Abstract
Chronic Obstructive Pulmonary Disease (COPD) is associated with frailty and ageing, but there is insufficient evidence from existing longitudinal studies. This research explored the longitudinal association between frailty, ageing and COPD. We used the China Health and Retirement Longitudinal Study (CHARLS) data to perform a cohort study. The study population was non-COPD patients in wave 1 (2011), and the outcome was the occurrence of COPD at the end of follow-up (wave 4-wave 5). Frailty was assessed using the CHARLS modified frailty index (CMFI), and ageing was evaluated using the biological age (BA). We used multivariate logistic regression to examine the longitudinal associations between CMFI and BA with COPD. Fitted curves were used to analyze the dose-response relationship of CMFI and BA with COPD. A 3D surface diagram was used to analyze the association between BA and CMFI with COPD. In addition, subgroup and sensitivity analyses were performed. 6452 non-COPD patients were enrolled in the study, and after follow-up, 616 participants were diagnosed with COPD. Logistic regression and fitted curves showed a positive correlation between CMFI and BA and the development of COPD. The risk of COPD increased by 19% for every one standard deviation (SD) increase in BA and 32% for every one SD increase in CMFI. A 3D surface diagram shows a joint association between CMFI and BA with the COPD. Subgroup and sensitivity analysis results are stable. This study found a joint association between CMFI and BA with COPD, suggesting that CMFI and BA are risk factors for the development of COPD.
Similar content being viewed by others
Introduction
As ageing accelerates, the impact of frailty on clinical practice and public health is increasing1. Frailty originates in geriatrics and is a state of reduced resistance and poor internal balance of the body due to deficiencies in the functioning of several systems2. This state involves neuromuscular, metabolic, and immune changes that significantly increase the risk of adverse events such as falls, disability, hospitalization, and all-cause mortality in older adults3,4,5. Frailty is a non-negligible part of the ageing process. The incidence and risk of frailty gradually increase with age6,7. Various chronic lung diseases are associated with ageing, and people with lung disease are more likely to become frail8.
Chronic Obstructive Pulmonary Disease (COPD) is a closely age-related lung disease that poses a significant challenge to the health system due to its high morbidity and mortality9,10. Frailty, ageing, and COPD do not exist in isolation from each other but interact with each other11. The lungs are critical respiratory organs that undergo structural and functional changes during ageing, which increases the risk of COPD12. Biological age, which represents ageing, has been linked to COPD and impaired lung function13,14. In addition, with ageing, the body’s muscle mass and physical activity decrease, increasing the risk of frailty15,16. Frailty is common in the COPD population, and several studies found that COPD patients are associated with higher rates of frailty17,18. However, the results on frailty and aging with COPD came from cross-sectional studies, so more longitudinal evidence is needed to explore this relationship13,14,19. Therefore, we plan to use a cohort study to analyze whether frailty and aging are risk factors for COPD.
We plan to use the China Health and Retirement Longitudinal Study (CHARLS) modified frailty index (CMFI) from CHARLS ‘previous studies to measure frailty20. The CMFI integrates multidimensional health variables such as somatic, functional, and psychological variables and can reflect the overall state of health21. In addition, we planned to use the Klemera-Doubal method (KDM) to calculate biological age (BA) to represent ageing22,23. Study data were obtained from the CHARLS. We plan to analyze the independent and combined associations of CMFI and BA with the risk of COPD occurrence.
Methods
Study population
This research was a prospective cohort study. Data on the research population were obtained from CHARLS, a longitudinal study focusing on middle-aged and older adults (≥ 45 years of age) in China24,25. The CHARLS study started in 2011 and covered 150 counties and included around 17,000 people per year. All data can be downloaded from the official website (https://charls.pku.edu.cn/index.htm) on request. Professionally trained interviewers and physicians collected data through structured questionnaires and examinations. The Ethics Committee of Peking University approved the survey, and all participants signed an informed consent form (IRB00001052-11014)20. All methods are carried out in accordance with the relevant guidelines and regulations of the official CHARLS website24. This study followed the STROBE guidelines.
Definition of COPD at baseline and after follow-up
In CHARLS, a diagnosis of COPD is a positive answer to the question, “Have you ever been diagnosed with COPD by a doctor?” while a negative answer is defined as a non-COPD population26,27. CHARLS conducts survey every 2–3 years, each called a wave. Questionnaires on COPD diagnosis were available in Wave 1 (2011), Wave 4 (2018) and Wave 5 (2020). The study population consisted of participants who answered non-COPD in Wave 1. Participants’ affirmative answers to the above questions in Waves 4 and 5 were defined as the occurrence of COPD after follow-up.
Definition of frailty
In this study, we exhibited frailty projects and formulas developed by Rockwood to assess frailty21. We assessed frailty using 34 indicators including activities of daily living, physical functioning, mental health and chronic conditions based on CHARLS data characteristics and previous relevant literature20. To avoid ambiguity with frailty index, we named it CHARLS modified frailty Index (CMFI) The CMFI contains 34 accessible items (Table S1). Specifically, the sum of the frailty items was divided by the total number of items measured (at least 20). The CMFI ranges from 0 to 1, with a higher CMFI indicating greater frailty.
Definition of biological age
We used the KDM-BA to indicate the degree of ageing22. The calculation of KDM-BA was based on 12 biomarkers, and 4 biomarkers (albumin, red blood cell count, ferritin, and transferrin) were missing in CHARLS. Liu showed through his study that the remaining 8 biomarkers are usually replicated in CHARLS, and have potential applications for early identification of aging and aging-related diseases in China23. We included the eight markers mentioned above: serum total cholesterol, triglycerides, glycosylated hemoglobin, urea nitrogen, creatinine, albumin, high-sensitivity c-reactive protein, and platelet count. The above data were log-transformed to ensure that they were normally distributed. The detailed formulae are shown in Table S2. Higher BA values indicate a higher degree of senescence23.
Inclusion and exclusion criteria
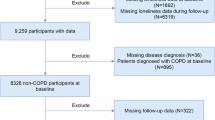
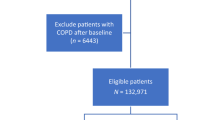
First, patients who had a diagnosis at baseline and were diagnosed with non-COPD were included in our research. Then, participants with missing follow-up data were excluded. Finally, after excluding participants with missing CMFI and BA variables and covariates, the final population was our study population.
Study covariates
This research incorporated several covariates, including gender, age, body mass index (BMI), marital status, place of residence, education, smoking and alcohol consumption. The population included in this study was middle-aged and older adults ≥ 45 years of age, which we categorized as middle-aged (< 65 years) and older adults (≥ 65 years). BMI was classified according to whether or not it was greater than 25 kg/m2. Smoking history was categorized as ever smoking (smoked more than 100 cigarettes but quit), current smoking, and never smoking. Alcohol consumption was classified into three groups based on whether participants drank alcohol and how often they drank: non-drinkers (no alcohol in the past year), infrequent drinkers (less than one drink per month in the past year), and frequent drinkers (more than one drink per month in the past year). C-reactive protein was included in the study. Comorbidities included cardiovascular disease, hypertension, diabetes mellitus, and asthma; see Supplementary file (Diagnostic Definitions of Diseases in Figure S1) for detailed diagnoses.
Statistical analysis
Continuous data conformed to normal distribution were expressed as mean (standard deviation, SD), and count data were expressed as N (%). Comparisons between variables were made using one-way ANOVA and chi-square tests.
We used multivariate logistic regression to examine the longitudinal relationship between CMFI and BA with COPD. Standard deviation (SD) was used as a quantification criterion, i.e., each change in SD resulted in a change in the ratio of ratios (OR). In addition, we categorized CMFI and BA into quartiles based on their magnitude. The quartiles for CMFI were Q1 (< 0.056), Q2 (0.056–0.096), Q3 (0.097–0.151), and Q4 (> 0.151), and the quartiles for BA were Q1 (< 50.44), Q2 (50.44–56.54), Q3 (56.55–63.30) and Q4 (> 63.30). P-trend was obtained using the linear term for quartiles, with P-trend < 0.05 indicating a linear relationship. The logistic regression was adjusted for three models: the crude model with unadjusted variables, model I, adjusted for gender and age, and model II, further adjusted for body mass index (BMI), marital status, residence place, education, smoking, drinking, CRP (only in CMFI), and comorbidities (only in BA). To further clarify the dose-response relationships of CMFI and BA with COPD, we plotted fitted curves incorporating the three models. In addition, we analyzed the relationship between CMFI and BA.
Subgroup analyses were used to analyze the longitudinal associations of CMFI and BA with COPD in different subgroups (sex, age, residence, BMI, smoking history, drinking history). P for interaction < 0.05 indicates an interaction in the group.
After CMFI (< 56.55, ≥ 56.55) and BA (< 0.097, ≥ 0.097) were classified as dichotomous variables by median, the joint association was analyzed by measuring the sum of the independent associations of the two factors in relation to COPD. We applied the relative excess risk due to interaction (RERI), the attributable proportion of interaction (AP), and the synergy index (SI) to assess additive interactions. When the confidence intervals for RERI and AP contain 0 and the confidence interval for SI contains 1, there is no additive interaction. In addition, we plotted a 3D surface map to better demonstrate the joint association of CMFI and BA with COPD.
We performed several sensitivity analyses. First, because 4,794 participants were excluded from the study due to missing data, we compared baseline information between the excluded and included populations. In addition, because of the potential association of asthma with COPD, we excluded the asthma population for regression analysis. Finally, participants diagnosed with COPD in 2018–2020 were the outcome variable, so we excluded participants who died during this period for regression analysis.
P < 0.05 was considered a statistically significant difference. This study used R version 4.2.2 and “FreeStatistics” software.
Results
Baseline characteristics
Figure 1 illustrates the detailed inclusion and exclusion results. Table 1 shows that 21.28% of participants were older (≥ 65), and 43.52% were male before follow-up. After at least seven years of follow-up, 616 participants reported COPD and 5836 were non-COPD. Compared to non-COPD, the COPD population was older, male, unmarried, and rural, and smokers were more prevalent. In addition, CMFI and BA were higher in the COPD population than in the non-COPD population. We also analyzed baseline information for the 4,794 participants who were excluded due to missing data compared with the 6,452 participants who were not excluded. Table S3 shows no significant difference in the percentage of COPD between the two groups before and after exclusion.
Longitudinal association of CMFI and BA with COPD
As shown in Table 2, elevated CMFI and BA were associated with an elevated risk of developing COPD, a stable result in all three models. In model 2, which was adjusted for more covariates, each 1 SD of elevated BA was associated with a 19% higher incidence of COPD, and each 1 SD of elevated CMFI was associated with a 32% higher risk of COPD. After quartiles, the logistic regression results remain stable.
Figure 2 shows that CMFI and BA were positively associated with the longitudinal occurrence of COPD in all three models. In particular, the relationship between CMFI and COPD was nonlinear (P-nonlinear < 0.05), whereas the relationship between BA and COPD was linear (P-nonlinear > 0.05). We did not find an inflection point in the nonlinear association between CMFI and COPD.
Table S4 shows that CMFI and BA were still associated with the occurrence of COPD after excluding the asthma population. Table S5 shows that the regression analysis results remain stable after excluding participants who died during 2018–2020.
Subgroup and joint analysis
Figure 3 shows the subgroup analyses of the association of CMFI and BA with COPD. After adjusting for covariates, the results for all subgroups in the forest plot remained stable (P for interaction > 0.05). Table 3 shows that high BA and low CMFI (OR, 1.37, 95%CI, 1.02,1.83, P = 0.034), high CMFI and low BA (OR, 1.88, 95%CI, 1.44,2.45, P < 0.001), and high CMFI and high BA (OR, 2.48, 95%CI, 1.89,3.26, P < 0.001) all have an increased risk of COPD compared to the combination of low CMFI and low BA. In addition, the results of the additive interaction showed that the interaction between CMFI and BA on COPD was not significant [adjusted RERI (95% CI): 0.23 (-0.34, 0.80); adjusted AP (95% CI): 0.09 (-0.13, 0.32); adjusted SI (95% CI): 1.19 (0.76, 1.86)]. Figure 4 demonstrates the joint association of CMFI and BA with COPD by means of a 3D surface map.
Discussion
Our study found that elevated CMFI and BA were associated with longitudinal COPD occurrence. Different models and fitted curves also confirmed this relationship, and the results of subgroup and sensitivity analyses remained stable. The present study provides longitudinal evidence for the association of CMFI and BA with COPD.
Multiple studies find indicators of aging and frailty linked to COPD17,18,13,14. Most of the studies mentioned above were cross-sectional, and the evidence from several longitudinal studies was inconsistent. Lee et al. found that the presence of COPD led to a greater likelihood of developing frailty in women, but the results were not significant in men28. Yee et al. found that weakness assessed by grip strength was not associated with all-cause hospitalization in patients with COPD in a longitudinal study29. In contrast, Kennedy et al. found that the Fried weakness phenotype was associated with more extended hospitalization and poorer quality of life in patients with COPD30. Scarlata et al. found a positive correlation between FI and the BODE index (BMI, Obstructive index, Dyspnea, Exercise Capacity) in patients with COPD in a study with a follow-up of 4 years31. The longitudinal association between BA and COPD is currently unclear. Therefore, we conducted this cohort study and found a longitudinal association between CMFI and BA with COPD in a Chinese population.
Researchers have used various tools in past studies to assess the relationship between frailty and COPD29,30,31. Commonly used tools include the Frailty index and Fried frailty phenotypes. We used the CMFI to assess frailty, which includes 34 indicators including activities of daily living, physical functioning, mental health and chronic conditions, and is more applicable to middle-aged and older adults. Currently, commonly used tools to quantify BA include the Klemera-Doubal method (KDM), the PhenoAge method, and homeostatic dysregulation22,32,33. In the CHARLS study, the KDM-BA was easily accessible and had a better ageing prediction; therefore, it was used in our research23,34.
We found a common association between CMFI and BA and COPD by stratified analyses and 3D surface diagram. This suggests that there is a strong relationship between frailty and ageing, which together promote COPD. Frailty, ageing, and COPD do not exist in isolation from each other but interact with each other. Ageing leads to decreased immune function and weakened defenses in the lungs, increasing the risk of COPD35. As ageing progresses, patients experience reduced muscle strength and mass in the extremities, decreased physical activity, and an elevated risk of frailty, further increasing the probability of COPD36,37. Respiratory muscle fatigue and sarcopenia are also an important factor in the development of COPD38,39. Muscle loss may be an external manifestation of the association between frailty, ageing, and COPD.
Reversing ageing is unrealistic, and slowing it down in various ways is a future research priority. Studies found that frailty is dynamic and can be reversed after effective interventions40,41. Maddocks et al. found that frail COPD patients responded well to pulmonary rehabilitation and could reverse their frailty in the short term42. Optimized nutrition and scientific training are positive for preventing sarcopenia, slowing down ageing, and reducing the risk of frailty43. In addition, inflammatory factors such as CRP and IL-6 are strongly associated with frailty and ageing44,45,46. Clearing chronic inflammation by targeting pro-inflammatory cytokines and inflammatory pathways may contribute to sarcopenia and thus improve frailty44. Smoking is significantly associated with adverse ageing phenotypes and frailty phenotypes, and smoking cessation is also an important way to improve frailty and ageing47,48. More longitudinal studies are needed to analyze the role of different strategies in COPD prevention and management.
This study has several strengths. First, the study used CHARLS, which has a large sample and high-quality data. Second, the prospective cohort study design allowed us to explore the association of frailty and ageing with COPD effectively. Undeniably, this study also has some limitations. First, the diagnosis of COPD is not made by pulmonary function tests but from self-report, which may be misdiagnosed or missed49. We look forward to further studies to enrich lung function diagnosis and address this deficiency. Secondly, although we adjusted for multiple confounding factors in this study, unmeasured and heavily missing factors such as diet, exercise, socioeconomic status, and medication history were not adjusted. Third, 4,696 people who lacked CMFI and BA data were excluded from the study, potentially leading to some potential bias. In addition, data related to cumulative smoking history (pack-years) were unavailable, and we expect future studies to be further refined. Finally, we only included CMFI and BA at baseline, and there was a lack of studies on the relationship between dynamic changes in CMFI and BA and COPD.
Conclusions
This study found a joint association between CMFI and BA with COPD. Frailty and ageing jointly contribute to the development of COPD. By further revealing the mechanisms of frailty and aging in COPD, it may provide new perspectives for personalized treatment of COPD.
Data availability
Data in the article can be obtained from the CHARLS database (https://charls.pku.edu.cn/). The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Hoogendijk, E. O. et al. Frailty: Implications for clinical practice and public health. Lancet Lond. Engl. 394(10206), 1365–1375 (2019).
Fried, L. P. et al. From bedside to bench: Research agenda for frailty. Sci. Aging Knowl. Environ. SAGE KE. 2005(31), pe24 (2005).
Si, H. et al. Predictive performance of 7 frailty instruments for short-term disability, falls and hospitalization among Chinese community-dwelling older adults: A prospective cohort study. Int. J. Nurs. Stud. 117, 103875 (2021).
Fan, J. et al. Frailty index and all-cause and cause-specific mortality in Chinese adults: A prospective cohort study. Lancet Public. Health 5(12), e650–e660 (2020).
Hanlon, P. et al. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: A prospective analysis of 493 737 UK biobank participants. Lancet Public. Health. 3(7), e323–e332 (2018).
Bellelli, F. et al. Relationship among age, education and frailty in older persons. J. Frailty Aging 12(4), 326–328 (2023).
Gordon, E. H. & Hubbard, R. E. Frailty: Understanding the difference between age and ageing. Age Ageing 51(8), afac185 (2022).
Cho, S. J. & Stout-Delgado, H. W. Aging and lung disease. Annu. Rev. Physiol. 82, 433–459 (2020).
2024 GOLD Report - Global Initiative for Chronic Obstructive Lung Disease - GOLD [Internet]. [cited 2024 Jul 23]; Available from https://goldcopd.org/2024-gold-report/.
Christenson, S. A., Smith, B. M., Bafadhel, M. & Putcha, N. Chronic obstructive pulmonary disease. Lancet Lond. Engl. 399(10342), 2227–2242 (2022).
Antoniu, S. A., Boiculese, L. V. & Prunoiu, V. Frailty, a dimension of impaired functional status in advanced COPD: Utility and clinical applicability. Med. Kaunas Lith. 57(5), 474 (2021).
Brandsma, C-A. et al. Lung ageing and COPD: is there a role for ageing in abnormal tissue repair? Eur. Respir Rev. Off J. Eur. Respir Soc. 26(146), 170073 (2017).
Campisi, M., Liviero, F., Maestrelli, P., Guarnieri, G. & Pavanello, S. DNA Methylation-Based age prediction and telomere length reveal an accelerated aging in induced sputum cells compared to blood leukocytes: A pilot study in COPD patients. Front. Med. 8, 690312 (2021).
Ruan, Z. et al. Relationship between an ageing measure and chronic obstructive pulmonary disease, lung function: A cross-sectional study of NHANES, 2007–2010. BMJ Open 13 (11), e076746 (2023).
Elliott, M. L. et al. Disparities in the pace of biological aging among midlife adults of the same chronological age have implications for future frailty risk and policy. Nat. Aging 1(3), 295–308 (2021).
Billot, M. et al. Preserving mobility in older adults with physical frailty and sarcopenia: Opportunities, challenges, and recommendations for physical activity interventions. Clin. Interv Aging 15, 1675–1690 (2020).
Lahousse, L. et al. Risk of frailty in elderly with COPD: A population-based study. J. Gerontol. Biol. Sci. Med. Sci. 71(5), 689–695 (2016).
Hanlon, P. et al. Frailty in COPD: An analysis of prevalence and clinical impact using UK biobank. BMJ Open. Respir Res. 9(1), e001314 (2022).
Wang, L., Zhang, X. & Liu, X. Prevalence and clinical impact of frailty in COPD: A systematic review and meta-analysis. BMC Pulm Med. 23(1), 164 (2023).
Qing, L. et al. Exploring the association between frailty index and low back pain in middle-aged and older Chinese adults: A cross-sectional analysis of data from the China health and retirement longitudinal study (CHARLS). BMJ Open 14(5), e085645 (2024).
Searle, S. D., Mitnitski, A., Gahbauer, E. A., Gill, T. M. & Rockwood, K. A standard procedure for creating a frailty index. BMC Geriatr. 8, 24 (2008).
Klemera, P. & Doubal, S. A new approach to the concept and computation of biological age. Mech. Ageing Dev. 127(3), 240–248 (2006).
Liu, Z. Development and validation of 2 composite aging measures using routine clinical biomarkers in the Chinese population: Analyses from 2 prospective cohort studies. J. Gerontol. Biol. Sci. Med. Sci. 76(9), 1627–1632 (2021).
Zhao, Y., Hu, Y., Smith, J. P., Strauss, J. & Yang, G. Cohort profile: The China health and retirement longitudinal study (CHARLS). Int. J. Epidemiol. 43(1), 61–68 (2014).
Sonnega, A. et al. Cohort profile: The health and retirement study (HRS). Int. J. Epidemiol. 43(2), 576–585 (2014).
Song, P., Zha, M., Xia, W., Zeng, C. & Zhu, Y. Asthma-chronic obstructive pulmonary disease overlap in China: Prevalence, associated factors and comorbidities in middle-aged and older adults. Curr. Med. Res. Opin. 36(4), 667–675 (2020).
Jin, C., Zhang, T., Li, Y. & Shi, W. Early-Life exposure to malnutrition from the Chinese famine on risk of asthma and chronic obstructive pulmonary disease in adulthood. Front. Nutr. 9, 848108 (2022).
Lee, J. S. W., Auyeung, T-W., Leung, J., Kwok, T. & Woo, J. Transitions in frailty states among community-living older adults and their associated factors. J. Am. Med. Dir. Assoc. 15(4), 281–286 (2014).
Yee, N. et al. Frailty in chronic obstructive pulmonary disease and risk of exacerbations and hospitalizations. Int. J. Chron. Obstruct Pulmon Dis. 15, 1967–1976 (2020).
Kennedy, C. C. et al. Frailty and clinical outcomes in chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 16(2), 217–224 (2019).
Scarlata, S. et al. Association between frailty index, lung function, and major clinical determinants in chronic obstructive pulmonary disease. Aging Clin. Exp. Res. 33(8), 2165–2173 (2021).
Levine, M. E. et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging 10(4), 573–591 (2018).
Cohen, A. A. et al. A novel statistical approach shows evidence for multi-system physiological dysregulation during aging. Mech. Ageing Dev. 134(3–4), 110–117 (2013).
Peng, S. et al. Association between kidney function and biological age: A China health and retirement longitudinal study. Front. Public. Health. 11, 1259074 (2023).
Schneider, J. L. et al. The aging lung: Physiology, disease, and immunity. Cell 184(8), 1990–2019 (2021).
Roig, M., Eng, J. J., MacIntyre, D. L., Road, J. D. & Reid, W. D. Deficits in muscle strength, mass, quality, and mobility in people with chronic obstructive pulmonary disease. J. Cardiopulm. Rehabil Prev. 31(2), 120–124 (2011).
Bone, A. E., Hepgul, N., Kon, S. & Maddocks, M. Sarcopenia and frailty in chronic respiratory disease. Chron. Respir Dis. 14(1), 85–99 (2017).
Bachasson, D. et al. Quadriceps and respiratory muscle fatigue following high-intensity cycling in COPD patients. PloS One. 8(12), e83432 (2013).
Wang, H., Qiu, H., Gu, X., Zhang, Y. & Wang, S. The association between sarcopenia and incident chronic lung disease in the general population: A longitudinal study based on CHARLS data. Exp. Gerontol. 180, 112257 (2023).
Reid, N., Young, A., Shafiee Hanjani, L., Hubbard, R. E. & Gordon, E. H. Sex-specific interventions to prevent and manage frailty. Maturitas 164, 23–30 (2022).
Albert, S. M. The dynamics of frailty among older adults. JAMA Netw. Open. 2(8), e198438 (2019).
Maddocks, M. et al. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax 71(11), 988–995 (2016).
Rogeri, P. S. et al. Strategies to prevent sarcopenia in the aging process: Role of protein intake and exercise. Nutrients 14(1), 52 (2021).
Soysal, P. et al. Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing Res. Rev. 31, 1–8 (2016).
Topinková, E. Aging, disability and frailty. Ann. Nutr. Metab. 52(Suppl 1), 6–11 (2008).
Tang, Y., Fung, E., Xu, A. & Lan, H-Y. C-reactive protein and ageing. Clin. Exp. Pharmacol. Physiol. 44(Suppl 1), 9–14 (2017).
Park, S. et al. Causal linkage of tobacco smoking with ageing: Mendelian randomization analysis towards telomere attrition and sarcopenia. J. Cachexia Sarcopenia Muscle. 14(2), 955–963 (2023).
Lv, J. et al. Smoking, alcohol consumption, and frailty: A Mendelian randomization study. Front. Genet. 14, 1092410 (2023).
Park, M-B., Lee, T. S., Lee, J-H. & Lee, J. Most patients with COPD are unaware of their health threats and are not diagnosed: a national-level study using pulmonary function test. Sci. Rep. 13(1), 5893 (2023).
Acknowledgements
There are no additional acknowledgements.
Funding
This research was supported by Research Project on Enhancing the Evidence Level of Traditional Chinese Medicine Clinical Evidence at Xiyuan Hospital (XYZX0201-13).
Author information
Authors and Affiliations
Contributions
Z.R., H.T., and D.L. participated in the research design and main manuscript writing. R.L., J.Z., and S.C. participated in data cleaning and statistical analysis. Z.Y. and X.C. participated in the manuscript revision. Q.M. participated in the research design and funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
The investigation was approved by the Ethics Committee of Peking University and all participants signed an informed consent form.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ruan, Z., Tian, H., Li, R. et al. Joint association of frailty index and biological age with chronic obstructive pulmonary disease: a cohort study from CHARLS. Sci Rep 15, 17616 (2025). https://doi.org/10.1038/s41598-025-99682-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-99682-6
Keywords
This article is cited by
-
Association between HDL-C variability and the risk of chronic obstructive pulmonary disease in the middle- and older Chinese: a cohort study
The Egyptian Journal of Bronchology (2025)