Abstract
Severe pediatric malaria remains a pressing global health issue. Laboratory parameters may provide early risk and severity stratification for better disease management, beyond current clinical severity scores. This study aimed to identify host biomarkers of immune and endothelial activation and parasite biomass in children with severe malaria (SM) compared to uncomplicated malaria (UM). We conducted a case–control study in a rural hospital in southern Mozambique from 2014 to 2016, recruiting patients under 10 years old with Plasmodium falciparum SM as cases, and patients with UM matched by age, sex, and parasitemia as controls. We compared plasma levels of biomarkers associated with total parasite mass (HRP-2), biomarkers of host response to infection (Angpt-1, Angpt-2, sTie-2, BDNF, CysC, sFlt-1, IL-6, IL-8, IP-10, sTNFR-1 and sTREM-1). All biomarker levels except Angpt-1, BDNF and CysC were significantly higher in children with SM. HRP-2 levels significantly differed between cases and controls, strongly correlating with Angpt-2, sTie-2, sFlt-1, TNRF, and sTREM-1, both in SM and UM. In conclusion, host biomarkers indicative of immune and endothelial activation were associated with malaria severity and HRP-2, even after controlling for matching variables, potentially offering targets for risk-stratification and adjuvant therapy.
Similar content being viewed by others
Introduction
Malaria is a parasitic disease with significant impact on global health. In 2022, it caused 249 million cases and 608,000 deaths in 85 malaria-endemic countries, most of them occurring in children under 5 years-old in sub-Saharan Africa (SSA)1. Of these, severe malaria (SM), mainly caused by Plasmodium falciparum, accounts for around 2–4 million cases per year2. SM is a complex multi-system disease which may present with many manifestations, although most patients can be identified by three overlapping syndromes: severe malarial anemia, acidosis, and cerebral malaria (CM)2. The onset and evolution of the disease depends on the intricate interaction between parasite, host, and socio-geographic factors, which determines the range of severity from asymptomatic patients to death3. Understanding the differential characteristics of uncomplicated malaria (UM) and SM cases is essential to identify children at a higher risk of SM and death, and to find new diagnostic, prognostic and therapeutic tools.
SM is characterized by high parasite burden; marked cytoadherence of parasitized erythrocytes to the microvasculature; impaired tissue perfusion; and activation of the immune system, complement, and the endothelium4. High parasite biomass is thought to trigger the pathological process leading to SM4. Previous studies have shown that parasite biomass is reflected by the levels of histidine-rich protein-2 (HRP-2), a protein released from erythrocytes into circulation by P. falciparum (and not other Plasmodium species) during infection, even more accurately than by peripheral blood parasitemia5,6. Higher concentrations of HRP-2 have been described in patients with SM in comparison with UM and associated with specific severe syndromes, disease progression, and mortality7,8,9,10,11,12,13,14. Moreover, high plasma levels of this parasite-based marker, in combination with other factors like transcript levels of the genes that codify the parasite molecules contributing to cythoadhesion (var genes), have been reported as good predictors of SM in children and adults, as confirmed by previous studies7,8,9,10,11,12,13,14,15,16.
Alternately, different markers of immune and endothelial activation have been linked to disease severity, including the angiopoietin-tyrosine kinase (Angpt-Tie) axis17 and soluble triggering receptor expressed on myeloid cells (sTREM-1)18. Several studies have shown that dysregulation of the Angpt-Tie system is associated with poor disease outcomes and can be quantified to differentiate between UM and SM. Likewise, low levels of Angpt-1, and high levels of Angpt-2 and soluble Tie-2 (sTie-2), have been associated with poor prognosis in malaria19,20,21,22,23,24,25,26. Changes in plasma levels of different immune activation biomarkers such as interleukin 6 (IL-6), interleukin 8 (IL-8), interferon γ-induced protein of 10 kDa (IP-10), soluble FMS-like tyrosine kinase-1 (sFlt-1), soluble tumor necrosis factor receptor-1 (sTNFR-1), brain-derived neurotrophic factor (BDNF), or levels of Cystatin C (CysC), a biomarker of kidney functional status, have been recently associated to SM and/or poor prognosis22,27,28,29,30,31,32,33,34.
Mortality in patients affected by malaria and other infectious diseases in low-resource settings can also be predicted through clinical severity scores35. Among them, the Lambaréné Organ Dysfunction Score (LODS) is a simple score that combines three features (coma, prostration, and deep breathing), and was developed to predict in-hospital deaths in African children with malaria35,36. Adding specific biomarkers such as sTREM-1, Angpt-2, and sFlt-1 to the evaluation of LODS, has shown to improve the model’s prognostic accuracy18,31. Thus, new approaches that combine clinical features with the measurement of plasma biomarkers have considerable potential to improve risk stratification and prognosticate children presenting with malaria.
In this study, we assessed the association of host biomarkers with malaria severity and parasite biomarkers using a matched case–control approach. The objectives of the study were: 1) to identify host and parasite biomarkers in plasma differentially expressed in children with UM and SM; and 2) to assess the relationship between host and parasite biomarkers in UM and SM. We hypothesized that higher levels of biomarkers would be present in SM cases, compared to UM cases. Finally, we expected to observe a significant correlation between biomarkers of parasite biomass and host biomarkers.
Methods
Study area
The study was conducted in Manhiça¸ a district in rural southern Mozambique, where the Centro de Investigação em Saúde de Manhiça (CISM; Manhiça Health Research Centre) has been running a health and demographic surveillance system since 1996. A full description of the Manhiça study area and population has been reported elsewhere37. Briefly, the area is a flat savannah with moderate vegetation. There are two distinct seasons, a hot and wet season from October to May, and a dry and cold season during the rest of the year. Two reference district hospitals in Manhiça and Xinavane, as well as 11 peripheral health facilities, constitute the government’s health network within the Manhiça district. Malaria transmission is perennial in this district of Mozambique, with highest incidence typically peaking between November and April. P. falciparum accounts for over 98% of all malaria cases in the region38. In 2003–2005, malaria comprised 30.5% of all pediatric outpatient visits38 and nearly half (49%) of all pediatric admissions39, 27% of which fulfilled the World Health Organization (WHO) criteria for being considered as SM cases. Almost 19% of all in-hospital pediatric deaths were due to malaria39. Among all SM admissions, prostration (55.0%), respiratory distress (41.1%), and severe anemia (17.3%) were the three most prevalent clinical presentations39. Recent changes in the epidemiology of malaria in SSA have encompassed a steady decline in malaria incidence in the Manhiça district, coupled with a decrease in severe disease, which reached its nadir in 201040. However, malaria incidence has increased since then, and severe cases have again become frequent at Manhiça Hospital. Furthermore, important differences in their mean age (shifted to older ages) and syndromic presentation (more CM cases, which were rare before) have been observed40.
Study design and population
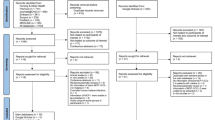
This was an individually matched prospective case–control study, with SM as cases and UM as controls. Matching factors included sex, age (± 3 months in children < 1-year-old and ± 6 months in children ≥ 1-year-old), and parasitemia (children were recruited by study staff guided by crosses, same or ± one cross level). Children ≤ 10 years of age presenting at the Manhiça District Hospital (MDH) with malaria were recruited between September 2014 and May 2016. SM cases were defined as patients with a clinical diagnosis of malaria, an asexual P. falciparum positive parasitemia by microscopic examination of Giemsa-stained blood smears, and fulfilling at least one of the following criteria: CM (deep coma with Blantyre coma score ≤ 2), severe malarial anemia (packed cell volume < 15% or hemoglobin < 5 g/dL), acute respiratory distress (chest indrawing and/or deep breathing), hyperlactatemia (lactate ≥ 5 mmol/L), prostration (inability to sit or breastfeed in children old enough to do so), hypoglycemia (blood glucose < 2.2 mmol/L) and multiple seizures (≥ 2 convulsions in the preceding 24 h). UM controls were defined as children attending MDH (admitted or not) with a clinical diagnosis of malaria with a P. falciparum asexual positive parasitemia and not fulfilling the criteria for SM. Patients were assessed by the study clinician to confirm the patient’s eligibility to participate in the study, and ensure malaria was the sole or principal cause of the disease. Considering the potential effect on the biomarker levels, patients were excluded from the study if they had a history of blood transfusion or use of antimalarials drugs in the preceding 15 days. They were also excluded if they had participated in any other study including the administration of antimalarial drugs or vaccines within the preceding 6 months. Patients with a positive blood culture were excluded from analysis. Human immunodeficiency virus (HIV) serostatus and nutritional status were ascertained among severe cases, but were not exclusion criteria, given that previous studies have shown that host response markers were still able to adequately risk stratify study participants irrespective of their HIV41 or nutritional status34. Patients were treated following the Mozambican national guidelines for malaria management, including parenteral artesunate for SM cases (until able to receive oral antimalarial medication) and artemether-lumefantrine for UM cases42. All methods were performed in accordance with the relevant guidelines and regulations.
Clinical and laboratory data
The procedures for identifying children with SM were those used in routine clinical practice at MDH. Capillary glycaemia and hematocrit were determined on admission to identify patients with hypoglycemia and anemia. A questionnaire for clinical and demographic data, together with a thorough clinical history of each child participating in the study (including information on use of antimalarial drugs before attending the hospital), was completed. Additionally, for each admitted SM case a summary sheet of information regarding the participant’s course during hospitalization was collected at discharge, regardless of outcome.
Hematological and biochemical parameters were performed for each patient using Vitros DT60 and Sysmex Kx21 analyzers. Thick and thin blood films for malaria diagnosis were processed as previously detailed39,43. As part of the routine clinical management at CISM, a semiquantitative “cross” system is used, classifying parasitemia levels from 0 (no malaria infection) to +++++ (“5 crosses”, high parasitemia infection) according to the number of trophozoites per field44. In addition, and after recruitment, the Lambaréné method45 and molecular methods (qPCR) were both used to calculate definitive peripheral parasitemia. However, for recruitment purposes, it was only feasible to pair cases and controls using the semiquantitative “cross” method.
Blood collection and processing
Whole blood was collected at recruitment by venipuncture (5 ml in children < 5 years and 10 ml in children between 5 and 10 years). Two drops of the blood were spotted onto a Whatman 903 filter paper. Blood was anticoagulated using acid citrate dextrose, centrifuged within 4 h of sample collection, and the resulting plasma was aliquoted, frozen, and stored at − 80 °C without thawing until analyzed. Samples were thawed overnight at 4 °C and aliquoted at room temperature immediately prior to each assay performance.
qPCR
Total genomic DNA (gDNA) was extracted from a blood drop spotted onto filter paper using QIAmp DNA Mini Kit (Qiagen) and tested in duplicate to measure the parasite density by real-time quantitative PCR (qPCR) targeting the P. falciparum 18S ribosomal RNA gene46. Parasitemia was quantified by extrapolation of cycle thresholds (Ct) from a standard curve of P. falciparum ring infected erythrocytes. Samples without amplification (no Ct detected) were considered negative. A negative control with no template DNA was run in all reactions.
Quantification of biomarkers in plasma
A commercial HRP-2 Enzyme-linked immunosorbent assay (ELISA) kit (Malaria Ag CELISA; Cellabs Pty. Ltd., Brookvale, New South Wales, Australia) was used to estimate HRP-2 levels at ISGlobal laboratory facilities in Barcelona, Spain. One hundred µL of each plasma sample were transferred to the ELISA plates in duplicate along with necessary controls and a standard curve, and the plates were incubated at room temperature for 1 h in a humid chamber followed by 5 washing steps with the washing solution provided in the kit. One hundred μl of the diluted antibody conjugate was added to each well after completion of 1 h of incubation, followed by 5 washing steps as stated above, 100 μL of the chromogen substrate (tetramethylbenzidine) were added to each well. Plates were incubated for 15 min in the dark, followed by addition of 50 μL of the stop solution. Spectrophotometric analysis was performed at 450 nm.
Host biomarkers were quantified at University Health Network, Canada, using two custom-developed Luminex panels from R&D Systems. Panel 1 included 3 high-abundance biomarkers, tested at a 1:20 dilution: BDNF, sTNFR-1 and CysC. Panel 2 included 7 low-abundance biomarkers, tested at a 1:2 dilution: IL-6, IL-8, IP-10, Angpt-2, Angpt-1, sFlt-1 and sTREM-1. sTie-2 was quantified by ELISA (R&D Systems, Minneapolis, MN) at a dilution of 1:20. Unfiltered plasma was diluted in assay diluents provided by the manufacturer. Each 96 well plate included a 7-point serial dilution of standards, in duplicate, and 72 patient samples, 8 of which were tested in duplicate. Assays were performed according to manufacturer’s Luminex or ELISA protocols.
Statistical analysis
Data were analyzed using R statistical software (v4.2 or higher). Descriptive statistics included arithmetic means with standard deviations (SD), geometric means with 95% confidence intervals (CIs), medians with interquartile ranges (IQRs), and percentages, as appropriate. Comparisons between matched cases and controls were performed using paired t-tests and Wilcoxon signed-rank tests for numerical variables, and McNemar’s and Cochran’s tests for categorical variables. Paired t-tests were also used to compare biomarker levels between uncomplicated malaria (UM) and severe malaria (SM) cases.
Conditional logistic regression models, stratified by matched pairs, were fitted to estimate odds ratios (ORs) and 95% CIs, assessing the association between HRP-2 levels, biomarkers, and SM. Biomarker data were log-transformed prior to modeling. To account for potential confounders, additional models adjusted for HRP-2 levels were fitted, although these results are not included in this manuscript. Both unadjusted and Benjamin-Hochberg-adjusted p-values are reported.
In children with SM, biomarker levels were compared across LODS score groups using Mann–Whitney tests, with Holm-adjusted p-values for multiple comparisons. Pairwise correlations between HRP-2 and biomarkers were assessed using Spearman’s correlation coefficients. An adjusted p-value of less than 0.05 was considered statistically significant.
Ethical considerations
This study was reviewed and approved by the Mozambican National Bioethics Committee (CNBS) (Ref. 71/CNBS/2014), the University Health Network Research Ethics Committee, Toronto, Canada (UHN REB Number 15-9013-AE), and the Clinical Research Ethics Committee of the Hospital Clínic, Barcelona, Spain (Ref. HCB/2013/8749). Children were recruited only after a written informed consent was signed by their parents/legal guardians.
Results
Characteristics of study participants
A total of 163 children were enrolled between September 2014 and May 2016. Of these, 79 presented with UM and 84 with SM. For the final analysis, children with negative qPCR, and positive blood cultures were excluded. After individual matching by sex, age, and parasitemia (based on the “cross” system), children with absence of a matched case or control were also excluded. Finally, 116 children were included in the study analysis (58 pairs of children with UM and SM matched by age, sex and parasitemia). Their demographic and clinical characteristics are presented in Table 1. Overall, mean age was 49.5 months (SD 25.81) and 63.8% were males. Eight children, 4 with SM and 4 with UM, were HIV positive.
Characteristics of children with SM
Among children admitted with SM, eight children (13.8%) presented with CM, 33 (56.9%) with multiple seizures, and 45 (77.6%) with prostration (Table 1). Twenty-one children (36.2%) had acidosis and/or acute respiratory distress. Six children (6.7%) presented with severe malarial anemia and 2 (3.4%) with hypoglycemia. The number of children with SM with a LODS score of 0, 1, 2, and 3, were 13 (22.4%), 37 (63.8%), 7 (12.1%) and 1 (1.7%), respectively. Fifty-three (91.4%) children with SM survived, 1 (1.8%) child died, 1 (1.8%) child absconded, and 2 (3.5%) children were transferred to a higher-level facility (Table 1). No deaths occurred in the UM group.
Biomarker levels in UM versus SM patients
Table 2 summarizes biomarker levels in UM when compared to SM patients. The median parasitemia measured by microscopy or by qPCR in children with SM was not significantly different compared to matched UM controls (Table 1). Plasma HRP-2 levels in children with UM [GeoMean (95% CI): 88.63 ng/mL (44.01 to 178.48)) were lower (OR = 1.42 (1.03 to 1.94), adjusted p-value = 0.056) than in children with SM [GeoMean (95% CI): 150.53 (70.57 to 321.1)) (Table 2). Biomarkers of immune and endothelial activation in SM and matched UM are depicted in Table 2 and Fig. 1. The ratio Angpt-2/Angpt-1 (OR 2.87, 95% CI [1.24, 6.61]), Angpt-2 (OR 7.97, 95% CI [1.84, 34.61]), sTie-2 (OR 4.62, 95% CI [1.39, 15.4]), sTNFR-1 (OR 4.79, 95% CI [1.5, 15.26]), sFlt-1 (OR 7.64, 95% CI [2.27, 25.69]), IL-6 (OR 1.83, 95% CI [1.23, 2.72]), IL-8 (OR 2.38, 95% CI [1.27, 4.46]), IP-10 (OR 2.05, 95% CI [1.2, 3.52]), and sTREM-1 (OR 3.13, 95% CI [1.13, 8.63) were significantly increased in SM compared to UM (adjusted p-value ≤ 0.05). Angpt-1, BDNF and CysC did not significantly differ between groups (adjusted p-value > 0.05) (Table 2 and Fig. 1).
Distribution of biomarker levels in severe malaria (SM) vs. uncomplicated malaria (UM). Boxplot represents median and interquartile range while violin plot is the density curve of the biomarker by group. The Angpt-2/Angpt-1 ratio and the levels of Angpt-2, sTie-2, sTNRF-1, sFlt-1, IL-6, IL-8, IP-10, and sTREM-1 were significantly higher in children with SM when compared with children with UM. P. falciparum histidine-rich protein 2 (HRP-2); angiopoietin 1 and 2 (Angpt-1, Angpt-2); ratio Angpt-2/Angpt-1 (Angpt-2/Angpt-1); brain-derived neurotrophic factor (BDNF); cystatin C (CysC); soluble FMS-like tyrosine kinase-1 (sFlt-1); interleukin (IL-6); interleukin (IL-8); 10 kDa interferon γ-induced protein (IP-10); soluble tyrosine-protein kinase receptor TEK (sTie-2); soluble tumor necrosis factor receptor 1 (sTNFR-1); soluble triggering receptor expressed on myeloid cells-1 (sTREM-1).
HRP-2 and host biomarkers levels
We also assessed the correlation between levels of HRP-2 with host biomarkers in children with SM and UM. Angpt-2, sTie-2, sFlt-1, sTNFR-1, and sTREM-1 were significantly correlated with HRP-2 levels for both SM and UM, with correlation coefficients ranging from 0.120 for sFlt-1 in UM, to 0.297 for sTNFR-1 in UM (Fig. 2).
Correlation between P. falciparum histidine-rich protein 2 (HRP-2) levels and host biomarker levels. Pairwise correlations between HRP-2 and biomarkers were assessed using Spearman’s correlation coefficients. An adjusted p-value of less than 0.05 was considered statistically significant. Angpt-2, sTie-2, sFlt-1, sTNFR-1, and sTREM-1 were significantly correlated with HRP-2 levels for both SM and UM. Angiopoietin 1 and 2 (Angpt-1, Angpt-2); ratio Angpt-2/Angpt-1 (Angpt-2/Angpt-1); brain-derived neurotrophic factor (BDNF); cystatin C (CysC); soluble FMS-like tyrosine kinase-1 (sFlt-1); interleukin (IL-6); interleukin (IL-8); 10 kDa interferon γ-induced protein (IP-10); soluble tyrosine-protein kinase receptor TEK (sTie-2); soluble tumor necrosis factor receptor 1 (sTNFR-1); soluble triggering receptor expressed on myeloid cells-1 (sTREM-1); severe malaria (SM); uncomplicated malaria (UM).
Discussion
In this matched case–control study we investigated the different associations between parasite and host biomarkers factors and disease severity in Mozambican children with UM and SM. Although different analyses have previously assessed host factors or parasite factors in relation to the severity in malaria, this is the first attempt to do so -simultaneously- in Mozambican children. The Angpt-2/Angpt-1 ratio and the levels of Angpt-2, sTie-2, sTNRF-1, sFlt-1, IL-6, IL-8, IP-10, and sTREM-1 were significantly higher in children with SM when compared with children with UM. On the other hand, HRP-2 levels were significantly correlated with levels of biomarkers (both in UM and SM cases) like Angpt-2, sTie-2, sFlt-1, sTNFR-1, and sTREM-1. These data show that host biomarkers of immune and endothelial activation measured at hospital presentation were correlated with HRP-2 in children with SM, which may help to better understand the physiopathology “puzzle” of severe malaria4.
Our findings regarding higher levels of biomarkers in SM cases align with current literature, as different members of the Angpt-Tie axis have been associated with the pathophysiology of SM17. sTie-2 is the receptor of both Angpt-1 and Angpt-2. When Angpt-1 bounds to sTie-2, it promotes endothelial stability and vascular quiescence, as well as anti-inflammatory and anti-apoptotic effects17. However, Angpt-2 antagonizes these actions and, when released from endothelial cells, triggers a pro-inflammatory and pro-coagulant state. In our study, we confirmed previous reports describing high levels of Angpt-2 in both adults and children as a biomarker of severity in malaria infection19,20,21,22,23,24,25,26. In addition, the ratio Angpt-2:Angpt-1 was also significantly higher in the SM group compared to UM. Finally, this study also confirmed previous findings where higher levels of sTie-2 were described in SM in comparison to UM21,22. These data provide further evidence that dysregulation of the angiopoietin-Tie axis is involved in the pathophysiology of SM47 and may be used as therapeutic target48,49.
SM, including severe malarial anemia and CM, has been correlated with a dysregulated pro-inflammatory state21,22,27,28,50. sFlt-1 binds the vascular endothelial growth factor (VEGF) and is expressed in monocytes and endothelium. Its expression is induced by hypoxia and VEGF, when imbalanced, is thought to contribute to vascular dysregulation. High levels of sFlt-1 have been associated to SM22,31 and our data confirm this association. On the other hand, IL-6 and IL-8 have been found to increase in children with SM27,28,29,50. This could be explained by the initiation of a pro-inflammatory state in severe children that could help to identify those who are at higher risk of progress to more severe forms of the disease. This study also confirmed findings that IP-10, a pro-inflammatory chemokine, is associated with CM and can accurately discriminate children with prolonged clinical recovery times and higher mortality22,31. Finally, this study also showed increased sTNFR-1 levels in children with SM. Tumour necrosis factor alpha (TNF) is another pro-inflammatory cytokine that is elevated in different severe diseases, including CM and severe malarial anemia51. That finding has triggered some attempts to find anti-TNF therapies for SM although without positive results52.
The activation of the soluble triggering receptor expressed on myeloid cells 1 (sTREM-1) is involved in pro-inflammatory responses. sTREM-1 negatively regulates TREM-1, and both molecules maintain a physiological balance53. High levels of circulating sTREM-1 reflects a dysregulated immune response. Elevated sTREM-1 have been observed in children with SM when compared with UM30,54 and sTREM-1 levels correlated well with poor prognosis and mortality, not only in malaria18,22,31,34, but also in the context of many other underlying infections18,41,55. Hence, our results confirm that sTREM-1 levels were higher in children with SM, evidencing its potential as a malaria severity biomarker.
Beyond host biomarkers, our findings also revealed that HRP-2 levels in children with SM [GeoMean (95% CI): 150.53 (70.57 to 321.1) were higher than in children with UM [GeoMean (95% CI): 88.63 ng/mL (44.01 to 178.48)). Although geometric means are not significantly different (P = 0.056) there is a clear trend towards higher HRP-2 in SM [OR = 1.42 (1.03 to 1.94), p-value = 0.031] (Table 2). Both UM and SM groups were matched by parasitemia (measured through microscopy), and our analysis through qPCR confirmed no significant differences between them (Table 1). A possible explanation for this is that HRP-2 is a more accurate indicator of the real parasite biomass of an infected case, as it considers sequestered parasites that are not detected in peripheral blood56. It would be interesting to confirm whether levels of HRP-2 among incidental parasitemia cases (malaria infections among asymptomatic children) were even lower, further helping to classify the different spectrum of disease in malaria, but our data do not allow us to explore this hypothesis. Importantly, data from 2010–201657, 201858 and 2023 (in press) show that hrp2/3 deletions are rare in Mozambique.
Lastly, our data found statistically significant associations between levels of HRP-2 and other biomarkers like Angpt-2, displaying the close interaction between these markers in the pathophysiology of SM. To our knowledge no previous studies have examined relationship between HRP-2 and host-biomarkers. It has previously been theorized that the physiological interaction between endothelial dysfunction and other pathways of severity may be triggered by a high parasite biomass; which is reinforced by our findings4. The association of HRP-2 with other host biomarkers makes it plausible to think about incorporating both biomarkers with quantitative measures to manage children with SM, guide risk stratification, and improve their outcome, as HRP-2 is the main antigen used in malaria rapid diagnostic tests. Further research is needed to better investigate these findings, but their future use as part of a rapid, point-of-care, low-cost prognostic test or as therapeutic targets might improve the management of this disease, whose impact in resource-constrained countries remains unacceptably high.
Despite the valuable insights gained from this study, it is crucial to recognize and address certain limitations to our findings. First, biomarker levels were only measured at hospital first encounter, where it was followed by immediate antimalarial treatment, and we did not evaluate the dynamics of biomarker levels in response to infection. Secondly, we did not analyze the levels of biomarkers in relation to specific SM syndromes or LODS score due to the low number of patients in each of those groups. Thirdly, low numbers of fatal malaria episodes hindered exploring variations in biomarker levels in relation to mortality. Also, we did not measure levels of host or parasite biomarkers in asymptomatic infected patients, which could have given further insights on the predictive capacity of those markers in risk-stratifying the entire spectrum of disease. A similar analysis of the same host-response biomarkers analyzed here, but conducted in Mozambican children with pneumonia, showed that all biomarkers were significantly elevated in 472 pneumonia cases versus 80 healthy community controls (p < 0.001)55. In addition, levels of host biomarkers were measured using citrate as anticoagulant, as many other studies have used EDTA as anticoagulant, this different methodology must be considered, as this can have an impact on biomarker levels. Finally, the particular matching methodology could also hinder comparison with other studies.
Conclusions
Our findings confirmed that levels of HRP-2 accurately indicate SM and is significantly correlated with other host biomarkers that present higher levels in SM cases, like Angpt-2, sTie-2, sFlt-1, sTNFR-1, and sTREM-1. This association was previously untested, and as host biomarkers associated with endothelial activation and inflammation are differently expressed in patients with greater severity, this allows for future studies to investigate the inclusion of quantitative measurements of parasite and host biomarkers in pediatric SM management to improve patient outcome.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
World malaria report 2023. (Geneva, 2023).
WHO. Severe malaria. Trop. Med. Int. Health 19(Suppl 1), 7–131. https://doi.org/10.1111/tmi.12313_2 (2014).
Miller, L. H., Baruch, D. I., Marsh, K. & Doumbo, O. K. The pathogenic basis of malaria. Nature 415, 673–679. https://doi.org/10.1038/415673a (2002).
Cunnington, A. J., Walther, M. & Riley, E. M. Piecing together the puzzle of severe malaria. Sci. Transl. Med. 5, 211ps218. https://doi.org/10.1126/scitranslmed.3007432 (2013).
Desakorn, V. et al. Stage-dependent production and release of histidine-rich protein 2 by Plasmodium falciparum. Trans. Roy. Soc. Trop. Med. Hyg. 99, 517–524. https://doi.org/10.1016/j.trstmh.2004.11.014 (2005).
Dondorp, A. M. et al. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med. 2, e204. https://doi.org/10.1371/journal.pmed.0020204 (2005).
Rubach, M. P. et al. Plasma Plasmodium falciparum histidine-rich protein-2 concentrations are associated with malaria severity and mortality in Tanzanian children. PLoS ONE 7, e35985. https://doi.org/10.1371/journal.pone.0035985 (2012).
Seydel, K. B. et al. Plasma concentrations of parasite histidine-rich protein 2 distinguish between retinopathy-positive and retinopathy-negative cerebral malaria in Malawian children. J. Infect. Dis. 206, 309–318. https://doi.org/10.1093/infdis/jis371 (2012).
Kariuki, S. M. et al. Value of Plasmodium falciparum histidine-rich protein 2 level and malaria retinopathy in distinguishing cerebral malaria from other acute encephalopathies in Kenyan children. J. Infect. Dis. 209, 600–609. https://doi.org/10.1093/infdis/jit500 (2014).
Boyce, R. et al. Use of a dual-antigen rapid diagnostic test to screen children for severe Plasmodium falciparum malaria in a high-transmission, resource-limited setting. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 65, 1509–1515. https://doi.org/10.1093/cid/cix592 (2017).
Park, G. S. et al. Plasmodium falciparum histidine-rich protein-2 plasma concentrations are higher in retinopathy-negative cerebral malaria than in severe malarial anemia. Open Forum Infect. Dis. 4, ofx151. https://doi.org/10.1093/ofid/ofx151 (2017).
Hendriksen, I. C. et al. Defining falciparum-malaria-attributable severe febrile illness in moderate-to-high transmission settings on the basis of plasma PfHRP2 concentration. J. Infect. Dis. 207, 351–361. https://doi.org/10.1093/infdis/jis675 (2013).
Hendriksen, I. C. et al. Diagnosing severe falciparum malaria in parasitaemic African children: A prospective evaluation of plasma PfHRP2 measurement. PLoS Med. 9, e1001297. https://doi.org/10.1371/journal.pmed.1001297 (2012).
Hendriksen, I. C. et al. Diagnosis, clinical presentation, and in-hospital mortality of severe malaria in HIV-coinfected children and adults in Mozambique. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 55, 1144–1153. https://doi.org/10.1093/cid/cis590 (2012).
Duffy, F. et al. Meta-analysis of Plasmodium falciparum var signatures contributing to severe malaria in African children and Indian adults. MBio 10, 10–1128. https://doi.org/10.1128/mBio.00217-19 (2019).
Walker, I. S. & Rogerson, S. J. Pathogenicity and virulence of malaria: Sticky problems and tricky solutions. Virulence 14, 2150456. https://doi.org/10.1080/21505594.2022.2150456 (2023).
de Jong, G. M., Slager, J. J., Verbon, A., van Hellemond, J. J. & van Genderen, P. J. Systematic review of the role of angiopoietin-1 and angiopoietin-2 in Plasmodium species infections: Biomarkers or therapeutic targets?. Malar. J. 15, 581. https://doi.org/10.1186/s12936-016-1624-8 (2016).
Leligdowicz, A. et al. Risk-stratification of febrile African children at risk of sepsis using sTREM-1 as basis for a rapid triage test. Nat. Commun. 12, 6832. https://doi.org/10.1038/s41467-021-27215-6 (2021).
Lovegrove, F. E. et al. Serum angiopoietin-1 and -2 levels discriminate cerebral malaria from uncomplicated malaria and predict clinical outcome in African children. PLoS ONE 4, e4912. https://doi.org/10.1371/journal.pone.0004912 (2009).
Conroy, A. L. et al. Whole blood angiopoietin-1 and -2 levels discriminate cerebral and severe (non-cerebral) malaria from uncomplicated malaria. Malar. J. 8, 295. https://doi.org/10.1186/1475-2875-8-295 (2009).
Conroy, A. L. et al. Endothelium-based biomarkers are associated with cerebral malaria in Malawian children: A retrospective case-control study. PLoS ONE 5, e15291. https://doi.org/10.1371/journal.pone.0015291 (2010).
Erdman, L. K. et al. Combinations of host biomarkers predict mortality among Ugandan children with severe malaria: A retrospective case-control study. PLoS ONE 6, e17440. https://doi.org/10.1371/journal.pone.0017440 (2011).
Conroy, A. L. et al. Angiopoietin-2 levels are associated with retinopathy and predict mortality in Malawian children with cerebral malaria: A retrospective case-control study*. Crit. Care Med. 40, 952–959. https://doi.org/10.1097/CCM.0b013e3182373157 (2012).
Weinberg, J. B. et al. Dimethylarginines: Endogenous inhibitors of nitric oxide synthesis in children with falciparum malaria. J. Infect. Dis. 210, 913–922. https://doi.org/10.1093/infdis/jiu156 (2014).
Abdi, A. I. et al. Plasmodium falciparum antigenic variation: relationships between widespread endothelial activation, parasite PfEMP1 expression and severe malaria. BMC Infect. Dis. 14, 170. https://doi.org/10.1186/1471-2334-14-170 (2014).
Moxon, C. A. et al. Persistent endothelial activation and inflammation after Plasmodium falciparum infection in Malawian children. J. Infect. Dis. 209, 610–615. https://doi.org/10.1093/infdis/jit419 (2014).
Ong’echa, J. M., Davenport, G. C., Vulule, J. M., Hittner, J. B. & Perkins, D. J. Identification of inflammatory biomarkers for pediatric malarial anemia severity using novel statistical methods. Infect. Immun. 79, 4674–4680. https://doi.org/10.1128/iai.05161-11 (2011).
Rovira-Vallbona, E. et al. Low antibodies against Plasmodium falciparum and imbalanced pro-inflammatory cytokines are associated with severe malaria in Mozambican children: a case-control study. Malar. J. 11, 181. https://doi.org/10.1186/1475-2875-11-181 (2012).
Oyegue-Liabagui, S. L. et al. Pro- and anti-inflammatory cytokines in children with malaria in Franceville, Gabon. Am. J. Clin. Exp. Immunol. 6, 9–20 (2017).
Adukpo, S. et al. Triggering receptor expressed on myeloid cells 1 (TREM-1) and cytokine gene variants in complicated and uncomplicated malaria. Trop. Med. Int. Health 21, 1592–1601. https://doi.org/10.1111/tmi.12787 (2016).
Conroy, A. L. et al. Host biomarkers are associated with response to therapy and long-term mortality in pediatric severe malaria. Open Forum Infect. Dis. 3, 134. https://doi.org/10.1093/ofid/ofw134 (2016).
Armah, H. B. et al. Cerebrospinal fluid and serum biomarkers of cerebral malaria mortality in Ghanaian children. Malar. J. 6, 147. https://doi.org/10.1186/1475-2875-6-147 (2007).
McDonald, C. R. et al. Brain-derived neurotrophic factor is associated with disease severity and clinical outcome in Ugandan children admitted to hospital with severe malaria. Pediatr. Infect. Dis. J. 36, 146–150. https://doi.org/10.1097/inf.0000000000001382 (2017).
Mufumba, I. et al. sTREM-1: A biomarker of mortality in severe malaria impacted by acute kidney injury. J. Infect. Dis. 229, 936–946. https://doi.org/10.1093/infdis/jiad561 (2024).
Conroy, A. L. et al. Prospective validation of pediatric disease severity scores to predict mortality in Ugandan children presenting with malaria and non-malaria febrile illness. Crit. Care (London, England) 19, 47. https://doi.org/10.1186/s13054-015-0773-4 (2015).
Helbok, R. et al. The Lambarene organ dysfunction score (LODS) is a simple clinical predictor of fatal malaria in African children. J. Infect. Dis. 200, 1834–1841. https://doi.org/10.1086/648409 (2009).
Sacoor, C. et al. Profile: Manhica health research centre (Manhica HDSS). Int. J. Epidemiol. 42, 1309–1318. https://doi.org/10.1093/ije/dyt148 (2013).
Guinovart, C. et al. Malaria in rural Mozambique. Part I: Children attending the outpatient clinic. Malar. J. 7, 36. https://doi.org/10.1186/1475-2875-7-36 (2008).
Bassat, Q. et al. Malaria in rural Mozambique. Part II: Children admitted to hospital. Malar. J. 7, 37. https://doi.org/10.1186/1475-2875-7-37 (2008).
Guinovart, C. et al. The epidemiology of severe malaria at Manhiça district hospital, Mozambique: A retrospective analysis of 20 years of malaria admissions surveillance data. Lancet Glob. Health 10, e873–e881. https://doi.org/10.1016/s2214-109x(22)00125-5 (2022).
Richard-Greenblatt, M. et al. Prognostic accuracy of soluble triggering receptor expressed on myeloid cells (sTREM-1)-based algorithms in febrile adults presenting to Tanzanian outpatient clinics. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 70, 1304–1312. https://doi.org/10.1093/cid/ciz419 (2020).
Malaria, P. N. d. C. d. (ed Ministry of Health) (Maputo, 2011).
Bassat, Q. et al. Severe malaria and concomitant bacteraemia in children admitted to a rural Mozambican hospital. Trop. Med. Int. Health 14, 1011–1019. https://doi.org/10.1111/j.1365-3156.2009.02326.x (2009).
WHO. Basic Laboratory Methods in Medical Parasitology. (1999).
Planche, T. et al. Comparison of methods for the rapid laboratory assessment of children with malaria. Am. J. Trop. Med. Hyg. 65, 599–602. https://doi.org/10.4269/ajtmh.2001.65.599 (2001).
Hermsen, C. C. et al. Detection of Plasmodium falciparum malaria parasites in vivo by real-time quantitative PCR. Mol. Biochem. Parasitol. 118, 247–251 (2001).
Higgins, S. J. et al. Dysregulation of angiopoietin-1 plays a mechanistic role in the pathogenesis of cerebral malaria. Sci. Transl. Med. 8, 358ra128. https://doi.org/10.1126/scitranslmed.aaf6812 (2016).
Varo, R. et al. Adjunctive rosiglitazone treatment for severe pediatric malaria: A randomized placebo-controlled trial in Mozambican children. Int. J. Infect. Dis. 139, 34–40. https://doi.org/10.1016/j.ijid.2023.11.031 (2024).
Hawkes, M. T. et al. Inhaled nitric oxide as adjunctive therapy for severe malaria: A randomized controlled trial. Malar. J. 14, 421. https://doi.org/10.1186/s12936-015-0946-2 (2015).
Lyke, K. E. et al. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect. Immun. 72, 5630–5637. https://doi.org/10.1128/iai.72.10.5630-5637.2004 (2004).
Shabani, E. et al. Elevated cerebrospinal fluid tumour necrosis factor is associated with acute and long-term neurocognitive impairment in cerebral malaria. Parasite Immunol. 39, e12438. https://doi.org/10.1111/pim.12438 (2017).
Varo, R. et al. Adjunctive therapy for severe malaria: A review and critical appraisal. Malar. J. 17, 47. https://doi.org/10.1186/s12936-018-2195-7 (2018).
Klesney-Tait, J., Turnbull, I. R. & Colonna, M. The TREM receptor family and signal integration. Nat. Immunol. 7, 1266–1273. https://doi.org/10.1038/ni1411 (2006).
Tahar, R. et al. Plasma levels of eight different mediators and their potential as biomarkers of various clinical malaria conditions in African children. Malar. J. 15, 337. https://doi.org/10.1186/s12936-016-1378-3 (2016).
Balanza, N. et al. Prognostic accuracy of biomarkers of immune and endothelial activation in Mozambican children hospitalized with pneumonia. PLOS Glob. Public Health 3, e0001553. https://doi.org/10.1371/journal.pgph.0001553 (2023).
Sinha, I., Ekapirat, N., Dondorp, A. M. & Woodrow, C. J. Use of a rapid test to assess plasma Plasmodium falciparum HRP2 and guide management of severe febrile illness. Malar. J. 14, 362. https://doi.org/10.1186/s12936-015-0900-3 (2015).
Gupta, H. et al. Molecular surveillance of pfhrp2 and pfhrp3 deletions in Plasmodium falciparum isolates from Mozambique. Malar. J. 16, 416. https://doi.org/10.1186/s12936-017-2061-z (2017).
Plucinski, M. M. et al. Assessing performance of HRP2 antigen detection for malaria diagnosis in Mozambique. J. Clin. Microbiol. 57, 10–1128. https://doi.org/10.1128/jcm.00875-19 (2019).
Author information
Authors and Affiliations
Contributions
QB, AM, KCK conceived of the study and QB, AM, KCK, LM and RV contributed to study design. RV and AS implemented the study. RV and AS acquired the clinical data. CJ, AC, IC participated in sample processing. RV and NB were involved in data management. AMW, KZ and VMC acquired the biomarker data. PC carried out the molecular analysis. DB and XMV performed the HRP-2 determinations. HG coordinated the sample processing and HRP-2 determinations. KCK, QB, AM, RV, LQ, NB and DO analyzed and interpreted the data. RV drafted the manuscript. QB, AM, KCK, RV, VMC, NB and DO critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
This work was partially funded by the Departament d’Universitats i Recerca de la Generalitat de Catalunya (AGAUR; 2021 SGR 01517). We also acknowledge support from the Spanish Ministry of Science and Innovation and State Research Agency through the “Centro de Excelencia Severo Ochoa 2019–2023” program (CEX2018-000806-S), and support from the Generalitat de Catalunya through the CERCA Program. This research is part of the ISGlobal’s Program on the Molecular Mechanisms of Malaria which is partially supported by the Fundación Ramón Areces. CISM is supported by the Government of Mozambique and the Spanish Agency for International Development (AECID). NB is supported by an FPU predoctoral fellowship from the Spanish Ministry of Universities (FPU18/04260). The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of this report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. The authors declare not having financial competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Varo, R., Sitoe, A., Madrid, L. et al. Host biomarkers and parasite biomass are associated with severe malaria in Mozambican children: a case–control study. Sci Rep 15, 14262 (2025). https://doi.org/10.1038/s41598-025-98154-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-98154-1
Keywords
This article is cited by
-
Potential of microRNAs as diagnostic markers for distinguishing malaria severity in samples from an Indian cohort
BMC Infectious Diseases (2025)