Abstract
BAM15 is a novel mitochondrial uncoupling agent derived from a synthetic source, that has been wildly explored for its ability to enhance mitochondrial respiration and metabolic flexibility. In this study, we investigated the underlying mechanisms of BAM15 on atherosclerosis (AS) through experimental validation, RNA-seq and molecular docking. The results showed that oral administration of BAM15 suppressed atherosclerosis in western diet (WD)-fed ApoE(−/−) mice and significantly improved the hyperlipidemia. And the increased serum ALT, AST and liver TC, TG, ALT, AST in ApoE(−/−) mice were reduced by BAM15 treatment. In in vitro experiments BAM15 inhibited RAW264.7 macrophages invasive ability and reduced palmitic acid-induced lipid accumulation. RNA-seq results confirmed the differential genes after BAM15 treatment and 140 common targets were identified by intersecting with AS-related targets. A protein–protein interaction (PPI) network analysis high-lighted IL1A, SRC and CSF3 as key targets of BAM15 against AS, which is further verified by molecular docking and western blot. Molecular dynamics analysis results confirmed that BAM15 exhibits strong affinity with the IL-1α, SRC and CSF3 proteins. This study indicates that BAM15 inhibits atherosclerosis through a multi-molecular mechanism, and we propose it as a novel anti-atherosclerotic drug.
Similar content being viewed by others
Introduction
Atherosclerosis is a chronic disease of the arterial wall, and a leading cause of death and loss of productive life years worldwide1. Atherosclerosis is considered to be caused by multiple factors, including genetic and environmental factors2,3. In humans, it takes several decades until the clinical complications develop. There are many known risk factors for atherosclerosis, including hypercholesterolemia, hypertension, diabetes and smoking, which are involved in the pathogenesis of atherosclerosis4,5. However, it is generally believed that atherosclerosis is vascular chronic inflammation initiated by interactions of these risk factors and arterial wall cells. In the past 30 years, the molecular mechanisms underlying the pathogenesis of atherosclerosis have been investigated extensively using genetically modified animals6, and lipid-reducing drugs, such as statins, have been demonstrated as the most effective for the prevention and treatment of atherosclerosis7. However, despite this progress, questions regarding the pathogenesis of atherosclerosis remain and there is a need to develop new animal models and novel therapeutics to treat patients who cannot be effectively treated by statins.
In recent years, with the continuous deepening of the exploration of mitochondrial uncouplers, people have gradually realized that mitochondrial uncouplers have great potential for the treatment of various diseases, such as cancer, obesity, diabetes, etc8,9. Among many mitochondrial uncouplers, BAM15 has outstanding advantages in disease treatment and clinical transformation due to its mild uncoupling effect, which reduced off-target effects, rendering it an attractive candidate for therapeutic intervention10. Studies have confirmed the beneficial effects of mild mitochondrial uncoupling, developing mitochondrial uncouplers to induce mild mitochondrial uncoupling is a strategy for treating not only the metabolic disorders but also the heart, vascular, and nerve injury11.
BAM15 is a novel mitochondrial uncoupling agent derived from a synthetic source10. The chemical formula is (N5,N6-bis(2-fluorophenyl)[1,2,5]oxadiazolo[3,4-b]pyrazine-5,6-diamine). Oral bioavailability of BAM15 in mice by comparing pharmacokinetics when delivered per oral (p.o.) or by intravenous (i.v.) tail vein injection. A dose-normalized area under the curve calculation showed that BAM15 was 67% orally bioavailable and p.o. delivery resulted in an average maximum plasma concentration (Cmax) of 8.2 µM with a 1.7 h half-life (t1/2) in C57BL/6 J mice. BAM15 tissue distribution was determined by orally administering BAM15 (50 mg/kg) and euthanizing mice at times 0.5, 1, 2, and 4 h. BAM15 measurement in tissue extracts revealed primary distribution to the liver with gradual clearance from tissues over 4 h12. Recent studies have identified that BAM15 increases nutrient oxidation, and decreases body fat mass without altering food intake, lean body mass, body temperature, or biochemical and haematological markers of toxicity12. BAM15 decreases hepatic fat, decreases inflammatory lipids, and has strong antioxidant effects13. In addition, our previous confirmed that BAM15 inhibited artery constriction and potently activates AMPK in vascular smooth muscle cells14. In macrophages, BAM15 suppresses the translocation of NF-κB into the nucleus, thereby inhibiting the expression of NLRP3 and IL-1β15. Since AMPK and NLRP3 are both important targets for combating AS15, the previous work indicated that BAM15 could be developed as a novel drug for AS. Therefore, we attempted to explore the anti-atherosclerosis effect and mechanism of BAM15 by experimental verification.
With the rapid development of bioinformatics, systems biology and pharmacology, network-based drug discovery is considered as a more efficient method to discover drug targets16,17. RNA sequencing (RNA-seq) is an emerging technology that enables qualitative and quantitative analysis of RNA to obtain mRNA expression profiles in cells and clinical samples, thus being widely employed for research on immune response, complex disease mechanisms, drug targets, and molecular marker screening in the medical field18. Moreover, molecular docking is an emerging technology that enables effectively determine the small molecule compound that matches the space, force and electrical characteristics the target receptor site and predict the possibility of binding to the target. Molecular dynamics (MD) simulations discerned ligand receptor interactions, evaluating active compound’s and therapeutic target’s binding sturdiness and flexibleness. In the present study, we applied RNA-seq technology, molecular docking and molecular dynamics simulations combined with experimental validation to identify the anti-atherosclerotic effects of BAM15 and reveal its therapeutic mechanism (Fig. 1).
Materials and methods
Animal maintenance and experiments
Wild-type male C57BL/6 J mice (18–23 g, 8 weeks old) and male ApoE(−/−) mice (18–23 g, eight weeks old) were purchased from the Corues Biotechnology (Nanjing, China, Certificate number: SCXK (Zhe) 2019-0004), and were kept in light/dark (12/12 h) cycle at 25℃ and given random food and water before the experiments. C57BL/6J mice as control group were fed normal diet and given deionized water. ApoE(−/−) mice were fed western diet (WD) (Xiaoshuyoutai Bioscience, China) to establish atherosclerosis model19. The drug treatment group based on this model were given BAM15 (CAS#:210302-17-3, purchased from MedChemExpress, USA) (85 mg kg−1d−1, dissolved in 0.5% sodium carboxymethyl cellulose) or atorvastatin (2 mg kg−1, dissolved in 0.5% sodium carboxymethyl cellulose) orally six times a week. The group of mice strictly followed the random principle. The mice were monitored daily for weight and food intake during the experiment. The experiment period was shown in the graph and/or corresponding legend.
Five mice were randomly selected from each group and euthanized by intraperitoneal injection of tribromoethanol. The aorta was removed by cutting off the minor branching arteries, followed by removal of excess tissue and fat outside the aorta, and immobilised in 10% phosphate-buffered formalin. After PBS washing, the aorta was incised longitudinally and stained with oil red O as previously described19. The plaque surface area was calculated using Image-Pro Plus 5.0 software (Media Cybernetics Inc, Silver Spring). Aorta image acquisition and lesion area analysis were carried out in a blinded manner.
The animal study protocol was approved by the Institutional Review Board of Jiangnan University Medical Center (protocol code 2021-Y-51, 31-Aug-2021). We confirm that all experiments were performed in accordance with relevant guidelines and regulations of Jiangnan University Medical Center. And all animal studies are reported in compliance with the ARRIVE guidelines.
Cell culture
The murine macrophage cell line RAW264.7 (ATCC, Cat# TIB-71, RRID: CVCL_0493, Shanghai Zhong Qiao Xin Zhou Biotechnology) was cultured in DMEM supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin at 37 °C and 5% CO2as described in our previous study19. Agent concentration and treatment time were shown in the figures and/or corresponding legends.
Cell viability
Cell viability was measured with the CCK8 assay20. Cells were incubated in advance on 96-well culture plates (5000 cells per well) and treated with different concentrations of BAM15 for 24 h, then 10 µL CCK-8 was added to each well and incubated for 1–4 h. Then the absorbance values of test wells (AS), control wells (AC), and blank wells (Ab) at 450 nm wavelength were obtained on a spectrophotometer (ThermoFisher Scientific). Inhibition ratio was calculated as follows: [(AC – AS)/(AC – Ab)] × 100%. IC50 values were calculated using GraphPad 8.0 software.
Scratch wound-healing assay
A horizontal line drawn on the back of the plate before cell seeding, 5 × 105cells were inoculated in each hole of the 6-well plate. Upon reaching 90% confluence, a 200 µL pipette tip was used to form a cell-free zone21. After drug treatment, gently wash with PBS to remove cell debris. Wound healing images were taken under an optical microscope (Olympus, CKX58). Cell migration was quantified by measuring cell-free area using ImagePro Plus image analysis software (Media Cybernetics Inc., Silver Spring).
Western blot
The protein levels were analyzed by Western blot. The methods in detail were described in our previous studies14,22. The antibodies used are as follows: CSF3 (#30415-1-AP, Proteintech, USA), IL-1α (#16765-1-AP, Proteintech, USA), SRC (#11097-1-AP, Proteintech, USA), PTX3 (#AFW12670, AiFang biological, China), β-actin (#66009-1-Ig, Proteintech, USA), β-Tubulin (#66240-1-Ig, Proteintech, USA), goat anti-rabbit IgG (#SA00002-1, Proteintech, USA), goat anti-mouse IgG (#SA00002-2, Proteintech, USA). Blots were visualized using Cytiva AI800 infrared imaging system and analyzed by Cytiva analysis software.
Flow cytometry
Flow cytometry was used to detect the cell cycle. Remove cells from growth media and wash out the serum of culture medium. The cells were suspended with 1 mL PBS buffer, gently swirled and slowly added with 3 mL pre-cooled anhydrous ethanol until the final concentration was 75%. Propidium iodide (PI) staining solution (20X): RNase A (50×) = 100:5:2, mix well to prepare the working solution, then add 500 ul of working solution to each sample and incubate at 37 °C in the dark for 30 min, and measure the absorbance value at 488 nm.
Histopathologic analysis
For histopathologic analysis, hematoxylineeosin (H&E) staining was performed using paraffin-prepared liver with procedures described in previous study23. Images were obtained using a light microscope (Olympus BX53 with DP80 camera, Japan) at 200× and 400× magnification. In addition, frozen liver tissues embedded with O.C.T Compound (Sakura Finetek USA Inc., Torrance, CA, USA) were cut into sections of 10 mm thickness, and then stained with oil red O staining solution (Sangon Biotech, Shanghai, China) to evaluate the degree of lipid deposition. Images were obtained using a light microscope (Olympus BX53 with DP80 camera, Japan) at 100× and 200× magnification.
Blood collection and analysis
The blood samples were left at room temperature for 4 h and then centrifuged at 4000×gfor 15 min to obtain serum21. The levels of serum total cholesterol (TC), triglycerides (TG), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), aspartate aminotransferase (AST), and alanine transaminase (ALT) were analyzed by enzymatic colorimetric methods using reagent kits (A110-1-1 for TG, A111-1-1 for TC, A112-1-1 for HDL-C, A113-1-1 for LDL-C, C010-2-1 for AST, C009-2-1 for ALT, njjcbio, Nanjing, China) following the instructions. The measured data were detected by BIOBASE-EL10 A (Quanzhou, China).
Oil red O staining
Under aseptic conditions, 0.1282 g palmitic acid (PA) powder was weighed and dissolved with 10 ml anhydrous ethanol to prepare 50 mM PA storage solution. The concentration of PA working solution was 0.5 mM and diluted with 0.5%BSA. Cells were treated with PA solution (0.5 mM) and various concentrations of BAM15 in 6-well culture plates. After 24 h, the culture medium in the plate was sucked away, rinsed with 60% isopropyl alcohol for 1 min, stained with oil red O for 15 min, rinsed with 60% isopropyl alcohol, and observed under microscope and photographed.
RNA-seq analysis
Total RNA was extracted with TRIzol reagent (TIANGEN, China) and detected on a 1% agarose gel. The purity, concentration, and integrity of total RNA samples were assessed24. After the generation of clusters, library preparations were sequenced on the Illumina NovaSeq 6000 platform, resulting in the production of raw reads. Subsequently, these raw reads underwent filtering to remove adapter sequences, poly-N sequences, and low-quality reads. The clean reads were aligned to the reference genome using HISAT2 tools. The levels of gene expression were quantified by calculating fragments per kilobase of transcript per million mapped fragments. StringTie was utilized for assembling the aligned reads. Gene expression analysis across different groups was conducted using DESeq2, with a fold change threshold set at ≥ 2 and a false discovery rate (FDR) < 0.05 as screening criteria.
Network construction
The AS-related targets were collected from the GeneCards database (https://www.genecards.org/) and DisGeNET (https://www.disgenet.org/search), and consistent targets for AS and BAM15 were screened. The relevant targets were utilized to construct the protein-protein interaction network using Cytoscape version 3.9.1 software.
Molecular docking
The 3D structures of the compound were obtained from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/). And retrieve the crystal structure of the key targets from the RCSB PDB database (https://www.rcsb.org/) and employ PyMol 2.3.4 to eliminate the native ligands, water molecules, and ions present in the downloaded complex25. Then, utilize AutoDock Tools 1.5.6 for the hydrogenation of the protein crystal structure, charge distribution, determination of docking box size and center (keeping the whole protein covered), as well as conversion of the protein into the ‘PDBQT’ format. Finally, flexible molecular docking was performed using AutoDock Vina 1.1.2, and the resulting combination was mapped and analyzed using PyMol to obtain improved outcomes.
Molecular dynamic simulation
To further investigate the molecular mechanism of protein-ligand binding in the CSF3-BAM15, IL-1α-BAM15, and SRC-BAM15 complexes, we performed molecular dynamics (MD) simulations using the GROMACS 2020 software package. The receptor proteins in the complexes were parameterized with the AMBER99SB-ILDN force field, while the small-molecule ligands were parameterized using the GAFF universal force field. The topology of the small molecules was generated using the sobtop program, with quantum calculations performed using the B3LYP-D3/6-31G(d, p) basis set, and charges were fitted using the RESP method. The TIP3P water model was selected for solvation, and the minimum distance between the atoms in the protein and the water box edge was set to 1.0 nm. Sodium or chloride ions were added to neutralize the system’s charge, based on docking results. The MD simulation workflow comprised four main steps: energy minimization, heating, equilibration, and production dynamics. Initially, heavy atoms of the protein (and small molecules) were restrained, and water molecules underwent 10,000 steps of energy minimization (5,000 steps of steepest descent and 5,000 steps of conjugate gradient). After the restraints were removed, the entire system underwent another 10,000 steps of energy minimization. Subsequently, the system was gradually heated to 300 K over 50 ps, and after heating, the system was equilibrated for 50 ps under the NPT ensemble. Finally, a 100 ns production MD simulation was performed under the NPT ensemble. Trajectory data was saved every 10 ps and analyzed using the trjconv module. The binding free energy of the ligands and proteins was calculated using the gmxMMPBSA method in GROMACS 2020.
Statistical analysis
Statistical analysis was conducted using GraphPad Prism version 8.0 software. Comparisons between two groups were conducted using the Student’s t-test, whereas multiple-group comparisons were executed through one-way ANOVA. For Student t-test and ANOVA P < 0.05 was considered statistically significant. Results are presented as means ± SEM.
Result
Validation of BAM15 against AS in animal models
The animal model of atherosclerosis was established by ApoE(−/−) mice with western diet (WD). As shown in Fig. 2A of the experiment design, BAM15 (85 mg kg−1 d−1) was intragastrically administered for 12 weeks. Meanwhile, we used atorvastatin (2 mg kg−1 d−1, p.o) equivalent to clinical dose as the positive control. The average body weight and food intake of ApoE(−/−) mice in each group during the period of treatment (84 days) were shown in Fig. 2B, C. The body weight of the ApoE(−/−) mice given oral administration of BAM15 and atorvastatin was significantly lower than that of the ApoE(−/−) model group at 12 weeks. And there was no difference of food intake among the groups. The thoracic part of aorta was collected and the aortic plaque lesion was quantified by using Oil Red O staining. As shown in Fig. 2D and E, ApoE(−/−) mice fed with WD for 12 weeks showed marked increase of plaque lesion, and BAM15 (85 mg kg−1 d−1) and atorvastatin (2 mg kg−1 d−1) treatment significantly diminished the increased plaque area. Wild type mice fed with normal chow diet showed no plaque lesion.
The histopathology of aortic valve region was observed by Movat’s pentachrome staining (MOVAT staining), and the representative images were shown in Fig. 3. Analysed data showed that BAM15 (85 mg kg−1) treatment significantly reduced the increased necrotic core area of plaque induced by WD feeding in ApoE(−/−) mice.
BAM15 inhibited the formation of atherosclerotic plaque in ApoE(−/−) mice fed with Western Diet. A Schematic diagram of the design of in vivo experiments. B, C The time course of body weight and average food intake changes. The food intake every three days was averaged. D, E Representative images of aorta oil red O staining and summarized data of plaque area ratio. n = 5 in WT, ApoE(−/−), ApoE(−/−) + BAM15 (85 mg kg−1) and ApoE(−/−) + Ato (2 mg kg−1), *P < 0.05 vs. ApoE(−/−). #P < 0.05 vs. WT. Ato, atorvastatin, ND normal diet, WD western diet, WT wild type.
Gavage administration of BAM15 improves Western diet (WD)-induced hyperlipidemia in ApoE(−/−) mice
Since the occurrence of AS is accompanied by lipid metabolism disorders, we examined the effect of BAM15 on blood lipids. WD feeding for 12 weeks induced hyperlipidemia in ApoE(−/−) mice and both BAM15 and atorvastatin treatment significantly improved the hyperlipidemia, as manifested by the analyzed lipid parameters including total cholesterol (TC), triglyceride (TG) in peritoneal macrophages (Fig. 4A), and TC, TG, HDL-C, and LDL-C in serum (Fig. 4B, C).
The livers of WD-fed ApoE(−/−) mice showed remarkable histopathologic changes. As shown by the hematoxylin/eosin (H&E)-stained liver paraffin sections in Fig. S1 A, B, there existed apparent hydropic degeneration of hepatocytes and clustered Kupffer cells with size enlargement in livers of WD-fed ApoE(−/−) mice. Since lipid droplets in the tissue are dissolved by the solvent during preparation of H&E-stained specimens, oil red O staining is a better method for determining lipid accumulation. As shown in Fig. S1 C, D, oil red O staining results showed that there existed significant lipid deposits in livers of WD-fed ApoE(−/−) mice which were mitigated by BAM15 treatment. BAM15 treatment significantly improved these pathological features induced by WD feeding. However, although atorvastatin treatment corrected WD-induced alteration of lipid profile, it did not improve WD-induced liver histopathologic changes. The increased serum ALT, AST and liver TC, TG, ALT, AST in WD-fed ApoE(−/−) mice were reduced by BAM15 treatment (Fig. S1E).
BAM15 inhibited the atherosclerosis in the aortic root of ApoE(−/−) mice fed on a Western diet for 8 weeks. A The representative MOVAT staining of aortic root at the level of the tricuspid valves, and the framed area was enlarged. B Statistical data of MOVAT staining, n = 3. *P < 0.05 vs. ApoE(−/−). #P < 0.05 vs.WT. Ato atorvastatin, ND normal diet, WD western diet, WT wild type.
Effects of BAM15 on cell physiological activity
Given the role of BAM15 in vivo experiments, we further verified the effect in vitro experiments. We chose RAW264.7 macrophages as the cell model. Firstly, we examined the cytotoxic effects of BAM15 on RAW264.7 macrophages. Results showed that the IC50 of BAM15 is 5.293µM, (Fig. 5A). By visualizing the state of the cells under a microscope, after BAM15 treatment, the cells maintained normal morphology at 1 µM, 2 µM and 4 µM. When BAM15 reaches 8 µM, the number of cells decreases and the cell morphology shrinks significantly (Fig. 5B). In addition, we found that BAM15 inhibited proliferation and migration of RAW264.7 macrophages by scratch wound-healing assay (Fig. 5C, D). In addition, we examined the effects of BAM15 on the cell cycle in vitro, as shown in Fig. 6, with the increase of BAM15 concentration, the S and G2M phases were gradually shortened, and the G1 phase was prolonged.
The effect of BAM15 on hyperlipidemia induced by WD feeding in ApoE(−/−) mice. A The total cholesterol and triglyceride content of peritoneal macrophages. n = 10 in WT, n = 15 in ApoE(−/−), ApoE(−/−) + BAM15 (85 mg kg−1) and ApoE(−/−) + Ato (2 mg kg−1). *P < 0.05 vs. ApoE(−/−). #P < 0.05 vs.WT. B, C BAM15 reduced the elevated blood lipids induced by WD feeding in ApoE(−/−) mice. n = 10 in WT, n = 15 in ApoE(−/−), ApoE(−/−) + BAM15 (85 mg kg−1) and ApoE(−/−) + Ato (2 mg kg−1). *P < 0.05 vs. ApoE(−/−). #P < 0.05 vs.WT. Ato, atorvastatin, TC total cholesterol, TG triglyceride, HDL-C high density lipoprotein cholesterol, LDL-C high density lipoprotein cholesterol.
The effect of BAM15 on the cell activity, proliferation and migration of RAW264.7 macrophages. A The proliferation of RAW264.7 macrophages was assessed by CCK-8 assay after 24 h of treatment with the different concentrations of BAM15. B Representative images of RAW264.7 macrophages cell state treated with BAM15 (1–8 µM) for 24 h. n = 3. C Representative images showed migration of RAW264.7 macrophages treated with BAM15 (2 µM) at the time 0 and 24 h measured by scratch wound-healing assay. D Statistical data of wound closure, n = 5. *P < 0.05 vs. FBS (10%), #P < 0.05 vs. Ctrl.
BAM15 inhibits PA-induced lipid accumulation
The persistence of cholesterol-engorged macrophages (foam cells) in the artery wall fuels the development of atherosclerosis. Therefore, the reduction of cellular cholesterol helps to inhibit the progression of atherosclerosis. We established PA-induced lipid accumulation cell model. As shown in Fig. 7, BAM15 treatment alleviated PA-induced lipid accumulation in RAW264.7 macrophages in a concentration-dependent manner, indicating that BAM15 has the potential to inhibit atherosclerosis.
BAM15 inhibited PA-induced increase of lipid in RAW264.7 macrophages. A Representative images of oil red O staining. B Statistical data of oil red O staining, n = 4 in oil red O staining (× 250 magnification) and n = 3 in oil red O staining (× 100 magnification). *P < 0.05 vs. PA (500 µM), #P < 0.05 vs. Ctrl.
Identification of BAM15-related targets and GO analysis
RNA sequencing was performed on three replicate cell samples from both the Ctrl group and the BAM15 group. A total of 2634 genes were identified, including 1341 up-regulated genes and 1293 down-regulated genes, as shown in the bar chart in Fig. 8A. Volcano plots were employed to visually depict the variation in gene expression between two groups, highlighting the statistical significance of these differences, as shown in Fig. 8B. Hierarchical clustering analysis was processed on differentially expressed gene, and genes with the same or similar expression patterns in different groups were clustered and displayed through heat maps in Fig. 8C.
GO annotation system is a directed acyclic graph containing three main branches: Biological Process, Molecular Function and Cellular Component. GO classification of DEGs between groups was shown as follows (Fig. 8D). (1) There were 21 biological processes (BP) associated with BAM15 in the treatment of AS, focusing largely on cellular processes, biological regulation, metabolic process and responses to stimulus. (2) Three cellular components (CC) were identified, focusing on cellular anatomical entity, intracellular and protein-containing complex. (3) A total of 12 molecular functions (MF) were identified, primarily linked to functions such as binding, catalytic activity, molecular function regulator and transcription regulator activity, etc.
To comprehend the Gene Ontology (GO) items significantly enriched relative to the entire genome background, we employed ClusterProfiler for conducting enrichment analyses of biological processes, molecular functions, and cellular components using hypergeometric testing on differential gene sets within each group. This approach aimed to infer the potential functions in which the differential genes within each group are involved. As shown in Fig. S2 A–C, the cellular reaction to DNA damage stimulus ranks foremost in the categorization of biological processes. Among the cellular components, nucleoplasm emerged as the predominant entity. Additionally, in terms of molecular functions, ATP binding exhibited a notable correlation. Based on this revelation, we concurrently identify the top 10 GO enrichment terms within each category for generating enrichment string plots as depicted in Fig. S2D–F.
Identification of DEGs and GO functional enrichment analysis. A The number of genes in each differentially expressed gene set. The horizontal coordinate represented different sets of differential genes, and vertical coordinates represented the number of differential genes. Blue represented all differential genes, orange represented up-regulated genes, green represented down-regulated genes. B In volcano plot, each dot represented a gene. X-axis: log2 Fold change of expression; Y-axis: – log10(FDR) or – log10(P-value). Green dots are down-regulated genes, while red dots were up-regulated ones and black dots were genes without significant difference. C Hierarchical cluster analysis of differentially expressed genes between Ctrl and BAM15 group, increasing in expression were indicated in red hues and decrease were in blue hues. Ctrl, control group. D GO functional enrichment analysis of targets in the treatment of BAM15. X-axis was Go terms and classifications; Y-axis represented the number of DEGs annotated to the term (right) and percentage of that in all DEGs (Left).
KEGG enrichment analysis on DEGs and PPI network analysis
In biological organisms, series of gene products collaboratively interact to execute essential biological functions, which is so called pathway. Annotating genes within pathway networks could largely benefit further analysis on biological functions. KEGG (Kyoto Encyclopedia of Genes and Genomes) is one of the major databases on pathways26,27,28. KEGG pathway annotation on DEGs was shown in the following Fig. 9A. The KEGG analysis revealed significant enrichment of 50 pathways associated primarily with the cellular processes, environmental information processing, human diseases, metabolism and organismal systems. Additionally, we employed the COG (Cluster of Orthologous Groups of proteins) database to classify the homologous data pertaining to gene products (Fig. 9B).
Enrichment factors and fisher test were applied in the determination of enrichment degree and significancy of the pathway. Enrichment of DEGs in KEGG pathways are shown in the Fig. 9C. Top 20 enriched pathways (with smallest Q-value) were shown. Based on the findings, the cell cycle emerged as the most significant factor. Figure 9D shows the five most enriched (q value) pathways and related genes, and the relationship between genes and pathways can be intuitively displayed through the network diagram.
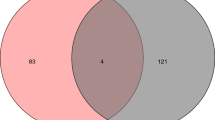
KEGG analysis and PPI network construction. A Taxonomic analysis of KEGG enrichment pathways. Y-axis represented KEGG pathway terms; X-axis represented the percentage of genes annotated to the KEGG pathway. B COG Classification on DEGs. X-axis: COG classification terms; Y-axis: Number of genes in the term. C The number of genes enriched in each KEGG pathway term was represented by a bar and the P-value was shown by different colors. D Network diagram of KEGG pathway. The color of the edge represented different pathways, and the color of the gene node represented the difference fold. E The potential target genes in BAM15 and AS in the intersection. BAM15 was shown as purple circle. AS was represented as yellow circle. F Protein interaction network by Cytoscape 3.9.1 software. The node size and color represent the size of the degree. Node size was proportional to its degree; node color was from yellow to red, and the corresponding degree gradually larger.
Gene Set Enrichment Analysis (GSEA) was processed on all genes based on expression level. In general, differential expression analysis tends to concentrate on genes that exhibit either upregulation or downregulation with statistical significance. However, this may mask the genes, which are altered slightly without significancy but play vital role in biological functions. As shown in Fig. S3, without setting threshold on fold change and significancy, GSEA is able to detect weak alterations in gene expression.
In order to identify potential novel therapeutic targets for AS, we collected 1696 AS-associated targets from GeneCards and DisGeNET database. To predict the potential pharmacological efficacy of BAM15 in treating atherosclerosis, it is essential to determine the shared targets between BAM15 and AS. As depicted in Fig. 9E, through overlap analysis, we identified 140 common targets between BAM15 and AS, representing 5.2% of the total targets. These findings indicate that the pharmacological profile of BAM15 involves multiple targets.
The 140 candidate targets were submitted to the STRING database to obtain their interactions and uploaded to Cytoscape for analysis and construction of the PPI network. As shown in Fig. 9F, core targets were identified by extracting the top 30 nodes based on closeness, betweenness and degree value. The intersection of these values resulted in 17 common elements serving as central targets. It is worth noting that in the PPI network, 17 targets are linked, of which IL1 A, SRC and CSF3 may be identified as hub genes, suggesting the potential targets of BAM15 in the treatment of AS.
Molecular docking validation analysis of core targets
Based on the previous results, IL-1α, SRC and CSF3 were selected as hub genes of BAM15 in AS treating. The binding energy of IL-1α-BAM15, SRC-BAM15 and CSF3-BAM15 were − 6.1 kcal/mol, −8.1 kcal/mol and − 7.1 kcal/mol respectively, which were regarded as good binding to further analyze the binding mode, binding affinity and critical interaction. As shown in Fig. 10, we observed slight changes in the binding modes of the small molecules to the protein active sites, but they still formed hydrogen bonds and hydrophobic interactions with the protein residues. For instance, BAM15 interacts with IL-1α protein at residue SER-75, forming two hydrogen bonds, and also engages in hydrophobic interactions with surrounding residues such as VAL-52, ILE-74, and THR-77 (Fig. 10A). BAM15 interacts with CSF3 protein at residue PRO-61, forming a strong hydrogen bond due to a short bond length, and also forms hydrophobic interactions with residues PRO-66, VAL-164, PHE-161, TRP-59, and PRO-61, which play a crucial role in stabilizing the small molecule (Fig. 10B). Furthermore, BAM15 fits well into the SRC protein active site pocket, forming a hydrogen bond with LYS-195 and hydrophobic interactions with surrounding residues such as LEU-393, ILE-336, ILE-294, VAL-281, LEU-273, and ALA-293 (Fig. 10C). These interactions help form a tight complex, which is essential for the biological activity. In summary, after molecular dynamics adjustment, the small molecule can still form a stable complex with the protein, maintaining its binding stability.
Molecular dynamics analysis
To further investigate the interactions between small molecules and proteins, molecular dynamics (MD) simulations of protein-small molecule complexes were performed for 100 ns. RMSD (Root Mean Square Deviation) measures the deviation of atomic positions from the initial conformation at a given time. Monitoring the RMSD of proteins throughout the simulation provides valuable insights into the structural conformation of the complex, the stability of the system, and the flexibility of the molecule. A wider distribution of RMSD values indicates more significant conformational changes of the molecule. If the protein’s RMSD continues to increase or decrease on average at the end of the simulation, it suggests that the system has not yet equilibrated, and the simulation time may be insufficient for rigorous analysis. From the RMSD plot in Fig. 11A, it is observed that the average RMSD of the complexes is below 3 Å, and dynamic equilibrium is generally reached around 40 ns. Additionally, we found that the RMSD fluctuations of the CSF3-BAM15 and IL-1α-BAM15 complexes were notably smaller than those of the SRC-BAM15 complex. This is primarily due to the high stability of the CSF3 and IL-1α proteins (close to spherical structures), with fewer flexible loop regions.
The root mean square fluctuation (RMSF) was used to characterize the conformational changes of each amino acid residue in the protein chain during the simulation, with peaks indicating regions of highest fluctuation. Larger RMSF values indicate greater conformational changes and higher flexibility of the amino acid residues. As seen in the RMSF plot in Fig. 11B, the protein residues in the CSF3-BAM15 and IL-1α-BAM15 complexes showed relatively small fluctuations, consistent with the RMSD results. In contrast, the SRC-BAM15 complex showed larger fluctuations in some regions (e.g., near residues 200 and 410), mainly due to the higher flexibility of the hinge region of the protein, where these residues are located. Despite these fluctuations, most conformational changes were within acceptable limits.
To analyze the relative compactness and stability induced by ligand binding at both primary and secondary sites, the radius of gyration (Rg) of the target proteins was measured, which reflects the mass-weighted distance between the receptor atoms and the center of mass. Rg provides an estimate of the compactness of the protein structure, a lower Rg indicates a more compact protein, while a higher Rg suggests higher conformational entropy and disorder. From the Rg plot in Fig. 11C, we observed a slight decrease in the Rg of the proteins in all three complexes, indicating that the binding of the compounds contributed to the stability of the proteins. This suggests that ligand binding promotes hydrophobic contacts within the protein, leading to more effective interactions and enhancing the stability of the complexes. The fluctuations in the solvent-accessible surface area (SASA) were consistent with the Rg fluctuations (Fig. 11D).
To further examine the binding interactions between the proteins and small molecules, we tracked the number of hydrogen bonds formed between them throughout the simulation. As shown in Fig. 11E, from the hydrogen bond network plots, it is clear that the compounds were able to form at least one hydrogen bond with the protein pocket amino acids, and these hydrogen bonds played a crucial role in stabilizing the protein-ligand complex.
We computed the binding free energies of the proteins and small molecules from the last 20 ns of the trajectory (Table 1). The van der Waals interactions showed the highest contribution to ligand stability, followed by electrostatic interactions. For example, the binding free energies of BAM15 with CSF3, IL-1α, and SRC proteins were − 15.74 ± 3.38 kcal/mol, − 18.6 ± 1.75 kcal/mol, and − 14.72 ± 1.45 kcal/mol, respectively. Energy decomposition analysis revealed that van der Waals forces played a major role in stabilizing the compounds at the protein active site pockets. Additionally, effective hydrogen bonding interactions with the protein active site also contributed significantly to the electrostatic interactions, as observed in BAM15’s interaction with SRC, where the electrostatic energy was − 19.57 ± 1.12 kcal/mol. Hydrophobic interactions also contributed to the binding stability due to the nonpolar solvation of the protein-ligand complex. In summary, CSF3, IL-1α, and SRC proteins exhibit strong binding affinity with BAM15, which promotes the formation of stable complexes, thereby contributing to the biological activity of the small molecule.
Verification results by Western blot
The above results showed that BAM15 has good docking activity with IL-1α, SRC and CSF3. Here, we further examined the effects of BAM15 on the expression of proteins encoded by IL1 A, SRC and CSF3 in RAW264.7 macrophages. As shown in Fig. 12, BAM15 treatment decreased IL-1α and CSF3 protein level and increased SRC protein expression in RAW264.7 macrophages.
We also detected the expression of IL-1α, SRC and CSF3 proteins in peritoneal macrophages of WD-fed ApoE(−/−) mice. As shown in Fig. 13, the level of IL-1α of peritoneal macrophages from WD-fed ApoE(−/−) mice was increased compared to group of WT mice. While IL-1α expression was significantly decreased treated with BAM15 in WD-fed ApoE(−/−) mice. BAM15-treated ApoE(−/−) mice showed a significant increase in SRC protein expression and a decrease in CSF3 protein expression in peritoneal macrophages compared with untreated mice.
The effect of BAM15 on IL-1α, SRC and CSF3 expression in peritoneal macrophages of ApoE(−/−) mice. A–F The representative western blots and analyzed data of IL-1α, SRC and CSF3 protein levels in peritoneal macrophages of ApoE(−/−) mice. n = 3 in each group. *P < 0.05 vs. ApoE(−/−). #P < 0.05 vs.WT. Ato atorvastatin, ND normal diet, WD western diet, WT wild type.
Discussion
Atherosclerosis is a chronic low-grade inflammatory disease that affects large and medium-sized arteries and is considered to be a major underlying cause of cardiovascular disease (CVD)6,29. The high risk of mortality by atherosclerosis has led to the development of new strategies for disease prevention and treatment. Our previous works systematically studied the cardiovascular effects of chemical mitochondrial uncouplers, especially BAM1514. We found that BAM15 relaxed phenylephrine (PE)-induced constricted rat mesenteric arteries with intact and denuded endothelium. Pretreatment with BAM15 inhibited PE-induced constriction of rat mesenteric arteries with intact and denuded endothelium. Moreover, BAM15 activates AMPK in vascular smooth muscle cells with higher potency than that of the known AMPK activators metformin and AICAR14. AMPK plays an important role in the vascular system and blood components including endothelial cells, smooth muscle cells30, platelets31and white blood cells32. In addition, BAM15 inhibited NLRP3 inflammasome activation through inhibiting NFκB nuclear translocation in RAW264.7 macrophages and THP-1 derived macrophages22. Therefore, we suggested that BAM15 has vascular anti-inflammatory action. In view of AMPK as the therapeutic target in multiple cardiovascular diseases33,34, and inhibition of NLRP3 inflammasome activation is also an important target for improving AS15, the previous work indicated that BAM15 could be developed as a novel drug for AS. Therefore, we attempted to explore the anti-atherosclerosis effect and mechanism of BAM15 by experimental verification. Herein, we demonstrated that: (i) Oral administration of BAM15 suppresses western diet (WD)-induced atherosclerosis in ApoE(−/−) mice. (ii) BAM15 regulated cell cycle and inhibited PA-induced lipid accumulation in RAW264.7 macrophages. (iii) The mechanism of BAM15 in alleviating AS may be through regulating IL1 A, SRC and CSF3. In addition, this study also summarizes the improvement effect of BAM15 on hyperlipidemia and liver pathological changes caused by WD in ApoE(−/−) mice.
Network approach can clarify the interaction of compound to multi-target. In present study, 3 key target genes were analyzed in BAM15 treating AS. Molecular docking results suggested that BAM15 could be stably combined with IL-1α, SRC, and CSF3, which is verified with the MD study. In the MD study, the binding free energy is a key tool for analyzing the changes in ligand binding modes by measuring the thermodynamic properties of the ligand. A negative binding free energy (ΔG_binding) indicates system stability, while a positive value suggests instability. The docking study and MD study results both showed that BAM15 could combine with IL-1α, SRC and CSF3 stably. In the in vitro experiment, BAM15 decreased IL-1α and CSF3 protein level and increased SRC protein expression in RAW264.7 macrophages, which is correspond with the in vivo experiment. The IL-1α activity was significantly decreased by BAM15 treatment in peritoneal macrophages from WD-fed ApoE(−/−) mice. SRC protein expression was increased, while CSF3 protein expression was decreased compared with untreated mice.
The protein encoded by IL1 A is a member of the interleukin 1 cytokine family. This cytokine is a pleiotropic cytokine involved in various immune responses, inflammatory processes, and hematopoiesis. A plethora of mediators involved in the processes of atherosclerosis are known to play crucial roles in the biology of the IL-1 cytokine family. More than two decades ago, IL-1α and IL-1β expression in atheroma was reported35. Macrophages were identified as the predominant IL-1α and IL-1β sources within the lesion in vivo and produced significantly more mRNA of these cytokines also in vitro when isolated from hyperlipidemic LDL receptor-deficient (Ldlr−/−) mice36. Recent studies targeted IL-1α with Xilonix, a specific human monoclonal antibody37. The intravenous dosing with Xilonix resulted in reduced rates of restenosis and supported use of Xilonix as safe and tolerate treatment to reduce inflammatory vascular processes leading to AS38. Therefore, IL-1 is an important target for improving AS.
SRC is a well-known protooncogene and a target for cancer treatment39. During cancer therapy, treatment with tyrosine kinase inhibitors, including dasatinib, an inhibitor of SRC, increases the risk for cardiovascular disease and heart failure40. There were studies observed that atherosclerosis in ApoE-KO mice was exacerbated in SRC-downregulated mice, which could underlie the increased risk in cancer patients treated with of SRC inhibitors41. SRC maintains cholesterol homeostasis and protects against atherosclerosis, that we suggested is consistent with our study. This also explains the role of BAM15 in regulating blood and liver cholesterol.
Another gene of interest identified in our study is the CSF3 gene, which encodes a member of the IL-6 superfamily of cytokines. The encoded cytokine controls the production, differentiation, and function of granulocytes. The importance of this gene in AS is described by M. Liu, et al., It has been shown that CSF3 therapy inhibits the atherosclerotic process in animal models42.
In addition to the key molecules mentioned above, PTX3 is strongly expressed during atherosclerosis progression. Especially in macrophages, PTX3 is produced in large quantities in response to inflammatory stimuli43. Previous studies have suggested that atherogenic lipoproteins induced local production of pro-inflammatory cytokines, including IL-1 and IL-6, and then up-regulate PTX3 expression by infiltrating monocytes, endothelial cells and SMCs44. In vitro experiments, we have confirmed that BAM15 reduce the protein expression of PTX3 in macrophages, indicating the role of BAM15 in reducing vascular inflammation. Moreover, PTX3 may serve as a mechanism of amplification of innate immunity45. Vessel wall elements produce high amounts of PTX3 during inflammation and the levels of circulating PTX3 increase in several pathological conditions affecting the cardiovascular system. The ability of PTX3 to interact with a variety of ligand, including microbial components, complement components C1q, apoptotic cells, ECM components, and angiogenic growth factors, suggests that PTX3 plays a role in vascular injury, angiogenesis, atherosclerosis, and restenosis46, and further illustrates the significance of BAM15 in the treatment of atherosclerosis.
BAM15 is orally available, selective to lipid-rich tissues, and protects against diet-induced obesity (DIO) in C57BL/6 J mice. Enhanced body weight regulation was observed concurrent with reductions in fat accrual, improvements in whole-body glucose clearance, and energy expenditure without altering body temperature. Notably, the effects of BAM15 on body composition and glycemic control were independent of reductions in body weight13. Moreover, BAM15 has been reported that has powerful insulin sensitizing effects without compromising lean mass or affecting food intake12. The previous study also discussed the role of BAM15 in adipose tissue expansion and lipid deposition to liver in C57BL/6 J mice12. C57BL/6 J mice were necropsied after 3 weeks of treatment and tissues were fixed, paraffin-embedded, and stained with hematoxylin and eosin (H&E) to determine whether any pathological features, off-target effects, or tissue-specific phenotypes of BAM15 administration were present in vivo. However, our research is different from previous studies. It is mainly reflected in: First, the purpose of research is different, we explore the possible treatment effect of BAM15 on the basis of establishing a clear model. Second, the animal models used are different. We use WD-induced ApoE(−/−) mice, which has also been used as an animal model of fatty liver.; third, the time of administration is different, we used WD-induced ApoE(−/−) mice to take BAM15 orally for 12 weeks to verify the therapeutic effect of the drug.
In the in vivo experiment, the BAM15 dose (85 mg kg−1 d−1) was determined as a possible effective dose based on previous results13. In addition, we used atorvastatin as the positive control drug. Although the action mechanisms of BAM15 and atorvastatin are not identical, it is reasonable to compare their protective effect against aortic plaque lesion in vivo. Atorvastatin treatment at the dose (2 mg kg−1 d−1, p.o) equivalent to clinical usage (10 mg d−1) in human.
Previous research has indicated that the antioxidant effect of BAM15 is due to the activation of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α). BAM15-induced uncoupling enhances the expression and activity of PGC-1α, a key regulator of mitochondrial biogenesis and oxidative metabolism47,48. The heightened activity of PGC-1α triggers the upregulation of genes involved in mitochondrial respiration, mitochondrial biogenesis, and antioxidant defenses, ultimately bolstering cellular energy metabolism and mitochondrial function48. However, in the process, we found that the ROS levels of the cells treated with BAM15 did not decrease, but increased (Fig. S5). This phenomenon was observed not only with BAM15 but also with FCCP and niclosamide. We believe that this is associated with the bidirectional regulatory mechanism of mitochondrial uncoupling agents, where mild mitochondrial uncoupling is protective, whereas excessive uncoupling is detrimental49,50. In our previous study on the characterization of mitochondrial uncoupling induced by chemical mitochondrial uncoupling agents in cardiomyocytes, it was found that low-dose mitochondrial uncoupling agents activate STAT3 and increase ATP production by enhancing the oxidative phosphorylation process and subsequent mitoROS production. However, high-dose mitochondrial uncoupling agents inhibit STAT3 and reduce ATP production through excessive uncoupling of oxidative phosphorylation and overproduction of mitoROS, resulting in myocardial cell damage. This finding explains the dual role of mitoROS in heart damage. Excessive ROS production during early reperfusion has been reported to have harmful effects, while small amounts of ROS production during ischemia and/or reperfusion have beneficial effects51,52. In vascular studies, we have demonstrated that BAM15 treatment increases the production of ROS in vascular smooth muscle cells, but BAM15 exhibits vascular protective properties due to its strong AMPK activation14. In this study, we found that BAM15 increased ROS in RAW264.7 macrophages, and a modest increase in ROS was considered beneficial. The mild mitochondrial uncoupling and ROS increase induced by BAM15 stimulated the expression of PGC-1α, further inducing the up-regulation of genes involved in antioxidant defense.
Analyzing the cross-section at the aortic valve region is another way to characterize the atherosclerotic plaque. In order to quantify the atherosclerotic lesion size accurately, we analyzed the vertical en face images of aorta with Oil Red O staining. We did not further analyze the composition of the atherosclerotic plaque, which was a limitation of the present study.
Conclusions
In summary, the in vivo experiments demonstrated that oral administration of BAM15 significantly reduced the increased plaque area and the increased necrotic core area of plaque induced by WD feeding in ApoE(−/−) mice. In in vitro experiments, cell biological assays confirmed that BAM15 inhibited RAW264.7 macrophages invasive ability and reduced PA-induced lipid accumulation. By combining RNA-seq techniques, molecular docking, and experimental validation, we found that IL-1α, SRC, and CSF3 are key anti-AS molecules of BAM15.
This study reveals that BAM15 may be a promising drug against AS, providing scientific evidence to reveal the pharmacological mechanism of BAM15 in the treatment of AS, and facilitating future clinical translational research.
.
Data availability
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Abbreviations
- AS:
-
Atherosclerosis
- BP:
-
Biological processes
- CC:
-
Cellular components
- CCK-8:
-
Cell Counting Kit-8
- CSF3:
-
Colony Stimulating Factor 3
- DAVID:
-
Database for Annotation Visualization and Integrated Discovery
- DEGs:
-
Identification of differentially expressed genes
- GO:
-
Gene Ontology
- IL1 A:
-
Interleukin 1 Alpha
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- MD:
-
Molecular dynamics
- MF:
-
Molecular function
- PA:
-
Palmitic acid
- PPI:
-
Protein–protein interactions
- RMSD:
-
Root mean square deviation
- RMSF:
-
Root mean square fluctuation
- TCMSP:
-
Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform
References
Björkegren, J., Lusis, A. J. C. & Atherosclerosis Recent developments. Cell 185 (10), 1630–1645 (2022).
Xu, S., Pelisek, J. & Jin, Z. J. Atherosclerosis is an epigenetic disease. Trends Endocrinol. Metab. 29(11), 739–742 (2018).
Libby, P. J. The changing landscape of atherosclerosis. Nature 592(7855), 524–533 (2021).
Lechner, K. et al. Lifestyle factors and high-risk atherosclerosis: pathways and mechanisms beyond traditional risk factors. Eur. J. Prev. Cardiol. 27 (4), 394–406 (2020).
Libby, P. et al. Atherosclerosis. Nat. Rev. Dis. Primers. 5 (1), 56 (2019).
Wolf, D. & Ley, K. J. Immunity and inflammation in atherosclerosis. Circ. Res. 124(2), 315–327 (2019).
Satny, M., Hubacek, J. & Vrablik, M. J. Statins and inflammation. Curr. Atheroscler Rep. 23(12), 80 (2021).
Demine, S., Renard, P. & Arnould, T. J. C. Mitochondrial uncoupling: A key controller of biological processes in physiology and diseases. Cells 8(8), (2019).
Cadenas, S. J. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim. Biophys. Acta Bioenerg 1859(9), 940–950 (2018).
Kenwood, B. et al. Identification of a novel mitochondrial uncoupler that does not depolarize the plasma membrane. Mol. Metab. 3(2), 114–123 (2014).
Gao, J. et al. Characterizations of mitochondrial uncoupling induced by chemical mitochondrial uncouplers in cardiomyocytes. Free Radic Biol. Med. 124, 288–298 (2018).
Alexopoulos, S. et al. Mitochondrial uncoupler BAM15 reverses diet-induced obesity and insulin resistance in mice. Nat. Commun. 11 (1), 2397 (2020).
Axelrod, C. et al. BAM15-mediated mitochondrial uncoupling protects against obesity and improves glycemic control. EMBO Mol. Med. 12 (7), e12088 (2020).
Tai, Y. et al. Mitochondrial uncoupler BAM15 inhibits artery constriction and potently activates AMPK in vascular smooth muscle cells. Acta Pharm. Sin B. 8 (6), 909–918 (2018).
Kong, P. et al. Inflammation and atherosclerosis: signaling pathways and therapeutic intervention. Signal. Transduct. Target. Ther. 7 (1), 131 (2022).
Keith, C., Borisy, A. & Stockwell, B. J. Multicomponent therapeutics for networked systems. Nat. Rev. Drug Discov 4(1), 71–78 (2005).
Schadt, E., Friend, S. & Shaywitz, D. J. A network view of disease and compound screening. Nat. Rev. Drug Discov 8(4), 286–295 (2009).
Jovic, D. et al. Single-cell RNA sequencing technologies and applications: A brief overview. Clin. Transl. Med. 12(3), (2022).
Ma, M. et al. Repurposing nitazoxanide as a novel anti-atherosclerotic drug based on mitochondrial uncoupling mechanisms. Br. J. Pharmacol. 180(1), 62–79 (2023).
Xiaoxue, W. et al. Novel EGFR inhibitors against resistant L858R/T790M/C797S mutant for intervention of non-small cell lung cancer. Eur. J. Med. Chem. 277, 116711 (2024).
Xiao, X. et al. Niclosamide inhibits vascular smooth muscle cell proliferation and migration and attenuates neointimal hyperplasia in injured rat carotid arteries. Br. J. Pharmacol. 175, 1707–1718 (2018).
Hu, N. et al. Chemical mitochondrial uncouplers share common inhibitory effect on NLRP3 inflammasome activation through inhibiting NFκB nuclear translocation. Toxicol. Appl. Pharmacol. 414, 115426 (2021).
Fengfeng, L. et al. Anthelmintics nitazoxanide protects against experimental hyperlipidemia and hepatic steatosis in hamsters and mice. Acta Pharm. Sin B 12(3), 1322–1338 (2022).
Liu, C. et al. An integrated network pharmacology and RNA-Seq approach for exploring the preventive effect of lonicerae japonicae Flos on LPS-induced acute lung injury. J. Ethnopharmacol. 264, 113364 (2021).
Tan, Y. & Lu, Y. J. Molecular mechanism of rhubarb in the treatment of non-small cell lung cancer based on network Pharmacology and molecular Docking technology. Mol. Divers. 27(3), 1437–1457 (2023).
Kanehisa, M. et al. KEGG: Biological systems database as a model of the real world. Nucleic Acids Res. 53, D672–D677 (2025).
Kanehisa, M. et al. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 28, 1947–1951 (2019).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30 (2000).
Doran, A. J. Inflammation resolution: implications for atherosclerosis. Circ. Res. 130(1), 130–148 (2022).
Fisslthaler, B. & Fleming, I. Activation and signaling by the AMP-activated protein kinase in endothelial cells. Circ. Res. 105 (2), 114–127 (2009).
Randriamboavonjy, V. et al. AMPK alpha2 subunit is involved in platelet signaling, clotretraction, and thrombus stability. Blood 116 (12), 2134–2140 (2010).
Sag, D. et al. Adenosine 5’-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J. Immunol. 181 (12), 8633–8641 (2008).
Li, J., Zhong, L., Wang, F. & Zhu, H. J. Dissecting the role of AMP-activated P.otein kinase in human diseases. Acta Pharm. Sin B 7(3), 249–259 (2017).
Grahame Hardie, D. J. Regulation of AMP-activated P.otein kinase by natural and synthetic activators. Acta Pharm. Sin B 6(1), 1–19 (2016).
Moyer, C., Sajuthi, D., Tulli, H. & Williams, J. J. Synthesis of IL-1 alpha and IL-1 beta by arterial cells in atherosclerosis. Am. J. Pathol. 138(4), 951–960 (1991).
Netea, M. et al. Increased interleukin-1alpha and interleukin-1beta production by macrophages of low-density lipoprotein receptor knock-out mice stimulated with lipopolysaccharide is CD11c/CD18-receptor mediated. Immunology 95 (3), 466–472 (1998).
Garrone, P. et al. Generation and characterization of a human monoclonal autoantibody that acts as a high affinity interleukin-1 alpha specific inhibitor. Mol. Immunol. 33 (7–8), 649–658 (1996).
El Sayed, H., Kerensky, R., Stecher, M., Mohanty, P. & Davies, M. J. J. A randomized phase II study of Xilonix, a targeted therapy against Interleukin 1α, for the prevention of superficial femoral artery restenosis after percutaneous revascularization. J. Vasc Surg. 63(1), 133-141e131 (2016).
Parsons, S. & Parsons, J. J. O. Src family kinases, key regulators of signal transduction. Oncogene 23 (48), 7906–7909 (2004).
Lenihan, D. & Kowey, P. J. T. Overview and management of cardiac adverse events associated with tyrosine kinase inhibitors. Oncologist 18(8), 900–908 (2013).
Byun, S. et al. Phosphorylation of hepatic farnesoid X receptor by FGF19 signaling-activated Src maintains cholesterol levels and protects from atherosclerosis. J. Biol. Chem. 294 (22), 8732–8744 (2019).
Liu, M. et al. The effect of granulocyte colony-stimulating factor on the progression of atherosclerosis in animal models: A meta-analysis. Biomed Res Int. 2017, 6705363 (2017).
Rolph, M. S. et al. Production of the long pentraxin PTX3 in advanced atherosclerotic plaques. Arterioscler. Thromb. Vasc Biol. 22, e10–e14 (2002).
Klouche, M. et al. Atherogenic properties of enzymatically degraded LDL: Selective induction of MCP-1 and cytotoxic effects on human macrophages. Arterioscler. Thromb. Vasc Biol. 18, 1376–1385 (1998).
Napoleone, E. et al. The long pentraxin PTX3 up-regulates tissue factor in activated monocytes: Another link between inflammation and clotting activation. J. Leukoc. Biol. 76, 203–209 (2004).
Presta, M. et al. Role of the soluble pattern recognition receptor PTX3 in vascular biology. J. Cell. Mol. Med. 11 (4), 723–738 (2007).
Tsuji, N. et al. BAM15 treats mouse sepsis and kidney injury, linking mortality, mitochondrial DNA, tubule damage, and neutrophils. J. Clin. Invest. 133(7), e152401 (2023).
Rius-Perez, S. et al. PGC-1a, inflammation, and oxidative stress: an integrative view in metabolism. Oxid. Med. Cell. Longev. 2020, 1452696 (2020).
R. Stoetzer OJpogrebniak Apelka-Fleischer. et al. Modulation of apoptosis by mitochondrial uncouplers: apoptosis-delaying features despite intrinsic cytotoxicity. Biochem Pharmacol. 63, 471–483 (2002).
Valmas, N. et al. Mitochondrial uncouplers act synergistically with the fumigant phosphine to disrupt mitochondrial membrane potential and cause cell death. Toxicology 252, 33–39 (2008).
Wang, Z. et al. Concentration-dependent wrestling between detrimental and protective effects of H2O2 during myocardial ischemia/reperfusion. Cell. Death Dis. 5, e1297 (2014).
Muntean, D. M. et al. The role of mitochondrial reactive oxygen species in cardiovascular injury and protective strategies. Oxid Med Cell Longev. 2016, 8254942 (2016).
Funding
This work was supported by the National Natural Science Foundation of China (82300510) and The Natural Science Foundation of Jiangsu Province (BK20210066) and Scientific Research Program of Wuxi Health Commission (Q202223, Q202227 and Q202329) and Science and Technology Development Funds of Wuxi (Y20222018, Y20222021, Y20212029, Y20212005 and Y20212031) and Top Talent Support Program for young and middle-aged people of Wuxi Health Committee (HB2023038) and Key Subject Medical Innovation Team of Wuxi (CXTDPY2021001).
Author information
Authors and Affiliations
Contributions
Conceptualization, Zejun Pei; Funding acquisition, Minghui Ma and Xin Wang; Investigation, Minghui Ma, Jiao Zhong, Yu Tai, Shuo Xu and Zejun Pei; Project administration, Xin Wang; Supervision, Zejun Pei and Xin Wang; Visualization, Minghui Ma, Jiao Zhong, Yu Tai, Shuo Xu and Zejun Pei; Writing – original draft, Minghui Ma; Writing – review & editing, Xin Wang.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
We confirmed that all experiments were performed in accordance with relevant named guidelines and regulations. And the authors complied with the ARRIVE guidelines for animal experiments. The animal study protocol was approved by the Institutional Review Board of Jiangnan University Medical Center (protocol code 2021-Y-51, 31-Aug-2021).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ma, M., Zhong, J., Tai, Y. et al. Combining RNA-seq, molecular docking and experimental verification to explore the mechanism of BAM15 as a potential drug for atherosclerosis. Sci Rep 15, 13347 (2025). https://doi.org/10.1038/s41598-025-98209-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-98209-3