Abstract
The growing prevalence of antimicrobial resistance poses a global threat to human and animal health. In this study, we investigated the occurrence and genetic basis of antimicrobial resistance in a Magellanic Penguin (Spheniscus magellanicus) rescued off the coast of Rio de Janeiro, Brazil. The penguin presented a bacterial infection, identified as Klebsiella pneumoniae. Molecular analysis revealed the presence of several resistance genes, including those that confer resistance to carbapenems, beta-lactams, quinolones, and other classes of antibiotics. The bacterial strain belonged to Sequence Type 70 (ST70), a clone previously associated with human nosocomial infections. This study highlights the potential of migratory penguins as vectors of antimicrobial-resistant microorganisms, emphasizing the need for a One Health approach to address the complex interaction between environmental factors, animal health, and human well-being. The findings underscore the urgency of implementing strategies to mitigate the spread of multidrug-resistant bacteria in natural and urban environments.
Similar content being viewed by others
Introduction
The genetic determinants of antimicrobial resistance are naturally present in bacteria1. The frequent use of these drugs creates selective pressure that contributes to the emergence of resistant bacteria, threatening human and animal health2,3.
Klebsiella pneumoniae demonstrates a progressive increase in multidrug resistance and may be naturally present in the intestinal tract of animals. It is a main bacterial species responsible for nosocomial infections, with a high prevalence of multidrug-resistant strains4,5. Recent studies have reported the isolation of antimicrobial-resistant K. pneumoniae in wild animals6,7. The closer contact between wild animals and the urban environment highlights the importance of a one-health approach in controlling zoonosis, food safety, pollution management, and antimicrobial resistance control8. The human-animal interaction contributes to the exchange of genetic material between microorganisms and reinforces the dissemination of multiresistant bacteria. Understanding the importance of zoonotic transmission of antimicrobial-resistant bacterial strains can slow their spread and ensure human and animal health.
Cases of different species of wild animals carrying these microorganisms in their respective habitats have already been reported9. The marine ecosystem is constantly affected by wastewater runoff containing bacteria carrying antibiotic-resistant genes or the genes themselves10. Aquatic species can act as sentinels and vectors of multiresistant microorganisms11,8. In this context, penguins are migratory seabirds, possibly capable of carrying different types of microorganisms throughout their journey9,12. Migratory species like the Magellanic Penguin (Spheniscus magellanicus) are fundamental for conservation, as these provide important information about changes in the marine environment13. These penguins are endemic to South America, inhabiting mainly along the coast of Patagonia, Argentina, and Chile14. Although individuals of this species do not reproduce in Brazil, after the reproductive period (September to March), these individuals start their pelagic season (April to September), when adults and juveniles leave their colonies and migrate to the north along the coast of Uruguay and Brazil, searching abundant food resources, and to avoid the adverse environmental conditions found in winter in the region of their colonies15. During this period of the year, individuals are seen stranded along several Brazilian beaches, showing mainly signs of exhaustion, drowning, and infectious processes caused by several factors like overfishing, fisheries action items, oil pollution, and climate change, so some projects carry out the rescue of these individuals for veterinary care, rehabilitation, and subsequent release.
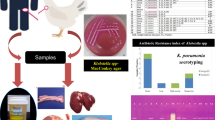
Thus, they have great potential to get infected and spread bacteria and antimicrobial resistance genes9,16. However, few studies relate the isolation of multiresistant bacteria in wild animals, particularly penguins. Therefore, we report a Magellanic-Penguin (Spheniscus magellanicus) case rescued on the Itacoatiara beach, located in the municipality of Niteroi, the coast of Rio de Janeiro, presenting a bacterial pneumoniae caused by K. pneumoniae.
Materials and methods
A single Magellanic penguin was rescued on the beach of Itacoatiara, located in the municipality of Niterói - RJ, through the Santos Basin Beach Monitoring Project (PMP-BS). The sample collection was approved by the Animal Use Ethics Committee of Fluminense Federal University, and an authorization for the Capture, Collection, and Transport of Biological Material No. 755/2016 by the Brazilian Institute of the Environment and Renewable Natural Resources (Portuguese: Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis, IBAMA) for the RJ Area, under the coordination of Econservation Environmental Studies and Projects. All experiments were performed following relevant guidelines and regulations. Also, this study aligns with Animal Research: Reporting of In Vivo Experiments (ARRIVE - Animal Research: Reporting of In Vivo Experiments) guidelines.
The penguin presented symptoms of “Stranded Penguin Syndrome”, being evidenced by lean body score, hypothermia, marked dehydration, pale mucous membranes, and caseous formation in the oral cavity due to the presence of fish spines that pierced its mucosa, in addition to characteristic symptoms of drowning. After the first care and screening, the animal stayed in a Poultry Treatment Unit, where it received appropriate treatment with intravenous fluids, osmotic diuretic, glucose, and bronchodilator for stabilization and treatment of clinical signs presented. The penguin received a feeding protocol with fish porridge and vitamin supplementation until the beginning of solid feeding. Complementary tests demonstrated marked leukopenia, suggestive of an acute infectious process. After seven days of stabilization, a radiographic examination revealed changes representative of pneumonia. An endotracheal swab was collected for better evaluation and clinical management.
After 90 days of treatment, during which the penguin showed clinical improvement and without respiratory symptoms, the animal began to present high respiratory rates during auscultation and died on the 99th day. At necropsy, diffuse caseous masses were evidenced, with yellowish mucus content in the lung parenchyma, characteristic of an infectious process. In addition, the penguin also presented alterations characteristic of infectious pericarditis and hepatomegaly.
Bacterial culture
A sample was collected by swabbing the tracheal region. The swab was transported to the Laboratory of Gram-Positive Cocos at Universidade Federal Fluminense and stored at 4ºC until processing. The sample was inoculated in TSB Broth (Tryptic Soy Broth) and incubated at 37ºC for 24 h. Next, after gram staining evaluation, the sample was inoculated onto Eosin Methylene Blue Agar (EMB), Mannitol Salt Agar, and Chromogenic Agar for KPC by the depletion technique. Blue-colored colonies, with a presumptive bright appearance of Klebsiella pneumoniae, were seeded on ATS Agar and incubated at 37 °C for 24 h. Identification was performed using the Matrix-Assisted Laser Desorption ionization-time of flight (MALDI–TOFms - Biotyper-Bruker) spectrometry method, as recommended by the manufacturer. A score ≥ 2000 was used for species-level identification.
Antimicrobial susceptibility test
We evaluated the isolate’s susceptibility by disk diffusion method, using CLSI standard17. The antimicrobials tested were ampicillin, amoxicillin with clavulanic acid, cefazolin, ceftazidime, cefotaxime, cefoxitin, ceftriaxone, ceftiofur, imipenem, aztreonam, ciprofloxacin, norfloxacin, enrofloxacin, gentamicin, azithromycin, tetracycline, chloramphenicol, fosfomycin, nitrofurantoin, and sulfamethoxazole. The commercial Policimbac® broth microdilution system determined Polymyxin B MIC (minimal inhibitory concentration). Resistance to three or more classes of antimicrobial agents defined the isolate as multi-drug resistant (MDR) according to the definition proposed by Magiorakos et al., 201218.
DNA extraction
Selected colonies were cultured on Tryptone Soy Agar (KASVI, Paraná, Brazil) and incubated aerobically at 37 ºC for 24 h. DNA was extracted using the Wizard Genomic DNA Kit (Promega, Madison, USA) according to the manufacturer’s instructions. The extracted DNA was quantified using the Quantus Fluorometer (Promega, Madison, EUA) according to the manufacturer’s instructions.
Carbapenemase screening
Modified carbapenem inactivation test (mCIM) combined with EDTA-modified carbapenem inactivation test (eCIM) was applied for detecting the carbapenemase, followed by NG-Test CARBA 5 immunochromatographic assay. Additionally, multiplex polymerase chain reaction (PCR) was performed. Genes encoding the main carbapenemases (blaNDM, blaKPC, and blaOXA48) were investigated using previously described primers18.
Whole-genome sequencing and genome assembly
Library preparation was performed using Illumina DNA prep (M) Tagmentation (96 samples) and Nextera DNA CD index (96 indexes, 96 samples) (Illumina, San Diego, California, USA), according to the manufacturer’s instructions. Libraries were subsequently quantified using Qubit and the dsDNA HS-kit (Thermo, Massachusetts, USA). Finally, the sample was loaded on a HiSeq2500 system and ran for 201 cycles (PE125), pair-end (500 bp library) using HiSeq Rapid SBS Kit v2 chemistry. The reads obtained were trimmed using BBDuk with default parameters. The assembly was performed with de novo and reference assembly protocols using Velvet 1.2.10 42 and Geneious 2022.0.2 (Biomatters, Auckland, New Zealand), using genome 6109 of Klebsiella pneumoniae as reference (CP051149).
Genome annotation was performed at the Pathosystems Resource Integration Center (PATRIC 3.6.12). MLST typing was performed in silico using the MLST tool from the Center for Genomic Epidemiology (CGE) (https://cge.food.dtu.dk/services/MLST/). The genetic determinants of resistance were searched using two tools: ResFinder from CGE (https://cge.food.dtu.dk/services/ResFinder/) and The Comprehensive Antibiotic Resistance Database – CARD (https://card.mcmaster.ca/home). The genetic determinants of virulence were analyzed using VirulenceFinder (CGE). The visualization of plasmid alignments was assessed with EasyFig v2.2.5. One hundred forty-three publicly available genomes were included in the phylogenetic tree reconstruction. The pathogen watch database was used to download 85 ST70 genomes, mostly isolated from humans, and the NCBI database to download 58 genomes isolated from birds and the environment (supplementary table). The sequences were aligned using bowtie2. Multiple sequence alignments were reconstructed, from which phylogenetic trees were inferred using PhyML on the REALPHY pipeline.
Results
All identifications confirmed that the isolated bacteria were Klebsiella pneumoniae. Regarding susceptibility to antimicrobials, it resisted all antibiotics tested except for Polymyxin B (MIC = 1 µg/mL). The K. pneumoniae isolate showed intermediate sensitivity to norfloxacin and enrofloxacin, while the mCIM/eCIM tests and the immunochromatographic assay suggested a metallo-carbapenemase production. The multiplex PCR assay was blaNDM-positive. No other carbapenemase gene was detected.
Sequencing identified K. pneumoniae subsp. pneumoniae with resistance genes for quinolones (oqxA; oqxB; qnrB1; aac(6)-Ib-cr_1), macrolides (mph(A); erm(B)), beta-lactams (blaCTX-M-15; blaOXA-1, blaSHV-32, blaTEM-1 C, blaNDM-1), chloramphenicol (floR, catB3), sulfonamides (sul2; sul1), rifampicin (ARR–3), aminoglycosides (aac(3)-IIa, aph(3’)-VIa, aph(3’’)-Ib, aph(6)-Id), streptogramins and lincosamides (erm(B)), fosfomycin (fosA), tetracycline (tetA), trimethoprim (dfrA14). Many of these genes were carried by plasmids, as shown in Figs. 1 and 2, and 3. The genes ble, blaNDM, sul, and quac are carried on a plasmid similar to pNDM-KN (accession number JN157804; Fig. 1), an IncA/C plasmid. The genes quac, sul, and mph are carried on a plasmid similar to pKPN-IT (accession number JN233704.1; Fig. 2). The genes aph(3), aph(6), and sul are carried on a plasmid similar to RSF1010 (accession number NC_001740; Fig. 3). Additionally, the studied isolate also presented virulence genes codifying for fimbriae (fimH and mrkA), siderophore receptor (fyuA), siderophore yersiniabactin (irp2), ferric aerobactin receptor (iutA) and complement resistance (traT).
MLST identified the bacterium as being from ST70. The phylogenetic tree showed that the sample branched together with other clinical isolates belonging to ST70, mainly those isolated in Europe (Fig. 4).
Discussion
The rise in antimicrobial resistance among bacterial pathogens represents a vital concern, and the role of wildlife in disseminating these resistant strains is becoming increasingly recognized. Our study unveils a multidrug-resistant Klebsiella pneumoniae in a Magellanic Penguin, a migratory seabird, implicating these animals as potential vectors for antimicrobial-resistant microorganisms. Notably, this study demonstrates the interconnection between environmental pollution, human-animal interactions, and the emergence of resistance.
Reports of isolation of resistant bacteria in wild animals are progressively increasing19,20. Analogously to this study, research carried out in wild hedgehogs highlighted Klebsiella spp. as the genus with the highest proportion of resistance genes21. A study in Senegal detected different clones of K. pneumoniae in wild chimpanzees and termites22. That same study reports the identification of clones harboring plasmids that carried the blaCTX-M-15 and blaOXA-1 genes, as well as the genes that confer resistance to aminoglycosides [aac(3)-Iia and aac(6’)Ib-cr], quinolone (qnrB1), sulfonamides (sul2) and chloramphenicol (floR), similar to what was found in this research. One of the resistance genes found in K. pneumoniae isolated in the present study was the blaNDM-1 gene, which refers to “New Delhi Metallo beta-lactamase” and confers resistance to broad-spectrum antibiotics such as carbapenems. Ahlstrom23 isolated enterobacteria resistant to carbapenems from wild birds in different world regions. K. pneumoniae carbapenemase (KPC) presenting the blaNDM-1 gene was isolated among these bacteria. Another study detected the presence of Escherichia coli bacteria carrying the blaNDM-1 gene in a penguin on the Brazilian coast24. Although there are few reports of the isolation of multidrug-resistant K. pneumoniae in penguins, previous studies reveal its appearance in wild animals, especially when dealing with migratory birds16,25.
The CTX-M group, which confers resistance to beta-lactams, is recognized as the most prevalent variant of Extended Spectrum Beta-Lactamase (ESBL), with the CTX-M-15 gene being particularly notable in bacteria found in both wild animals and humans9. In Brazil, researchers isolated K. pneumoniae containing blaNDM-1 and blaCTX-M genes from hospitalized patients26. The present work reports, for the first time, the occurrence of K. pneumoniae that carries CTX-M-15 isolated from a free-living penguin. Thus, identifying these resistance genes in the urban and wild environment reveals the danger of the emergence of multidrug-resistant bacteria due to the excessive use of antimicrobials and consequent dissemination through migratory animals.
In this study, multilocus sequence typing identified K. pneumoniae ST70, a clone previously reported in nosocomial infections in humans in Brazil27,28 and India29. A recent study revealed the presence of this clone in a blue and yellow macaw (Ara ararauna) in the central-west region of Brazil30. This ST70 is more associated with nosocomial infections in humans. Other studies with different species of penguins found several STs differing from our results. The isolation of K. pneumoniae ST70 in distant continents and different species is alarming worldwide. When we evaluated studies that conducted WGS analyses, this was the first study that assessed the ST70 from animal origin in South America. Since this ST has already been reported in Hospitals in Rio de Janeiro27, one possible explantion is that the penguin got in contact with bacteria from hospital waste.
In conclusion, our study highlights the emergence of multidrug-resistant Klebsiella pneumoniae in migratory penguins, underscoring the importance of a One-Health approach to combating antimicrobial resistance. Collective efforts are required to mitigate the spread of antimicrobial-resistant microorganisms, encompassing environmental conservation, responsible antimicrobial use, and collaborative research across disciplines. By addressing these challenges, we can safeguard both animal and human health in the face of the escalating threat of antimicrobial resistance.
Data availability
Sequence data that support the findings of this study have been deposited in the Gene bank with the primary accession code JBDLPA000000000; BioSample SAMN41189574.
References
Read, A. F. & Woods, R. J. Antibiotic resistance management. Evolution, medicine, and public health. ; 2014(1):147. (2014).
Zhang, R. et al. Occurrence, sources and transport of antibiotics in the surface water of coral reef regions in the South China Sea: potential risk to coral growth. Environ. Pollut. 232, 450–457 (2018).
Bag, S. et al. Molecular insights into antimicrobial resistance traits of commensal human gut microbiota. Microb. Ecol. 77 (2), 546–557 (2019).
Cassettari, V. C., Silveira, I. R., Balsamo, A. C. & Franco, F. Outbreak of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae in an intermediate-risk neonatal unit linked to onychomycosis in a healthcare worker. Jornal De Pediatria. 82, 313–316 (2006).
Martínez-Aguilar, G. et al. Outbreak of nosocomial sepsis and pneumonia in a newborn intensive care unit by multiresistant Extended-Spectrum β-Lactamase-Producing Klebsiella pneumoniae high impact on mortality. Infect. Control Hosp. Epidemiol. 22 (11), 725–728 (2001).
Sousa, A. T. H. I. et al. [Antimicrobial resistance profile of Klebsiella pneumoniae isolated from domestic and wild animals]. Arquivo Brasileiro De Med. Veterinária E Zootecnia. 71, 584–593 (2019). Portuguese.
Lundbäck, I. C. et al. Into the Sea: antimicrobial resistance determinants in the microbiota of little Penguins (Eudyptula minor). Infect. Genet. Evol. 88, 104697 (2021).
Rocha, M. F. G. et al. One health implications of antimicrobial resistance in bacteria from Amazon river dolphins. EcoHealth 18 (3), 383–396 (2021).
Wang, J. et al. The role of wildlife (wild birds) in the global transmission of antimicrobial resistance genes. Zool. Res. 38 (2), 55–80 (2017).
Nava, A. R., Daneshian, L. & Sarma, H. Antibiotic resistant genes in the environment-exploring surveillance methods and sustainable remediation strategies of antibiotics and ARGs. Environ. Res. 215 (1), 114212 (2022).
Stewart, J. R. et al. The coastal environment and human health: microbial indicators, pathogens, sentinels and reservoirs. Environ. Health. 7, 1–14 (2008).
Fornells, L. A. M. G. et al. Detection of paramyxoviruses in Magellanic Penguins (Spheniscus magellanicus) on the Brazilian tropical Coast. Vet. Microbiol. 156 (3–4), 429–433 (2012).
Boersma, P. D. Penguins as Mar. Sentinels BioScience ;58(7):597–607. (2008).
Bingham, M. The decline of Falklands Penguins in the presence of a commercial fishing industry. Revista Chil. De Historia Nat. 75, 805–818 (2002).
Pütz, K., Schiavini, A., Rey, A. R. & Lüthi, B. H. Winter migration of Magellanic Penguins (Spheniscus magellanicus) from the southernmost distributional range. Mar. Biol. 152, 1227–1235 (2007).
Raza, S. et al. First report of Bla CTX-M-15-Type ESBL-Producing Klebsiella pneumoniae in wild migratory birds in Pakistan. EcoHealth 14 (1), 182–186 (2017).
CLSI (Clinical and Laboratory Standards Institute). Performance standards for antimicrobial susceptibility testing. Clinical and Laboratory Standards Institute Guidelines, 31ª ed., (2021).
Magiorakos, A-P. et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infection: Official Publication Eur. Soc. Clin. Microbiol. Infect. Dis. 18 (3), 268–281. https://doi.org/10.1111/j.14690691.2011.03570.x (2012).
Monteiro, J., Widen, R. H., Pignatari, A. C., Kubasek, C. & Silbert, S. Rapid detection of carbapenemase genes by multiplex real-time PCR. J. Antimicrob. Chemother. 67 (4), 906–909. https://doi.org/10.1093/jac/dkr563 (2012).
Garcias, B. et al. Extended-spectrum β-Lactam resistant Klebsiella pneumoniae and Escherichia coli in wild European hedgehogs (Erinaceus europeus) living in populated areas. Anim. (basel). 28 (10), 2837 (2021).
Stewart, J. R. et al. Survey of antibiotic-resistant bacteria isolated from bottlenose dolphins tursiops truncatus in the southeastern USA. Dis. Aquat. Organ. 108 (2), 91–102 (2014).
Baron, S. A. et al. Multidrug-resistant Klebsiella pneumoniae clones from wild chimpanzees and termites in Senegal. Antimicrob. Agents Chemother. 65 (9), e0255720 (2021).
Ahlstrom, C. A. et al. Genomically diverse carbapenem resistant Enterobacteriaceae from wild birds provide insight into global patterns of Spatiotemporal dissemination. Sci. Total Environ. 824, 153632 (2022).
Wink, P. L., Lima-Morales, D., Meurer, R. & Barth, A. L. Escherichia coli carrying blaNDM-1 obtained from a migratory Penguin (Spheniscus magellanicus) in the Brazilian seacoast. Braz J. Microbiol. 53 (1), 499–502 (2022).
Hernández, J. & González-Acuña, D. Anthropogenic antibiotic resistance genes mobilization to the Polar regions. Infect. Ecol. Epidemiol. 6, 32112 (2016).
Nava, R. G., Oliveira-Silva, M., Nakamura-Silva, R., Pitondo-Silva, A. & Vespero, E. C. New sequence type in multidrug-resistant Klebsiella pneumoniae harboring the blaNDM-1-encoding gene in Brazil. Int. J. Infect. Dis. 79, 101–103 (2019).
Seki, L. M. et al. Molecular epidemiology of KPC-2- producing Klebsiella pneumoniae isolates in Brazil: the predominance of sequence type 437. Diagn. Microbiol. Infect. Dis. 70 (2), 274–277 (2011).
Fehlberg, L. C. C., Carvalho, A. M. C., Campana, E. H., Gontijo-Filho, P. P. & Gales, A. C. Emergence of Klebsiella pneumoniae-producing KPC-2 carbapenemase in Paraíba, Northeastern Brazil. Brazilian J. Infect. Dis. 16 (6), 577–580 (2012).
Sahoo, R. K. et al. The first report of colistin–carbapenem resistance in Klebsiella pneumoniae ST70 isolated from the pediatric unit in India. Brazilian J. Microbiol. 51 (1), 1–3 (2020).
Sousa, A. T. H. I. et al. Determination of multidrug-resistant populations and molecular characterization of complex Klebsiella spp. In wild animals by multilocus sequence typing. Vet. World. 15 (7), 1691–1698 (2022).
Acknowledgements
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq (406057/2016-8; 443764/2018-2), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro-FAPERJ (Projeto REDES: E26/211.554/2019; E-26/201.328/2021) and by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES) Finance Code 001.
Author information
Authors and Affiliations
Contributions
Conceptualization, Methodology, and Writing – Original Draft Preparation: Sandryelle M. Freire, Annelise Kylar. Formal analysis: Marina F. Côrtes, Thiago P. G. Chagas, Renata Freire Alves Pereira, Felipe R. Pinheiro, Fabio Alves-Aguiar, Bruno Penna. Writing – Review & Editing: Marina F. Côrtes2, Thiago P. G. Chagas, Renata Freire Alves Pereira, Bruno Penna. Resources, project administration , and supervision: Fabio Aguiar-Alves, Bruno Penna.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Freire, S.M., Kyllar, A., Côrtes, M. et al. Multidrug-resistant Klebsiella pneumoniae ST70 harboring blaNDM in a migratory Penguin. Sci Rep 15, 20814 (2025). https://doi.org/10.1038/s41598-025-97816-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-97816-4