Abstract
This study investigated the correlation between metformin use and diabetic peripheral neuropathy (DPN) risk in patients with type 2 diabetes mellitus (T2DM) and its dose-dependent relationship. The study included new-onset T2DM patients from 2002 to 2013. Patients were divided into two groups based on metformin treatment, and DPN risk was assessed at 2- and 5-year follow-ups. After adjusting for various factors, two logistic models, metformin cumulative defined daily dose (cDDD) and metformin treatment intensity (defined daily dose [DDD]/month), evaluated the metformin-DPN risk association. Results showed that patients with metformin cDDD < 300, 300–500, and > 500 had higher DPN risk at both follow-ups. Odds ratios (ORs) and confidence intervals (CIs) for DPN were 1.74 (1.69–1.79), 2.05 (1.81–2.32), and 2.36 (1.34–4.16) at 2 years and 1.63 (1.60–1.65), 1.82 (1.69–1.96), and 2.17 (1.56–3.03) at 5 years. Similarly, patients with < 10, 10–25, and > 25 DDD/month had higher DPN risk at both follow-ups. Metformin use correlated with DPN risk in T2DM patients, with a dose-dependent relationship. Higher metformin cDDD or treatment intensity increased DPN risk. However, the absence of vitamin B12 data limits the understanding of the underlying mechanisms. Well-designed, large-scale studies are required to evaluate the potential risks of metformin therapy for DPN in patients with T2DM.
Similar content being viewed by others
Introduction
Peripheral neuropathy (PN) comprises a wide range of clinical syndromes potentially presenting with peripheral nervous system disorder1. Some individuals with diabetes mellitus (DM) develop diabetic neuropathy, which is characterized by both positive symptoms (e.g., burning, pain, and tingling in the extremities) and negative symptoms (e.g., numbness and dysesthesia in the extremities and stumbling)2. Diabetic PN (DPN) is the most common complication of DM. Its prevalence increases with the disease duration of DM, and approximately 50% of patients with type 2 DM (T2DM) develop neuropathy in their lifetime2. In patients with T2DM, the DPN prevalence ranges from 21.3% to 34.5%, and up to 45% of patients with T2DM with DPN may be asymptomatic3.
Hyperglycemia is the most common risk factor for nerve cell damage through several physiological mechanisms, including the oxidative stress and polyol accumulation pathways4. Metformin has been noted to attenuate diabetic neuropathic pain in a rodent model. It was reported may also reduce serum vitamin B12 levels, potentially leading to nerve injury5. However, the correlation between metformin use and the risk of DPN remains unclear.
Because relevant epidemiological studies thus far have reported inconsistent findings6,7, we conducted a study to determine whether metformin use is associated with increased odds of DPN development by using data from the Taiwan National Health Insurance (NHI) Research Database (NHIRD), a large-scale, cross-sectional, nationwide database; we also investigated whether this association is dose-dependent.
Results
Table 1 presents the baseline characteristics of all patients. The average age of all patients was 56.20 ± 12.46 years, and 47.24% of all patients were women. Moreover, 29.00%, 16.00%, 15.90%, 12.64%, and 25.60% were aged 20–49, 50–54, 55–59, 60–64, and > 65 years, respectively.
In the patients treated with metformin, the average age was 54.94 ± 12.19 years. Among all patients, 3,412 (0.71%) had hyperuricemia, 16,181 (3.39%) had CVD, 2,973 (0.62%) had RA, 85,803 (17.96%) had sleep disturbance, 228 (0.05%) had SLE, 2,237 (0.47%) had migraine, 28,447 (5.96%) had CAD, 2,328 (0.49%) had PAD, 4,122 (0.86%) had depression, 3,328 (0.70%) had obesity, 5,450 (1.14%) had diabetic retinopathy, and 1,525 (0.32%) had CKD. The distribution of each comorbidity, with the exception of alcoholism, differed significantly between the patients treated and not treated with metformin (P < 0.05).
Table 2 presents the DPN incidence rates and risk at the 2-year follow-up. In total, 22,137 (2.48%) patients developed DPN 2 years after their initial DM diagnosis. The incidence rate of DPN in patients not treated with metformin was 1.79%. In the patients treated with metformin at a cDDD of < 300, 300–500, and > 500, the DPN incidence rates were 3.07%, 3.56%, and 4.26%, respectively; moreover, in those treated with < 10, 10–25, and > 25 DDDs/month of metformin, the rates were 3.03%, 3.18%, and 3.59%, respectively. Furthermore, at the 2-year follow-up, the ORs (95% CIs) for DPN after treatment with metformin at a cDDD of < 300, 300–500, and > 500 were 1.82 (1.72–1.93), 2.22 (1.90–2.59), and 2.48 (1.39–4.46), respectively. Moreover, the ORs (95% CIs) for DPN after treatment with > 25, 10–25, and < 10 DDDs/month of metformin were 1.79 (1.69–1.90), 1.90 (1.78–2.02), and 2.22 (1.90–2.59), respectively—indicating that the DPN risk was higher after 2 years of metformin treatment at > 25 DDDs/month than at ≤ 25 DDDs/month.
Table 3 presents the DPN risk at the 5-year follow-up. After adjusting for the relevant variables, we discovered that the ORs (95% CIs) for DPN after treatment with metformin at a cDDD of < 300, 300–500, and > 500 DPN were 1.66 (1.61–1.71), 1.86 (1.70–2.04), and 2.07 (1.47–2.92), respectively. Moreover, the ORs (95% CIs) for DPN after treatment with < 10, 10–25, and > 25 DDDs/month of metformin were 1.64 (1.59–1.70), 1.69 (1.63–1.75), and 1.87 (1.71–2.05), respectively. Compared with patients aged 20–49 years, those aged ≥ 65 years had a higher DPN risk (OR: 1.27; 95% CI: 1.22–1.31). Moreover, patients with comorbid CVD (OR: 1.05, 95% CI: 1.01–1.09) had a higher DPN risk, whereas those with comorbid hyperuricemia (OR: 0.73, 95% CI: 0.72–0.75), CAD (OR: 0.94, 95% CI: 0.91–0.97), and obesity (OR: 0.83, 95% CI: 0.75–0.93) had a lower DPN risk. Finally, patients with comorbid alcoholism, RA, sleep disturbance, SLE, migraine, PAD, depression, sarcopenia, and diabetic retinopathy did not exhibit a notable DPN risk.
Table 4 presents the association between metformin use and DPN in different cohorts. At the 2-year follow-up, there was a higher risk of developing DPN in female patients using metformin at a cDDD of < 300 (OR: 1.99, 95% CI: 1.85–2.15) and 300–500 (OR: 2.21, 95% CI: 1.77–2.77). The ORs (95% CIs) for DPN after treatment with < 10, 10–25, and > 25 DDDs/month of metformin were 1.96 (1.82–2.12), 2.08 (1.92–2.26), and 2.16 (1.73–2.71), respectively. Similarly, in male patients, the risk of DPN was higher with metformin at a cDDD of < 300 (OR: 1.57, 95% CI: 1.48–1.67), 300–500 (OR: 1.86, 95% CI: 1.55–2.23), and > 500 (OR: 2.84, 95% CI: 1.50–5.38). Among patients aged 20–49, the risk of developing DPN was higher with metformin at a cDDD of < 300 (OR: 1.85, 95% CI: 1.68–2.03), 300–500 (OR: 2.45, 95% CI: 1.92–3.14) and > 500 (OR: 3.29, 95% CI: 1.34–8.11). Furthermore, in patients with a DCSI score 0, the risk of developing DPN was also higher with metformin at a cDDD of < 300 (OR: 1.83, 95% CI: 1.71–1.96), 300–500 (OR: 2.39, 95% CI: 1.98–2.88), and > 500 (OR: 3.96, 95% CI: 1.95–8.05). The results of the risk of developing DPN obtained from the 5-year follow-up are similar to those observed at the 2-year follow-up.
Discussion
Few large-scale retrospective cohort epidemiology studies have evaluated the association between T2DM patients who are receiving metformin and the risk of DPN. In our study, we can find that metformin use was associated with DPN risk among T2DM patients in a dose-dependent association manner. The results suggest that T2DM patients received < 300, 300–500, ≥ 300 cDDD of metformin or use intensity of < 10, 10 ~ 25, > 25 DDD/month experienced higher risk of DPN at 2 and 5 years. The higher cDDD of metformin or use intensity, the higher DPN risk was found in this study. Our findings also indicated that among T2DM patients receiving metformin, being elderly and having a higher DCSI score were associated with an increased risk of DPN. In addition, T2DM patients comorbid with cerebrovascular disease also had a higher risk for the development of DPN.
The mechanisms underlying the pathogenesis of DPN are not fully understood. Multiple hypotheses have been proposed. The most generally accepted theory regarding DPN is the multifactorial process that involves several metabolic pathways, induced by hyperglycemia, which associate with nerve injury and dysfunction8. Oxidative stress, alternation in mitochondrial dysfunction, neuroinflammation and changes in the patterns of gene expression may be involved in the development of DPN9. Hyperglycemia is a major pathophysiologic risk factor that contributes to the development of DPN in T2DM patients. Hyperglycemia acts as an inducer to the endothelial cells through increasing oxidative stress and increasing the production of vasoconstrictor compounds, which lead to hypoxia, which is a strong inducer of VEGF expression10.
Over the last few years, the clinical symptoms of vitamin B12 deficiency have shown notable trends towards signs and symptoms of nervous system disorders. Vitamin B12 deficiency is associated with multiple neurological and neurocognitive symptoms, including peripheral and autonomic neuropathy11,12. Peripheral neuropathy can be asymptomatic and could likely exacerbate DPN in T2DM patients13.
In our large-scale retrospective cohort study, we report that metformin use was associated with DPN risk among T2DM patients in a dose-dependent association manner. The results suggest that T2DM patients received < 300, 300–500, ≥ 300 cDDD of metformin or use intensity of < 10, 10 ~ 25, > 25 DDD/month experienced higher risk of DPN after 2-year and 5-year follow-up period. The higher cDDD of metformin or use intensity, the higher DPN risk was found in this study. Several studies showed that T2DM patients using metformin was associated with higher incidence of DPN development7,14,15. The key factors predicting the occurrence of DPN in T2DM patients treated with metformin include a higher dosage and prolonged use of metformin, low vitamin B12 levels, and elevated homocysteine levels15. Several studies have generally indicated that metformin use was associated with lower plasma vitamin B12 levels16,17. A meta-analysis study also showed that metformin use is a risk factor for vitamin B12 deficiency in T2DM subjects18. The dosage of metformin also influences the likelihood of vitamin B12 deficiency. A positive correlation exists between metformin dosage and the risk of DPN, likely because higher metformin doses contribute to more severe vitamin B12 deficiency14. Over a period of at least 6 months, patients taking between 1000 and 1500 mg per day had a 72% higher risk of developing vitamin B12 deficiency compared to those taking 1000 mg or less daily. Those consuming between 1500 and 2000 mg per day experienced a 334% increase in risk, while individuals taking more than 2000 mg daily faced an 867% higher risk19. A logistic regression analysis also showed that T2DM patients treated with metformin for prolonged duration and higher metformin dose > 2000 mg were associated with lower vitamin B12 level and more severe DPN15. Although metformin can attenuate diabetic neuropathic pain via AMPK/NF-κB signaling pathway in diabetic rats model20. However, prolonged use of metformin can cause a deficiency in serum vitamin B12, which may exacerbate signs of peripheral nerve damage14. The occurrence of DPN in T2DM patients receiving metformin treatment is influenced by factors such as vitamin B12 and homocysteine levels15. However, the lack of this data restricts the ability to understand the underlying mechanisms. The relationship between cumulative metformin dosage and DPN risk remains uncertain, emphasizing the need for well-designed, large-scale studies to assess the potential risks of metformin therapy for DPN in patients with T2DM.
Vitamin B12 plays an important role in the conversion of homocysteine to methionine in methionine cycle21. Deficiency of vitamin B12 can impair the remethylation of homocysteine, and metformin-induced vitamin B12 deficiency could be associated with hyperhomocysteinemia22. While et al. showed that T2DM patients with exposure to metformin more than 6 months had lower serum vitamin B12 and higher serum homocysteine, which may be an iatrogenic cause for exacerbation of peripheral neuropathy. This study highlight that these abnormalities were correlated strongly with cumulative metformin exposure7. Plasma homocysteine levels may be independently associated with the prevalence and severity of diabetic neuropathy in T2DM patients23,24. A larger and prospective study in larger population would be suitable to clarify the role of homocysteine in the pathogenesis of DPN.
The underlying mechanism by which vitamin B12 deficiency occurs in patients with long-term metformin use is unclear. However, proposed mechanisms accounting for metformin-induced vitamin B12 deficiency have been proposed, include alteration of the small intestine motility leading to small intestinal bacterial overgrowth and subsequent inhibition of vitamin B12 calcium-dependent intrinsic factor complex absorption25,26. Competitive inhibition of vitamin B12 absorption and alterations of the intrinsic factor and cubilin receptor have also been proposed27. This malabsorption basically leads to a decrease of serum vitamin B12 plasma level. Metformin reduces hepatic vitamin B12 storage, eventually leading to vitamin B12 deficiency, which may contribute to distal symmetrical or autonomic neuropathy, spinal subacute combined degeneration, or the progression of pre-existing neuropathies associated with diabetes28.
Our findings show that T2DM patients receiving metformin, being elderly and having a higher DCSI score were associated with an increased odds of DPN. Several risk factors of DPN have been identified, including aging and duration of DM are correlated with significantly higher risk for DPN in T2DM patients29. DPN prevalence increased with age from 11.9% aged below 40 years up to > 50% aged above 70 years30. The Diabetes Complications and Severity Index (DCSI) is a useful tool for prediction of risk of mortality and hospitalization in patients with DM31. Our study showed that T2DM patients receiving metformin with higher DCSI scores had a higher odds for developing DPN. DCSI was associated with the risk of DPN. DCSI may be used an indicator for estimating the risk of DPN. In our study, we can find that T2DM patients comorbid with cerebrovascular disease was associated with higher risk for the development of DPN. Our study results is consistent with a previous study showed that the risk factors for PAD including: age above 40 years and cerebrovascular disease32. Ischemic damage is caused by an interruption in cerebral blood flow, which causes a dramatic alteration of the complex neural network and induces severe neural injuries within the affected area33. Persistent changes following stroke can occur in lower motor neurons when central pathways are interrupted by stroke34. Regarding the association between chronic kidney disease (CKD) and DPN, available data do not demonstrate a clear correlation35. Some studies showed that patients with CKD are at higher risk of diabetic foot disease, and lower extremity amputation is at least two to six times greater among patients with both CKD and DM than DM alone36,37. However, our study discovered that DM patients comorbid with CKD had a lower risk for developing DPN. Further research is needed to better understand the relationship between CKD and DPN among DM patients.
This study has several strengths. First, the primary strength of our study is its population-based design performed using Taiwan’s NHIRD. We included the entire Taiwanese population in our study; thus, the qualitative sample sizes are large enough that can provide high-quality database to reduce the selection bias. Second, the characteristics of the database can provide sufficient statistical power to investigate the association between metformin use and DPN risk among patients with T2DM. Third, the follow-up period of metformin use in our study is divided into 2 years and 5 years. cDDD of metformin use is divided into ≤ 300, > 300, > 500, intensity of metformin use (DDD/month) is divided into ≤ 10, 10–25, > 25/month to investigate the relationship between T2DM patients and the risk of DPN development.
However, there were also several limitations in this population-based cohort study. First, we had no information regarding family histories of DPN among T2DM patients. Second, lifestyle personal data such as those related to cigarette, smoking habits, alcohol consumption, HbA1c, body mass index, physical activity, personal history and dietary habits could not be accessed, all of these factors were associated with DPN risk, which was not available. The prediction of DPN occurrence in T2DM patients undergoing metformin treatment is influenced by factors such as vitamin B12 or homocysteine levels. However, the absence of this data limits the ability to interpret the underlying mechanisms. Third, the diagnoses of DPN and other comorbidities are completely dependent on ICD- 9-CM codes and ICD- 10-CM code. Nonetheless, the NHI Bureau of Taiwan randomly reviews the charts and interviews patients to verify the accuracy of the diagnoses. These processes improve the accuracy and validity of NHIRD. We agree that it is possible there are some uncontrolled confounding variables not solved. However, we proved that the results were approximately similar to prospective cohort trials. Last, this study was conducted using data from the Taiwanese population. However, ethnic and genetic factors may also play a role in the development of DPN, which could limit the generalizability of our findings to other populations.
Conclusions
To sum up, this study provides large-scale population-based longitudinal evidence that metformin use was associated with DPN odds among T2DM patients in a dose-dependent manner. The results suggest that T2DM patients received < 300, 300–500, ≥ 300 cDDD of metformin or use intensity of < 10, 10 ~ 25, > 25 DDD/month experienced higher risk of DPN at 2 and 5 years. The higher cDDD of metformin or use intensity, the higher DPN risk was found in this study. T2DM patients receiving metformin, being elderly and having a higher DCSI score were associated with an increased risk of DPN. In addition, T2DM patients comorbid with cerebrovascular disease also had a higher risk for the development of DPN. However, the lack of vitamin B12 data hinders a clear understanding of the underlying mechanisms. Comprehensive, large-scale studies are needed to assess the potential risks of metformin therapy for DPN in T2DM.patients.
Methods
Data source
This study used secondary data from the Longitudinal Health Insurance Database (LHID), a subset of the NHIRD, from 2001 to 2018 provided by the Health and Welfare Data Science Center (HWDC) of the Ministry of Health and Welfare in Taiwan. The LHID contains information on all beneficiaries enrolled in Taiwan’s National Health Insurance (NHI) program, which is a government-run, single-payer national social insurance program that has operated since 1995. The NHI contains health insurance claims data for 99% of Taiwan’s 23 million residents. Disease diagnoses were coded according to the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) and ICD, 10th Revision, Clinical Modification (ICD-10-CM). The NHIRD can be used to obtain real-world evidence to support clinical decisions and health-care policy making38,39. This study was conducted in accordance with the Declaration of Helsinki. The patient data were extracted from the LHID released by the Health and Welfare Data Science Center, in which NHI beneficiaries are represented using scrambled random identification numbers to protect their privacy. Our study protocol was approved by the Central Regional Research Ethics Committee of China Medical University, Taiwan (No. CRREC-109–011). Because we used only de-identified data in the current study, the requirement for informed consent was waived.
Study subjects
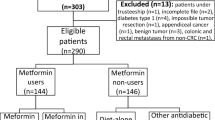
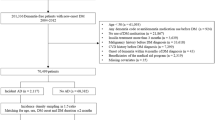
We enrolled ≥ 20-year-old patients given a diagnosis of new-onset DM (ICD-9-CM: 250) over 2002–2013. A DM diagnosis was considered to be the presence of three outpatient DM diagnoses. Metformin use was coded using the Anatomical Therapeutic Chemical code A10BA02. To reduce bias, we excluded (1) patients with type 1 DM, (2) patients given a diagnosis of DPN before or within the first year of their DM diagnosis, and (3) patients hospitalized within 1 year of their DM diagnosis.
Figure 1 presents a flowchart of the patient selection process. We included 892,836 patients given a diagnosis of new-onset DM over 2002–2013. The case group comprised 477,644 patients treated with metformin within the first year of their DM diagnosis, whereas the comparison group comprised 415,192 patients not treated with metformin.
Study designs
We measured metformin intake in terms of the defined daily dose (DDD) of metformin—which is a standard method for measuring drug use and exposure. According to the World Health Organization, the DDD is defined as the assumed average maintenance dose per day in adults. However, the DDD does not necessarily reflect the recommended or prescribed daily dose40. One unit of DDD of metformin is equal to 2 g, which was used to measure the medication unit41. Several predictors were used to examine the correlation between metformin use and the risk of DPN. The first predictor was years of metformin use compared with metformin no use. The second predictor was the cumulative dose of metformin use, which was measured using the cumulative defined daily dose (cDDD).
In the current study, the observation period before metformin treatment was 1 year after initial DM diagnosis. A metformin DDD of 2 g was considered the baseline dose41. Based on the study design from several researches, we used 2 criteria for analyzing the dose-dependency of the metformin treatment–DPN risk association: The first criterion was defined using 3 ranges of cumulative DDD (cDDD) of metformin in the first year: < 300, 300–500, and > 500. The second criterion was defined using 3 ranges of the average monthly intensity of metformin use: < 10, 10–25, and > 2511,42. All patients were observed at their 2- and 5-year follow-ups after initial DM diagnosis.
The presence of DPN (ICD-9-CM: 250.6, 250.7, and 250.9; ICD-10-CM: E10.4, E11.4, E12.4, E13.4, E14.4, G63.2, and G62.9) was defined as a patient receiving ≥ 3 DPN diagnoses within 1 year. Sex, age, income level, urbanization level, diabetes severity, and comorbidities were considered control variables. We used the Diabetes Complications Severity Index (DCSI) to evaluate diabetes severity and assess adverse outcome risk; the index was calculated using the information from seven diabetes complication categories (retinopathy, nephropathy, neuropathy, cerebrovascular, cardiovascular, peripheral vascular disease, and metabolic)31,43.
We also considered the following comorbidities: hyperuricemia (ICD-9-CM: 790.6), cerebrovascular disease (CVD; ICD-9-CM: 430–438), alcoholism (ICD-9-CM: 303), rheumatoid arthritis (RA; ICD-9-CM: 714), sleep disturbance (ICD-9-CM: 780), systematic lupus erythematosus (SLE; ICD-9-CM: 710.0), migraine (ICD-9-CM: 346.90), coronary artery disease (CAD; ICD-9-CM: 410–414), peripheral arterial disease (PAD; ICD-9-CM: 443.9), depression (ICD-9-CM: 296.2 and 296.3), sarcopenia (ICD-9-CM: 724.8, 728.3, 728.8, and 728.9), obesity (ICD-9-CM: 278.0), diabetic retinopathy (ICD-9-CM: 362.0), and chronic kidney disease (CKD; ICD-9-CM: 585).
Statistical analysis
All analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC, USA). The chi-square test was used to evaluate differences in the baseline characteristics between the patients treated and not treated with metformin. The difference in DPN risk among the patients treated with metformin was estimated using multiple logistic regression with adjustments for sex, age, income level, urbanization level, diabetes severity, and comorbidities. Odds ratios (ORs) with their 95% confidence intervals (CIs) were calculated. Two adjusted models were developed to estimate metformin cDDD and metformin treatment intensities (expressed as DDDs/month). Furthermore, we conducted a stratified analysis to investigate the association between metformin use and DPN, containing sex, age, and DCSI. A P value of < 0.05 was considered to indicate statistical significance.
Data availability
The data that support the findings of this study are available from Health and Welfare Data Science Center, Ministry of Health and Welfare (HWDC, MOHW) but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the corresponding authors upon reasonable request and with permission of Health and Welfare Data Science Center, Ministry of Health and Welfare (HWDC, MOHW, https://dep.mohw.gov.tw/dos/np-2497–113.html).
References
Bodman, M.A., Dreyer, M.A. & Varacallo, M.A. Diabetic Peripheral Neuropathy. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK442009/ (2024).
Callaghan, B. C., Cheng, H. T., Stables, C. L., Smith, A. L. & Feldman, E. L. Diabetic neuropathy: Clinical manifestations and current treatments. Lancet Neurol. 11, 521–534. https://doi.org/10.1016/S1474-4422(12)70065-0 (2012).
Feldman, E. L. et al. Diabetic neuropathy. Nat. Rev. Dis. Primers 5, 41. https://doi.org/10.1038/s41572-019-0092-1 (2019).
Vinik, A. I., Park, T. S., Stansberry, K. B. & Pittenger, G. L. Diabetic neuropathies. Diabetologia 43, 957–973. https://doi.org/10.1007/s001250051477 (2000).
Rodriguez-Gutierrez, R. et al. Metformin use and vitamin B12 deficiency: Untangling the association. Am. J. Med. Sci. 354, 165–171. https://doi.org/10.1016/j.amjms.2017.04.010 (2017).
Alharbi, T. J. et al. The association of metformin use with vitamin B12 deficiency and peripheral neuropathy in Saudi individuals with type 2 diabetes mellitus. PLoS ONE 13, e0204420. https://doi.org/10.1371/journal.pone.0204420 (2018).
Wile, D. J. & Toth, C. Association of metformin, elevated homocysteine, and methylmalonic acid levels and clinically worsened diabetic peripheral neuropathy. Diabetes Care 33, 156–161. https://doi.org/10.2337/dc09-0606 (2010).
Sloan, G. et al. A new look at painful diabetic neuropathy. Diabetes Res. Clin. Pract. 144, 177–191. https://doi.org/10.1016/j.diabres.2018.08.020 (2018).
Feldman, E. L., Nave, K. A., Jensen, T. S. & Bennett, D. L. H. New Horizons in Diabetic Neuropathy: Mechanisms, Bioenergetics, and Pain. Neuron 93, 1296–1313. https://doi.org/10.1016/j.neuron.2017.02.005 (2017).
Zhang, Q. et al. VEGF levels in plasma in relation to metabolic control, inflammation, and microvascular complications in type-2 diabetes: A cohort study. Medicine (Baltimore) 97, e0415. https://doi.org/10.1097/MD.0000000000010415 (2018).
Huang, K. H., Chang, Y. L., Gau, S. Y., Tsai, T. H. & Lee, C. Y. Dose-response association of metformin with Parkinson’s disease odds in type 2 diabetes mellitus. Pharmaceutics https://doi.org/10.3390/pharmaceutics14050946 (2022).
Kibirige, D. & Mwebaze, R. Vitamin B12 deficiency among patients with diabetes mellitus: Is routine screening and supplementation justified?. J. Diabetes Metab. Disord. 12, 17. https://doi.org/10.1186/2251-6581-12-17 (2013).
Ahmed, M. A., Muntingh, G. L. & Rheeder, P. Perspectives on peripheral neuropathy as a consequence of metformin-induced vitamin B12 deficiency in T2DM. Int. J. Endocrinol. 2017, 2452853. https://doi.org/10.1155/2017/2452853 (2017).
Yang, R. et al. Metformin treatment and risk of diabetic peripheral neuropathy in patients with type 2 diabetes mellitus in Beijing, China. Front. Endocrinol. (Lausanne) 14, 1082720. https://doi.org/10.3389/fendo.2023.1082720 (2023).
Hashem, M. M., Esmael, A., Nassar, A. K. & El-Sherif, M. The relationship between exacerbated diabetic peripheral neuropathy and metformin treatment in type 2 diabetes mellitus. Sci. Rep. 11, 1940. https://doi.org/10.1038/s41598-021-81631-8 (2021).
Chapman, L. E., Darling, A. L. & Brown, J. E. Association between metformin and vitamin B(12) deficiency in patients with type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab. 42, 316–327. https://doi.org/10.1016/j.diabet.2016.03.008 (2016).
Liu, Q., Li, S., Quan, H. & Li, J. Vitamin B12 status in metformin treated patients: Systematic review. PLoS ONE 9, e100379. https://doi.org/10.1371/journal.pone.0100379 (2014).
Yang, W., Cai, X., Wu, H. & Ji, L. Associations between metformin use and vitamin B(12) levels, anemia, and neuropathy in patients with diabetes: A meta-analysis. J. Diabetes 11, 729–743. https://doi.org/10.1111/1753-0407.12900 (2019).
Kim, J., Ahn, C. W., Fang, S., Lee, H. S. & Park, J. S. Association between metformin dose and vitamin B12 deficiency in patients with type 2 diabetes. Medicine (Baltimore) 98, e17918. https://doi.org/10.1097/MD.0000000000017918 (2019).
Cao, X. J. et al. Metformin attenuates diabetic neuropathic pain via AMPK/NF-κB signaling pathway in dorsal root ganglion of diabetic rats. Brain Res. 1772, 147663. https://doi.org/10.1016/j.brainres.2021.147663 (2021).
Allen, L. H. Vitamin B-12. Adv. Nutr. 3, 54–55. https://doi.org/10.3945/an.111.001370 (2012).
Russo, G. T. et al. Mild hyperhomocysteinemia, C677T polymorphism on methylenetetrahydrofolate reductase gene and the risk of macroangiopathy in type 2 diabetes: a prospective study. Acta Diabetol. 48, 95–101. https://doi.org/10.1007/s00592-009-0169-5 (2011).
Ambrosch, A. et al. Relation between homocysteinaemia and diabetic neuropathy in patients with Type 2 diabetes mellitus. Diabet. Med. 18, 185–192. https://doi.org/10.1046/j.1464-5491.2001.00445.x (2001).
Gonzalez, R. et al. Plasma homocysteine levels are independently associated with the severity of peripheral polyneuropathy in type 2 diabetic subjects. J. Peripher. Nerv. Syst. 17, 191–196. https://doi.org/10.1111/j.1529-8027.2012.00408.x (2012).
Alvarez, M., Sierra, O. R., Saavedra, G. & Moreno, S. Vitamin B12 deficiency and diabetic neuropathy in patients taking metformin: A cross-sectional study. Endocr. Connect 8, 1324–1329. https://doi.org/10.1530/EC-19-0382 (2019).
Damiao, C. P. et al. Prevalence of vitamin B12 deficiency in type 2 diabetic patients using metformin: A cross-sectional study. Sao Paulo Med. J. 134, 473–479. https://doi.org/10.1590/1516-3180.2015.01382111 (2016).
Infante, M., Leoni, M., Caprio, M. & Fabbri, A. Long-term metformin therapy and vitamin B12 deficiency: An association to bear in mind. World J. Diabetes 12, 916–931. https://doi.org/10.4239/wjd.v12.i7.916 (2021).
Bell, D. S. H. Metformin-induced vitamin B12 deficiency can cause or worsen distal symmetrical, autonomic and cardiac neuropathy in the patient with diabetes. Diabetes Obes. Metab. 24, 1423–1428. https://doi.org/10.1111/dom.14734 (2022).
Liu, X., Xu, Y., An, M. & Zeng, Q. The risk factors for diabetic peripheral neuropathy: A meta-analysis. PLoS ONE 14, e0212574. https://doi.org/10.1371/journal.pone.0212574 (2019).
Aleidan, F. A. S. et al. Prevalence and risk factors for diabetic peripheral neuropathy among Saudi hospitalized diabetic patients: A nested case-control study. Int. J. Gen. Med. 13, 881–889. https://doi.org/10.2147/IJGM.S273807 (2020).
Young, B. A. et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am. J. Manag. Care 14, 15–23 (2008).
Liu, J. et al. Risk factors for diabetic peripheral neuropathy, peripheral artery disease, and foot deformity among the population with diabetes in Beijing, China: A multicenter, Cross-Sectional Study. Front. Endocrinol. (Lausanne) 13, 824215. https://doi.org/10.3389/fendo.2022.824215 (2022).
Qin, C. et al. Signaling pathways involved in ischemic stroke: molecular mechanisms and therapeutic interventions. Signal Transduct. Target Ther. 7, 215. https://doi.org/10.1038/s41392-022-01064-1 (2022).
Ivanova, T. D., Knorr, S., MacDonell, C. W., Pollock, C. L. & Garland, S. J. Motoneurone afterhyperpolarisation time-course following stroke. Clin. Neurophysiol. 125, 544–551. https://doi.org/10.1016/j.clinph.2013.08.017 (2014).
Mayeda, L. et al. Glucose time in range and peripheral neuropathy in type 2 diabetes mellitus and chronic kidney disease. BMJ Open Diabetes Res. Care https://doi.org/10.1136/bmjdrc-2019-000991 (2020).
Hoffstad, O., Mitra, N., Walsh, J. & Margolis, D. J. Diabetes, lower-extremity amputation, and death. Diabetes Care 38, 1852–1857. https://doi.org/10.2337/dc15-0536 (2015).
Margolis, D. J., Hofstad, O. & Feldman, H. I. Association between renal failure and foot ulcer or lower-extremity amputation in patients with diabetes. Diabetes Care 31, 1331–1336. https://doi.org/10.2337/dc07-2244 (2008).
Hsieh, C. Y. et al. Taiwan’s National Health Insurance Research Database: Past and future. Clin. Epidemiol. 11, 349–358. https://doi.org/10.2147/CLEP.S196293 (2019).
Lai, S. W., Liao, K. F., Lin, C. L., Lin, C. C. & Lin, C. H. Longitudinal data of multimorbidity and polypharmacy in older adults in Taiwan from 2000 to 2013. Biomedicine (Taipei) 10, 1–4. https://doi.org/10.37796/2211-8039.1013 (2020).
Grimmsmann, T. & Himmel, W. Discrepancies between prescribed and defined daily doses: A matter of patients or drug classes?. Eur. J. Clin. Pharmacol. 67, 847–854. https://doi.org/10.1007/s00228-011-1014-7 (2011).
Wellington, K. Rosiglitazone/Metformin. Drugs 65, 1581–1592. https://doi.org/10.2165/00003495-200565110-00013 (2005).
Huang, K. H. et al. Correlation between long-term use of metformin and incidence of NAFLD among patients with type 2 diabetes mellitus: A real-world cohort study. Front. Endocrinol. (Lausanne) 13, 1027484. https://doi.org/10.3389/fendo.2022.1027484 (2022).
Chang, H. Y., Weiner, J. P., Richards, T. M., Bleich, S. N. & Segal, J. B. Validating the adapted Diabetes Complications Severity Index in claims data. Am. J. Manag. Care 18, 721–726 (2012).
Acknowledgements
We are grateful to Chung Shan Medical University Taiwan, China Medical University Taiwan, and the Health Data Science Center, China Medical University Hospital, for providing administrative, technical, and funding support that has contributed to the completion of this study. This study is based, in part, on data released by the Health and Welfare Data Science Center, Ministry of Health and Welfare. The interpretation and conclusions contained herein do not represent those of the Ministry of Health and Welfare.
Funding
This research was funded by the Chung Shan Medical University, Taiwan (CSMU-INT-112-02), Chung Shan Medical University Hospital, Taiwan (CSH-2023-C-003, CSH-2024 C-002), China Medical University Taiwan (CMU113-MF-120) and the Ministry of Science and Technology Taiwan (MOST 111-2410-H-040-002).
Author information
Authors and Affiliations
Contributions
All authors have participated in this study and have reviewed and agree with the final manuscript. Kuang-Hua Huang: Conceptualization, Funding acquisition, Methodology, Validation, Writing – original draft, and Writing – review & editing. Shiang-Wen Huang: Formal analysis and Writing – review & editing. Yih Yang: Formal analysis, and Writing – review & editing. Shuo-Yan Gau: Formal analysis and Writing – review & editing. Tung-Han Tsai: Formal analysis, and Writing – review & editing. Ya-Lan Chang: Data curation, Formal analysis, and Writing – review & editing. Chien-Ying Lee: Conceptualization, Methodology, Validation, Writing – original draft, and Writing – review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, KH., Huang, SW., Yang, Y. et al. Dose dependent relationship of metformin use and diabetic peripheral neuropathy risk in patients with type 2 diabetes mellitus. Sci Rep 15, 12040 (2025). https://doi.org/10.1038/s41598-025-96445-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-96445-1