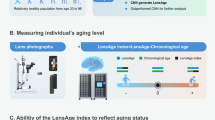
Abstract
Herein we developed age clocks that predict biological age from fundus photography and optical coherence tomography. We evaluated our multimodal models’ clinical relevance by examining their associations between predicted biological age and the Charlson Comorbidity Index (CCI). Study 1 assessed how models trained on normal eyes generalize to diseased eyes, and Study 2 tested whether incorporating disease labels improves performance and systemic associations. Models were fine-tuned to the imaging dataset to predict biological age. Linear regressors were trained on chronological and biological features to infer CCI. Gradient-weighted regression activation mapping also generated heatmaps to identify the model’s region of focus. Prediction performance improved when trained on both normal and diseased eyes. Predicted biological age showed significantly stronger correlations with CCI than chronological age across both studies, supporting our algorithm’s association with this validated measure of mortality. Thus, our algorithm may provide insight into systemic health burdens beyond that of traditional risk assessments.
Similar content being viewed by others
Data availability
The data analyzed in the current study is available from the corresponding author on reasonable request.
References
Rollandi, G. A. et al. Biological age versus chronological age in the prevention of age associated diseases. OBM Geriatr. 3, 1–11 (2019).
Salih, A., Nichols, T., Szabo, L. & Petersen, S. E. Raisi-Estabragh, Z. Conceptual overview of biological age Estimation. Aging Disease. 14, 583 (2023).
Chen, B. H. et al. DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging (Albany NY). 8, 1844 (2016).
Wolf, J. et al. Liquid-biopsy proteomics combined with AI identifies cellular drivers of eye aging and disease in vivo. Cell 186, 4868–4884 (2023).
Le Goallec, A., Diai, S., Collin, S., Vincent, T. & Patel, C. J. Deep learning of fundus and optical coherence tomography images enables identification of diverse genetic and environmental factors associated with eye aging. medRxiv, 2006. 2024.21259471 (2021).
Ahadi, S. et al. Longitudinal fundus imaging and its genome-wide association analysis provide evidence for a human retinal aging clock. Elife 12, e82364 (2023).
Wong, T. Y. et al. Retinal microvascular abnormalities and incident stroke: the atherosclerosis risk in communities study. Lancet 358, 1134–1140 (2001).
Wong, T. Y. et al. Retinal arteriolar narrowing and risk of coronary heart disease in men and women: the atherosclerosis risk in communities study. Jama 287, 1153–1159 (2002).
Salobrar-Garcia, E. et al. Ocular vascular changes in mild alzheimer’s disease patients: foveal avascular zone, choroidal thickness, and ONH hemoglobin analysis. J. Personalized Med. 10, 231 (2020).
Poplin, R. et al. Prediction of cardiovascular risk factors from retinal fundus photographs via deep learning. Nat. Biomedical Eng. 2, 158–164 (2018).
Hassan, O. N. et al. in 2021 IEEE 18th International Symposium on Biomedical Imaging (ISBI). 238–242 (IEEE).
Peng, Q. et al. Predictive potential of Retina-Based biological age in assessing chronic obstructive pulmonary disease risk. Clin. Exp. Ophthalmol. 53, 402–408 (2025).
Wu, G. et al. Association of retinal age gap with chronic kidney disease and subsequent cardiovascular disease sequelae: a cross-sectional and longitudinal study from the UK biobank. Clin. Kidney J. 17, sfae088 (2024).
Chang, J. et al. in. IEEE International Conference on Bioinformatics and Biomedicine (BIBM). 1179–1181 (IEEE). (2019).
Zhu, Z. et al. The association of retinal age gap with metabolic syndrome and inflammation. J. Diabetes. 15, 237–245 (2023).
Wong, W. L. et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Global Health. 2, e106–e116 (2014).
Teo, Z. L. et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology 128, 1580–1591 (2021).
Holden, B. A. et al. Global prevalence of myopia and high myopia and Temporal trends from 2000 through 2050. Ophthalmology 123, 1036–1042 (2016).
Bosworth, B. P. & Keys, B. Increased life expectancy: a global perspective. In Coping with Methuselah: The Impact ofMolecular Biology on Medicine and Society (eds Cutler, D. M. & Liebman, J. B.) 247–283 (Brookings Institution Press, 2004).
Xiao, W., Chen, X., Yan, W., Zhu, Z. & He, M. Prevalence and risk factors of epiretinal membranes: a systematic review and meta-analysis of population-based studies. BMJ open. 7, e014644 (2017).
Cho, B. J., Shin, J. Y. & Yu, H. G. Complications of pathologic myopia. Eye Contact Lens. 42, 9–15 (2016).
Garcia-Garcia, J. et al. Pathophysiology of age-related macular degeneration: implications for treatment. Ophthalmic Res. 65, 615–636 (2022).
Ożóg, M. K., Nowak-Wąs, M. & Rokicki, W. Pathophysiology and clinical aspects of epiretinal membrane–review. Front. Med. 10, 1121270 (2023).
Wang, W. & Lo, A. C. Diabetic retinopathy: pathophysiology and treatments. Int. J. Mol. Sci. 19, 1816 (2018).
D’Hoore, W., Bouckaert, A. & Tilquin, C. Practical considerations on the use of the Charlson comorbidity index with administrative data bases. J. Clin. Epidemiol. 49, 1429–1433 (1996).
Charlson, M. E., Carrozzino, D., Guidi, J. & Patierno, C. Charlson comorbidity index: a critical review of clinimetric properties. Psychother. Psychosom. 91, 8–35 (2022).
Bannay, A. et al. The best use of the Charlson comorbidity index with electronic health care database to predict mortality. Med. Care. 54, 188–194 (2016).
Coleman-Belin, J. et al. Aging effects on optic nerve neurodegeneration. Int. J. Mol. Sci. 24, 2573 (2023).
Albon, J., Karwatowski, W., Avery, N., Easty, D. L. & Duance, V. C. Changes in the collagenous matrix of the aging human lamina cribrosa. Br. J. Ophthalmol. 79, 368–375 (1995).
Wang, J. Glaucoma and myopia: risk factors, pathophysiology, and treatment. Can. Eye Care Today. 30-35, 30–35 (2022).
Downs, J. C. Optic nerve head biomechanics in aging and disease. Exp. Eye Res. 133, 19–29 (2015).
Parikh, R. S. et al. Normal age-related decay of retinal nerve fiber layer thickness. Ophthalmology 114, 921–926 (2007).
Chirco, K., Sohn, E., Stone, E., Tucker, B. & Mullins, R. Structural and molecular changes in the aging choroid: implications for age-related macular degeneration. Eye 31, 10–25 (2017).
Wakatsuki, Y., Shinojima, A., Kawamura, A. & Yuzawa, M. Correlation of aging and segmental choroidal thickness measurement using swept source optical coherence tomography in healthy eyes. PloS One. 10, e0144156 (2015).
Ikuno, Y., Kawaguchi, K., Nouchi, T. & Yasuno, Y. Choroidal thickness in healthy Japanese subjects. Investig. Ophthalmol. Vis. Sci. 51, 2173–2176 (2010).
Nivison-Smith, L. et al. Normal aging changes in the choroidal angioarchitecture of the macula. Sci. Rep. 10, 10810 (2020).
Gartner, S. & Henkind, P. Aging and degeneration of the human macula. 1. Outer nuclear layer and photoreceptors. Br. J. Ophthalmol. 65, 23–28 (1981).
Hogan, M. J. & Alvarado, J. Studies on the human macula: IV. Aging changes in bruch’s membrane. Arch. Ophthalmol. 77, 410–420 (1967).
Bhagat, N., Grigorian, R. A., Tutela, A. & Zarbin, M. A. Diabetic macular edema: pathogenesis and treatment. Surv. Ophthalmol. 54, 1–32 (2009).
Gottlieb, J. L. Age-related macular degeneration. Jama 288, 2233–2236 (2002).
Drosdowsky, A. & Gough, K. The Charlson comorbidity index: problems with use in epidemiological research. J. Clin. Epidemiol. 148, 174–177 (2022).
Szegedy, C., Ioffe, S., Vanhoucke, V. & Alemi, A. in Proceedings of the AAAI conference on artificial intelligence.
Dosovitskiy, A. et al. An image is worth 16x16 words: Transformers for image recognition at scale. arXiv preprint arXiv:.11929 (2020). (2020). (2010).
Selvaraju, R. R. et al. Grad-CAM: visual explanations from deep networks via gradient-based localization. Int. J. Comput. Vision. 128, 336–359 (2020).
Deng, J. et al. in 2009 IEEE conference on computer vision and pattern recognition. 248–255 (Ieee).
Funding
This work was supported by the National Eye Institute K23 Grant, K23EY035741 and E. Matilda Ziegler Foundation for the Blind Grant awarded to Chase A. Ludwig as well as the Stanford P30 Vision Research Core Grant, NEI P30-EY026877, and Research to Prevent Blindness, Inc.
Author information
Authors and Affiliations
Contributions
CAL contributed to the conception, design, data acquisition, analysis, and interpretation of the present work, and the writing and revision of the manuscript; AS contributed to the design, analysis, and interpretation of the present work and writing and revision of the manuscript; YM contributed to the interpretation of the present work and the writing and revision of the manuscript; LA contributed to the revision of manuscript; CL contributed to the writing and revision of the manuscript; VM contributed to the design and writing and revision of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ludwig, C.A., Salvi, A., Mesfin, Y. et al. A multimodal retinal aging clock for biological age prediction and systemic health assessment via OCT and fundus imaging. Sci Rep (2026). https://doi.org/10.1038/s41598-026-36518-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-36518-x