Abstract
The effective management of pollutants in waste incineration flue gas remains a critical challenge in environmental protection. This study develops a novel vanadium (V), molybdenum (Mo)/cerium (Ce), titanium (Ti)–polytetrafluoroethylene (VMo/CeTi-PTFE) composite catalytic filtration material to address the simultaneous removal of multiple pollutants in waste incineration flue gas purification. A systematic investigation of its performance and reaction mechanisms reveals that the coating process optimizes the pore structure of the filtration material. While the specific surface area is slightly reduced, the increased pore volume and diameter facilitate gas diffusion and enhance reaction efficiency. Experimental results demonstrate that under high catalyst loading conditions, this material exhibits outstanding performance in denitrification, dioxin degradation, and particulate removal, maintaining a consistently high dust removal efficiency of over 99.97%. Additionally, a high binder content enhances mechanical stability, while water and sulfur resistance tests confirm its exceptional durability. Mechanistic analysis indicates a significant synergistic effect between the denitrification reaction and dioxin degradation. Specifically, surface − OH groups promote the cleavage of C–Cl bonds, enabling efficient dioxin degradation while simultaneously improving nitrogen oxide (NOx) reduction efficiency and suppressing the formation of the byproduct nitrous oxide (N2O). This study provides a solid theoretical foundation and technical support for the design of multifunctional flue gas purification materials and underscores their broad application potential in managing complex pollutants. The findings have important implications for enhancing the efficiency and environmental benefits of waste incineration flue gas purification, representing a significant step toward more cost-effective, efficient, and environmentally friendly flue gas treatment solutions.
Similar content being viewed by others
Introduction
Waste incineration is widely used worldwide as an effective method of waste disposal. However, the harmful gases generated during the incineration process, including nitrogen oxides, particulate matter, and dioxins, pose serious threats to both the environment and human health1,2,3. Consequently, the development of efficient flue gas purification technologies has become a critical issue in controlling pollutants from waste incineration. Over the years, significant progress has been made in the purification of waste incineration flue gas. Traditional treatment methods primarily include Selective Catalytic Reduction (SCR) and Electrostatic Precipitators (ESP)4,5. SCR technology is widely used for nitrogen oxide removal but exhibits poor adaptability in environments with high concentrations of sulfur dioxide (SO2) and deionized water (H2O), along with issues such as catalyst poisoning6,7,8. ESP technology is highly efficient in removing particulate matter; however, its ability to address gaseous pollutants is limited9. Additionally, dioxins, which are persistent organic pollutants generated during waste incineration, are particularly challenging to degrade. Existing treatment methods, such as activated carbon adsorption and chemical reduction, suffer from high costs and low efficiency10,11.
With advancements in solid waste management technologies, various treatment strategies have been proposed for different types of waste. For example, Karimi et al.12 explored the feasibility of producing activated carbon from municipal solid waste (MSW) for CO2 adsorption, demonstrating that waste materials can be transformed into highly efficient adsorbents through carbonization and activation processes. Additionally, they investigated the use of MSW compost as a source of biochar for CO2 capture13. These approaches not only improve resource recovery efficiency but also help mitigate the environmental pollution associated with conventional landfill disposal and incineration. Compared with traditional methods, the application of activated carbon and biochar provides an alternative pathway for waste management, highlighting their potential for sustainable development.
In recent years, research on the efficient integration of inorganic catalysts with organic fiber filter materials has gained significant attention. Zhou et al.14 investigated the effects of in situ fibrillated polytetrafluoroethylene (PTFE) on the rheological, mechanical, and foaming properties of polystyrene-based nanocomposite foams, highlighting its potential in catalysis and composite materials. Abidin et al.15 optimized the PTFE coating surface using oxygen plasma treatment and applied response surface methodology to analyze the influence of various parameters on the performance of food processing membranes, providing new pathways for material modification and composite optimization. Hou et al.16 developed a hydrophilic modification strategy for PTFE capillary membranes by constructing a three-dimensional hydrophilic network, preserving their chemical resistance while offering novel design strategies for catalytic filtration composite materials. Kim et al.17 examined the impact of 1T/2H-MoS2 heterophase control on the SCR performance of VMo/Ti catalysts, advancing research on the efficient integration of catalysts with filter materials. These studies indicate that most filter material carriers exhibit relatively weak surface inertness, which facilitates catalyst loading. However, under complex flue gas conditions, these materials often fail to maintain high efficiency and stability. Consequently, the development of catalytic filter bags using PTFE filter media, which possesses strong chemical inertness, has become a key research focus. Additionally, current studies primarily emphasize the integrated removal of nitrogen oxides and particulate matter, while research on the synergistic removal of dioxins remains relatively limited. Municipal solid waste incineration flue gas purification presents particular challenges, including high humidity, elevated sulfur content, and the coexistence of multiple pollutants. These factors impose stringent requirements on materials capable of achieving simultaneous nitrogen oxide reduction, dioxin degradation, and particulate matter filtration.
Against this backdrop, this study aims to develop a composite catalytic filtration material capable of integrating denitrification, dioxin degradation, and particulate matter removal to address the challenges of synergistic pollutant treatment under complex waste incineration flue gas conditions. This research proposes an innovative composite catalytic filtration material—vanadium-molybdenum/cerium-titanium-polytetrafluoroethylene (VMo/CeTi-PTFE)—and conducts an in-depth investigation into its multifunctional synergistic efficiency in waste incineration flue gas purification. This material not only combines excellent filtration performance with high catalytic activity, simplifying the flue gas purification process and reducing operational costs but also significantly enhances pollutant removal efficiency. Experimental results demonstrate that the composite catalytic filtration material exhibits outstanding performance in nitrogen oxide (NOx) reduction, dioxin degradation, and particulate matter filtration, with particularly notable improvements under high catalyst loading conditions. Furthermore, stability tests confirm that a high binder content effectively enhances the mechanical stability of the catalytic filtration material, while water and sulfur resistance tests indicate that the VMo/CeTi filter media possess strong durability. Mechanistic analysis reveals a synergistic effect between the denitrification reaction and dioxin degradation, wherein surface − OH groups facilitate the cleavage of C–Cl bonds, enabling efficient dioxin degradation while simultaneously improving NOx reduction efficiency and suppressing the formation of nitrous oxide (N2O) byproducts. This study provides a theoretical foundation and technical support for the development of multifunctional flue gas purification materials, highlighting their promising applications in the comprehensive management of waste incineration flue gas pollution.
The contributions of this study are as follows:
-
Development of a novel composite catalytic filtration material: An innovative vanadium-molybdenum/cerium-titanium-polytetrafluoroethylene (VMo/CeTi-PTFE) composite catalytic filtration material is proposed, integrating excellent filtration performance with high catalytic activity.
-
Enhancement of treatment efficiency: Experimental results demonstrate that the composite catalytic filtration material exhibits outstanding performance in denitrification, dioxin degradation, and particulate matter filtration, with particularly significant improvements under high catalyst loading conditions.
-
Improvement of mechanical stability and durability: Stability tests confirm that a high binder content effectively enhances the mechanical stability of the catalytic filtration material. Water and sulfur resistance tests show that the VMo/CeTi filter media possess strong durability, making them suitable for harsh environmental conditions in practical applications.
-
Synergistic effect mechanism: Mechanistic analysis reveals a synergistic effect between the denitrification reaction and dioxin degradation. Surface − OH groups facilitate the cleavage of C–Cl bonds, enabling efficient dioxin degradation while simultaneously enhancing NOx reduction efficiency and suppressing the formation of nitrous oxide (N2O) byproducts.
-
Theoretical foundation and technical support: This study provides both a theoretical foundation and technical support for the development of multifunctional flue gas purification materials. Additionally, it demonstrates the broad application prospects of these materials in the comprehensive management of waste incineration flue gas pollution.
Materials and methods
Materials and reagents
The main raw materials used to synthesize the VMo/CeTi catalyst include ammonium metavanadate (NH4VO3), ammonium heptamolybdate tetrahydrate ((NH4)6Mo7O24·4H2O), cerium (III) nitrate hexahydrate (Ce(NO3)3·6H2O), and titanium isopropoxide (Ti{OCH(CH3)2}4). Auxiliary reagents include anhydrous ethanol (C6H5OH), water (H2O), ammonia water (NH3·H2O), and citric acid (C6H₈O7·H2O). Polyvinyl alcohol ([C2H4O]n) is also added to the catalyst coating solution. This stabilizes the catalyst dispersion system and improves the binding strength between the catalyst particles and the PTFE filter material18,19.
To evaluate the catalytic filter material’s performance, the experiment uses high-purity gases, including nitrogen (N2), oxygen (O2), and mixed standard gases such as nitric oxide (NO/N2), ammonia (NH3/N2), and sulfur dioxide (SO2/N2). These gases simulate the complex composition of waste incineration flue gas. The experiment tests the synergistic efficiency of the catalytic filter material in denitrification, dust removal, and dioxin degradation.
Table 1 lists the main materials used in the experiment and their specifications.
Synthesis and coating of catalyst
Synthesis of VMo/CeTi catalyst
The VMo/CeTi catalyst is synthesized using the co-precipitation method to ensure the uniform dispersion and stability of the active components20,21,22. The specific steps are as follows:
First, 50 mL of 0.01 mol/L NH4VO3, 0.01 mol/L (NH4)6Mo7O24·4H2O, 0.02 mol/L Ce(NO3)3·6H2O, and 0.05 mol/L Ti{OCH(CH3)2}4 are each dissolved in a mixed solution of H2O and C6H5OH. To prevent the rapid hydrolysis of Ti{OCH(CH3)2}4, an appropriate amount of citric acid complexing agent is added at a molar ratio of 1:1. The mixture is stirred at room temperature for 30 min to form a homogeneous solution.
Next, the mixed solution of V, Mo, Ce, and Ti is heated to 50 °C. 25% NH3·H2O is slowly added under continuous stirring to adjust the pH of the solution to 8.5. The addition rate of NH3·H2O is controlled at 1 mL per minute to ensure the uniformity of the precipitate. Meanwhile, the solution is maintained at a constant temperature and stirred for 2 h to promote the complete precipitation of metal ions. After precipitation, the mixture is left to stand for 12 h to enhance the crystallization of the precipitate.
The precipitate is then separated by vacuum filtration and repeatedly washed with H2O until no residual ions remain in the filtrate. The washed precipitate is dried at 105 °C for 12 h and calcined at 500 °C for 4 h in a tubular furnace with a heating rate of 5 °C/min. The final product is a highly dispersed VMo/CeTi composite catalyst.
Coating of catalyst
The catalyst coating is performed using a cyclic ultrasonic-vibration coating technique to ensure the uniform dispersion and strong adhesion of the catalyst on the surface of the PTFE filter material. First, the synthesized VMo/CeTi catalyst powder is dispersed in a mixed solution of C6H5OH and H2O at a volume ratio of 1:1, with a catalyst-to-solvent mass ratio of 1:10 to prepare the coating solution. To enhance the stability of the suspension, 1% polyvinyl alcohol ([C2H4O]n) is added as a dispersant. After cutting the PTFE filter material to the required size, it is placed in an ultrasonic cleaner to remove surface impurities. The cleaned filter material is then immersed in the catalyst coating solution and treated in an ultrasonic bath (frequency 40 kHz) for 30 min to ensure the catalyst enters the microporous structure of the filter material. After ultrasonic treatment, the sample is transferred to a constant-temperature shaker and oscillated at 120 rpm for 1 h to further promote the uniform attachment of catalyst particles.
After coating, the sample is removed and dried at 80 °C for 2 h to remove the solvent. It is then calcined at 300 °C for 1 h (heating rate of 3 °C/min) to enhance the bonding strength between the catalyst and the filter material surface, optimizing the catalyst’s structural properties. The resulting VMo/CeTi-PTFE composite catalytic filter material exhibits uniform catalyst distribution, good gas permeability, and mechanical strength.
Sample characterization
Several advanced characterization techniques are employed to comprehensively analyze the physicochemical properties and structural performance of the prepared VMo/CeTi-PTFE composite catalytic filter material. These include tests for crystal structure, specific surface area and pore characteristics, surface morphology, and the catalyst’s redox and acid–base surface properties. The characterization methods and instrument models used are outlined in Table 2.
The specific surface area and pore characteristics are determined by nitrogen adsorption/desorption isotherms. The experiments are conducted using an ASAP 2460 analyzer from Quantachrome Instruments for measuring adsorption and a BELSORP-mini II fully automated analyzer for calculating pore size and pore volume. The specific surface area is calculated using the Brunauer–Emmett–Teller (BET) theory model, while pore size and pore volume are derived from adsorption data using the Barrett-Joyner-Halenda (BJH) method. Prior to testing, the samples are degassed at 300 °C under vacuum for 10 h to remove surface moisture and volatile impurities.
XRD is employed to study the crystal structure and crystallinity of the catalyst. A Bruker D8 X-ray diffractometer, equipped with a Cu Kα radiation source (λ = 1.5406 nm) and a nickel filter, is used for the analysis. The testing conditions are set to a 2θ range of 5° to 50°, with a step size of 0.1° and a scan speed of 1 s/step.
SEM is employed to analyze the micro-morphology and elemental distribution of the catalyst surface. The S-4800 scanning electron microscope provides high-resolution imaging and a wide magnification range, allowing for detailed morphological analysis of the samples.
H2-temperature programmed reduction (H2-TPR) is utilized to examine the catalyst’s redox properties. The tests are conducted using an AutoChem II 2920 fully automated chemisorption analyzer. A 5 vol.% H2/Ar mixed gas is used as the reduction gas, with a flow rate of 30 mL/min and a heating rate of 10 °C/min. The temperature is ramped from room temperature to 800 °C.
NH3-temperature programmed desorption (NH3-TPD) is employed to characterize the surface acidity distribution of the catalyst. The experiment is carried out using the AutoChem II 2920 fully automated chemisorption analyzer. A 10 vol.% NH3/He mixed gas is used to adsorb onto the sample. After adsorption, unbound NH3 molecules are purged with high-purity He gas. The heating rate is set at 10 °C/min, and the temperature range is from room temperature to 700 °C.
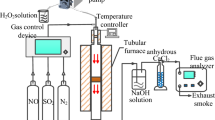
Performance test experiment of the catalytic filter material
The performance of the catalytic filter material is evaluated using a laboratory-scale simulated flue gas reaction system. The simulated flue gas comprises the following components: NO (500 ppm), NH3 (500 ppm), O2 (10 vol.%), SO2 (200 ppm), H2O (10 vol.%), and N2 (balance gas). Furan and 1,2-Dichlorobenzene (1,2-DCB) are chosen as model compounds for dioxin, serving as reference substances to assess dioxin degradation efficiency23,24. Quantitative analysis of reactant and product concentration changes during the NH3-Selective Catalytic Reduction (NH3-SCR) denitrification reaction and the degradation of dioxin model compounds is carried out using the external standard method. Standard gases at different concentrations are used to create a standard curve that relates peak area to concentration through linear regression. This curve, along with real-time monitoring data from the reaction process, allows for the calculation of conversion rates for NO, furan, and 1,2-DCB. The conversion rates or removal efficiencies are calculated using the following equations, based on the inlet and outlet concentrations of each component during the reaction:
\({[NO]}_{in}\) and \({[NO]}_{out}\) represent the inlet and outlet concentrations of NO (ppm), respectively; \({[furan]}_{in}\) and \({[furan]}_{out}\) refer to the furan’s inlet and outlet concentrations (mg/L), respectively; \({[DCB]}_{in}\) and \({[DCB]}_{out}\) denote the inlet and outlet concentrations of 1,2-DCB (mg/L), respectively; \({[dust]}_{in}\) and \({[dust]}_{out}\) indicate the dust’s inlet and outlet concentrations (g/m3), respectively. Furthermore, the system’s stability under complex conditions, such as high humidity and high SO2 concentration, is evaluated to ensure its feasibility and long-term performance in the treatment of actual municipal waste incineration flue gas.
Results and discussion
Characterization results of the catalytic filter material
Specific surface area and pore structure analysis
To comprehensively assess the impact of the catalyst coating on the PTFE substrate, the physical properties of the untreated PTFE material, particularly its BET surface area, are first determined. The test results indicate that the original PTFE material possesses relatively large inter-fiber voids and a relatively smooth surface, with a BET surface area of approximately 10 m2/g. This suggests that PTFE inherently has good gas permeability, but relatively few surface active sites. The specific surface area, pore volume, and pore size distribution of the VMo/CeTi catalyst and the VMo/CeTi-PTFE composite catalytic filter material are analyzed using the BET and BJH methods. The results are summarized in Table 3.
As shown in Table 3, comparing the BET surface areas of the VMo/CeTi catalyst and the VMo/CeTi-PTFE composite catalytic filtration material (85.934 m2/g and 61.117 m2/g, respectively), it can be observed that, although the specific surface area of the composite material is reduced by approximately 28.88%, the large pore structure of the PTFE substrate allows for maintaining good gas diffusion performance even after coating. Moreover, with the increase in pore volume from 0.333 to 0.352 cm3/g and the average pore diameter expanding from 15.341 to 21.831 nm, the diffusion rate of large molecular pollutants through the composite material is enhanced. This effectively compensates for the potential decrease in diffusion efficiency caused by the reduction in specific surface area. Therefore, by optimizing the coating process, the loss of the original diffusion performance of the PTFE substrate can be minimized, while simultaneously enhancing its ability to process complex pollutants.
Crystal structure analysis
The crystal structures of TiO2, CeO2, V/Ti, VMo/Ti, VMo/CeTi catalysts, and their composite material VMo/CeTi-PTFE are studied using XRD, as displayed in Fig. 1.
Figure 1 reveals that the main diffraction peaks of all catalyst samples align well with the standard diffraction peaks of anatase-type TiO2. This indicates that the primary structure of the catalyst is based on TiO2. In the VMo/CeTi catalyst, a weak diffraction peak corresponding to CeO2 can be observed, indicating the successful incorporation of the CeO2 crystal structure into the catalyst. For the VMo/CeTi-PTFE catalytic filter material, distinct peaks for PTFE and TiO2 are also visible in the XRD pattern. The XRD results suggest that the VMo/CeTi catalyst is effectively integrated with the PTFE filter material through the coating technique, and the main crystalline structure of the catalyst remains largely unaffected.
Surface morphology and elemental distribution analysis
Figure 2 shows the surface morphology of the VMo/CeTi catalyst, PTFE filter material, and VMo/CeTi-PTFE composite catalytic filter material.
Figure 2 shows that the surface of the VMo/CeTi catalyst exhibits a typical granular morphology with a relatively uniform particle distribution. The surface structure of the PTFE filter material is fibrous, with loosely arranged fibers and large pore structures, which promote gas penetration and diffusion. The surface morphology of the VMo/CeTi-PTFE composite catalytic filter material indicates that the catalyst particles are evenly distributed on the PTFE fiber surface, forming a continuous coating structure. Further elemental distribution analysis of the VMo/CeTi-PTFE composite catalytic filter material is shown in Fig. 3.
Figure 3 shows that the main elements (Ce, V, Mo, Ti) are evenly distributed on the surface of the filter material, indicating that the catalyst components are uniformly coated onto the PTFE fiber surface. The distribution of fluorine aligns with the morphology of the PTFE fibers, suggesting that the structural characteristics of the PTFE filter material remain largely intact during the coating process. Additionally, the uniform dispersion of the catalyst’s active elements (Ce, V, Mo) further confirms their high distribution stability, which is crucial for enhancing catalytic activity and long-term performance. To better assess the impact of this modification on gas permeation and diffusion efficiency in practical applications, the filtration efficiency and permeability of the base PTFE material and the catalyst-loaded PTFE are compared in Table 4.
As shown in Table 4, although the catalyst coating partially shields the PTFE fiber structure, optimization of the coating process results in an increased pore diameter (from 15.341 nm to 21.831 nm) and a higher pore volume (from 0.333 to 0.352 cm3/g). These changes contribute to improved gas diffusion efficiency. Despite some reduction in fiber exposure, the VMo/CeTi-PTFE composite catalytic filtration material maintains a filtration efficiency exceeding 99.97%, demonstrating its ability to effectively filter particulates while preserving good gas permeability. More importantly, under challenging conditions with 200 ppm SO2 and 10% H2O, the N2 yield of the composite material reaches 97%, significantly higher than the 80% observed for the base PTFE material. This indicates that the composite material exhibits superior adaptability and stability under complex operational environments.
Analysis of H2-TPR and NH3-TPD
The H2-TPR and NH3-TPD analysis results of the catalytic filter material are presented in Fig. 4, with the thermal conductivity detector signal recorded as the output value.
As shown in Fig. 4a, the H2-TPR analysis indicates that the reduction behavior of VMo/CeTi and VMo/CeTi-PTFE exhibits similarities. However, the content of high-valent vanadium ions in VMo/CeTi-PTFE is slightly reduced, resulting in a lower reduction peak intensity. This suggests that while the oxidation capacity of high-valent vanadium is somewhat diminished, the VMo/CeTi-PTFE catalytic filter material still demonstrates superior redox performance compared to the VMo/CeTi catalyst. In Fig. 4b, the NH3-TPD analysis reveals that both VMo/CeTi and VMo/CeTi-PTFE exhibit weak acid adsorption peaks in the 150–200 °C range and medium-to-strong acid adsorption peaks in the 240–400 °C range. After the coating treatment, the distribution of weak and medium-to-strong acid sites remains largely unchanged. However, the total acidity of the catalytic filter material decreases, primarily due to the loss of Mo and the increase in defect sites within the crystal structure during the coating process.
In the investigation of the redox and acidic properties of the VMo/CeTi catalyst and the VMo/CeTi-PTFE catalytic filtration material, H2-TPR (Fig. 4a) and NH3-TPD (Fig. 4b) analyses provide critical insights. The H2-TPR curve, after deconvolution, reveals multiple reduction peaks corresponding to different reduction steps of various metal oxides or their composites. For both VMo/CeTi and VMo/CeTi-PTFE samples, the primary reduction peaks are concentrated in the 350–650 °C range, indicating similar redox characteristics. Notably, despite a slight decrease in the oxidation ability of vanadium, the VMo/CeTi-PTFE sample exhibits enhanced redox capability. This improvement is likely attributed to the presence of CeO2, which facilitates the formation of oxygen vacancies and promotes the generation of chemisorbed oxygen. Additionally, deconvolution of the NH3-TPD results allows for more precise identification of weak and moderate-to-strong acidic sites. Peaks in the 150–200 °C range correspond to weak acid adsorption, while those between 240 and 400 °C are associated with moderate-to-strong acid adsorption. The deconvoluted NH3-TPD spectra confirm that the partial loss of Mo and Ce during the coating process leads to a reduction in acidic sites, thereby affecting the NH3 adsorption capacity on the catalyst surface. This finding is crucial for understanding variations in catalytic activity during the low-temperature SCR reaction. By integrating these deconvolution analyses, the study provides a more detailed differentiation of redox processes and acidic sites. Furthermore, it establishes a theoretical foundation for optimizing catalyst design to enhance performance in practical applications.
The results indicate that the reduction temperatures of both VMo/CeTi and VMo/CeTi-PTFE exceed 560 °C. However, denitrification, dioxin degradation, and dust removal tests are conducted within a temperature range of 140–260 °C in the presence of 8% O2. To better understand these differences and their impact on catalytic performance, the relationship between the H2-TPR results and actual catalytic activity is further examined, as summarized in Table 5.
As shown in Table 5, although both VMo/CeTi and VMo/CeTi-PTFE exhibit reduction temperatures above 560 °C, their catalytic activities within the actual operating temperature range (140–260 °C) differ significantly. The VMo/CeTi-PTFE composite material demonstrates superior NOx conversion and furan degradation rates at lower temperatures compared to the standalone VMo/CeTi catalyst. This observation suggests that H2-TPR results provide insights into high-temperature reduction behavior but do not fully capture catalytic performance under practical conditions. The enhanced low-temperature activity of the VMo/CeTi-PTFE composite can be attributed to the presence of low-valence vanadium ions (e.g., V4+), which play a critical role in improving catalytic performance. These species facilitate dioxin degradation by cleaving the C–Cl bond via surface –OH groups, while simultaneously enhancing NOx reduction efficiency and suppressing N2O formation. Moreover, even without high-temperature pre-reduction, low-valence vanadium ions remain capable of activating reactant molecules at lower temperatures, particularly by promoting redox reactions through surface –OH groups. This mechanism significantly improves dioxin degradation efficiency and NOx reduction capacity, enabling highly efficient flue gas purification. Therefore, despite the high reduction temperatures observed in H2-TPR results, the VMo/CeTi-PTFE composite material exhibits outstanding catalytic performance under practical conditions, owing to its unique microstructure and chemical composition.
Synergistic effect analysis
To further illustrate the synergistic effect between the catalyst and PTFE material, investigations were conducted from the perspectives of thermal stability (mass loss at 400 °C, %), long-term operational performance (NOx conversion rate, %), and resistance to humidity and SO2 (N2 yield, %). The detailed results are summarized in Table 6.
As shown in Table 6, a detailed analysis of the performance differences and synergistic effects between the VMo/CeTi catalyst and the VMo/CeTi-PTFE composite catalytic filter material reveals significant improvements across multiple aspects. In the thermal stability test, the mass loss at 400 °C for the standalone VMo/CeTi catalyst reaches 15%, whereas it is substantially reduced to 2% when combined with PTFE to form a composite catalytic filter material. This significant reduction indicates that the composite material exhibits superior thermal stability, maintaining structural integrity under high-temperature conditions. Regarding NOx conversion efficiency, the VMo/CeTi-PTFE composite material achieves an initial NOx conversion rate of 97%, markedly higher than the 85% observed for the standalone VMo/CeTi catalyst. More importantly, after 360 min (6 h) of continuous operation, the composite material retains a high NOx conversion efficiency of 95%, whereas the efficiency of the standalone catalyst declines to 60%. These findings not only confirm the composite material’s higher initial catalytic activity but also demonstrate its outstanding long-term stability. Furthermore, under simulated harsh environmental conditions, the VMo/CeTi-PTFE composite catalytic filter material achieves an N2 yield of 97%, significantly exceeding the 80% yield of the standalone VMo/CeTi catalyst. This result underscores the composite material’s exceptional resistance to high humidity and elevated SO2 concentrations, ensuring stable performance in challenging industrial environments. Collectively, these findings provide strong evidence of the synergistic effect between the VMo/CeTi catalyst and the PTFE substrate, leading to enhanced thermal stability, catalytic efficiency, and environmental adaptability. These improvements reinforce the material’s potential for industrial flue gas purification applications, offering a high-performance and durable solution for air pollution control.
Performance test results of catalytic filter materials
Comparison of denitrification performance of different catalysts
The denitrification performance of the NH3-SCR (Selective Catalytic Reduction) reaction is evaluated by loading V/Ti, VMo/Ti, VMoCe/Ti, and VMo/CeTi catalysts onto the PTFE filter surface. The tests are conducted under conditions of 8% O2, 1000 ppm NH3, 1000 ppm NO, and a gas flow rate of 0.8 m/min, as shown in Fig. 5.
As illustrated in Fig. 5, at 140 °C, the NOx conversion efficiency and N2 yield of VMo/CeTi-PTFE are 42.3%, significantly higher than those of the other catalysts. As the temperature increases to 220 °C, both the N2 yield and NOx conversion efficiency of VMo/CeTi-PTFE reach 100%, with its selectivity at high temperatures markedly superior to the other catalysts. This indicates that the catalytic activity and selectivity of VMo/CeTi-PTFE are notably enhanced through the synergistic effect of Ce and Ti, making it an ideal material for denitrification reactions. Given that the actual flue gas temperature in waste incineration plants typically ranges from 850 to 900 °C, the NOx conversion efficiency of different catalysts, including V/Ti, VMo/Ti, VMoCe/Ti, and VMo/CeTi-PTFE, was further analyzed at both 850 °C and 900 °C, as presented in Table 7.
As shown in Table 7, at higher temperatures (850 °C and 900 °C), the NOx conversion efficiency of all catalysts decreases. However, the VMo/CeTi-PTFE catalyst still demonstrates the highest conversion efficiency. Specifically, at 850 °C, the NOx conversion rate of the VMo/CeTi-PTFE catalyst is 82.5%, while the conversion rates for the other catalysts (V/Ti, VMo/Ti, and VMoCe/Ti) are 55.2%, 62.4%, and 70.1%, respectively. At 900 °C, the VMo/CeTi-PTFE catalyst maintains a conversion rate of 78.9%, compared to 50.1%, 58.3%, and 65.4% for the other catalysts. This superior performance can be attributed to the unique microstructure and compositional combination of the VMo/CeTi-PTFE composite material, which offers enhanced thermal stability and retains higher activity at elevated temperatures. Furthermore, the large pore structure provided by the PTFE substrate facilitates effective gas diffusion, enabling better molecular contact and reactions even under high-temperature conditions. These factors collectively contribute to the enhanced overall catalytic efficiency of the VMo/CeTi-PTFE catalyst.
To ensure the reliability and validity of the experimental data, at least three independent replicate experiments were conducted to assess the denitrification performance of different catalysts, including V/Ti, VMo/Ti, VMoCe/Ti, and VMo/CeTi-PTFE. Statistical methods were employed to analyze the results, yielding average values and their standard deviations. The NOx conversion efficiency and statistical analysis results for the different catalysts at 140 °C are presented in Table 8.
As shown in Table 8, the t-test comparison of the NOx conversion rates for different catalysts under the same conditions reveals significant differences between the VMo/CeTi-PTFE catalyst and the other catalysts (p < 0.05). This indicates that the VMo/CeTi-PTFE catalyst exhibits superior denitrification performance under low-temperature conditions.
Influence of catalyst loadings on the performance of catalytic filter materials
The denitrification, dioxin degradation, and dust removal performance of the VMo/CeTi-PTFE catalytic filter material with varying catalyst loadings are illustrated in Figs. 6, 7, and 8, respectively.
Figure 6 demonstrates that as the catalyst loading increases from 250 to 500 g/m2, the catalytic filter material’s NOx conversion efficiency and N2 yield are significantly enhanced across all temperature ranges. Specifically, at 140 °C, the NOx conversion efficiency for the 500 g/m2 loading is 42.3%, a marked improvement compared to the 23.87% observed for the 250 g/m2 loading. At 220 °C, the 500 g/m2 loading achieves 100% NOx conversion efficiency and N2 yield, while the 250 g/m2 loading only reaches 87.29%. These results indicate that increasing the catalyst loading effectively increases the number of active sites in the catalytic filter material, significantly improving its denitrification performance. Notably, under high loading conditions, the material exhibits excellent low-temperature and high-temperature denitrification performance.
Figure 7 demonstrates that as the catalyst loading increases, the VMo/CeTi-PTFE catalytic filter material’s performance in dioxin degradation significantly improves. For furan degradation, at the low temperature of 160 °C, the degradation rate increases from 53.78% at 250 g/m2 loading to 94.92% at 500 g/m2 loading. At the higher temperature of 260 °C, the degradation rate increases from 75.19 to 99.57%. A similar trend is observed for the degradation rate of 1,2-DCB, which also increases substantially with higher catalyst loading. Overall, these results show that increasing the catalyst loading and reaction temperature notably enhances the catalytic filter material’s ability to degrade dioxins. Further analysis is conducted to investigate the impact of different catalyst loadings (250 g/m2 and 500 g/m2) on NOx conversion efficiency, furan degradation rate, and 1,2-dichlorobenzene (1,2-DCB) degradation rate at temperatures ranging from 850 to 900 °C, as shown in Table 9.
As shown in Table 9, under high-temperature conditions (850 °C and 900 °C), the impact of catalyst loading on NOx conversion efficiency, furan degradation rate, and 1,2-DCB degradation rate was assessed. The experimental results indicate that, at 850 °C, the catalyst with a 250 g/m2 loading showed a 45.1% NOx conversion efficiency, 45.2% furan degradation rate, and 42.1% 1,2-DCB degradation rate. However, increasing the catalyst loading to 500 g/m2 improved the performance, with 65.2% NOx conversion efficiency, 60.3% furan degradation, and 58.9% 1,2-DCB degradation. At 900 °C, the catalyst with 250 g/m2 loading exhibited lower performance, with 40.3% NOx conversion efficiency, 42.5% furan degradation, and 39.8% 1,2-DCB degradation. In contrast, when the catalyst loading was increased to 500 g/m2, the performance was notably higher, with 60.5% NOx conversion efficiency, 57.6% furan degradation, and 56.4% 1,2-DCB degradation. These results indicate that while catalytic performance decreases at higher temperatures, the VMo/CeTi-PTFE composite catalyst filter material with higher catalyst loading significantly improves both NOx conversion efficiency and dioxin degradation (e.g., furan and 1,2-DCB). This highlights its potential for high-temperature exhaust gas purification, providing a strong foundation for further optimization of catalyst formulations to enhance their performance under practical industrial conditions.
The experimental results shown in Fig. 8 indicate that the dynamic dust removal efficiency of the VMo/CeTi-PTFE catalytic filter material remains consistently above 99.97% across various catalyst loadings. The lowest particulate emission concentration recorded is 0.049 mg/m3, which meets industrial particulate emission standards. These results demonstrate the excellent dust removal performance of the filter material, further supporting its potential for industrial flue gas treatment applications. Further analysis is conducted to compare the dust removal efficiency of the base PTFE material and the VMo/CeTi-PTFE composite catalytic filter material under different temperature conditions (140 °C and 220 °C) and catalyst loading conditions (250 g/m2 and 500 g/m2), as shown in Table 10.
As shown in Table 10, there are notable differences in the dust removal efficiency between the base PTFE material and the VMo/CeTi-PTFE composite catalytic filter material under varying temperatures and catalyst loading conditions. Specifically, at 140 °C, the dust removal efficiency of the base PTFE material is 99.61%, whereas the VMo/CeTi-PTFE catalyst achieves an impressive 99.98% efficiency. At 220 °C, the dust removal efficiency of the base PTFE material slightly decreases to 99.55%, while the VMo/CeTi-PTFE catalyst maintains a high level of 99.97% efficiency. Further analysis under varying catalyst loadings (250 g/m2 and 500 g/m2) reveals that the VMo/CeTi-PTFE catalyst consistently demonstrates excellent dust removal performance. At a loading of 250 g/m2, the dust removal efficiency is 99.975%, and when the loading increases to 500 g/m2, the efficiency further improves to 99.981%. In contrast, the base PTFE material maintains relatively stable dust removal efficiency across all test conditions, with values of 99.60% (250 g/m2) and 99.63% (500 g/m2), respectively. These results clearly highlight the significant advantage of the VMo/CeTi-PTFE composite catalytic filter material in enhancing dust removal efficiency. Even under higher temperature conditions and varying catalyst loadings, the VMo/CeTi-PTFE catalyst consistently maintains exceptionally high purification efficiency. Notably, at higher catalyst loadings, the dust removal efficiency approaches the theoretical limit, further confirming its potential for use in complex pollutant treatment. This finding provides valuable insights for optimizing catalyst formulations and coating processes, which enhances the effectiveness of industrial waste gas purification.
In studying the effect of catalyst loading on the performance of catalytic filter materials, statistical analysis methods were applied to verify the consistency and reliability of the results. As the catalyst loading increased from 250 to 500 g/m2, a significant improvement in NOx conversion efficiency and N2 yield was observed. All experiments were repeated more than three times to calculate the mean and standard error. Table 11 presents the NOx conversion efficiency and its statistical analysis results under different catalyst loadings.
As shown in Table 11, the Analysis of Variance (ANOVA) confirms significant differences in catalytic performance at different loadings (p < 0.01). This demonstrates that increasing the catalyst loading effectively enhances catalytic activity.
Stability testing of catalytic filter materials
Under 0.5 MPa compressed air, after 500 pulses, the mass changes of the VMo/CeTi-PTFE catalytic filter material with different binder contents are illustrated in Fig. 9.
The results suggest that binder content significantly affects the mass stability of the filter material. When the binder content is 60%, the mass of the filter material decreases from 45.41 to 38.54 g after pulsing, with a mass loss rate of 15.13%. This indicates that insufficient binder content leads to poor adhesion of the coating, resulting in significant material loss after pulsing. As the binder content increases, the mass loss rate decreases noticeably. At 80% binder content, the mass loss rate is only 0.91%, and at 85% and 90% binder content, the mass loss rates further decrease to 0.044% and 0.022%, respectively, which can be considered negligible. These findings demonstrate that when the binder content exceeds 80%, the coating’s adhesive strength is sufficient to withstand the impact of high-intensity pulsing, thus exhibiting excellent mechanical stability.
Analysis of the water and sulfur resistance of catalytic filter materials
The NOx conversion rate and N2 yield of the catalytic filter materials are measured at 200 °C in the presence of 200 ppm SO2 and 10% H2O in the reaction gas, as shown in Fig. 10.
As shown in Fig. 10, after introducing 200 ppm SO2 and 10% H2O into the reaction gas, both the NOx conversion rate and N2 yield of the VMo/CeTi and VMo/CeTi-PTFE catalytic filter materials at 200 °C exhibit a certain degree of stability and attenuation over time. In the initial phase (0–60 min), the N2 yield and NOx conversion rate for both catalysts remain above 97%, suggesting that SO2 and H2O have minimal impact on catalytic performance during the early reaction phase. However, as the reaction time progresses, catalytic performance begins to degrade. By 360 min, the performance of VMo/CeTi shows some recovery, while the performance of VMo/CeTi-PTFE recovers to a lesser extent. This phenomenon may be attributed to sulfate deposition caused by SO2 and competitive adsorption of active sites due to H2O. The introduction of PTFE significantly affects the catalyst’s resistance to sulfur and water, which in turn influences the overall catalytic performance.
Reaction mechanism analysis of catalytic filter materials
Denitrification reaction mechanism
In the denitrification reaction, NH3 is first adsorbed onto the acidic sites on the catalyst surface, forming NH4+ and coordinated NH3 species. These adsorbed species then interact with the active sites on the catalyst surface, facilitating the reduction of NOx. The adsorption of NH3 occurs through the following reactions, where it binds to the surface active sites:
Simultaneously, NOx on the catalyst surface generates mono-coordinated nitrate and nitrite species:
Subsequently, the adsorbed NH3 reacts with the active intermediate species of NOx, producing N2 and H2O, while regenerating the catalyst’s active sites:
Dioxin degradation mechanism
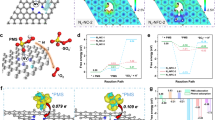
In the dioxin degradation reaction, the cleavage of the C–Cl bond and the dechlorination process are key steps. The dioxin molecule is initially adsorbed onto the acidic sites of the catalyst, where the − OH species on the catalyst surface break the C–Cl bond through a nucleophilic substitution reaction. Compared to C–O and C–C bonds, the C–Cl bond has lower bond energy and is more susceptible to cleavage. This process can be described by the following reaction:
In this reaction, Cl⁻ is released from the molecule, and hydroxyl species (OH⁻) are generated. The ring-opening reaction of the benzene ring follows, where the benzene ring, having lost its chlorine substituent, undergoes further cleavage of C–O and C–C bonds, releasing organic intermediates. These intermediates are subsequently oxidized to H2O and CO2 through the redox centers on the catalyst surface, ultimately leading to the degradation of dioxin. The reaction can be represented as Eq. (11):
These reaction mechanisms demonstrate that the catalyst effectively degrades dioxin by breaking the C–Cl bond and oxidizing the intermediate species in the benzene ring during degradation.
Reaction synergy effect
In the application of the VMo/CeTi-PTFE catalytic filter material, a significant synergistic effect is observed between the NH3-SCR (Selective Catalytic Reduction) reaction and the dioxin degradation reaction. During the reduction of NOx, the − OH species generated on the catalyst surface not only promote the reduction of NOx but also accelerate the dechlorination and ring-opening oxidation of dioxin molecules. Specifically, the hydroxide and oxidized species produced during the NOx reduction process interact with the dioxin molecules, disrupting their chlorine-substituted structure, which facilitates the degradation of dioxin. Moreover, the degradation of dioxin helps inhibit the formation of by-products, as this process consumes some of the oxygen species on the catalyst surface, reducing the availability of reactants for by-product formation. As a result, dioxin degradation mitigates the environmental harm caused by dioxin-related pollutants and enhances the overall efficiency of the NOx reduction reaction.
Building upon the previously discussed reaction mechanism, the roles of Mo and Ti in the catalytic process were further examined. The VMo/CeTi catalyst, through the cleavage of the C–Cl bond by surface − OH groups, efficiently degrades dioxins while simultaneously enhancing NOx reduction efficiency and suppressing the formation of the byproduct N2O. Moreover, both Mo and Ti play vital roles in this catalytic process. Mo, acting as a co-catalyst, significantly improves the redox performance of the catalyst. Its variable valence characteristics (Mo6+⁺/Mo5+) facilitate electron transfer, which increases the number and stability of active sites. This property not only enhances NOx reduction efficiency but also effectively inhibits the formation of N2O, a harmful byproduct. In contrast, Ti, as the primary support material, provides a stable framework structure, contributing to the catalyst’s thermal and chemical stability. This stability ensures the catalyst’s long-term operation under high-temperature conditions. Additionally, the acidic sites on the surface of TiO2 offer an ideal environment for the adsorption and activation of reactant molecules. This feature promotes the effective conversion of NOx and other pollutants. In conclusion, the synergistic effect between Mo and Ti not only enhances the overall activity and stability of the catalyst but also optimizes the reaction pathway. As a result, the VMo/CeTi-PTFE composite catalytic filter material exhibits exceptional performance in treating complex exhaust pollutants.
Discussion
The VMo/CeTi-PTFE composite catalytic filter material proposed in this study offers notable advantages in the field of waste incineration flue gas purification. Compared to recent studies, such as those by Wu et al.25 and Wei et al.26, which focused on activated carbon preparation from municipal solid waste for CO2 adsorption and improving filtration performance using in situ microfibering technology, respectively, these approaches did not effectively address the simultaneous removal of complex pollutants like NOx, dioxins, and particulate matter. In contrast, the VMo/CeTi-PTFE composite material in this study excels by achieving efficient de-NOx, dioxin degradation, and dust removal, while also demonstrating excellent stability and durability.
From the perspectives of adaptability and innovative development, the proposed method is especially suited for treating exhaust gases containing multiple pollutants, particularly flue gases from waste incineration under high-temperature and high-humidity conditions. Compared with conventional SCR and ESP technologies, the VMo/CeTi-PTFE composite material improves the number and distribution of catalyst active sites through its unique microstructure and composition, thereby enhancing overall reaction efficiency. Furthermore, the introduction of PTFE as the substrate not only maintains good gas permeability but also significantly strengthens the mechanical properties and durability of the material. This design effectively addresses the challenge of maintaining both high efficiency and stable performance under complex flue gas conditions, outperforming traditional filter media supports.
A deeper analysis of the experimental results reveals that the VMo/CeTi-PTFE composite catalytic filter material exhibits excellent catalytic performance across a wide temperature range. This is primarily attributed to the presence of CeO2, which promotes the formation of oxygen vacancies and enhances the generation of chemisorbed oxygen, facilitating the ability of surface − OH groups to cleave C–Cl bonds. This mechanism not only enables efficient dioxin degradation but also improves NOx reduction efficiency and suppresses the formation of the harmful byproduct N2O. Additionally, while the specific surface area of the material is slightly reduced due to the optimization of the coating process, the increase in pore volume and diameter enhances the gas diffusion rate, compensating for the potential reduction in diffusion efficiency due to the decreased surface area. Consequently, under practical operating conditions, even without high-temperature pre-reduction treatment, the VMo/CeTi-PTFE composite material retains outstanding catalytic performance, owing to its unique microstructure and chemical composition.
From the perspective of waste sustainability, Karimi and Shirzad27 proposed a sustainable industrial process design for extracting CO2 adsorbents from MSW, focusing on process scalability, techno-economic feasibility, and parameter optimization. Their study demonstrated that activated carbon derived from MSW can effectively capture CO2, enabling resource recovery, reducing treatment costs, and mitigating environmental pollution. In contrast, the composite catalytic filter material technology presented in this study not only optimizes waste resource utilization for the transformation of waste and efficient removal of pollutants (such as NOx, dioxins, and particulate matter) but also offers significant advantages in the integration of multiple functions for flue gas purification. While Karimi and Shirzad’s research emphasized the techno-economic benefits of converting waste into CO2 adsorbents, the composite catalytic filter material proposed in this study presents a more comprehensive waste resource recovery pathway. It specifically focuses on the synergistic removal of multiple pollutants. Karimi and Shirzad’s research centers on CO2 capture alone, whereas the present study transforms waste into composite catalysts and filter materials, integrating denitrification, dioxin degradation, and dust removal. This multifaceted approach not only improves flue gas purification efficiency but also promotes environmental protection and energy recovery through waste resource recovery. This technology enhances treatment efficiency, reduces industrial operation costs, and drives the sustainable development of waste management. Therefore, compared to existing single waste conversion technologies, the composite catalytic filter material in this study offers significant advantages in terms of application scope and comprehensive benefits, providing an innovative solution for the comprehensive utilization of waste and environmental protection.
In conclusion, this study offers an effective solution to address the challenges of synergistically treating complex pollutants. Through a deep understanding of material properties and innovative design, it opens a new direction for multifunctional flue gas purification materials. Future research will focus on further enhancing the thermal stability and toxicity resistance of the catalyst to meet the more demanding requirements of practical applications.
Conclusion
This study develops an innovative composite catalytic filter material—Vanadium Molybdenum/Cerium Titanium-Polytetrafluoroethylene (VMo/CeTi-PTFE)—designed to effectively address the challenges of synergistically treating multiple pollutants in waste incineration flue gas purification under complex conditions. The key aspects of this study are summarized as follows:
-
1.
Material Design and Synthesis: The VMo/CeTi catalyst was successfully synthesized using the co-precipitation method, and the catalyst was uniformly coated onto PTFE filter media using a cyclic ultrasonic vibration coating technique. This resulted in the formation of the VMo/CeTi-PTFE composite catalytic filter material, which exhibited outstanding filtration performance and catalytic activity.
-
2.
Material Characterization and Analysis: The physical, chemical, and structural properties of the synthesized material were comprehensively analyzed using advanced characterization techniques, including nitrogen adsorption/desorption isotherms, XRD, scanning electron microscopy (SEM), hydrogen temperature-programmed reduction (H2-TPR), and ammonia temperature-programmed desorption (NH3-TPD). The results indicated that, although the composite material’s specific surface area was slightly reduced, the increase in pore volume and diameter enhanced the gas diffusion rate, compensating for any potential efficiency loss due to the decrease in specific surface area.
-
3.
Performance Testing and Evaluation: Experimental results demonstrated that the VMo/CeTi-PTFE composite catalytic filter material achieved exceptional performance in denitrification, dioxin degradation, and dust removal, with significant performance improvements under high catalyst loading conditions. Additionally, stability tests confirmed that the high binder content effectively enhanced mechanical stability. Water and sulfur resistance experiments showed that the VMo/CeTi filter media exhibited strong durability.
-
4.
Synergistic Effects and Mechanism Analysis: The study revealed a synergistic effect between the denitrification reaction and dioxin degradation. The surface -OH groups cleave the C–Cl bonds, facilitating the effective degradation of dioxins while simultaneously enhancing NOx reduction efficiency and suppressing the formation of the byproduct N2O.
-
5.
Comparison with Other Studies and Unique Contributions: Compared to existing research, the proposed VMo/CeTi-PTFE composite material not only achieved the simultaneous removal of NOx, dioxins, and particulate matter but also demonstrated excellent stability and durability under complex conditions such as high temperature and high humidity. This provides valuable insights and technical support for the design of multifunctional flue gas purification materials.
In conclusion, this study presents an efficient, economical, and environmentally friendly solution for managing complex pollutants in waste incineration flue gas.
However, this study also has certain limitations. Firstly, the water and sulfur resistance of the catalytic filter material has been preliminarily explored. Still, the catalytic filter material’s long-term stability and poisoning resistance under actual industrial operations with more complex flue gas components need further verification. Secondly, the partial loss of acidic sites during the coating process may limit its performance enhancement. This requires improvement through optimization of the coating process and catalyst component design. Future work can be conducted in the following aspects. The structure and active components of the catalytic filter material are further optimized to enhance its durability under complex environments such as high humidity and high sulfur; In-situ characterization techniques and theoretical simulations are combined to deeply reveal the catalytic reaction mechanisms, providing more precise design guidance for the catalytic filter material’s performance enhancement; The development of large-scale preparation and industrial application technical schemes are explored to accelerate its promotion and application in actual waste incineration flue gas treatment.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Luo, Y. et al. Assessing the environmental impact of municipal waste on energy incineration technology for power generation using life cycle assessment methodology. Toxics 12(11), 786 (2024).
Li, E. et al. Performance of asphalt mastic and asphalt mixture with harmless municipal solid waste incineration fly ash. Buildings 13(2), 498 (2023).
Chen, J., Chen, Z. & Wang, Z. Research and application of detoxification and harm reduction technology for fly ash from refuse incineration. Acad. J. Environ. Earth Sci. 5(1), 21–32 (2023).
Kumar, M. S. et al. A review on zeolite catalyst for deNOx performance in ammonia–selective catalytic reduction. Fuel 334, 126828 (2023).
Molchanov, O. et al. Specifics of electrostatic precipitation of fly ash from small-scale fossil fuel combustion. Processes 11(3), 808 (2023).
Cai, Q. et al. Core-shell materials for selective catalytic reducing of NOx with ammonia: Synthesis, anti-poisoning performance, and remaining challenges. Fuel Process. Technol. 243, 107675 (2023).
Kumar, M. S. et al. A review of comparison between the traditional catalyst and zeolite catalyst for ammonia-selective catalytic reduction of NOx. Fuel 344, 128125 (2023).
Zhang, C. et al. Establishment of a novel Fenton-like enhanced low-temperature selective catalytic reduction over FeVO4 catalysts. J. Environ. Chem. Eng. 11(3), 109634 (2023).
Holubčík, M., Trnka, J. & Kantová, N. Č. Using heat exchanger for construction of electrostatic precipitator in a small heat source. J. Electrostatics 128, 103884 (2024).
Li, W. et al. Review of thermal treatments for the degradation of dioxins in municipal solid waste incineration fly ash: Proposing a suitable method for large-scale processing. Sci. Total Environ. 875, 162565 (2023).
Li, Q., Cui, Y., Wang, Z., et al. Toxicity assessment of dioxins and their transformation by-products from inferred degradation pathways. Sci. Total Environ. 2024: 173416.
Karimi, M. et al. Novel insights into activated carbon derived from municipal solid waste for CO2 uptake: Synthesis, adsorption isotherms and scale-up. J. Environ. Chem. Eng. 8(5), 104069 (2020).
Karimi M, Diaz de Tuesta JL, Gonçalves CNdP, et al. Compost from municipal solid wastes as a source of biochar for CO2 capture. Chem. Eng. Technol. 2020, 43(7): 1336–1349.
Zhou, M., Chen, S. & Huang, A. The effect of in-situ fibrillated polytetrafluoroethylene on the rheological, mechanical, and foaming properties of polystyrene based nanocomposite foams. J. Polym. Sci. 62(20), 4742–4752 (2024).
Abidin, N. Z. et al. Enhancing polytetrafluoroethylene (PTFE) coated film for food processing: Unveiling surface transformations through oxygenated plasma treatment and parameter optimization using response surface methodology. Plos one 19(5), e0303931 (2024).
Hou, M., Li, Q. & Che, Y. Hydrophilic modification of polytetrafluoroethylene (PTFE) capillary membranes with chemical resistance by constructing three-dimensional hydrophilic networks. Polymers 16(8), 1154 (2024).
Kim, S. J. et al. Phase control of heterogeneous 1T/2H-MoS2 to improve the selective catalytic reduction activity of VMo/Ti. Surf. Interfaces 46, 103780 (2024).
Vineeth, S. K., Gadhave, R. V. & Gadekar, P. T. Polyvinyl alcohol–cellulose blend wood adhesive modified by citric acid and its effect on physical, thermal, mechanical and performance properties. Polym. Bull. 80(7), 8013–8030 (2023).
Gadhave, R. V. et al. Effect of addition of boric acid on thermo-mechanical properties of microcrystalline cellulose/polyvinyl alcohol blend and applicability as wood adhesive. J. Adhesion Sci. Technol. 35(10), 1072–1086 (2021).
Qiu, J. et al. Catalytic activity, selectivity, and stability of co-precipitation synthesized Mn-Ce mixed oxides for the oxidation of 1, 2-dichlorobenzene. Environ. Sci. Pollut. Res. 28, 65416–65427 (2021).
Zonarsaghar, A., Mousavi-Kamazani, M. & Zinatloo-Ajabshir, S. RETRACTED ARTICLE: Co-precipitation synthesis of CeVO4 nanoparticles for electrochemical hydrogen storage. J. Mater. Sci.: Mater. Electron. 33(9), 6549–6554 (2022).
Wolski, L. et al. Influence of co-precipitation agent on the structure, texture and catalytic activity of Au-CeO2 catalysts in low-temperature oxidation of benzyl alcohol. Catalysts 11(5), 641 (2021).
Liu, X. et al. Formation and inventory of polychlorinated dibenzo-p-dioxins and dibenzofurans and other byproducts along manufacturing processes of chlorobenzene and chloroethylene. Environ. Sci. Technol. 57(4), 1646–1657 (2023).
Qiu, J. et al. Solvothermal preparation of Mn-based catalysts for simultaneous removal of 1, 2-dichlorobenzene and furan. Waste Disposal Sustain. Energy 4(2), 105–116 (2022).
Wu, H. et al. Based on machine learning model for prediction of CO2 adsorption of synthetic zeolite in two-step solid waste treatment. Arab. J. Chem. 17(2), 105507 (2024).
Wei, X. et al. Hydrophobic and oleophilic open-cell foams from in-situ microfibrillation blends of poly (lactic acid) and polytetrafluoroethylene: Selective oil-adsorption behaviors. Int. J. Biol. Macromol. 227, 273–284 (2023).
Karimi, M. & Shirzad, M. Sustainable industrial process design for derived CO2 adsorbent from municipal solid wastes: Scale-up, techno-economic and parametric assessment. Sustain. Mater. Technol. 41, e01091 (2024).
Author information
Authors and Affiliations
Contributions
Chen Songxuan, Wang Hao and Yao Liang wrote the main manuscript text . Sun Zhi and Cao Hongbin prepared figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Songxuan, C., Hao, W., Liang, Y. et al. Application of PTFE composite catalytic filter material in synergistic purification of multiple pollutants in waste incineration flue gas. Sci Rep 15, 10221 (2025). https://doi.org/10.1038/s41598-025-94704-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-94704-9