Abstract
Purpose The study aimed to assess the value of event-related potentials (ERP) of executive functions in distinguishing between attention deficit hyperactivity disorder (ADHD) and ADHD combined with autism spectrum disorder (ADHD&ASD). Methods This retrospective analysis included 204 participants divided into three groups: ADHD&ASD (64/204), ADHD (70/204), and typical development (TD) (70/204). Neuropsychological tests, including the Digit Span Test, Behavior Rating Inventory of Executive Function (BRIEF), Trail Making Test at its Chinese equivalent version, the Shape Trail Test (STT), and the Stroop Color Word Interference Test, were compared across groups. ERP measures, such as latency and amplitude at the frontal zone (Fz), central zone (Cz), and parietal zone (Pz), along with reaction times and target stimulus hits using an oddball paradigm, were analyzed. Post-hoc analyses and receiver operating characteristic (ROC) curves identified key indicators for distinguishing ADHD from ADHD&ASD. Results Significant differences were found in ERP and BRIEF indicators (P < 0.001), as well as selected TMT and Stroop parameters (P < 0.05) across the groups. The ADHD&ASD group exhibited notably higher Fz, Cz, and Pz latencies, interference times, and reaction times compared to the ADHD group (P < 0.05). With the exception of word meaning interference time, the area under ROC curves (AUC) of the remaining five indicators exceeds 0.8. Particularly noteworthy is the combined ROC curve of Fz latency and average reaction time, which demonstrated a sensitivity, specificity, and AUC of 88.68%, 97.14%, and 0.938 (95%CI: 0.885–0.990, P < 0.001), respectively. Conclusion ERP measurements using oddball paradigms hold strong potential for distinguishing ADHD from ADHD&ASD, enabling the development of more targeted treatment approaches through precise neurophysiological markers.
Similar content being viewed by others
Introduction
Attention deficit hyperactivity disorder (ADHD) is a prevalent neurodevelopmental condition among children, typically emerging before the age of 12 and primarily characterized by inattention, hyperactivity and impulsivity with their developmental stage1. Widely recognized as a lifelong condition, ADHD persists into adulthood for 30–50% of individuals2. Notably, a study involving 1,496 participants aged 2–17 revealed that children initially diagnosed with ADHD were nearly 30 times more likely to be diagnosed with autism spectrum disorder (ASD) after the age of 6 compared to their non-ADHD counterparts3. Further examination of ASD symptoms in children with ADHD showed that 21% of them met the diagnostic criteria for ASD according to the Autism Diagnostic Observation Schedule (ADOS)4. Specifically, comorbidity of ASD and ADHD (ADHD&ASD) exhibit more pronounced cognitive impairments compared to those with ADHD alone5. Recent research has suggested that factors such as shared genetic markers and overlapping environmental influences could contribute to the high rates of ADHD-ASD comorbidity. In addition, both ADHD and ASD often show similar deficits in executive functioning, which are controlled by overlapping brain networks, including the prefrontal cortex, parietal cortex, and cerebellum6.
Executive functions, which include cognitive processes such as working memory, attention control, cognitive flexibility, and inhibitory control, are often impaired in both ADHD and ASD7,8,9,10,11. Recent meta-analyses have provided an updated overview of executive function deficits in these populations, indicating that individuals with ADHD and ASD show significant executive function impairments compared to typically developing (TD) individuals. However, the specific nature of these deficits varies between the disorders and their comorbidity12. Studies examining executive function using neuropsychological assessments (e.g., Digit Span, Stroop, and Wisconsin Card Sorting Tests) have consistently shown differences in executive function profiles between ADHD, ASD, and ASD&ADHD, with ADHD showing greater impairments in attention regulation and inhibitory control, and ASD demonstrating more deficits in cognitive flexibility and social cognition13,14,15. Moreover, studies have shown that gender is one of the key factors in the neuropsychological characteristics and executive function of ADHD and ADHD&ASD, and understanding these gender differences is crucial for developing gender-sensitive diagnostic criteria and intervention measures16,17,18,19,20. However, using subjective scales for diagnosing ADHD and ADHD comorbid with ASD is time-consuming and often relies on evaluations by parents and school teachers, which introduces the potential for subjective bias and existing research lacks a thorough comparative analysis of executive function impairments across specific age groups in ADHD and ASD and effective identification methodologies21. Thus, determining how to promptly and accurately identify ADHD&ASD within children with ADHD could offer valuable assistance for diagnosis, treatment, and prognosis22.
While both disorders share some common neural features, particularly in regions related to attention and sensory processing, they also show distinct patterns of brain activity, particularly in social processing and reward systems. Prior studies the use of electroencephalogram (EEG)-imaging and other neuroimaging techniques have begun to uncover both the shared and distinct neural mechanisms underlying these disorders, which may offer valuable insights into ADHD&ASD co-occurrence6. Event-related potential (ERP) offer a non-invasive approach for gathering quantitative neurobiological data by monitoring voltage fluctuations in EEG recordings and are widely employed in investigating childhood psychiatric disorders23. In the exploration of the pathophysiological mechanisms of ADHD, ERP is recognized as the most suitable theoretical framework reflecting the clinical symptoms and behavioral deficits of ADHD13,24. Prior research indicates that individuals with ADHD exhibit ERP alterations during neurocognitive tasks related to attention, inhibitory control, information processing, and reward processing, underscoring deficits in executive functions25.
Notably, the P300 stimulation task has been extensively employed in both ADHD and ASD research to examine attentional deficits. Recent meta-analyses have provided a more robust understanding of ERP markers in ADHD by comparing early versus late cognitive ERP components, revealing consistent alterations in P300 markers, with reduced amplitude and increased latency observed across studies that emphasized the importance of considering timing in ERP components for a more comprehensive view of ADHD’s cognitive deficits26. In ASD, studies on the P300 have similarly shown alterations in amplitude and latency, which are thought to reflect deficits in sensory processing and attentional control. A meta-analysis by Cui et al. found that P300 amplitudes were significantly reduced in individuals with ASD, suggesting impairments in cognitive processing related to attention and sensory integration27. These findings are consistent with the hypothesis that ASD-related attentional deficits may differ from those observed in ADHD, despite the overlapping attentional symptoms in both populations. Researchers focusing on P300 in task states have identified a positive correlation between ADHD Rating Scale-IV-Japanese version hyperactivity-impulsivity subscale scores and ERP P300 latency28. Additionally, other EEG assessments in the early stages of ASD highlight differences from the TD group, potentially reflecting ASD’s socialization function and prognostic implications29. The oddball paradigm, which requires participants to discriminate between rare and frequent stimuli, is ideal for studying attentional shifts, inhibition, and response control in ADHD and ASD. ERP components, particularly the P300, are sensitive to these processes and are altered in both ADHD and ASD, allowing for the examination of cognitive differences between these groups30,31. Nonetheless, current research lacks comparative analyses of executive function disparities between ADHD and co-occurring groups, underscoring the critical need for establishing reliable diagnostic and identification biomarkers.
The aims of this study is to elucidate the specific indicators and advantages of ERP in identifying ADHD and ADHD&ASD as follows: (1) to comprehensively compare neuropsychological scales and ERP indexes across three groups: ADHD, ADHD&ASD, and TD individuals; (2) to identify reliable indicators that can distinguish between ADHD and ADHD&ASD among those indicators showing differences in the overall comparison; (3) to assess the diagnostic accuracy of the identified indicators through the establishment of receiver operating characteristic (ROC) curves.
Materials and methods
Patients
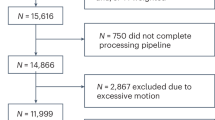
Between December 2022 and December 2023, a total of 236 consecutive children, aged 6 to 12 years, were recruited from outpatient services at the local hospital. All participants were admitted to the Child Health Department of the local children’s hospital. All participants were all native Chinese speakers. Two experienced pediatricians diagnosed all participants based on the criteria outlined in the Diagnostic and Statistical Manual of Mental Disorders – Fifth Edition (DSM-V) for ADHD, ASD and ADHD&ASD. Exclusion criteria included intellectual disability (intelligence quotient [IQ] < 70), epilepsy, and other organic disorders that could potentially impact attention deficit, learning difficulties, or cognitive test performance. Additionally, children with visual or hearing impairments were excluded. Based on this operationalization, 64 participants were determined to be co-morbid ASD&ADHD (6 females), 70 participants met criteria for ADHD (6 females), and 70 TD (4 females). The enrollment process is detailed in Fig. 1. Further clinical assessment procedures were conducted, incorporating the ADOS32and a cognitive assessment using the Wechsler Intelligence Scale for Children in Chinese (WISC-C)33. The informed consent was obtained from each participant. This study was approved by the institutional review board (2022-021-01) and was in accordance with the Declaration of Helsinki.
Flowchart of the patient’s cohort. Notes: ADHD = attention-deficit/hyperactivity disorder; ASD = autism spectrum disorder; ADHD&ASD = Comorbidity of ASD and ADHD; TD = typical development; DQ = disqualified from study; ADOS-2 = Autism Diagnostic Observation Study, Second Edition; C-WISC = Wechsler Intelligence Scale for Children in Chinese.
Neuropsychological assessment
Researchers conducted a comprehensive neuropsychological assessment of participants, including the following measures: (1) Digit Span Test (DST): The DST assesses attention, concentration, executive functioning, and short-term memory in children. It involves listening to a sequence of numbers read aloud and repeating the sequence verbatim (DS Forwards) or in reverse order (DS Backwards). Scores reflect the longest correctly repeated sequence34. (2) Trail Making Test (TMT): This test requires connecting numbers or numbers and letters in proper order on a page. Due to language differences, the Shape Trail Test (STT) was utilized in China, which involves connecting numbers presented in different shapes35. The TMT consists of two parts, Part A and Part B, which are designed to assess different aspects of cognitive functioning: Part A primarily measures processing speed and visual attention, while Part B is designed to assess cognitive flexibility and the ability to shift attention between different sets of rules. (3) Stroop Color Word Interference Test: This test measures interference inhibition. Participants are presented with cards containing color names or colors and are asked to either read the color names or name the ink colors and consists of four parts, each assessing different aspects of cognitive function: Color Naming: Participants name the color of printed words, measuring processing speed and basic attention. Word Reading: Participants read a list of color words, assessing cognitive processing speed without interference. Color-Word Interference: Participants name the color of a word that conflicts with its meaning, measuring cognitive flexibility and inhibitory control. Color-Word Interference with Switching: Participants alternate between naming colors and reading words, assessing cognitive set-shifting and attentional control. Time and error rates are recorded to calculate color and word interference times36. (4) The Behavior Rating Inventory of Executive Function (BRIEF) is a questionnaire for parents and teachers of school age children that enables professionals to assess executive function behaviors in the home and school environments37. These assessments provide insights into various cognitive functions, including attention, memory, inhibition, and executive functioning.
ERP acquisition
For ERP recording, participants were seated comfortably in a tranquil environment, relaxed their entire body, and focused both eyes on the center point of the screen. Before the commencement of the experiment, clear explanations of the experimental instructions and test requirements were provided to the participants. The distance between the eyes and the screen should be 80 cm, with a horizontal viewing angle of 0.7° and a vertical viewing angle of 1.4°. Since the experimental results are closely linked to the psychological state of the subjects, their cooperation is essential. For participants who experienced difficulty completing the tasks due to non-cooperation, the researcher or clinician in charge conducted a brief reassessment to understand the cause of the issue (e.g., anxiety, confusion, fatigue). Depending on the situation, the test was either rescheduled or if non-cooperation was persistent or the participant was unable to complete the task despite reassurance, their data was excluded from the analysis to avoid any bias or inaccuracies in the results.
Following the international 10/20 system for electrode placement, disc-shaped silver/silver chloride electrodes were utilized, and the EEG was recorded using a 32-channel electrode cap with placements based on this system. These electrodes were positioned along the midline of the scalp in the frontal zone (Fz), central zone (Cz), and parietal area (Parietal zone, Pz) to record brain electrical activity. Additional electrodes were placed to monitor vertical eye movements (VEOG) for artifact correction. The reference electrode is positioned on the left ear (A1) and the right ear (A2), while the ground wire is attached to the forehead. The impedance between each electrode and the skin was below 5Ω. Prior to attaching the electrode pad, it was cleaned with medical alcohol to remove any grease. During the pre-test, subjects must be briefed on the test requirements. They are instructed to press the button upon viewing the target stimulus pattern and refrain from pressing the button for the non-target stimulus pattern. The formal test can only commence once the pre-test has been successfully completed.
ERP measurements
The peak-to-coordinate distance was softly calculated to ensure precision in the analysis. The original EEG data, collected by the amplifier, was retained. For analysis, a bandpass filter using FIR filtering with a Hamming window was applied to isolate signals within the 1–70 Hz range38. Additionally, a Butterworth notch filter was used to remove 50 Hz power line noise. The device operated with a sampling rate of 1024 Hz and a sampling range of DC-256 Hz, recording raw data while using both earlobes as reference points with an impedance below 10 kΩ. Mark information was synchronously collected and sent to the Rainjet amplifier by the host computer, ensuring low latency in data upload. Baseline correction was performed using the 100 ms pre-stimulation period, and any data with peak-to-peak amplitudes exceeding 120 µV were excluded. Mismatch negativity recording was conducted before P300 recording for all children and adolescents in our study. Data analysis excluded averages with counting errors exceeding 10%.
Our study used peak detection algorithms to identify the maximal positive deflections in the EEG signal. We defined the P300 as the largest positive peak in the 250–500 ms window, encompassing both P3a and P3b components. The P300 latency was the time interval between stimulus presentation and the peak of the positive deflection. P300 amplitude was measured as the difference between the baseline (pre-stimulus period) and the peak of the positive deflection, both of which typically occur between 300 ms and 500 ms after stimulus presentation. After preprocessing the EEG data using EEGLAB and applying ICA, we averaged the EEG signals across trials for each subject. This averaging method helps to increase the signal-to-noise ratio by minimizing random fluctuations and focusing on the consistent neural response to the stimuli. The latency and amplitude of the P300 components were then measured using peak detection algorithms within the EEGLAB toolbox, which automatically identifies the most prominent peaks in the waveform. This study recorded several indicators, including latency, amplitude, maximum reaction time, minimum reaction time, average reaction time, and the number of correct responses (hits) for target stimuli.
ERP paradigm and quality control
Our experiment followed a typical oddball paradigm depicted in Fig. 2, and was executed using E-prime software for both compilation and control39. All subjects completed the experiment using only one hand (right-handed). In this task, the “french fries” pattern served as the target stimulus, while other patterns acted as non-target stimuli. Each stimulus was displayed for 800 ms, with intervals of 1000–1200 ms between presentations. A total of 300 stimuli were shown randomly, with target stimuli comprising 20% and non-target stimuli making up 80%. The stimuli were categorized based on their frequency: target stimuli appeared occasionally, while non-target stimuli appeared frequently. Participants were instructed to respond by pressing a key when a target stimulus appeared and refraining from pressing when a non-target stimulus was shown. Data were recorded with a 1000 ms analysis window, including a baseline period of 100–150 ms before stimulus onset.
EEGLAB was used to preprocess the EEG data, which included bandpass filtering to remove unwanted frequencies (1–30 Hz). Independent Component Analysis (ICA) was applied to remove eye blinks, muscle artifacts, and other non-neuronal signals from the EEG data40. In this study, we set the amplitude threshold for artifact detection at ± 100 µV ensuring that only clean data were included in the analysis. To reduce artifacts, electrooculographic (EOG) signals were removed through correction procedures, and digital low-pass filtering was applied with a cutoff frequency of 12 Hz38. Following the artifact removal process, the ERP data were averaged across valid trials for each condition (e.g., target vs. non-target) for each participant. We analyzed the data separately for each electrode site (Fz, Cz, Pz) to capture the regional brain activity. After averaging across trials, group-level ERPs were calculated by averaging the individual participant waveforms within each group. This study recorded several indicators, including latency, amplitude, maximum reaction time, minimum reaction time, average reaction time, and the number of correct responses (hits) for target stimuli. After applying the artifact exclusion criteria, 8 participants (3.4%) were excluded due to artifacts.
Statistical analysis
All demographic and clinical data were assessed for normality and the presence of outliers prior to statistical analysis using IBM SPSS Statistics version 26.0. Descriptive statistics was used to display participants’ data as median, mean, standard deviation range or percentages, where applicable. Clinical data and ERP results were compared across groups (ADHD&ASD, ADHD and TD) using one-way analysis of variance (ANOVA) and Bonferroni correction was used for post hoc comparisons or Wilcoxon- Mann-Whitney U test. Basing on the results above, receiver operating characteristic (ROC) analysis was all assessing the prediction value for ADHD and ADHD&ASD discrimination, and area under the curve (AUC) value was generated. An AUC above 0.8 is generally considered excellent, suggesting that the model is highly effective in distinguishing between the two groups. For instance, in a case where the symptoms of ADHD overlap with ADHD&ASD, the model’s high AUC value could guide clinicians in making a more accurate diagnosis, thereby leading to better-targeted interventions. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
The Table 1 provide a comprehensive overview of the clinical and demographic characteristics across the three groups. Nonparametric tests demonstrated statistically significant differences among these groups across various variables. To be specific, all indicators of BRIEF showed significant differences among the three groups (p < 0.001). The Digit Span Test yielded no statistically significant differences among the three groups (p = 0.441, p = 0.400, respectively). Part B error of TMT, Part 2 time, Part 4 time, Part 4 error and word interference time in Stroop also had significant differences among the three groups (p = 0.014, p = 0.023, p = 0.004, p = 0.027 and p = 0.033, respectively). In the ADHD&ASD group, there were greater levels of inhibition, switching difficulties, challenges in affective control, task initiation, organization, and behavioral management as evidenced by higher scores on the BRIEF scale. Additionally, the ADHD&ASD group exhibited more prompts in connected test A, more errors in connected test B, longer time required for coloring the color blocks, and a higher number of colored letter color errors compared to the TD group. In the ADHD group, there were significantly greater levels of inhibition, switching difficulties, emotional control challenges, and deficits in task initiation, organization, and behavioral management as indicated by higher scores on the BRIEF scale, compared to the TD group.
The ERP indicators were also significant differences among the three groups (p < 0.001) (Table 2).
Subsequent post-hoc analyses revealed that compared to the ADHD group, the ADHD&ASD group had significantly higher Fz latency (p<0.001), Cz latency (p<0.001), Pz latency (p<0.001), word meaning interference time (p = 0.031), minimum reaction time (p<0.001) and mean reaction time (p<0.001) with statistically significant differences (see Fig. 3). In the ADHD&ASD group, there were significantly longer latency in Fz, Cz, and Pz, maximal reaction times. Additionally, the ADHD&ASD group exhibited longer minimum and average reaction times compared to the TD group. In the ADHD group, there were significantly longer Pz latency and maximal reaction times compared to the TD group. In contrast to the TD group, the ADHD&ASD group exhibited fewer target stimulus hits, lower amplitudes of Pz, Fz, and Cz waves. This difference was also found to be statistically significant. The Pz wave amplitude, Fz wave amplitude, Cz wave amplitude, and target stimulus hits were all significantly lower compared to the TD group.
Based on this foundation, to enhance the validation of the aforementioned indicators in distinguishing ADHD & ASD within ADHD, we found that five ERP indicators AUC exceeded 0.8 with the sensitivity, specificity, positive predictive value(PPV), negative predictive value(NPV) as follows: Fz latency (75.47%, 97.14%, 96.02%, 81.24% and AUC: 0.878, 95%CI: 0. 860 to 0.990, p<0.001), Cz latency (77.36%, 81.43%, 79.20%, 79.73% and AUC: 0.843, 95%CI: 0.766 to 0.902, p < 0.001), Pz latency (75.47%, 85.71%, 82.84%, 79.26& and AUC: 0.851, 95%CI: 0.775–0.908, p < 0.001), minimum reaction time (73.58%, 87.14%, 83.95%, 78.30% and AUC: 0.832, 95%CI: 0.753–0.893, p < 0.001), and average reaction time (84.91%, 82.86%, 81.91%, 85.73% and AUC: 0.892, 95%CI: 0.823–0.940, p < 0.001), respectively (Fig. 4, a-e). Additionally, word meaning interference time showed the sensitivity, specificity, PPV, NPV, and AUC as follow: 72.13%, 49.18%, 56.48%, 65.87% and AUC: 0.630 (95%CI: 0.538–0.715, p = 0.010). In order to further improve the diagnostic performance, the ROC curve of the Fz latency and average reaction time was combined to obtain the maximum AUC result with sensitivity 88.68%, specificity 97.14% and AUC: 0.938(95%CI: 0.885–0.990, p < 0.001) (Fig. 4, f).
Discussion
This study aimed to assess potential differences among existing neuropsychological scales and ERP in children with distinct diagnoses of ADHD, ADHD&ASD, and TD children, however, the constructs captured by these different methods—neural markers versus neuropsychological tests and questionnaires—may not overlap entirely. Neuroimaging studies focus on brain activity patterns, tests primarily capture behavioral manifestations of executive function. These differences in methods are crucial for understanding how executive function deficits are expressed and measured in these populations. Recent studies show executive function deficits in children and adolescents with ADHD and ASD are not uniform and must be analyzed from multiple perspectives to better differentiate these groups from each other and from TD individuals41. As far, as a direct comparison of these deficits using ERP has not been conducted between ADHD and cases with comorbidity yet.
Our findings revealed that ERP measures could effectively detect children with ADHD&ASD within the ADHD population, demonstrating high diagnostic accuracy, specificity, and sensitivity. This suggests that ERP technology can serve as a reliable screening tool for ADHD, particularly in identifying cases with co-occurring ASD. A previous study revealed that individuals with ADHD (ADHD/ASD + ADHD) committed more ERP related task errors, greater variability in reaction times, and showed diminished amplitudes of the P300 compared to participants with TD42. Our study further analyzed the differences in executive functions between ADHD and ADHD combined with ASD groups. Although there were differences in neuropsychological scales and ERP between ADHD and ADHD&ASD patients compared with the TD group, post- results showed ERP indicators can be used to identify ADHD and comorbidity groups better than the neuropsychological scales.
A previous study used the no-go paradigm to investigate ERP patterns in children with ADHD, finding that their P300 latency was significantly prolonged in the parietal lobe compared to typically developing children43,44. Prolonged latency, which relates to cognitive response speed, indicates impaired cognitive flexibility, potentially associated with ASD. Our findings showed that the co-occurring ADHD&ASD group had both prolonged latency and amplitude compared to the ADHD-only group, underscoring more severe executive dysfunction in those with combined ADHD and ASD. Our ERP findings, which include increased latency and reduced amplitude, indicate significant cognitive impairments in both ADHD and ADHD + ASD, primarily linked to deficits in attention networks and executive control. Previous research has demonstrated that targeted training in working memory and cognitive flexibility can strengthen attention networks in adults with ADHD, leading to improvements in academic, social, and career outcomes45. These findings support the potential effectiveness of similar interventions for both ADHD and ADHD + ASD children’s populations, providing a neurophysiological basis for their application.
Using ROC curve analysis, we found that the latency of Fz, Cz, and Pz had superior diagnostic efficacy for distinguishing ADHD from ADHD&ASD compared to amplitude, suggesting that latency measurements may enhance diagnostic precision. This may indicate that ADHD-related executive dysfunctions are more prominent in the co-occurring group, while different patterns are present in the ADHD-only group. Delayed Fz latency reflects neural inefficiency in processing cognitive tasks, a hallmark feature of these conditions. Studies have demonstrated that individuals with ADHD&ASD often show significantly prolonged latencies in the frontal region, indicating more severe deficits in executive control compared to those with ADHD alone and average reaction time42. On the other hand, captures the behavioral manifestation of these delays, reflecting not only cognitive speed but also motor response efficiency. Longer reaction times are indicative of deficits in attention allocation and response preparation28. This holistic approach leverages their complementary strengths to provide a comprehensive measure of executive dysfunction, improving the model’s ability to distinguish ADHD&ASD from ADHD25. While the ROC curve and AUC are commonly used to evaluate model performance, they can be less informative or even misleading when applied to small sample sizes, particularly when the dataset is imbalanced46. We will continue to expand the sample size, ensure the balance between samples, and further verify the reliability of the data in future research. Additionally, Balanced Average Accuracy and Deep ROC Analysis have been suggested as more robust methods for small sample sizes and imbalanced data47. This can be further considered and applied in future analysis.
Our study revealed significant differences among the three groups in TMT Part B performance, particularly regarding error rates. TMT Part B, which demands cognitive flexibility and attentional shifting, poses specific challenges for children with ADHD, who show slower processing speeds and difficulty switching between cognitive sets, reflected in higher error rates. Children with comorbid ADHD&ASD exhibit even greater difficulties, including weaker visuomotor coordination, intensifying the challenges in Part B compared to TD children48. These deficits are more pronounced in children with ASD, who may also experience sensory processing and social cognition difficulties that further affect their performance. Part B is inherently more difficult than Part A due to the need for shifting attention between cognitive sets, a skill commonly impaired in ADHD and ASD. Thus, the performance differences in Part B reflect deficits in cognitive flexibility, attentional control, visuomotor coordination, and set-shifting, which are particularly prominent in ADHD and ASD, especially when comorbid. In contrast, the Digit Span Test did not show significant group differences. Although working memory deficits are common in ADHD, the Digit Span Test may not fully capture these impairments, as it primarily assesses short-term memory and attention rather than broader working memory aspects49,50. More complex tasks with higher working memory demands may be needed to detect group differences, and gender-based symptom variations could also influence results51,52,53.
A key limitation of this study is the underrepresentation of females, which may lead to gender bias in our findings. Females with ADHD or ADHD&ASD often present with subtler symptoms (e.g., inattention, emotional dysregulation), making them more likely to be underdiagnosed compared to males who typically display more overt symptoms. This gender imbalance in research samples can skew results, making them more reflective of male-dominant profiles54,55. Future studies should ensure gender-balanced samples to enhance diagnostic accuracy and capture gender-specific presentations of ADHD and ADHD&ASD. This study’s small sample size limits its ability to detect significant interactions between ERP indicators and ASD/ADHD, though similar results have been reported in other small-sample studies51,52,56. Future research with larger samples should explore and directly compare ASD with combined groups to enhance ERP applications. Our study did not employ advanced clustering techniques, such as fuzzy clustering, which could have provided deeper insights into complex ERP patterns and overlapping characteristics in the ADHD and ADHD&ASD groups57. Additionally, investigating other brain regions, such as the occipital and temporal areas, and incorporating additional electrode sites could provide a more comprehensive understanding of sensory and attentional processing differences in ADHD and ADHD&ASD populations. False positive rates in this study were consistent with larger studies58.
In conclusion, our study demonstrated utilizing ERP characteristic parameters to distinguish between ADHD and ADHD-co-occurring ASD. Our findings reveal that features such as latency, average reaction time and minimum reaction time, particularly when combined with Fz latency and average reaction time, exhibit strong diagnostic performance in identifying children with ADHD&ASD within the ADHD population, a capability not demonstrated by executive function neuropsychological scales. The incorporation of ERP measurements employing oddball designs holds promise in identifying comorbidities of ADHD and ASD, thereby facilitating the implementation of more targeted treatment strategies.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ADHD:
-
attention deficit hyperactivity disorder
- ADOS:
-
Autism Diagnostic Observation Schedule
- ASD:
-
autism spectrum disorder
- AUC:
-
area under the curve
- BRIEF:
-
Behavior Rating Inventory of Executive Function
- Cz:
-
central zone
- DSM-V:
-
Diagnostic and Statistical Manual of Mental Disorders – Fifth Edition
- DST:
-
Digit Span Test
- EEG:
-
electroencephalogram
- ERP:
-
event-related potentials
- Fz:
-
frontal zone
- IQ:
-
intelligence quotient
- Pz:
-
parietal zone
- ROC:
-
receiver operating characteristic
- STT:
-
Shape Trail Test
- TD:
-
typical development
- TMT:
-
Trail Making Test
- WISC-C:
-
Wechsler Intelligence Scale for Children in Chinese
References
Faraone, S. V. et al. Attention-deficit/hyperactivity disorder. Nat. Rev. Dis. Primers. 10, 11 (2024).
Wolraich, M. L. et al. Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 144, e20192528 (2019).
Miodovnik, A. et al. Timing of the diagnosis of attention-deficit/hyperactivity disorder and autism spectrum disorder. Pediatrics 136, e830–e837 (2015).
Grzadzinski, R. et al. Parent-reported and clinician-observed autism spectrum disorder (ASD) symptoms in children with attention deficit/hyperactivity disorder (ADHD): implications for practice under DSM-5. Mol. Autism. 7, 7 (2016).
Rao, P. A. & Landa, R. J. Association between severity of behavioral phenotype and comorbid attention deficit hyperactivity disorder symptoms in children with autism spectrum disorders. Autism: Int. J. Res. Pract. 18, 272–280 (2014).
Lau-Zhu, A., Fritz, A. & McLoughlin, G. Overlaps and distinctions between attention deficit/hyperactivity disorder and autism spectrum disorder in young adulthood: systematic review and guiding framework for EEG-imaging research. Neurosci. Biobehav Rev. 96, 93–115 (2019).
Diamond, A. Executive functions. Annu. Rev. Psychol. 64, 135–168 (2013).
Townes, P. et al. Do ASD and ADHD have distinct executive function deficits? A systematic review and meta-analysis of direct comparison studies. J. Atten. Disord. 27, 1571–1582 (2023).
Perone, S., Simmering, V. R. & Buss, A. T. A dynamical reconceptualization of executive function development. Perspect. Psychol. Sci. 16, 1198–1208 (2021).
Funahashi, S. Neuronal mechanisms of executive control by the prefrontal cortex. Neurosci. Res. 39, 147–165 (2001).
Fuster, J. M. The Prefrontal Cortex: Anatomy, Physiology, and Neuropsychology of the Frontal Lobe (Lippincott-Raven, 1997).
Miyake, A. et al. The unity and diversity of executive functions and their contributions to complex frontal lobe tasks: A latent variable analysis. Cogn. Psychol. 41, 49–100 (2000).
Suarez, I. et al. Two sides of the same coin: ADHD affects reactive but not proactive Inhibition in children. Cogn. Neuropsychol. 38, 349–363 (2021).
Uddin, L. Q. Brain mechanisms supporting flexible cognition and behavior in adolescents with autism spectrum disorder. Biol. Psychiatry. 89, 172–183 (2021).
Craig, F. et al. A review of executive function deficits in autism spectrum disorder and attention-deficit/hyperactivity disorder. Neuropsychiatr Dis. Treat. 12, 1191–1202 (2016).
Gaub, M. & Carlson, C. L. Gender differences in ADHD: A meta-analysis and critical review. J. Am. Acad. Child. Adolesc. Psychiatry. 36, 1036–1045 (1997).
Reinke, W. M. & Ostrander, R. Heterotypic and homotypic continuity: the moderating effects of age and gender. J. Abnorm. Child. Psychol. 36, 1109–1121 (2008).
Loomes, R. et al. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J. Am. Acad. Child. Adolesc. Psychiatry. 56, 466–474 (2017).
Hull, L. et al. Behavioral and cognitive sex/gender differences in autism spectrum condition and typically developing males and females. Autism 21, 706–727 (2017).
Doi, H. et al. Transdiagnostic and sex differences in cognitive profiles of autism spectrum disorder and attention-deficit/hyperactivity disorder. Autism Res. 15, 1130–1141 (2022).
Emser, T. S. et al. Assessing ADHD symptoms in children and adults: evaluating the role of objective measures. Behav. Brain Funct. 14, 11 (2018).
Antshel, K. M. & Russo, N. Autism spectrum disorders and ADHD: overlapping phenomenology, diagnostic issues, and treatment considerations. Curr. Psychiatry Rep. 21, 34 (2019).
Loo, S. K., Lenartowicz, A. & Makeig, S. Use of EEG biomarkers in child psychiatry research: current state and future directions. J. Child. Psychol. Psychiatry. 57, 4–17 (2016).
Nigg, J. T. Annual research review: on the relations among self-regulation, self-control, executive functioning, effortful control, cognitive control, impulsivity, risk-taking, and Inhibition for developmental psychopathology. J. Child. Psychol. Psychiatry. 58, 361–383 (2017).
Marquardt, L. et al. Event-related-potential (ERP) correlates of performance monitoring in adults with attention-deficit hyperactivity disorder (ADHD). Front. Psychol. 9, 485 (2018).
Kaiser, A. et al. Earlier versus later cognitive event-related potentials (ERPs) in attention-deficit/hyperactivity disorder (ADHD): A meta-analysis. Neurosci. Biobehav Rev. 112, 117–134 (2020).
Cui, T. et al. P300 amplitude and latency in autism spectrum disorder: A meta-analysis. Eur. Child. Adolesc. Psychiatry. 26, 177–190 (2017).
Yamamuro, K. et al. Event-related potentials correlate with the severity of child and adolescent patients with attention deficit/hyperactivity disorder. Neuropsychobiology 73, 131–138 (2016).
Mason, L. et al. Stratifying the autistic phenotype using electrophysiological indices of social perception. Sci. Transl Med. 14, eabf8987 (2022).
Carlo, R. & Rueda, M. R. ERP responses to oddball stimuli in children with autism spectrum disorder. Neuropsychology 31, 444–453 (2017).
Kozlowski, A. D. & Woodward, H. J. Event-related potentials (ERPs) as a tool for studying ADHD and ASD. J. Clin. Child. Adolesc. Psychol. 41, 784–795 (2021).
Lord, C. et al. Autism diagnostic observation schedule: A standardized observation of communicative and social behavior. J. Autism Dev. Disord. 19, 185–212 (1989).
Li, D. et al. Report on Shanghai norms for the Chinese translation of the Wechsler intelligence scale for Children-Revised. Psychol. Rep. 67, 531–541 (1990).
Taylor, A. F. & Kuo, F. E. Children with attention deficits concentrate better after walk in the park. J. Atten. Disord. 12, 402–409 (2009).
Zhao, Q. et al. The shape trail test: application of a new variant of the trail making test. PLoS ONE. 8, e57333 (2013).
Shuai, L. et al. The effect of methylphenidate on executive function for children with attention deficit hyperactivity disorder. J. Peking Univ. 39, 293–298 (2007).
Biederman, J., DiSalvo, M. L. & Vater, H. Toward operationalizing executive function deficits in adults with ADHD using the behavior rating inventory of executive function-adult version (BRIEF-A). J. Clin. Psychiatry. 84, 22m14530 (2022).
Jiang, X., Bian, G. B. & Tian, Z. Removal of artifacts from EEG signals: A review. Sens. (Basel). 19, 987 (2019).
Janssen, T. W. P. et al. An ERP source imaging study of the oddball task in children with attention deficit/hyperactivity disorder. Clin. Neurophysiol. 127, 1351–1357 (2016).
Delorme, A. & Makeig, S. E. E. G. L. A. B. An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 134, 9–21 (2004).
Ceruti, C. et al. Comparing executive functions in children and adolescents with autism and ADHD—A systematic review and meta-analysis. Child. (Basel). 15, 473 (2024).
Tye, C. et al. Attention and Inhibition in children with ASD, ADHD, and co-morbid ASD + ADHD: an event-related potential study. Psychol. Med. 44, 1101–1116 (2014).
Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 4, 863 (2013).
Liu, C., Liang, X. & Sit, C. H. P. Physical activity and mental health in children and adolescents with neurodevelopmental disorders: A systematic review and meta-analysis. JAMA Pediatr. e236251 (2024).
Dotare, M. et al. Attention networks in ADHD adults after working memory training with a dual n-back task. Brain Sci. 10, 715 (2020).
Saito, T. & Rehmsmeier, M. The precision-recall plot is more informative than the ROC plot when evaluating binary classifiers on imbalanced datasets. PLoS ONE. 10, e0118432 (2015).
Carrington, A. M. et al. Deep ROC analysis and AUC as balanced average accuracy for improved classifier selection, audit, and explanation. IEEE Trans. Pattern Anal. Mach. Intell. 45, 329–341 (2023).
Ashwood, K. L. et al. Brief report: adaptive functioning in children with ASD, ADHD, and ASD + ADHD. J. Autism Dev. Disord. 45, 2235–2242 (2015).
Tye, C. et al. Callous-unemotional traits moderate executive function in children with ASD and ADHD: A pilot event-related potential study. Dev. Cogn. Neurosci. 26, 84–90 (2017).
Elosúa, M. R., Olmo, D. & Contreras, S. Differences in executive functioning in children with attention deficit and hyperactivity disorder (ADHD). Front. Psychol. 8, 976 (2017).
Fabio, R. et al. Working memory and decision making in children with ADHD: an analysis of delay discounting with the use of the dual-task paradigm. BMC Psychiatry. 20, 272 (2020).
Oguchi, M. et al. The moderating effect of attention-deficit hyperactivity disorder symptoms on the relationship between procrastination and internalizing symptoms in the general adult population. Front. Psychol. 12, 708579 (2021).
Dupuy, F. E. et al. EEG differences between the combined and inattentive types of attention-deficit/hyperactivity disorder in girls: A further investigation. Clin. EEG Neurosci. 45, 231–237 (2014).
Gershon, J. A meta-analytic review of gender differences in ADHD. Psychol. Bull. 128, 3–14 (2002).
Quinn, P. O. & Madhoo, M. ADHD in women: the hidden gender gap. Curr. Psychiatry. 13, 47–51 (2014).
Ghanizadeh, A., Salehi, A. & Seyed, M. D. Clinical presentation of attention-deficit hyperactivity disorder symptoms in terms of gender and chronological age. Int. J. Community Based Nurs. Midwifery. 7, 241 (2019).
Masulli, P. et al. Fuzzy clustering for exploratory analysis of EEG event-related potentials. IEEE Trans. Fuzzy Syst. 28, 28–38 (2020).
Patros, C. H. et al. Greater delay discounting among girls, but not boys, with ADHD correlates with cognitive control. Child. Neuropsychol. 24, 1026–1046 (2018).
Acknowledgements
Nothing to disclose.
Funding
This research was supported by the Natural Science Foundation of China (81771223), Chief Medical Expert Studio of Chongqing (YWBF [2018]263), and Natural Science Foundation of Shaanxi Province, China (2018SF-229).
Author information
Authors and Affiliations
Contributions
Chuanxue Tan drafted the manuscript, contributed to the conception and design, analysis and interpretation of data. Huimin Zhou and Huanli Chen contributed to the analysis and interpretation of data. Miao Yang and Chunyang Li contributed to acquisition of data. Li He, Ting Yang and Jie Chen contributed to the conception and design. Jie Zhang and Tingyu Li made significant contributions to the critical revision of the manuscript, focusing on important intellectual content.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of The Children’s Hospital Affiliated of Xi ‘an Jiaotong University.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Statements & Declarations
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tan, C., Zhou, H., Yang, M. et al. The discriminate value of event-related potentials in executive function of ADHD and comorbidity of ADHD and ASD. Sci Rep 15, 9802 (2025). https://doi.org/10.1038/s41598-025-94156-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-94156-1