Abstract
Pachychoroid neovasculopathy (PNV) is characterized by increased choroidal thickness, choroidal hyperpermeability, and fluid accumulation. Given its distinct pathophysiology, PNV requires management with anti-VEGF therapy, as its treatment response differs from typical neovascular age-related macular degeneration (nAMD). This study aimed to compare the one-year visual and anatomic outcomes between aflibercept and brolucizumab for treatment-naive PNV. A retrospective medical chart review was performed for consecutive 45 eyes from 45 patients with treatment-naive PNV initially treated with thee monthly intravitreal aflibercept (n = 28, 2.0 mg/0.05 ml) or brolucizumab (n = 17, 6.0 mg/0.05 ml) followed by as-needed regimen, followed up for at least 12 months. Best corrected visual acuity (BCVA) and OCT parameters including central macular thickness (CMT), subfoveal choroidal thickness (SFCT), changes in thickness of choriocapillaris (CC)/Sattler and Haller layer, choroidal vascularity index (CVI) were evaluated at baseline, 3-month, 6-months, and 12-month visits. At the 12-month visit, BCVA improved and CMT decreased significantly in both brolucizumab-treated group and in the aflibercept-treated group, suggesting comparable visual improvement in both groups (p < 0.05 for all). Mean SFCT were significantly reduced through 12 months of treatment in both aflibercept and brolucizumab groups (p = 0.001 for both). Decrease in CMT from the baseline for the brolucizumab-treated group was significantly greater than for the aflibercept group at month 12 (p = 0.038). Decrease in the mean SFCT and Haller layer thickness were significantly greater for the brolucizumab-treated group than that for the aflibercept-treated group at month 3 and 6 (p = 0.013 and p = 0.035). An increase of the CVI from baseline was observed only in the brolucizumab group at month 12 (p = 0.041). CC flow density did not change after 12 months in both groups. The rate of dry macula at month 12 did not differ significantly between the two groups (78.6% in aflibercept group vs. 82.4% in brolucizumab group, p = 0.104). These findings suggested that as-needed administration of brolucizumab demonstrated comparable visual outcomes to aflibercept in treatment-naïve PNV eyes. Additionally, over 12 months, brolucizumab showed a greater effect in reducing CMT, SFCT, and Haller layer thickness, as well as increasing CVI, suggesting potential choroidal morphology remodeling.
Similar content being viewed by others
Introduction
Pachychoroid neovasculopathy (PNV) is a distinct subset of neovascular age-related macular degeneration (nAMD) characterized by pachychoroid features such as increased choroidal thickness and choroidal hyperpermeability1,2. This condition is frequently misclassified as nAMD, with studies estimating that at least 20% of wet AMD cases are actually PNV. This misclassification has significant clinical implications, as PNV responds differently to conventional treatments for nAMD2. Recent advances in imaging modalities and machine learning algorithms have refined our understanding of PNV, revealing that nearly half (46.2%) of previously diagnosed nAMD cases in Japanese populations were reclassified as PNV3. This highlights the necessity of accurate diagnosis to guide appropriate therapeutic strategies.
PNV is characterized by its hallmark features of increased choroidal thickness, choroidal vascular hyperpermeability, and fluid accumulation, often in the absence of drusen. These features are known to influence the disease’s natural course and its response to treatment. Several studies showed that PNV needed fewer anti-vascular endothelial growth factor (VEGF) injections compared to nAMD4,5,6,7. However, there are reports that adjunctive photodynamic therapy (PDT) was required in 6 of 54 (11.1%) eyes of PNV treated with aflibercept or ranibizumab and the treatment response of PNV varies depending on choroidal features8,9. Moreover, recent studies on Japanese patients who were non-responders to three-monthly injections of anti-VEGF drugs suggested that non-responders included more patients with choroidal hyperpermeability7. Therefore, aflibercept and brolucizumab, known to affect the choroid, could be promising treatment options. Aflibercept is well-documented for its effectiveness in achieving macular dryness and reducing choroidal thickness, while brolucizumab, characterized by its smaller molecular size and higher tissue penetration, demonstrates promising outcomes, particularly in cases requiring rapid regression of fluid and the activity of choroidal neovascularization (CNV). Previous studies have largely focused on their application in nAMD populations, which may not fully demonstrate the unique characteristics and treatment responses of PNV3,4,5,6. Furthermore, despite the use of aflibercept and brolucizumab in clinical practice, comparative data on their efficacy and safety in treatment-naïve PNV patients remain limited.
Therefore, this study aims to compare the one-year outcomes of aflibercept and brolucizumab in treatment-naïve PNV patients. By assessing visual acuity improvements and anatomical changes, this research seeks to provide clinicians with evidence-based guidance for selecting appropriate therapeutic approaches for PNV. Additionally, the findings may contribute to a deeper understanding of the disease process and inform treatment strategies for managing this challenging condition.
Results
Demographics and clinical characteristics
A total of 45 eyes of 45 patients with treatment naïve PNV were included in the study. 28 eyes were treated with intravitreal aflibercept injection, and 17 eyes were treated with intravitreal brolucizumab injection. The mean age was 74.7 ± 7.9 years in the aflibercept group and 73.4 ± 8.2 years in the brolucizumab group, with no statistically significant difference (p = 0.156). Male patients constituted 67.8% of the aflibercept group and 70.5% of the brolucizumab group and there was no difference between two groups (p = 0.590). Baseline BCVA was 0.24 ± 0.21 in the aflibercept group and 0.29 ± 0.25 in the brolucizumab group, with no statistically significant difference (p = 0.402). Similarly, CMT, SFCT, CVI, CNV lesion area, and CC flow density were comparable between the two groups at baseline (p > 0.05 for all). A summary of patients’ characteristics is shown in Table 1.
Visual and anatomic outcomes at month 12
The aflibercept group required a mean of 5.4 ± 1.7 injections over the 12-month follow-up period, while the brolucizumab group required 4.7 ± 1.5 injections (p = 0.112). By month 12, 78.6% showed a dry macula in aflibercept group and 82.4% showed dry macula in brolucizumab group (p = 0.219). Mean BCVA increased significantly in aflibercpet-treated eyes from baseline to the last visit from 0.24 ± 0.21 logMAR to 0.14 ± 0.12 logMAR (p = 0.020). In the brolucizumab group, the mean BCVA improved from 0.29 ± 0.25 logMAR at baseline to 0.16 ± 0.14 logMAR at the final visit (p = 0.030). The CMT significantly decreased in both groups, with the mean CMT reducing to 231.4 ± 48.6 μm at 12 months following aflibercept injection (p = 0.010). In brolucizumab group, the mean CMT showed a significant reduction after 12 months (342.9 ± 84.8 μm to 227.8 ± 46.2 μm, p = 0.012). After 12 months of treatment, the SFCT significantly reduced in both groups. The SFCT significantly decreased to 279.1 ± 30.8 μm in aflibercept group (p = 0.001), while the SFCT decreased to 221.7 ± 64.8 μm in brolucizumab group (p = 0.001). The Haller layer thickness significantly reduced in both groups. However, CC/Sattler layer thickness did not change in both groups. The brolucizumab group exhibited a significant increase in CVI from 69.2 to 75.4% (p = 0.026), while the CVI of aflibercept group did not change. The CNV lesion area was reduced in both groups, while the CC flow density did not change in both groups 12 months after treatment (Table 2). During the follow-up period, no intraocular inflammation or other adverse events were found in both groups.
Comparison of mean changes of clinical parameters between aflibercept and brolucizumab group
The aflibercept group demonstrated a mean improvement of -0.07 ± 0.17 logMAR, while the brolucizumab group showed a similar mean improvement of -0.09 ± 0.10 logMAR. However, the changes of BCVA did not different between two groups (p = 0.214). In terms of anatomical outcomes, both treatment groups showed substantial reductions in CMT, with the brolucizumab group achieving a more pronounced effect. At 12 months, the mean amount of reduction in CMT was − 108.4 ± 21.6 μm in the aflibercept group and − 120.7 ± 22.4 μm in the brolucizumab group, with the difference being statistically significant (p = 0.038). As for the SFCT, reductions were also more pronounced in the brolucizumab group (-75.6 ± 34.3 μm in the brolucizumab group and − 60.2 ± 38.7 μm in the aflibercept group, p = 0.011). Especially, brolucizumab group showed more reduction of Haller layer thickness than aflibercept group (-59.4 ± 18.2 μm in brolucizumab group and − 43.2 ± 12.7 μm in aflibercept group, p = 0.028). The brolucizumab group exhibited a significantly higher increase in CVI compared to the aflibercept group (6.7 ± 2.7% vs. 1.7 ± 1.3%, p = 0.041). The reduction in lesion area was more pronounced in the brolucizumab group (-1.1 ± 0.8 mm2) compared to the aflibercept group (-0.8 ± 0.4 mm2, p = 0.046). No significant differences were noted in the CC/Sattler layer thickness (p = 0.783) or flow density (p = 0.652) between the two groups (Table 3).
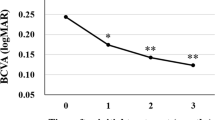
The changes of visual and anatomical outcomes over 1 year are shown in Fig. 1. Although the BCVA tended to improve substantially, there was no significant difference between two groups. CMT, SFCT, and Haller layer thickness showed consistent decreases throughout the treatment period. The decrease in CMT from baseline in the brolucizumab-treated group was significantly greater than that in the aflibercept group at month 12. Additionally, the reductions in SFCT and Haller layer thickness were greater in the brolucizumab group compared to the aflibercept group at months 3 and 6. An increase of the CVI from baseline was observed only in the brolucizumab group at month 12.
Discussion
This study demonstrated that while both aflibercept and brolucizumab reduced choroidal thickness in treatment-naive PNV eyes, choroidal remodeling was observed only in eyes treated with brolucizumab. Additionally, brolucizumab demonstrated superior efficacy with greater reductions in CMT, SFCT, and Haller layer thickness, alongside an increase in the CVI over 12 months, achieving visual outcomes comparable to aflibercept.
Both aflibercept group and brolucizumab group showed significant improvement of BCVA after 12 months, while the BCVA did not significantly differ between the two groups at the 12-month follow-up. This finding suggests that both aflibercept and brolucizumab are comparably effective in improving vision in PNV patients, which aligns with prior studies highlighting the strong anti-VEGF properties of both agents10,11,12,13.
Brolucizumab, as a single-chain antibody fragment, possesses the smallest molecular size among anti-VEGF agents. This compact size enhances its ability to penetrate the retina and choroid, making it more effective in reducing retinal fluid and choroidal thickness. Several studies have demonstrated a significant reduction in choroidal thickness following brolucizumab treatment in patients with PNV10,14,15 This study also showed that the reductions in CMT, SFCT, and Haller layer thickness in brolucizumab group were significantly greater when compared to aflibercept group, supporting prior evidence of its enhanced choroidal penetration and higher molar concentration10,16,17 These properties allow brolucizumab to target pachyvessels effectively and alleviate mechanical stress on the inner choroidal layers, a critical factor in the pathogenesis of PNV. This aligns with findings from earlier studies emphasizing the role of choroidal abnormalities in disease progression and the potential of targeted therapies to modulate these changes3,4,5,6,7,8,9.
Notably, changes in SFCT and CVI highlighted the unique effect of brolucizumab in inducing choroidal morphology remodeling. In the brolucizumab group, significant decreases in SFCT and Haller’s layer thickness were observed at month 3 and 6, followed by an increase in CVI at month 12. This pattern can be explained by Brolucizumab’s smaller molecular size and its ability to penetrate deeper into the tissue, which allows for efficient reabsorption of VEGF-related stromal transudation and a reduction in hydrostatic pressure within the choroid. This mechanism addresses the primary cause of retinal pigment epithelial detachment (PED) development, with brolucizumab inducing significant reduction of the transudate and effectively decreasing PED volume. These findings suggest that brolucizumab not only reduces disease activity but also facilitates structural stabilization of the choroid, an essential aspect of PNV management. In contrast, while aflibercept also reduced SFCT, it did not demonstrate a similar modification of choroidal morphology, indicating a mechanistic distinction between the two agents.
Fluid resolution rates were high in both groups, with the majority of patients achieving a dry macula by 12 months. Although the brolucizumab group showed a slightly higher resolution rate than the aflibercept group, this difference was not statistically significant. Both agents effectively controlled disease activity and reduced retinal fluid accumulation—a critical contributor to visual impairment in PNV. In terms of treatment burden, the mean number of injections over 12 months was slightly lower in the brolucizumab group. Although the difference was not statistically significant, the potential for extended dosing intervals with brolucizumab, as reported in prior studies, suggests a practical advantage for selected patients in real-world settings where frequent visits pose challenges10,15,16,17,18,19. This feature enhances its applicability for long-term disease management.
The results of this study have important clinical implications for the management of PNV. PNV is a heterogeneous condition, often complicated by variable choroidal abnormalities and fluid dynamics. The ability of brolucizumab to induce choroidal morphology remodeling and achieve substantial reductions in disease-related anatomical markers suggests that it may be particularly suited for addressing the underlying pathophysiology of pachychoroid diseases. This unique mechanism could make brolucizumab an attractive option for patients with refractory or severe choroidal abnormalities.
Despite its strengths, this study has several limitations. The retrospective design and relatively small sample size may restrict the generalizability of the findings. Additionally, the 12-month follow-up period may be insufficient to fully assess long-term efficacy and safety. Future research should focus on the durability of anatomical improvements and their impact on long-term visual outcomes. While this study evaluated the choroidal changes using OCT, advances in imaging technologies such as wide-field OCT and quantitative choroidal analysis hold promise for enhancing our understanding of PNV and optimizing treatment strategies. Unraveling the molecular mechanisms underlying choroidal remodeling also could pave the way for novel therapeutic approaches in PNV management.
In conclusion, both aflibercept and brolucizumab effectively improved BCVA and reduced retinal fluid, brolucizumab exhibited distinct advantages in anatomical outcomes, including greater reductions in CMT and SFCT and a unique increase in CVI. These findings suggest that brolucizumab may provide additional benefits in managing pachychoroid disease, particularly in promoting choroidal morphology remodeling and reducing pachyvessel-related stress.
Methods
Study design
This retrospective, comparative study was conducted at the Yeungnam University Hospital. Ethical approval was obtained from the Institutional Review Board of Yeungnam University hospital (IRB No. 2024-12-036), and all study procedures adhered to the Declaration of Helsinki. Due to the retrospective nature of the study, the requirement for obtaining informed consent was waived by the Institutional Review Board of Yeungnam University hospital. Data were collected from electronic medical records of patients diagnosed with treatment-naïve PNV between January 2020 and December 2022. Diagnosis was confirmed using multimodal imaging, including spectral-domain optical coherence tomography (SD-OCT), fluorescein angiography (FA), and indocyanine green angiography (ICGA). Enhanced depth imaging OCT (EDI-OCT) was used to identify pachychoroid-specific features such as pachyvessels, thickened choroidal layers, and associated subretinal or intraretinal fluid.
Study population
To ensure a homogeneous study population, strict inclusion and exclusion criteria were applied. Inclusion criteria required treatment-naïve PNV patients to be 50 years or older and a minimum follow-up period of 12 months. Exclusion criteria included any history of prior retinal treatments such as anti-VEGF therapy, photodynamic therapy (PDT), or vitreoretinal surgery. Patients with history of central serous chorioretinopathy (CSC) or retinal pigment epithelial atrophic tracts secondary to CSC were excluded. Patients with coexisting retinal conditions, including diabetic retinopathy, retinal vein occlusion, pathologic myopia, or uveitis, were excluded, as were those with systemic diseases such as uncontrolled diabetes or autoimmune disorders. Cases with significant media opacity, poor imaging quality, or incomplete clinical records were also excluded to maintain the integrity of the data.
Clinical examinations
At each follow-up visit, the patients underwent a complete ophthalmic examination, which included measurement of the best-corrected visual acuity (BCVA), slit-lamp biomicroscopy, dilated funduscopy, OCT (Spectralis®, Heidelberg Engineering, Heidelberg, Germany), and OCT angiography (RTVue XR Avanti, Optovue Inc., Fremont, USA) at all visits during the 12-month follow-up. The decimal BCVA was converted to a logarithm of the minimal angle resolution (logMAR) units for statistical analysis. Angiography was performed with FA and ICGA at baseline using the Heidelberg Retina Angiograph + OCT (Heidelberg Engineering, Heidelberg, Germany) before treatment to exclude CSC and polypoidal choroidal vasculopathy.
Treatment strategies
After administering topical anesthesia, a 30-gauge needle was used to inject an anti-VEGF drug into the vitreous cavity at a point 3.5–4.0 mm posterior to the corneal limbus. All patients received intravitreal injections of either aflibercept (2.0 mg/0.05 ml) or brolucizumab (6.0 mg/0.05 ml). The treatment protocol consisted of an initial loading phase of three-monthly injections, followed by pro re nata (PRN) dosing based on disease activity. Disease activity was assessed through clinical examination and imaging findings, including the retinal fluid and thickness on OCT.
Outcome measures
The outcomes of the study included changes in BCVA measured in logMAR, central macular thickness (CMT), subfoveal choroidal thickness (SFCT), Haller’s layer and CC/Sattler layer thickness. CMT was automatically measured using the macular thickness map protocol of the Heidelberg Spectralis OCT. CMT was defined as the mean retinal thickness within the central 1-mm diameter circle of the Early Treatment Diabetic Retinopathy Study (ETDRS) grid centered on the fovea. Applying the Heidelberg Eye Explorer software, we measured the thicknesses of choroidal layers on horizontally orientated OCT sections at the foveal region. The SFCT was defined as the vertical distance from Bruch’s membrane to the choroidal-scleral interface. Haller’s layer was defined as the outer choroidal layer containing large choroidal vessels. CC/Sattler layer was calculated by subtracting the Haller’s layer thickness from the SFCT. The choroidal vascularity index (CVI) was defined as the ratio of luminal area (LA) to total choroidal area (TCA) within a 1500 μm-wide subfoveal region. A single EDI-OCT B-scan was binarized using the modified Niblack method in ImageJ (National Institutes of Health, Bethesda, MD, USA) to segment the luminal and stromal components. The TCA was outlined from the outer border of the RPE to the inner scleral boundary, and LA was calculated as the area occupied by dark pixels20. CNV lesion area was measured using the AngioVue (RTVue XR Avanti, Optovue Inc., Fremont, CA, USA) built-in analysis software. The lesion boundary was automatically outlined on the outer retina slab and manually adjusted if necessary. CC flow density was measured using the AngioVue built-in software on a 3 × 3 mm OCTA image of the CC slab. The area corresponding to the CNV lesion was manually excluded from the analysis to prevent artifacts and ensure accurate assessment of the surrounding CC perfusion. The proportion of patients achieving dry macula, defined as the absence of fluid on OCT, and the total number of injections administered over the 12-month follow-up period were also evaluated.
Statistical analysis
Statistical analyses were conducted using Statistical Product and Service Solution (SPSS) statistical software V24.0. Continuous variables were expressed as mean ± standard deviation. Qualitative data were compared using Fisher’s exact test. The Wilcoxon t-test was used for nonparametric numerical data. The Bonferroni correction is used when several dependent or independent statistical tests are performed simultaneously. Longitudinal changes in BCVA, CMT, SFCT, CVI, and CC flow density were assessed using repeated measures ANOVA to evaluate trends over time. A p-value of < 0.05 was considered statistically significant for all analyses.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Pang, C. E. & Freund, K. B. Pachychoroid neovasculopathy. Retina 35, 1–9 (2015).
Farvardin, M. et al. Pachychoroid neovasculopathy can mimic wet type age-related macular degeneration. Int. J. Retina Vitreous. 8, 78 (2022).
Hosoda, Y. et al. Deep phenotype unsupervised machine learning revealed the significance of pachychoroid features in etiology and visual prognosis of age-related macular degeneration. Sci. Rep. 10, 18423 (2020).
Kuranami, A., Maruko, R., Maruko, I., Hasegawa, T. & Iida, T. Pachychoroid neovasculopathy has clinical properties that differ from conventional neovascular age-related macular degeneration. Sci. Rep. 13, 7379 (2023).
Sartini, F., Figus, M., Casini, G., Nardi, M. & Posarelli, C. Pachychoroid neovasculopathy: A type-1 choroidal neovascularization belonging to the pachychoroid spectrum—pathogenesis, imaging and available treatment options. Int. Ophthalmol. 40, 3577–3589 (2020).
Matsumoto, H. et al. Chronic choriocapillaris ischemia in dilated vortex vein region in pachychoroid neovasculopathy. Sci. Rep. 11, 16274 (2021).
Hara, C. et al. Characteristics of patients with neovascular age-related macular degeneration who are non-responders to intravitreal Aflibercept. Br. J. Ophthalmol. 102, 1–6 (2018).
Jung, B. J. et al. Intravitreal Aflibercept and Ranibizumab for pachychoroid neovasculopathy. Sci. Rep. 9, 2055 (2019).
Jeong, A., Kang, W. & Sagong, M. Multimodal imaging features and treatment responses of choroidal neovascularization secondary to central serous chorioretinopathy. Retina 42, 2326–2335 (2022).
Carosielli, M. et al. Intravitreal Brolucizumab for pachychoroid neovasculopathy associated with chronic central serous chorioretinopathy. Transl. Vis. Sci. Technol. 12, 17 (2023).
Chang, Y. C. & Cheng, C. K. Difference between pachychoroid and non-pachychoroid polypoidal choroidal vasculopathy and their response to anti-vascular endothelial growth factor therapy. Retina 40, 1403–1411 (2020).
Kitajima, Y. et al. One-year outcome of combination therapy with intravitreal anti-vascular endothelial growth factor and photodynamic therapy in patients with pachychoroid neovasculopathy. Graefes Arch. Clin. Exp. Ophthalmol. 258, 1279–1285 (2020).
Miki, A. et al. Photodynamic therapy combined with anti-vascular endothelial growth factor therapy for pachychoroid neovasculopathy. PLoS One 16, e0248760 (2021).
Hikichi, T., Kubo, N. & Yamauchi, M. One-year comparison of anti-vascular endothelial growth factor and half-dose photodynamic therapies for pachychoroid neovasculopathy. Eye 35, 3367–3375 (2021).
Yoon, J. et al. Long-term outcome of intravitreal anti-vascular endothelial growth factor treatment for pachychoroid neovasculopathy. Sci. Rep. 11, 12052 (2021).
Azuma, K. et al. The association of choroidal structure and its response to anti-VEGF treatment with the short-time outcome in pachychoroid neovasculopathy. PLoS One 14, e0212055 (2019).
Boscia, G. et al. Choroidal remodeling following different anti-VEGF therapies in neovascular AMD. Sci. Rep. 14, 1941 (2024).
Abdin, A. D. et al. Long-term choroidal thickness changes based on the subtype of macular neovascularization in neovascular age-related macular degeneration (5-year follow-up). Graefes Arch. Clin. Exp. Ophthalmol. 262, 457–468 (2024).
Viggiano, P. et al. Short-term morphofunctional changes in previously treated neovascular AMD eyes switched to Brolucizumab. J. Clin. Med. 11, 5517 (2022).
Sonoda, S. et al. Choroidal structure in normal eyes and after photodynamic therapy determined by binarization of optical coherence tomographic images. Investig. Ophthalmol. Vis. Sci. 55, 3893–3899. https://doi.org/10.1167/iovs.14-14447 (2014).
Author information
Authors and Affiliations
Contributions
Concept and design: M.S. Data collection and statistical analysis: A.J. and S.C.B. Drafting of the manuscript: A.J., S.C.B., and F.R.d.J. Critical revision of the manuscript for important intellectual content: A.J. and M.S. Supervision: M.S.
Corresponding author
Ethics declarations
Competing interests
M. Sagong reported being a consultant for and receiving grant support from Samsung Bioepis, Novartis, Bayer, Roche, Allergan/Abbvie, Celltrion, Alteogen, Alcon and Curacle, and receiving lecture fee from Novartis, Bayer, Roche, and Allergan/Abbvie.All other authors declare no competing interests. All other authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jeong, A., de Jesus, F.R., Baek, S.C. et al. Comparison of one-year outcomes between aflibercept and brolucizumab for treatment-naïve pachychoroid neovasculopathy. Sci Rep 15, 13674 (2025). https://doi.org/10.1038/s41598-025-98402-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-98402-4