Abstract
This study aimed to evaluate the causal effect of sodium-glucose cotransporter protein 2 (SGLT2) inhibition on primary open-angle glaucoma (POAG) and explore potential mechanisms. A drug-targeted Mendelian randomization (MR) study was conducted using genetic variation related to SGLT2 inhibition, based on SGLT2 gene expression and glycated hemoglobin levels. Genetic summary statistics for POAG were obtained from the FinnGen consortium and a multi-ancestry genome-wide association study. Glaucomatous endophenotype data were also incorporated. A two-step MR analysis was performed to examine whether pathways related to obesity, blood pressure, lipid levels, oxidative stress, and inflammation mediated the association between SGLT2 inhibition and POAG. Genetically predicted SGLT2 inhibition was associated with a reduced risk of POAG (OR: 0.28; 95% CI: 0.12 to 0.63; P = 2.22 × 10− 3), confirmed in a multi-ancestry validation cohort. It was also associated with decreased optic cup area, reduced vertical cup-disc ratio, and increased optic disc area. Mediation analysis indicated that the effect of SGLT2 inhibition on POAG was partly mediated by diastolic blood pressure (4.8%). This study suggests that SGLT2 inhibition is a promising therapeutic target for POAG. However, further large-scale randomized controlled trials are required to confirm these findings.
Similar content being viewed by others
Introduction
Glaucoma is a progressive optic neuropathy representing the primary cause of irreversible blindness globally. It is characterized by the progressive degeneration of retinal ganglion cells and axons, which causes optic nerve head cupping and subsequent visual impairment1,2. The most common glaucoma subtype is the primary open-angle glaucoma (POAG)3. The biological basis of POAG has not been comprehensively elucidated, and the factors involved in its development have not been thoroughly characterized1. Intraocular pressure (IOP) constitutes a major independent and modifiable risk factor for POAG. IOP reduction through the administration of topical eye drops reportedly decelerates the progression of POAG4. However, these topical treatments necessitate lifelong adherence and are usually accompanied by numerous ocular side effects5. Moreover, they may not be suitable for patients with glaucoma who do not have elevated IOP. Systemic diseases, including type 2 diabetes mellitus (T2DM), are reportedly associated with glaucomatous optic nerve damage6. Chronic hyperglycemia contributes to endothelial dysfunction and impairs blood flow to the optic nerve head, potentially causing glaucomatous damage to the optic nerve and retinal nerve fiber layer. Diabetes-induced oxidative stress and chronic inflammation can produce analogous outcomes7. Additionally, numerous observational studies have revealed metabolic characteristics, such as obesity, systemic arterial hypertension, and dyslipidemia, as potential risk factors for the development of glaucomatous optic nerve damage7,8.
The sodium-glucose cotransporter protein 2 inhibitor (SGLT2i) represents a novel class of antidiabetic agents. This pharmacological intervention involves specifically targeting the sodium-glucose cotransporter protein 2 (SGLT2) to impede glucose reabsorption in the renal proximal tubules, enhancing urinary glucose excretion and contributing to maintaining glucose homeostasis9. In addition to its hypoglycemic properties, SGLT2i reportedly lowers blood pressure, modulates lipid metabolism, reduces obesity, and mitigates tissue oxidative stress and inflammation10,11. Extensive clinical trials have shown that SGLT2i significantly improves major renal outcomes and cardiovascular event outcomes in patients with T2DM12,13. Similarly, SGLT2i improved retinal hypoxia and optic neuroprotection, and a population-based cohort study by Shao et al. showed that SGLT2 inhibitors significantly reduced glaucoma risk in patients with type 2 diabetes compared with that in patients with T2DM taking GLP-1 RA14,15. The latest meta-analysis has confirmed that the incidence of glaucoma is significantly lower in T2DM patients receiving GLP-1 RA therapy16. Therefore, SGLT2i may hold substantial clinical potential in reducing the risk of glaucoma and could potentially serve as the first-line antidiabetic agents for diabetic patients at high risk of glaucoma. However, current clinical evidence is limited to a single study conducted in Taiwan, and the causal relationship as well as the precise underlying mechanisms remain unclear, necessitating further investigation.
Mendelian randomization (MR) study is a new epidemiological method for making causal inferences, which involves using genetic variation as an instrumental variable (IV)17. Given that genetic variation is subject to the random assignment of alleles during meiosis, similar to that in a randomized controlled experiment, and germline genotypes remain unaltered by disease onset and progression, MR markedly diminishes the influence of confounding factors and reverse causality18. This advantage of MR overcomes the inherent limitations of observational studies.
Investigating multi-target long-term oral medications is crucial for the early prevention of POAG. In this study, we initially conducted a two-sample MR analysis to examine the causal effect of SGLT2 inhibition on POAG, using genetic variations in drug targets as IVs. Subsequently, mediation analyses were performed based on the pharmacological effects of SGLT2is in a two-step MR framework to identify potential pathways by which they affect POAG risk. This analysis provides new insights into the repurposing value of SGLT2i in POAG.
Materials and methods
Study design
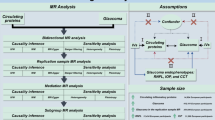
Figure 1 shows a comprehensive overview of the study’s framework. First, genetic variants associated with SGLT2 inhibition were identified. Second, a drug-target MR analysis was conducted using a TSMR method to investigate the effect of SGLT2 inhibition on POAG and glaucoma endophenotypes. Third, we evaluated the causal impact of fasting glucose and glycated hemoglobin (HbA1c) levels on POAG to confirm whether the causal effect of SGLT2 inhibition on POAG could be attributed to its glucose-lowering effect. Moreover, we included validation cohorts for external validation and used meta-analyses to amalgamate non-significant findings to enhance the robustness and credibility of our results. Finally, we used mediation analyses within a two-step MR framework to elucidate whether the established pharmacological effects of SGLT2is could act as potential mechanisms for mitigating POAG risk.
Our MR analysis was based on three fundamental assumptions that the genetic variation: (1) is strongly associated with the exposure; (2) is independent of potential confounders; and (3) influences the outcome solely through the exposure19. Furthermore, the study was designed following the Strengthening the Reporting of Observational Studies in Epidemiology using MR (STROBE-MR) reporting guidelines20. The genome-wide association study (GWAS) summary data used in our study were approved by the appropriate ethics committees in the original studies. A comprehensive characterization of the data sources is presented in Supplementary Table S1.
Selection and validation for IVs of SGLT2 Inhibition
We identified IVs for SGLT2 inhibition through a five-step process based on the methods of Li et al., aiming to establish a genetic proxy that bridges gene expression-associated loci with drug inhibition effects21. (1) Using data from the Genotype-Tissue Expression (GTEx) project22 and the eQTLGen consortium23, we identified single nucleotide polymorphisms (SNPs) associated with SLC5A2 (the gene encoding SGLT2) expression. (2) Given that SGLT2i lower blood glucose by inhibiting renal glucose reabsorption, we conducted a MR analysis to evaluate the association between each SLC5A2 variant and HbA1c levels. SLC5A2 variants with a significant negative association with HbA1c (P < 1 × 10− 4) were selected to simulate the long-term pharmacological effect of SGLT2 inhibition. This step identified SLC5A2 variants naturally corresponding to reduced HbA1c levels, effectively serving as a genetic proxy for SGLT2 inhibition. The GWAS summary data for HbA1c were obtained from a cohort of 344,182 non-diabetic European individuals in the UK Biobank. (3) We performed Bayesian colocalization analysis to determine whether the selected genetic variants within the SLC5A2 region simultaneously influence SLC5A2 expression and HbA1c levels, selecting colocalized variants with a posterior probability of H4 (PPH4) greater than 0.724,25. (4) A standard clumping procedure was used to eliminate SNPs in linkage disequilibrium, with parameters set at r² = 0.8 and a distance of 250 kb. (5) The F-statistic of each IV was calculated to ensure a value of > 10 to mitigate bias owing to weak IVs26. We identified 10 SNPs as IVs for SGLT2 inhibition after the selection and validation procedures for subsequent MR analysis (Supplementary Table S2).
Data source for POAG and glaucoma endophenotypes
Summary genetic data for POAG were obtained from two large GWAS datasets. During the discovery phase, we procured summary statistics for POAG from the FinnGen R11 research consortium (https://r11.finngen.fi/), which included 9,565 cases and 430,250 controls, all of Finnish descent. POAG was classified according to the International Classification of Diseases, 10th Revision, under the code H40.127. During the external validation phase, summary data for POAG were sourced from Zhou et al.28. These summary data were derived from a GWAS meta-analysis encompassing six distinct ancestry groups, which was used to conduct a sensitivity test of the association between SGLT2 inhibition and POAG across trans-ethnic populations, mitigating the bias associated with reliance on a single European ancestry. Additionally, we extracted GWAS summary data for six glaucoma endophenotypes, namely retinal nerve fiber layer thickness29, ganglion cell inner plexiform layer thickness29, IOP30, optic cup area30, optic disc area30, and vertical cup-disc ratio30. Detailed information on each dataset is presented in Supplementary Table S1.
Exclusion of outcome-associated Snps and confounders
Based on the three core assumptions of MR studies, we excluded SNPs strongly associated with the outcome before each MR analysis to ensure that the selected SNPs influence the outcome solely through the exposure31. Since blood pressure, blood glucose, lipid traits, oxidative stress, and inflammatory responses are potential confounders of POAG, we examined the GWAS data of potential mediators listed in Supplementary Table S1 before performing the MR analysis of SGLT2 inhibition and POAG. After scanning these data, we confirmed that all 10 identified SNPs for SGLT2 inhibition were not strongly associated with these potential confounders. Both steps were conducted using a threshold of P < 5 × 10− 8.
MR analysis
We evaluated the causal relationship between SGLT2 inhibition and POAG using four MR techniques, namely inverse variance weighted (IVW), weighted median, MR-Egger, and maximum likelihood. Among these, the IVW method was the principal analytical approach, and the other three were used to complement and enhance the IVW findings. The IVW method necessitates the validity of all IVs, yielding unbiased causal estimates in the absence of horizontal pleiotropy32. The weighted median method, which yields unbiased estimates and sustains high statistical efficiency even in the presence of approximately 50% invalid IVs, is a significant complement to the IVW results33. The MR-Egger method relaxes the “no horizontal pleiotropy assumption” by allowing all IVs to be invalid. While this method is less efficient than the others, it provides causal estimates that consider horizontal pleiotropy effects34.
In addition, further sensitivity analyses were conducted to investigate potential heterogeneity and horizontal pleiotropy, enhancing the reliability of the findings. Cochran’s Q test based on MR-Egger and IVW methods was performed to assess the presence of heterogeneity within the IVs. When P > 0.05, it indicated the absence of significant heterogeneity, and the fixed-effects IVW model was selected; otherwise, the random-effects IVW model was selected. The presence of horizontal pleiotropy was determined using MR-Egger regression analysis, with P < 0.05 indicating significant horizontal pleiotropy26.
To mitigate the probability of Type I errors, the Benjamini–Hochberg procedure was used to calculate the false discovery rate (FDR) to correct IVW results after conducting multiple MR tests. Results exhibiting a PFDR < 0.05 indicated a significant causal relationship. Conversely, associations that met the criterion of PIVW < 0.05 but were not corrected using FDR were considered to suggest potential causality only35. In our study, a robust causal relationship should meet the following criteria: (i) a PFDR of < 0.05 after multiple MR tests; (ii) consistent directional estimates across four MR methods; (iii) the absence of horizontal pleiotropy. Additionally, we have external validation for the replication of the association between SGLT2 inhibition and POAG. The MR analyses were performed using R software (version 4.3.2) and the “TwoSampleMR” package (version 0.5.8).
Effect of glucose traits on the risk of POAG
To evaluate whether the effect of SGLT2 inhibition on POAG risk is attributable to its glucose-lowering effects, we examined the impact of fasting glucose and HbA1c levels on POAG risk using TSMR.
Mediation analysis based on the Pharmacological effects of SGLT2is
In addition to direct hypoglycemic effects, the potential influence of other pathways associated with SGLT2i on POAG remains uncertain. To elucidate the genetically predicted pharmacological mechanisms by which SGLT2i may prevent POAG, we conducted a mediation analysis using a two-step MR design. Initially, we evaluated the causal relationship between genetic proxies for SGLT2i and its established regulatory pathways, including obesity indicators36, blood pressure37, lipid traits38, oxidative stress39, and inflammation-related pathways40. MR analyses were conducted using the IVW method, with analytical models selected based on heterogeneity assessments. P-values from multiple tests were adjusted using the FDR technique. Significant results were subsequently incorporated into mediation analysis to investigate the pharmacological pathways underlying the association between SGLT2i and POAG. Detailed information regarding the GWAS datasets used for the mediation analysis is presented in Supplementary Table S1. We used the “product of coefficients” method to calculate the indirect effect of SGLT2 on POAG via the mediator (β1 × β2). The proportion of the mediator variable in the total effect was calculated using the indirect effect divided by the total effect (β1 × β2/β3). β1, β2, and β3 represent the effect of SGLT2 inhibition on the mediators, the effect of the mediators on POAG, and the effect of SGLT2 inhibition on POAG, respectively. Finally, we used the delta method to calculate the 95% confidence intervals (CIs) for the mediator proportions41.
Ethics approval and consent to participate
This study conducted an analysis utilizing previously GWAS summary data and did not involve the recruitment of new human participants or the use of animals. Ethical approval and consent to participate were secured for all original studies from which the data were derived. Consequently, the application of these data for MR analyses typically did not necessitate additional ethical approval.
Results
Effect of SGLT2 Inhibition on the risk of POAG
We identified 10 sufficiently robust SNPs as genetic instruments for SGLT2 inhibition, as detailed in the publication by Li et al. (Supplementary Table S2). Each SNP exhibited an F-statistical value exceeding 10, minimizing the potential bias associated with weak IVs. In the MR analysis conducted during the discovery phase, the primary results obtained through the IVW method demonstrated a significant association between SGLT2 inhibition and a reduced POAG risk (OR: 0.28; 95% CI: 0.12 to 0.63; P = 2.22 × 10− 3), corresponding to a 1 standard deviation decrease in HbA1c owing to SGLT2 inhibition (Fig. 2; Supplementary Table S3). The robustness of the results was enhanced using other statistical methods that consistently confirmed a causal relationship between SGLT2 inhibition and POAG. Among them, weighted median and maximum likelihood methods also yielded the causal association as significant. In addition, Cochran’s Q test showed no significant heterogeneity among SNPs, and MR-Egger regression analysis did not reveal significant horizontal pleiotropy (Supplementary Table S3). To further validate the causality, we selected POAG data from other data sources as external validation. IVW results showed that SGLT2 inhibition significantly reduced POAG risk (OR: 0.45; 95% CI: 0.24 to 0.84; P = 0.013). Furthermore, consistent directions of association were obtained for the four MR methods, and the sensitivity analyses did not detect significant heterogeneity or horizontal pleiotropy (Fig. 2; Supplementary Table S3). Additional tests and sensitivity analyses reinforced the association of SGLT2 inhibition with POAG.
Effect of SGLT2 Inhibition on glaucoma endophenotypes
Glaucoma endophenotypes demonstrated a statistically significant correlation with SGLT2 inhibition (Fig. 3; Supplementary Table S4). Under the IVW approach, FDR-corrected, SGLT2 inhibition was significantly associated with decreased optic cup area (OR: 0.78; 95% CI: 0.70 to 0.88; P = 2.47 × 10− 5), decreased vertical cup-disc ratio (OR: 0.90; 95% CI: 0.84 to 0.96; P = 1.48 × 10− 3), and increased optic disc area (OR: 1.24, 95% CI: 1.02 to 1.52, P = 0.033). Other MR methods also produced consistent correlation directions. The sensitivity analyses showed no heterogeneity or horizontal pleiotropy.
Effect of glucose traits on the risk of POAG
To determine whether the protective effect of SGLT2 inhibition on POAG risk is mediated by its glucose-lowering properties, we conducted a TSMR analysis to explore the association between glucose-related traits (fasting glucose and HbA1c) and POAG. The findings indicate that in the discovery phase, genetically predicted HbA1c levels were significantly associated with an increased POAG risk (Fig. 4; Supplementary Table S5; IVW OR: 1.13; 95% CI: 1.01 to 1.27; P = 0.034), whereas no significant association was observed between fasting glucose levels and POAG. During the validation phase, genetically predicted fasting glucose levels were associated with an increased POAG risk (IVW OR: 1.34; 95% CI: 1.01 to 1.78; P = 0.046), whereas HbA1c levels and POAG showed no significant associations. Subsequently, data from the discovery and validation phases were combined in a meta-analysis. The meta-analysis revealed a positive correlation between genetic susceptibility to POAG and elevated levels of fasting glucose and HbA1c (both p < 0.05; Fig. 4. Although each glycemic trait demonstrated significant results exclusively in the discovery or validation phase, the potent glucose-lowering effects of SGLT2is necessitate the careful consideration of glucose regulatory role in the intermediate pathway between SGLT2 inhibition and POAG. To enhance statistical power, a meta-analysis was conducted on the results obtained from the two data sources regarding POAG. The findings indicate that blood glucose levels contribute to increased susceptibility to POAG.
Mediation analysis based on the Pharmacological effects of SGLT2is
In addition to its glucose-lowering effects, we aimed to identify other pharmacological mechanisms by which SGLT2is reduce POAG risk using a two-step MR mediation analysis. In the first step, we explored the causal association between genetically proxied SGLT2 inhibition and 58 traits across five known pharmacological pathways of SGLT2i action. Following FDR correction, we identified 20 traits associated with genetically predicted SGLT2 inhibition. These traits were broadly related to indicators of obesity, blood pressure, lipid profiles, oxidative stress, and inflammation-related pathways within the five SGLT2i pharmacological pathways (Supplementary Table S6). In the second step, we explored the causal relationship between these 20 traits and POAG. Our results demonstrated that diastolic blood pressure (DBP), corrected for FDR, could significantly increase POAG risk (Supplementary Table S7; IVW OR: 1.27; 95% CI: 1.07 to 1.50; P = 6.38 × 10− 3). Subsequently, combining two-step MR results and a “product of coefficients” method, we evaluated the role of DBP in mediating the causal association between SGLT2 inhibition and POAG. We observed (Table 1) that the total effect of SGLT2 inhibition on POAG was indirectly mediated by DBP (β: -0.06; 95% CI: -0.11 to -0.01; P = 0.022), with a mediated proportion of 4.8% (95% CI: 0.3–9.9%).
Discussion
In this study, we confirmed the causal effect of SGLT2 inhibition on POAG risk. Our findings provide robust evidence that SGLT2 inhibition is a promising therapeutic target with a protective effect against POAG, a conclusion that was corroborated in another trans-ancestral dataset. Genetic evidence further supports a causal relationship between SGLT2 inhibition and a decreased optic cup area, an increased optic disc area, and a decreased vertical cup-to-disc ratio. This suggests that SGLT2 inhibition significantly reduces the risk of optic nerve damage. In addition, lifelong elevated fasting glucose and HbA1c levels were associated with an increased POAG risk, revealing that the therapeutic action of SGLT2 inhibition in POAG may be related to its hypoglycemic effect. A two-step MR mediation analysis further indicated that the risk-reduction effect of SGLT2 inhibition on POAG was partially mediated by DBP (4.8%).
The protective effect of SGLT2i on POAG remains uncertain. To date, only one multi-institutional cohort study conducted in Taiwan has suggested that, among patients with T2DM, those newly prescribed SGLT2i had a lower risk of developing glaucoma compared to those newly prescribed GLP-1 RA15. However, this observational study has several limitations, including a sample restricted to the East Asian population, a small sample size, a short study duration, and a lack of adjustment for potential confounders. These limitations hinder further exploration of this topic. Currently, the therapeutic role of SGLT2i in glaucoma remains unexplored. No studies have investigated whether SGLT2i can reduce the risk of glaucoma in non-diabetic populations, and the potential mechanisms of action remain unclear. Our study, from a genetic perspective, utilized large-scale GWAS summary data to establish a causal relationship between SGLT2 inhibition and POAG and explored the potential mechanisms underlying the drug’s effects.
We posit that the primary mechanism by which SGLT2i mitigates POAG risk is its direct glucose-lowering effect. Previous observational studies have indicated that oral SGLT2i significantly decreased the risk of diabetic retinopathy and macular edema compared with other oral hypoglycemic agents in patients with T2DM14,42. Diabetes frequently elevates POAG risk through the dysfunction of small blood vessels supplying the optic nerve and oxidative damage. Effective oral hypoglycemic control is crucial to prevent POAG7,43. Matthews et al. demonstrated that dapagliflozin attenuated retinal microvascular and neural abnormalities in a diabetic rat model44. Ipragliflozin ameliorated neuromodulatory dysfunction in the retina by blocking the latency prolongation of electroretinographic oscillatory potentials in rats after oral drug administration45.
SGLT2i also confer protective effects against glaucoma through non-glycemic mechanisms, providing a theoretical basis for their potential application in non-diabetic patients. In POAG, disruption of the blood-retinal barrier (BRB) leads to the extravasation of harmful plasma components into the retina and optic nerve, which triggers inflammation, immune cell infiltration, and retinal ganglion cells (RGCs) death46,47. SGLT2 is specifically expressed in retinal microvascular endothelial cells, and its excessive activation can disrupt osmotic balance in pericytes, leading to cellular swelling, loss of contractile function, and ultimately cell death48,49. By modulating these pathological processes, SGLT2i may help preserve the structural and functional integrity of the BRB, thereby exerting neuroprotective effects against POAG. Oxidative stress also plays a significant role in glaucoma progression, as it promotes extracellular matrix remodeling in the trabecular meshwork, leading to increased IOP50. Additionally, excessive reactive oxygen species (ROS) production activates autophagy and mitochondrial apoptosis, contributing to the degeneration of RGCs and irreversible optic nerve damage51,52. By mitigating oxidative stress and reducing ROS accumulation, SGLT2i may help regulate IOP homeostasis and protect the optic nerve from degeneration. Studies have shown that dapagliflozin, for example, alleviates high glucose-induced apoptosis in retinal microvascular endothelial cells through the ERK1/2/cPLA2/AA/ROS pathway53. SGLT2i have also been shown to regulate lipid metabolism, increasing HDL-C levels, which are reduced in POAG patients54,55. Our study found that SGLT2 inhibition significantly elevated HDL-C levels, suggesting a potential role for these agents in glaucoma prevention and treatment by enhancing reverse cholesterol transport and modulating lipid-related processes.
We used mediated MR methods to find that the protective effect of SGLT2 inhibition against POAG is facilitated through DBP reduction. The relationship between blood pressure and glaucoma is intricate, suggesting that antihypertensive therapy may play a preventive role in the development of glaucoma. Marshall et al. observed in a cohort study that elevated DBP and systolic blood pressure were associated with structural damage in glaucoma56. Furthermore, in patients with glaucoma, aggressive antihypertensive therapy may be advantageous in delaying the onset of the disease57. A meta-analysis encompassing 60 studies demonstrated that systolic blood pressure and DBP were consistently positively correlated with IOP58. However, the relationship between blood pressure and glaucoma may be nonlinear, potentially exhibiting a U-shaped association59. Hypotension and hypertension are considered risk factors for glaucoma, although through distinct mechanisms. Hypertension can elevate IOP by enhancing aqueous humor production. Additionally, chronic hypertension can cause increased peripheral resistance and abnormalities in small blood vessels, reducing perfusion to the optic nerve head and causing ischemic damage to retinal ganglion cells60. Conversely, hypotension directly diminishes the perfusion pressure to the optic nerve head61. Kim et al. used data from the National Health and Nutrition Examination Survey to determine that the prevalence of glaucoma was lowest at DBP levels ranging from 81 to 90 mmHg59. SGLT2i exhibits a significant hypotensive effect, with an average blood pressure reduction of 11.9 mmHg62. The hypotensive action of SGLT2i is primarily attributed to osmotic diuresis induced by enhanced glucose excretion. Moreover, SGLT2i can lower blood pressure by inhibiting sympathetic nerve activity and promoting weight reduction11.
The pleiotropic mechanisms of SGLT2i complement the pathophysiological processes of glaucoma, offering a novel therapeutic perspective. Further research is needed to assess their safety and benefits in ophthalmic applications. Currently, first-line POAG treatment focuses on topical IOP-lowering medications, including β-blockers, carbonic anhydrase inhibitors, prostaglandin analogs, and cholinergic agonists63,64. However, monotherapy often faces challenges such as inadequate response and side effects(e.g., ocular irritation and conjunctival hyperemia)65,66. SGLT2i, with their multi-target properties, have shown therapeutic value in systemic diseases like diabetes and cardiovascular disease. Their unique advantage lies in their potential to provide both systemic benefits and neuroprotection for glaucoma patients with comorbid conditions.
Furthermore, SGLT2i have a favorable safety profile, with common adverse effects being limited to reversible symptoms such as genitourinary infections, rashes, and thirst67,68. No increased risk of severe hypoglycemia has been observed compared to placebo69,70. While their effects on blood pressure and lipid parameters are generally beneficial, they may cause unintended physiological fluctuations in individuals with normal baseline metabolic indices. The therapeutic value of SGLT2i in glaucoma patients without systemic comorbidities requires further validation. However, for high-risk or diagnosed glaucoma patients with systemic conditions, SGLT2i offer unique clinical advantages, providing both systemic and ocular benefits.
However, our study has several limitations. First, IVs selection for SGLT2i was based on SLC5A2 variants that naturally correspond to a targeted reduction in HbA1c levels. This approach may not fully capture the precise clinical therapeutic effects of SGLT2 inhibitors. Second, the present study found a higher effect strength of SGLT2 inhibition in association with POAG, significantly higher than conventionally expected, which may be related to the methodological properties of the study. The simulation of SGLT2 inhibition effects based on genetic variants reflects the lifelong regulatory effects at the gene level, which differs from the pharmacological effects of SGLT2i with a limited regimen of medication in the real world of clinical use. In real-world scenarios, drug efficacy is influenced by multiple factors—including dosage, medication adherence, interindividual metabolic differences, and other pharmacokinetic parameters. It is difficult to systematically assess the modulation of observed effects by these dosing regimen-related variables at the level of the existing study design. Third, The primary objective of this drug-target MR study is to simulate the inhibitory effect of the drug on the SGLT2 protein, rather than merely identifying genetic loci associated with the phenotype. IVs selection must meet two criteria: specificity for the target gene (SLC5A2) and association with the phenotype (reduction in HbA1c levels). Using a stringent threshold of P < 5 × 10− 8 for HbA1c association could result in an insufficient number of SNPs, potentially failing to capture a sufficient range of functional loci within the target gene. Therefore, we applied a more lenient threshold of P < 1 × 10− 4. Despite performing colocalization analysis, removing linkage disequilibrium, and ensuring an F-statistic > 10, there remains a risk of weak IVs. Fourth, the POAG validation cohort in this study included 4,433 Finnish individuals, with partial sample overlap with the POAG discovery cohort. This overlap may violate the assumption of independence in meta-analysis, potentially increasing the Type I error rate. However, our original intention in utilizing the GWAS data provided by Zhou et al. for external validation was to assess the generalizability of our findings across ancestrally diverse POAG populations. Meta-analysis was only conducted when the primary results from the two datasets showed inconsistency. Furthermore, despite conducting sensitivity analyses, we could not entirely eliminate the bias attributable to potential horizontal pleiotropy. Finally, the study population was predominantly of European ancestry. While this study design effectively controlled for genetic heterogeneity within the population, it may limit the transethnic generalizability of our findings. Although we validated the MR results using GWAS data from multiethnic POAG patient cohorts, there is currently a lack of GWAS data on SLC5A2 gene expression in non-European populations. To confirm the generalizability of our findings, future translational studies are urgently needed in diverse ethnic groups, incorporating both clinical phenotype validation and mechanistic exploration.
Conclusion
Genetic evidence indicates that SGLT2 inhibition represents a promising therapeutic target for POAG. The protective effect of SGLT2 inhibition against POAG may be mediated through the modulation of DBP, in addition to its hypoglycemic properties. This offers novel insights into the preventive and therapeutic potential of SGLT2i for POAG, suggesting recommendations to optimize their clinical application.
Overview of the research design in this study. (A) Flowchart for assessing the effect of SGLT2 inhibition on POAG risk using MR. (B) The utilization of MR to investigate the impact of SGLT2 inhibition on POAG (total effect). (C) The application of a two-step MR framework was employed to investigate potential mediators in the SGLT2 inhibition and POAG association pathways, and to calculate indirect effects.
Data availability
The datasets generated and analyzed in this study are all available in the article/supplementary material. Contact the corresponding author for additional information.
References
Weinreb, R. N. & Khaw, P. T. Primary open-angle glaucoma. Lancet 363 (9422), 1711–1720 (2004).
Weinreb, R. N., Aung, T. & Medeiros, F. A. The pathophysiology and treatment of glaucoma: a review. Jama 311 (18), 1901–1911 (2014).
Tham, Y. C. et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 121 (11), 2081–2090 (2014).
Weinreb, R. N. et al. Primary open-angle glaucoma. Nat. Rev. Dis. Primers. 2, 16067 (2016).
Beckers, H. J., Schouten, J. S., Webers, C. A., van der Valk, R. & Hendrikse, F. Side effects of commonly used glaucoma medications: comparison of tolerability, chance of discontinuation, and patient satisfaction. Graefes Arch. Clin. Exp. Ophthalmol. 246 (10), 1485–1490 (2008).
Poh, S., Mohamed Abdul, R. B., Lamoureux, E. L., Wong, T. Y. & Sabanayagam, C. Metabolic syndrome and eye diseases. Diabetes Res. Clin. Pract. 113, 86–100 (2016).
Newman-Casey, P. A., Talwar, N., Nan, B., Musch, D. C. & Stein, J. D. The relationship between components of metabolic syndrome and open-angle glaucoma. Ophthalmology 118 (7), 1318–1326 (2011).
Kim, H. A., Han, K., Lee, Y. A., Choi, J. A. & Park, Y. M. Differential association of metabolic risk factors with open angle Glaucoma according to obesity in a Korean population. Sci. Rep. 6, 38283 (2016).
Heerspink, H. J., Perkins, B. A., Fitchett, D. H., Husain, M. & Cherney, D. Z. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 134 (10), 752–772 (2016).
Dharia, A., Khan, A., Sridhar, V. S. & Cherney, D. Z. I. SGLT2 inhibitors: the sweet success for kidneys. Annu. Rev. Med. 74, 369–384 (2023).
Salvatore, T. et al. An overview of the cardiorenal protective mechanisms of SGLT2 inhibitors. Int. J. Mol. Sci. 23(7). (2022).
Wiviott, S. D. et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl. J. Med. 380 (4), 347–357 (2019).
Bhatt, D. L. et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl. J. Med. 384 (2), 129–139 (2021).
Su, Y. C. et al. Risk of diabetic macular oedema with sodium-glucose cotransporter-2 inhibitors in type 2 diabetes patients: A multi-institutional cohort study in Taiwan. Diabetes Obes. Metab. 23 (9), 2067–2076 (2021).
Shao, S-C. et al. Association between sodium glucose co-transporter 2 inhibitors and incident glaucoma in patients with type 2 diabetes: A multi-institutional cohort study in Taiwan. Diabetes Metab. 48 (1), 101318 (2022).
Amaral, D. C. et al. GLP-1 receptor agonists use and incidence of glaucoma: A systematic review and Meta-Analysis. Am. J. Ophthalmol. 271, 488–497 (2025).
Burgess, S. & Thompson, S. G. Mendelian Randomization: Methods for Using Genetic Variants in Causal Estimation (CRC, 2015).
Yuan, S. et al. Lifestyle and metabolic factors for nonalcoholic fatty liver disease: Mendelian randomization study. Eur. J. Epidemiol. 37 (7), 723–733 (2022).
Emdin, C. A., Khera, A. V. & Kathiresan, S. Mendelian randomization. Jama 318 (19), 1925–1926 (2017).
Skrivankova, V. W. et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. Jama 326 (16), 1614–1621 (2021).
Li, J. et al. SGLT2 Inhibition, Circulating metabolites, and atrial fibrillation: a Mendelian randomization study. Cardiovasc. Diabetol. 22 (1), 278 (2023).
The GTEx. Consortium atlas of genetic regulatory effects across human tissues. Science 369 (6509), 1318–1330 (2020).
Võsa, U. et al. Large-scale cis- and trans-eQTL analyses identify thousands of genetic loci and polygenic scores that regulate blood gene expression. Nat. Genet. 53 (9), 1300–1310 (2021).
Chen, J. et al. Multi-omic insight into the molecular networks of mitochondrial dysfunction in the pathogenesis of inflammatory bowel disease. EBioMedicine 99, 104934 (2024).
Chen, Z. et al. Sodium-glucose cotransporter protein 2 Inhibition, plasma proteins, and ischemic stroke: A mediation Mendelian randomization and colocalization study. J. Stroke Cerebrovasc. Dis. 34 (1), 108136 (2025).
Chen, Z. et al. Exploration of the causal associations between Circulating inflammatory proteins, immune cells, and neuromyelitis Optica spectrum disorder: a bidirectional Mendelian randomization study and mediation analysis. Front. Aging Neurosci. 16, 1394738 (2024).
Kurki, M. I. et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 613 (7944), 508–518 (2023).
Zhou, W. et al. Global biobank Meta-analysis initiative: powering genetic discovery across human disease. Cell. Genom. 2 (10), 100192 (2022).
Currant, H. et al. Genetic variation affects morphological retinal phenotypes extracted from UK biobank optical coherence tomography images. PLoS Genet. 17 (5), e1009497 (2021).
Bonnemaijer, P. W. M. et al. Multi-trait genome-wide association study identifies new loci associated with optic disc parameters. Commun. Biol. 2, 435 (2019).
Chen, Z. et al. Exploring correlations between immune cell phenotypes and the risk of epilepsy: A bidirectional Mendelian randomization study. Epilepsy Behav. 157, 109896 (2024).
Yuan, S. & Larsson, S. C. Coffee and caffeine consumption and risk of kidney stones: A Mendelian randomization study. Am. J. Kidney Dis. 79 (1), 9–14e11 (2022).
Yavorska, O. O. & Burgess, S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int. J. Epidemiol. 46 (6), 1734–1739 (2017).
Burgess, S. & Thompson, S. G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 32 (5), 377–389 (2017).
Zhang, T., Cao, Y., Zhao, J., Yao, J. & Liu, G. Assessing the causal effect of genetically predicted metabolites and metabolic pathways on stroke. J. Transl Med. 21 (1), 822 (2023).
Rajeev, S. P., Cuthbertson, D. J. & Wilding, J. P. Energy balance and metabolic changes with sodium-glucose co-transporter 2 Inhibition. Diabetes Obes. Metab. 18 (2), 125–134 (2016).
Mazidi, M., Rezaie, P., Gao, H. K. & Kengne, A. P. Effect of Sodium-Glucose Cotransport-2 inhibitors on blood pressure in people with type 2 diabetes mellitus: A systematic review and Meta-Analysis of 43 randomized control trials with 22 528 patients. J. Am. Heart Assoc. 6(6). (2017).
Lazarte, J., Kanagalingam, T. & Hegele, R. A. Lipid effects of sodium-glucose cotransporter 2 inhibitors. Curr. Opin. Lipidol. 32 (3), 183–190 (2021).
Kolijn, D. et al. Empagliflozin improves endothelial and cardiomyocyte function in human heart failure with preserved ejection fraction via reduced pro-inflammatory-oxidative pathways and protein kinase Gα oxidation. Cardiovasc. Res. 117 (2), 495–507 (2021).
Elrakaybi, A., Laubner, K., Zhou, Q., Hug, M. J. & Seufert, J. Cardiovascular protection by SGLT2 inhibitors - Do anti-inflammatory mechanisms play a role? Mol. Metab. 64, 101549 (2022).
MacKinnon, D. P., Lockwood, C. M., Hoffman, J. M., West, S. G. & Sheets, V. A comparison of methods to test mediation and other intervening variable effects. Psychol. Methods. 7 (1), 83–104 (2002).
Chung, Y. R., Ha, K. H., Lee, K. & Kim, D. J. Effects of sodium-glucose cotransporter-2 inhibitors and dipeptidyl peptidase-4 inhibitors on diabetic retinopathy and its progression: A real-world Korean study. PLoS One. 14 (10), e0224549 (2019).
Szaflik, J. P. et al. Reactive oxygen species promote localized DNA damage in glaucoma-iris tissues of elderly patients vulnerable to diabetic injury. Mutat. Res. 697 (1–2), 19–23 (2010).
Mudaliar, S., Hupfeld, C. & Chao, D. L. SGLT2 Inhibitor-Induced Low-Grade ketonemia ameliorates retinal hypoxia in diabetic Retinopathy-A novel hypothesis. J. Clin. Endocrinol. Metab. 106 (5), 1235–1244 (2021).
Takakura, S., Toyoshi, T., Hayashizaki, Y. & Takasu, T. Effect of Ipragliflozin, an SGLT2 inhibitor, on progression of diabetic microvascular complications in spontaneously diabetic Torii fatty rats. Life Sci. 147, 125–131 (2016).
Alarcon-Martinez, L. et al. Neurovascular dysfunction in glaucoma. Prog Retin Eye Res. 97, 101217 (2023).
O’Leary, F. & Campbell, M. The blood-retina barrier in health and disease. Febs J. 290 (4), 878–891 (2023).
Wakisaka, M. et al. Suppression of sodium-dependent glucose uptake by Captopril improves high-glucose-induced morphological and functional changes of cultured bovine retinal pericytes. Microvasc Res. 58 (3), 215–223 (1999).
Roy, S., Bae, E., Amin, S. & Kim, D. Extracellular matrix, gap junctions, and retinal vascular homeostasis in diabetic retinopathy. Exp. Eye Res. 133, 58–68 (2015).
Hsueh, Y. J. et al. The pathomechanism, antioxidant biomarkers, and treatment of oxidative Stress-Related eye diseases. Int. J. Mol. Sci. 23(3). (2022).
Tezel, G. Multifactorial pathogenic processes of retinal ganglion cell degeneration in Glaucoma towards Multi-Target strategies for broader treatment effects. Cells 10(6). (2021).
Lin, W. J. & Kuang, H. Y. Oxidative stress induces autophagy in response to multiple noxious stimuli in retinal ganglion cells. Autophagy 10 (10), 1692–1701 (2014).
Hu, Y. et al. Dapagliflozin reduces apoptosis of diabetic retina and human retinal microvascular endothelial cells through ERK1/2/cPLA2/AA/ROS pathway independent of hypoglycemic. Front. Pharmacol. 13, 827896 (2022).
Posch-Pertl, L. et al. Cholesterol and glaucoma: a systematic review and meta-analysis. Acta Ophthalmol. 100 (2), 148–158 (2022).
Filippas-Ntekouan, S., Tsimihodimos, V., Filippatos, T., Dimitriou, T. & Elisaf, M. SGLT-2 inhibitors: pharmacokinetics characteristics and effects on lipids. Expert Opin. Drug Metab. Toxicol. 14 (11), 1113–1121 (2018).
Marshall, H. et al. Cardiovascular disease predicts structural and functional progression in early Glaucoma. Ophthalmology 128 (1), 58–69 (2021).
Horwitz, A. et al. Antihypertensive medication postpones the onset of glaucoma: evidence from a nationwide study. Hypertension 69 (2), 202–210 (2017).
Zhao, D., Cho, J., Kim, M. H. & Guallar, E. The association of blood pressure and primary open-angle glaucoma: a meta-analysis. Am. J. Ophthalmol. 158 (3), 615–627e619 (2014).
Kim, H. & Choi, B. Nonlinear relationship between blood pressure and Glaucoma in US adults. Am. J. Hypertens. 32 (3), 308–316 (2019).
Memarzadeh, F., Ying-Lai, M., Chung, J., Azen, S. P. & Varma, R. Blood pressure, perfusion pressure, and open-angle glaucoma: the Los Angeles Latino eye study. Invest. Ophthalmol. Vis. Sci. 51 (6), 2872–2877 (2010).
Flammer, J. et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 21 (4), 359–393 (2002).
Sha, W. et al. The Role of SGLT2 Inhibitor on the Treatment of Diabetic Retinopathy. J Diabetes Res 2020:8867875. (2020).
Lu, L. J., Tsai, J. C. & Liu, J. Novel Pharmacologic candidates for treatment of primary Open-Angle Glaucoma. Yale J. Biol. Med. 90 (1), 111–118 (2017).
Fishman, P., Cohen, S. & Bar-Yehuda, S. Targeting the A3 adenosine receptor for glaucoma treatment (review). Mol. Med. Rep. 7 (6), 1723–1725 (2013).
Feldman, R. M. Conjunctival hyperemia and the use of topical prostaglandins in glaucoma and ocular hypertension. J. Ocul Pharmacol. Ther. 19 (1), 23–35 (2003).
Pisella, P. J. et al. Conjunctival Proinflammatory and proapoptotic effects of Latanoprost and preserved and unpreserved Timolol: an ex vivo and in vitro study. Invest. Ophthalmol. Vis. Sci. 45 (5), 1360–1368 (2004).
Bellido, V. et al. Beyond the glycaemic control of Dapagliflozin: microangiopathy and Non-classical complications. Diabetes Ther. 13 (5), 873–888 (2022).
Mirabelli, M. et al. Long-Term Effectiveness and Safety of SGLT-2 Inhibitors in an Italian Cohort of Patients with Type 2 Diabetes Mellitus. J Diabetes Res 2019:3971060. (2019).
Hao, Z. et al. Effects and mechanisms of Dapagliflozin treatment on ambulatory blood pressure in diabetic patients with hypertension. Med. Sci. Monit. 26, e925987 (2020).
Heerspink, H. J. L. et al. Dapagliflozin in patients with chronic kidney disease. N Engl. J. Med. 383 (15), 1436–1446 (2020).
Acknowledgements
This study’s analysis was conducted using publicly available GWAS summary data. We extend our sincere gratitude to all participants and data managers for their invaluable contributions. Furthermore, we acknowledge Editage (www.editage.cn) for their assistance with English language editing.
Author information
Authors and Affiliations
Contributions
Y.G. conceptualized and conducted the study, and wrote the manuscript. J.Z. contributed to the statistical analyses. S.H. assisted in data collection. Z.C. provided critical review and editing of the manuscript. All authors actively participated in the research project and gave final approval to the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Guo, Y., Zhao, J., Hou, S. et al. Exploring the effect of SGLT2 inhibitors on the risk of primary open-angle glaucoma using Mendelian randomization analysis. Sci Rep 15, 13946 (2025). https://doi.org/10.1038/s41598-025-98997-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-98997-8