risk factors identified include male sex, black race/skin color, TB relapse, HIV positivity, alcoholism, illicit drug use, and smoking. Additionally, social factors such as experiencing homelessness and government program beneficiary status were also associated with unfavorable outcomes.
Globally, TB shows higher incidence among men than women (WHO). Gender differences may be attributed to economic, cultural, and social factors that result in greater exposure of men to infection sources, as well as risk factors and lower adherence to health services49,50,51. In the case of TB-DM, this disparity may be even greater, as there is evidence that underreporting of DM is higher among men than women 52, primarily due to differences in health service-seeking behaviors.
Moreover, men exhibit higher prevalence of risk factors for developing TB, such as alcoholism and smoking, which were also associated with unfavorable outcomes in DM-TB comorbidity in this study53. These findings suggest an overlap of risk factors that significantly contribute to adverse clinical outcomes and the occurrence of both diseases.
Literature findings reveal different rates of TB relapse among individuals with DM-TB comorbidity. In Brazil, there is evidence of higher relapse rates in this population, similar to the findings in this study54,55. The association of the two diseases may increase the risk of reactivation of TB infection after initial treatment, particularly due to immune system impairment in people with DM. This is a particularly important issue to consider as part of the control of TB-DM in order to prevent development of drug-resistant TB and death.
Regarding race/skin color, unfavorable outcomes were associated with cases among the Black population. Although this population does not represent the majority of cases of TB and DM separately, they experience some of the highest mortality rates from both diseases56,57. This can be explained by the historical vulnerabilities of this group, related to lower incomes, more limited access to healthcare services, and lower levels of education compared to other race/colors58. The pandemic may have exacerbated these vulnerabilities, further intensifying the challenges faced by this population.
In DM-COVID-19 comorbidity, male sex again emerged as a significant risk factor. Additionally, factors associated with unfavorable outcomes included ICU admissions, diagnoses based on clinical-epidemiological, laboratory, and imaging criteria, as well as age from 50 years onward. These findings are consistent with existing literature, which demonstrates that men and older individuals have higher risks of complications and mortality associated with COVID-1959.
Other comorbidities such as heart disease, neurological disorders, kidney disease, and obesity were identified as aggravating factors for unfavorable outcomes of DM-COVID-19. These underlying conditions can complicate the course of COVID-19, hindering recovery and increasing mortality and symptom severity60. Furthermore, association of multiple comorbidities and aging are highly relevant for developing clinically severe forms of COVID-1961, thus increasing the risk of death.
Patients with diabetes often experience worse COVID-19-related complications, including respiratory failure and acute respiratory distress syndrome, which can lead to ICU admission61. In the ICU, patients typically face heightened vulnerability due to their pre-existing health conditions, making them more susceptible to additional complications such as infections, organ failure, and prolonged hospitalization62. As a result, ICU admissions are associated with an increase in unfavorable outcomes for DM-COVID-19 patients.
Regarding male sex, this difference in incidence has been associated with biological, behavioral, cultural, and occupational factors. An important biological factor is that the SARS-CoV-2 virus uses the Angiotensin-Converting Enzyme 2 as a mechanism for entry into human cells63, with this enzyme showing nearly three times higher expression in men compared to women. Additionally, factors such as men’s greater resistance to seeking healthcare services and higher smoking may also contribute to increased disease severity in men55.
A major limitation of this study arises from its ecological design, which restricts the generalization of results to the individual level, a phenomenon known as ecological fallacy. Additionally, the use of secondary retrospective data sources may introduce reporting biases and incomplete records. It is worth noting that the data regarding DM diagnosis in both datasets lack information about the diagnostic criteria for DM and are often self-reported by patients. Consequently, the occurrence of DM as a comorbidity of TB and COVID-19 may be underreported. Furthermore, in the TB notification form in SINAN, the field for comorbidities associated with TB is not mandatory, leading to a high percentage of missing data related to DM diagnosis and, thus, affecting the incidence estimate of DM-TB.
Conclusion
This study highlights the magnitude and spatial distribution of DM-TB and DM-COVID-19 comorbidities in Brazil, identifying high-risk areas and factors associated with adverse clinical outcomes. The results indicate that individual and sociodemographic factors, such as male sex, black race/skin color, and disadvantaged socioeconomic conditions, play significant roles in the severity of clinical outcomes. These findings underscore the need for an integrated approach at the primary, secondary, and tertiary care levels, essential for effective and continuous management, improving early detection and treatment of associated conditions.
Furthermore, collaboration between health sectors and social policies is vital to address inequalities negatively impacting clinical outcomes. Identifying factors such as homelessness and government program beneficiary status underscores the need for public policies that integrate social support with healthcare. Training of healthcare professionals and the implementation of educational programs focused on risk behaviors are also important for improving clinical outcomes. Partnership networks can promote collaborative research and innovation, facilitating the development of evidence-based interventions that more effectively address the needs of vulnerable populations and reduce barriers to access and adherence to treatment.
Data availability
The data presented in this study are available on request from the corresponding author on reasonable request.
Abbreviations
- ACE2:
-
Angiotensin-Converting Enzyme 2
- AIC:
-
Akaike Information Criterion
- CAAE:
-
Certificate of Ethical Appreciation Presentation
- CI95%:
-
95% Confidence Intervals
- DATASUS:
-
Department of Informatics of the Unified Health System
- DM:
-
Diabetes mellitus
- DM-COVID-19:
-
Diabetes-COVID-19
- DM-TB:
-
Diabetes-Tuberculosis
- DOT:
-
Directly Observed Treatment
- EERP-USP:
-
Ribeirão Preto School of Nursing at the University of São Paulo
- HDI:
-
Human Development Index
- IBGE:
-
Brazilian Institute of Geography and Statistics
- ICU:
-
Intensive Care Unit
- LLR:
-
Log Likelihood Ratio
- MERS:
-
Middle East Respiratory Syndrome
- MT:
-
Mato Grosso
- OR:
-
Odds Ratio
- PAHO:
-
Pan American Health Organization
- RJ:
-
Rio de Janeiro
- ROC:
-
Receiver Operating Characteristic
- SARS:
-
Severe Acute Respiratory Syndrome
- SINAN:
-
Notification Diseases Information System
- SIVEP-GRIPE:
-
Influenza Epidemiological Surveillance Information System
- SP:
-
São Paulo
- SRR:
-
Spatial Relative Risk
- TB:
-
Tuberculosis
- TRM-TB:
-
Molecular Rapid Test for Tuberculosis
- VIF:
-
Variance Inflation Factor
References
World Health Organization (Who). Global diabetes mellitus report 2016 World Health Organization, Geneva (2016).
Krug, E. G. Trends in diabetes: Sounding the alarm. Lancet 387(10027), 1485–1486 (2016).
International Diabetes Federation (IDF) Diabetes Atlas 10th edition. IDF; 2021
Brasil. Plano de contingência datasus situação de crise provocada pelo novo coronavírus (COVID-19) Coordenação-Geral De Inovação Em Sistemas Digitais. 2020
Zhou, F. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 395(10229), 1054–1062 (2020).
Yang, J. et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int. J. Infect. Dis. 94, 91–95. https://doi.org/10.1016/j.ijid.2020.03.017 (2020).
Lönnroth, K., Roglic, G. & Harries, A. D. Improving tuberculosis prevention and care through addressing the global diabetes epidemic: From evidence to policy and practice. Lancet Diabetes Endocrinol. 2(9), 730–739. https://doi.org/10.1016/S2213-8587(14)70109-3 (2014).
Baker, M. A. et al. The impact of diabetes on tuberculosis treatment outcomes: A systematic review. BMC Med. 9, 81. https://doi.org/10.1186/1741-7015-9-81 (2011).
Harries, A. D. et al. The looming epidemic of diabetes-associated tuberculosis: Learning lessons from HIV-associated tuberculosis. Int. J. Tuberc. Lung Dis. 15(11), 1436–1444. https://doi.org/10.5588/ijtld.11.0503 (2011).
Organização Pan-Americana da Saúde. Panorama da resposta do setor saúde ao tratamento e controle da diabetes no Brasil. OPAS 2020.
World Health Oraganization. Coranavírus Dashboard. [Internet]. Geneva 2022
Brasil. Boletim Epidemiológico da Tuberculose. Secretaria de Vigilância em Saúde. Ministério da Saúde. 2022
World Health Organization. Global tuberculosis report 2020, World Health Organization, 2020 Geneva.
Dara, M. et al. New diseases and old threats: Lessons from tuberculosis for the COVID-19 response. Int. J. Tuberc. & Lung Dis. 24(5), 1 (2020).
BispoJúnior, J. P. & dos Santos, D. B. COVID-19 como sindemia: modelo teórico e fundamentos para a abordagem abrangente em saúde. Cadernos De Saúde Pública. 37(10), e00119021. https://doi.org/10.1590/0102-311X00119021 (2021).
Aguillón-Durán, G. P. et al. COVID-19 and chronic diabetes: the perfect storm for reactivation tuberculosis?: A case series. J. Med. Case Rep. 15(1), 621. https://doi.org/10.1186/s13256-021-03193-7.PMID:34915933;PMCID:PMC8674832 (2021).
Comitê Gestor da Internet no Brasil. TIC SAÚDE 20118. Pesquisa sobre o Uso das Tecnologias de Informação e Comunicação nos Estabelecimentos de Saúde Brasileiros, 2018. Available from: https://www.cetic.br/media/docs/publicacoes/2/15303120191017-tic_saude_2018_livro_eletronico.pdf.
Instituto Brasileiro de Geografia e Estatística. Áreas Territoriais - Brasil, Grandes Regiões, Unidades da Federação e Municípios. 2022. Available from: https://www.ibge.gov.br/geociencias/organizacao-do-territorio/estrutura-territorial/15761-areas-dos-municipios.html.
Instituto Brasileiro de Geografia e Estatística. Portal do IBGE. População, Inflação, PIB, Desemprego e outros indicadores. 2022. Available from: https://www.ibge.gov.br/apps/populacao/projecao/.
Instituto Brasileiro de Geografia e Estatística. Portal do IBGE. Ibge Cidades. Instituto Brasileiro de Geografia e Estatística (IBGE), Brasilia. 2022. https://cidades.ibge.gov.br/.
Apunike, A.C. et al. Analyses of Public Health Databases via Clinical Pathway Modelling: TBWEB. In: International Conference on Computational Science. Springer Cham 2020. 550–562
Kulldorff, M. & Nagarwalla, N. Spatial disease clusters: Detection and inference. Stat. Med. 14, 799–810 (1995).
Olfatifar, M. et al. Clustering of pulmonary tuberculosis in Hamadan province, west of Iran: A population based cross sectional study (2005–2013). J. Res. Health Sci. 16(3), 166 (2016).
Lucena, S. E. F. & Moraes, R. M. Detecção de agrupamentos espaço-temporais para identificação de áreas de risco de homicídios por arma branca em João Pessoa, PB. Boletim de Ciências Geodésicas, Curitiba 18(4), 605–623 (2012).
Waller, Lance A., and Carol A. Gotway. Applied spatial statistics for public health data. John Wiley & Sons, 2004.
Monken, M. Desenvolvimento de tecnologia educacional a partir de uma abordagem geográfica para a aprendizagem da territorialização em vigilância da saúde. Rio de Janeiro, 170p. v. 164, 2003 (Dissertação). Escola Nacional de Saúde Pública. Disponível em: < https://thesis.icict.fiocruz.br/pdf/monkenmd.pdf >. Acesso em: 05 jan. 2018.
Lawson A.B. et al. Disease mapping models na empirical evaluation. Disease Mapping Collaborative Group. Stat Med, v. 19, p.2217–1141, 2000. Disponível em: http://www.personal.soton.ac.uk/dab1f10/lawsonetal.pdf.
Gao, F. et al. Fine scale Spatial-temporal cluster analysis for the infection risk of Schistosomiasis japonica using space-time scan statistics. Parasites & vectors, v. 7, n. 1, p. 578, 2014. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4273478/pdf/13071_2014_Article_57 8.pdf.
Prates, M.O.; Kulldorff, M.; Assunção, R.M. Relative risk estimates from spatial and space–time scan statistics: are they biased?. Statistics in medicine, v. 33, n. 15, p. 2634–2644, 2014. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4047196/pdf/nihms572329.pdf.
Gordis, L. Colocar Medidas de ocorrência das doenças Epidemiology. 31–62 Saunders 2009
Wagner, M.B; Callegari-Jacques, S.M. Medidas de associação em estudos epidemiológicos: risco relativo e odds ratio. Jornal de pediatria, Rio de Janeiro. 74 3 247–251 Available from: http://www.lume.ufrgs.br/bitstream/handle/10183/54354/000246332.pdf?sequence=1 1998
Mccullagh, P. Regression models for ordinal data. J. Royal Stat. Soc.: Ser. B (Methodol.) 42(2), 109–127 (1980).
Agresti, A. Categorical data analysis (John Wiley & Sons, 2012).
Grove, D., Sakamoto, Y., Ishiguro, M. & Kitagawa, G. Akaike information criterion statistics. Stat. https://doi.org/10.2307/2348776 (1988).
Gunst, R. F. Response surface methodology: process and product optimization using designed experiments. 1996
Agresti, A. Analysis of ordinal categorical data (John Wiley & Sons, 2010).
Brazil. Ministry of Health. Manual de Recomendações para o Controle da Tuberculose no Brasil Brasília: Ministry of Health 2019 https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/t/tuberculose/publicacoes/manual-de-recomendacoes-para-o-controle-da-tuberculose-no-brasil.pdf/@@download/file. Accessed 10 Jul 2024. (in Portuguese)
Da Soeiro, V. M. & S., Caldas, A. De J. M., Ferreira, T. F.,. Abandono do tratamento da tuberculose no Brasil, 2012–2018: tendência e distribuição espaço-temporal. Ciência & saúde coletiva 27(3), 825–836 (2022).
Brazil. Brazilian Institute of Geography and Statistics (IBGE), Brasil em Síntese. Brasília: 2022. https://brasilemsintese.ibge.gov.br/ Accessed in 30 October 2024.
Toledo, R. F., Koury, A. P., Carvalho, C. M. & Santos, F. N. P. Participatory process for mapping socio-environmental determinants of health by community agents: Contributions to urban management and planning. Rev. Bras. Ciênc. Ambient. https://doi.org/10.5327/z217694781035 (2021).
Rocha, T. A. H. et al. Addressing geographic access barriers to emergency care services: a national ecologic study of hospitals in Brazil. Int. J. Equity Health 16(1), 10 (2017).
Szwarcwald, C. L. et al. Inequalities in healthy life expectancy by Brazilian geographic regions: Findings from the National Health Survey, 2013. Int. J. Equity Health 15, 1–9 (2016).
Brazil. Brazilian Institute of Geography and Statistics (IBGE), Pesquisa nacional de saúde 2019: percepção do estado de saúde, estilos de vida, doenças crônicas e saúde bucal. Rio de Janeiro: 2019. https://www.pns.icict.fiocruz.br/wp-content/uploads/2021/02/liv101764.pdf. Accessed in 30 October 2024
Paiva, M. N. D. et al. Factors associated with poor access to health services in Brazil. Revista brasileira de Epidemiologia 24, e210004 (2020).
Niquini, R. P. et al. SRAG por COVID-19 no Brasil: Descrição e comparação de características demográficas e comorbidades com SRAG por influenza e com a população geral. Cadernos de Saúde Pública 36, e00149420 (2020).
Baqui, P. et al. Ethnic and regional variations in hospital mortality from COVID-19 in Brazil: a cross-sectional observational study. Lancet Global Health 8(8), e1018–e1026 (2020).
Beese, F. et al. Temporal dynamics of socioeconomic inequalities in COVID-19 outcomes over the course of the pandemic—a scoping review. Int. J. public health 67, 1605128 (2022).
Nutakor, Jonathan Aseye et al. Socioeconomic status and quality of life: an assessment of the mediating effect of social capital. In: Healthcare. MDPI 749 (2023)
Ferrer, G. C. N. et al. The burden of disease due to tuberculosis in the state of Santa Catarina, Brazil. J. Brasileiro de Pneumologia, São Paulo 40(1), 61–68 (2014).
Dale, K. et al. Mortality among tuberculosis cases in Victoria, 2002–2013: Case fatality and factors associated with death. Int. J. Tuberc. & Lung Dis. 20(4), 515–523 (2016).
Beyene, Y., Geresu, B. & Mulu, A. Mortality among tuberculosis patients under DOTS programme: A historical cohort study. BMC public health 16(1), 883 (2016).
Goldenberg, P., Schenkman, S. & Franco, L. J. Prevalência de diabetes mellitus: diferenças de gênero e igualdade entre os sexos. Rev. bras. epidemiol. 6(1), 18–28. https://doi.org/10.1590/S1415-790X2003000100004 (2003).
Chidambaram, V. et al. Male sex is associated with worse microbiological and clinical outcomes following tuberculosis treatment: A retrospective cohort study, a systematic review of the literature, and meta-analysis. Clinical infectious diseases: An official publication of the Infectious Diseases Society of America 73(9), 1580–1588 (2021).
Abreu, R. G. & de LRS Freitas de AIA Sousa de MRF Oliveira de,. Tuberculose e diabetes: Associação com características sociodemográficas e de diagnóstico e tratamento Brasil 2007-2011. Revista Brasileira De Epidemiologia 23, e200009 (2020).
Nascimento, C.V.; Soares, S.M. Manejo integrado de tuberculose e diabetes: uma revisão integrativa. Rev Panam Salud Publica, 2019
Santos Fiuza, E. D. et al. Perfil epidemiológico dos pacientes diagnosticados com Tuberculose pulmonar, residentes no Município De Salvador, entre o Ano de, E 2014. Seminário Estudantil de Produção Acadêmica 14, 2015 (2010).
Oliveira, R. G. et al. Desigualdades raciais e a morte como horizonte: considerações sobre a COVID-19 e o racismo estrutural. Cadernos de Saúde Pública 36, e00150120 (2020).
Chiavegatto Filho ADP, Laurenti R. Disparidades étnico-raciais em saúde autoavaliada: análise multinível de 2.697 indivíduos residentes em 145 municípios brasileiros. Cad Saúde Pública. Aug;29(8):1572–82. https://doi.org/10.1590/0102-311X00139012. (2013)
Williamson, E. J. et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 584(7821), 430–436 (2020).
Petrilli, Christopher M. et al. 2020 Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. bmj, 369
Nunes, B. P. et al. Multimorbidity and population at risk for severe COVID-19 in the Brazilian Longitudinal Study of Aging. Cadernos de saude publica 36, e00129620 (2020).
Corrêa TD, Midega TD, Timenetsky KT, Cordioli RL, Barbas CSV, Silva Júnior M, et al.. 2021 Clinical characteristics and outcomes of COVID-19 patients admitted to the intensive care unit during the first year of the pandemic in Brazil: a single center retrospective cohort study. einstein (São Paulo) https://doi.org/10.31744/einstein_journal/2021AO6739.
Zhu, N. et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 382(8), 727–733 (2020).
Acknowledgements
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES) – Finance Code 001
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES) – Finance Code 001. This work was supported by the São Paulo Research Foundation [process: 2022/08510–7]; National Council for Scientific and Technological Development (CNPq) [Grant 445741/2023-6] and the Research Productivity Grant from the National Council for Scientific and Technological Development [process: 307014/2022–3].
Author information
Authors and Affiliations
Contributions
Conceptualization, L.S.A., T.Z.B. and R.A.A.; methodology, L.S.A., T.Z.B., Y.M.A., D.G. and R.A.A.; formal analysis, L.S.A., T.Z.B., D.G. and R.A.A.; investigation, L.S.A.; data curation, L.S.A., T.Z.B. and R.A.A.; writing—original draft preparation, L.S.A., T.Z.B., Y.M.A., L.P.F., A.L.T.V. and R.A.A.; writing—review and editing, L.S.A., T.Z.B., Y.M.A., L.P.F., A.L.T.V., R.B.V.T., A.F.T., and D.G.; supervision, R.A.A.; project administration, L.S.A; All authors have read and agreed to the published version of the manuscript. I also declare that all authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. Additionally, I declare that , L.S.A., T.Z.B., Y.M.A., L.P.F., A.L.T.V and R.A.A. have accessed and verified the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Institutional review board statement
This study was approved by the Research Ethics Committee of the Ribeirão Preto School of Nursing, University of São Paulo, in accordance with the Guidelines and Regulatory Standards for Research with Human Subjects, Resolution No. 466/2012 of the National Health Council, under Certificate of Presentation for Ethical Appreciation No. 57933622.4.0000.5393, issued on June, 06th 2022. There was no consent required to participate in the study, as the study was conducted using secondary data from registered in the SINAN and SIVEP-GRIPE databases. The databases used for the study are anonymous, so it is not possible to identify the subjects included in the study. All methods in the study were performed in accordance with relevant guidelines and regulations.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Alves, L.S., Berra, T.Z., Alves, Y.M. et al. Geographic inequalities and factors associated with unfavorable outcomes in diabetes-tuberculosis and diabetes-covid comorbidities in Brazil. Sci Rep 15, 8353 (2025). https://doi.org/10.1038/s41598-025-93476-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-93476-6
Keywords
This article is cited by
-
Review: missed tuberculosis cases in India: a systematic analysis of diagnostic, treatment, and reporting gaps
BMC Health Services Research (2025)