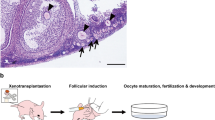
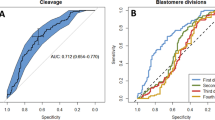
Abstract
Marmosets are a valuable model for studying human disorders, but developing specific genetic models requires efficient protocols for embryo genomic manipulation. Although marmoset embryos have been produced in vitro, oocyte retrieval traditionally involves extended recombinant human follicle-stimulating hormone (FSH) treatment (9–10 days), surgical laparotomy and ovarian exposure, limiting repeat procedures. Here we evaluated a simpler, cost-effective ovarian stimulation protocol using porcine pituitary FSH, combined with laparoscopic ovum pick-up (LOPU), for in vitro production of marmoset embryos via in vitro fertilization (IVF) and somatic cell nuclear transfer. A total of 3,922 oocytes were retrieved from 129 LOPU procedures, averaging 30.4 oocytes per LOPU, with 85.5% (26.0 per LOPU) graded as viable. The procedure was safe, with no adverse effects observed in ovaries or fimbria after up to nine LOPU sessions. Most collected oocytes were meiotically immature and matured in vitro before use. Following IVF, approximately 40% of oocytes cleaved, and 40% of cleaved embryos developed to the expanded blastocyst stage. LOPU-derived oocytes also supported somatic cell nuclear transfer, with 69.8% of embryos cleaving and 21.8% forming blastocysts. IVF-derived blastocyst quality was assessed by total cell count, immunodetection of SOX2 (inner cell mass) and CDX2 (trophectoderm), and cryotolerance. Embryo transfer to recipients resulted in successful live births. These findings demonstrate that a simplified pituitary FSH protocol followed by LOPU is an efficient, less invasive and safe method for retrieving developmentally competent marmoset oocytes, offering a promising approach for advancing marmoset-based research in disease modeling and reproductive biotechnology.
This is a preview of subscription content, access via your institution
Access options




Similar content being viewed by others
Data availability
Derived data supporting the findings of this study are available from the corresponding authors on request. Source data are provided with this paper.
References
Mitchell, J. F. & Leopold, D. A. The marmoset monkey as a model for visual neuroscience. Neurosci. Res. 93, 20–46 (2015).
Philippens, I. H. C. H. M. in The Common Marmoset in Captivity and Biomedical Research Vol. 1. (eds Marini, R. P. et al.) 415–435 (Academic Press, 2019).
Kap, Y. S. D. J. & ‘t Hart, B. A. in The Common Marmoset in Captivity and Biomedical Research Vol. 1. (eds Marini, R. P. et al.) 437–449 (Academic Press, 2019).
French, J. A. in The Common Marmoset in Captivity and Biomedical Research Vol. 1. (eds Marini, R. P. et al.) 477–491 (Academic Press, 2019).
Inoue, T., Yurimoto, T., Seki, F., Sato, K. & Sasaki, E. The common marmoset in biomedical research: experimental disease models and veterinary management. Exp. Anim. 72, 140–150 (2023).
Okano, H., Hikishima, K., Iriki, A. & Sasaki, E. The common marmoset as a novel animal model system for biomedical and neuroscience research applications. Semin. Fetal Neonatal Med. 17, 336–340 (2012).
Marini, R. P., Wachtman, L. M., Tardif, S. D., Mansfield, K. & Fox, J, G. The Common Marmoset in Captivity and Biomedical Research 1st edn, Vol. 1 (Academic Press, 2019).
Chan, A. W. et al. Progressive cognitive deficit, motor impairment and striatal pathology in a transgenic Huntington disease monkey model from infancy to adulthood. PLoS ONE 10, e0122335 (2015).
Niu, Y. et al. Early Parkinson’s disease symptoms in alpha-synuclein transgenic monkeys. Hum. Mol. Genet. 24, 2308–2317 (2015).
Niu, Y. et al. Transgenic rhesus monkeys produced by gene transfer into early-cleavage-stage embryos using a simian immunodeficiency virus-based vector. Proc. Natl Acad. Sci. USA 107, 17663–17667 (2010).
Niu, Y. et al. Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell 156, 836–843 (2014).
Yang, S. H. et al. Towards a transgenic model of Huntington’s disease in a non-human primate. Nature 453, 921–924 (2008).
Sasaki, E. et al. Generation of transgenic non-human primates with germline transmission. Nature 459, 523–527 (2009).
Takahashi, T. et al. Birth of healthy offspring following ICSI in in vitro-matured common marmoset (Callithrix jacchus) oocytes. PLoS ONE 9, e95560 (2014).
Sato, K. S. et al. A non-human primate model of familial Alzheimer’s disease. Preprint at bioRxiv https://doi.org/10.1101/2020.08.24.264259 (2020).
Marshall, V. S., Browne, M. A., Knowles, L., Golos, T. G. & Thomson, J. A. Ovarian stimulation of marmoset monkeys (Callithrix jacchus) using recombinant human follicle stimulating hormone. J. Med. Primatol. 32, 57–66 (2003).
Kurotaki, Y. S. E. Practical reproductive techniques for the common marmoset. J. Mamm. Ova Res. 34, 9 (2017).
Park, J. E. et al. Generation of transgenic marmosets expressing genetically encoded calcium indicators. Sci. Rep. 6, 34931 (2016).
Kanda, A., Nobukiyo, A., Yoshioka, M., Hatakeyama, T. & Sotomaru, Y. Quality of common marmoset (Callithrix jacchus) oocytes collected after ovarian stimulation. Theriogenology 106, 221–226 (2018).
Tomioka, I., Takahashi, T., Shimada, A., Yoshioka, K. & Sasaki, E. Birth of common marmoset (Callithrix jacchus) offspring derived from in vitro-matured oocytes in chemically defined medium. Theriogenology 78, 1487–1493 (2012).
Grupen, C. G. et al. Effects of ovarian stimulation, with and without human chorionic gonadotrophin, on oocyte meiotic and developmental competence in the marmoset monkey (Callithrix jacchus). Theriogenology 68, 861–872 (2007).
Tkachenko, O. Y. et al. In vitro matured oocytes have a higher developmental potential than in vivo matured oocytes after hormonal ovarian stimulation in Callithrix jacchus. J. Ovarian Res. 17, 120 (2024).
Baldassarre, H., de Matos, D. G., Furnus, C. C., Castro, T. E. & Cabrera Fischer, E. I. Technique for efficient recovery of sheep oocytes by laparoscopic folliculocentesis. Anim. Reprod. Sci. 35, 145–150 (1994).
Baldassarre, H. et al. Advances in the production and propagation of transgenic goats using laparoscopic ovum pick-up and in vitro embryo production technologies. Theriogenology 57, 275–284 (2002).
Baldassarre, H. et al. Interval of gonadotropin administration for in vitro embryo production from oocytes collected from Holstein calves between 2 and 6 months of age by repeated laparoscopy. Theriogenology 116, 64–70 (2018).
Currin, L. et al. Optimization of gonadotropin stimulation protocols for in vitro embryo production in prepubertal Mediterranean water buffalo. Theriogenology 197, 84–93 (2023).
Baldassarre, H. et al. Laparoscopic ovum-pick up and in vitro embryo production in gonadotropin-stimulated gilts: preliminary results and envisioned applications. Theriogenology 214, 141–147 (2024).
Tardif, S. D., Lacker, H. M. & Feuer, M. Follicular development and ovulation in the marmoset monkey as determined by repeated laparoscopic examination. Biol. Reprod. 48, 1113–1119 (1993).
Eom, H. et al. Laparoscopic ovum pick up in common marmoset (Callithrix jacchus). Theriogenol. Wild 7, 1–6 (2025).
Blondin, P., Coenen, K., Guilbault, L. A. & Sirard, M. A. In vitro production of bovine embryos: developmental competence is acquired before maturation. Theriogenology 47, 1061–1075 (1997).
Landry, D. A. et al. Comparative analysis of granulosa cell gene expression in association with oocyte competence in FSH-stimulated Holstein cows. Reprod. Fertil. Dev. 29, 2324–2335 (2017).
Grynberg, M. et al. Comparative effectiveness of gonadotropins used for ovarian stimulation during assisted reproductive technologies (ART) in France: a real-world observational study from the French nationwide claims database (SNDS). Best Pract. Res. Clin. Obstet. Gynaecol. 88, 102308 (2023).
Summers, P. M., Shephard, A. M., Taylor, C. T. & Hearn, J. P. The effects of cryopreservation and transfer on embryonic development in the common marmoset monkey, Callithrix jacchus. J. Reprod. Fertil. 79, 241–250 (1987).
Lopata, A., Summers, P. M. & Hearn, J. P. Births following the transfer of cultured embryos obtained by in vitro and in vivo fertilization in the marmoset monkey (Callithrix jacchus). Fertil. Steril. 50, 503–509 (1988).
Kropp, J., Di Marzo, A. & Golos, T. Assisted reproductive technologies in the common marmoset: an integral species for developing nonhuman primate models of human diseases. Biol. Reprod. 96, 277–287 (2017).
Sotomaru, Y. et al. Preimplantation development of somatic cell cloned embryos in the common marmoset (Callithrix jacchus). Cloning Stem Cells 11, 575–583 (2009).
Ishibashi, H. et al. Efficient embryo transfer in the common marmoset monkey (Callithrix jacchus) with a reduced transfer volume: a non-surgical approach with cryopreserved late-stage embryos. Biol. Reprod. 88, 115 (2013).
Sato, K. et al. Production of a heterozygous exon skipping model of common marmosets using gene-editing technology. Lab Anim. 53, 244–251 (2024).
Drummer, C. et al. Generation and breeding of EGFP-transgenic marmoset monkeys: cell chimerism and implications for disease modeling. Cells 10, 505 (2021).
Fortune, J. E. Ovarian follicular growth and development in mammals. Biol. Reprod. 50, 225–232 (1994).
Gougeon, A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr. Rev. 17, 121–155 (1996).
Ginther, O. J., Beg, M. A., Donadeu, F. X. & Bergfelt, D. R. Mechanism of follicle deviation in monovular farm species. Anim. Reprod. Sci. 78, 239–257 (2003).
Eppig, J. J., Wigglesworth, K. & Pendola, F. L. The mammalian oocyte orchestrates the rate of ovarian follicular development. Proc. Natl Acad. Sci. USA 99, 2890–2894 (2002).
Seita, Y. et al. Poor second ovarian stimulation in cynomolgus monkeys (Macaca fascicularis) is associated with the production of antibodies against human follicle-stimulating hormone. J. Reprod. Dev. 65, 267–273 (2019).
Muller, T. et al. Chorionic gonadotrophin beta subunit mRNA but not luteinising hormone beta subunit mRNA is expressed in the pituitary of the common marmoset (Callithrix jacchus). J. Mol. Endocrinol. 32, 115–128 (2004).
Gilchrist, R. B., Nayudu, P. L. & Hodges, J. K. Maturation, fertilization, and development of marmoset monkey oocytes in vitro. Biol. Reprod. 56, 238–246 (1997).
Russell, W. M. S. & Burch, R. L. The Principles of Humane Experimental Technique (Methuen & Co, London, 1959).
Liu, Z. et al. Cloning of macaque monkeys by somatic cell nuclear transfer. Cell 172, 881–887 (2018).
Liao, Z. et al. Reprogramming mechanism dissection and trophoblast replacement application in monkey somatic cell nuclear transfer. Nat. Commun. 15, 5 (2024).
Rizos, D., Ward, F., Boland, M. P. & Lonergan, P. Effect of culture system on the yield and quality of bovine blastocysts as assessed by survival after vitrification. Theriogenology 56, 1–16 (2001).
Kocyigit, A. & Cevik, M. Correlation between the cryosurvival, cell number and diameter in bovine in vitro produced embryos. Cryobiology 73, 203–208 (2016).
Baldassarre, H. Laparoscopic ovum pick-up followed by in vitro embryo production and transfer in assisted breeding programs for ruminants. Animals 11, 216 (2021).
Bo, G. A., Cedeno, A. & Mapletoft, R. J. Strategies to increment in vivo and in vitro embryo production and transfer in cattle. Anim. Reprod. 16, 411–422 (2019).
Currin, L. et al. Factors affecting the efficiency of in vitro embryo production in prepubertal Mediterranean water buffalo. Animals 12, 3549 (2022).
Gonzalez-Bulnes, A., Garcia-Garcia, R. M., Santiago-Moreno, J., Lopez-Sebastian, A. & Cocero, M. J. Effect of follicular status on superovulatory response in ewes is influenced by presence of corpus luteum at first FSH dose. Theriogenology 58, 1607–1614 (2002).
Mapletoft, R. J., Steward, K. B. & Adams, G. P. Recent advances in the superovulation in cattle. Reprod. Nutr. Dev. 42, 601–611 (2002).
Piekarski, N. et al. A comparison of oocyte yield between ultrasound-guided and laparoscopic oocyte retrieval in rhesus macaques. Animals 13, 3017 (2023).
Yoshioka, K., Suzuki, C. & Onishi, A. Defined system for in vitro production of porcine embryos using a single basic medium. J. Reprod. Dev. 54, 208–213 (2008).
Schneiders, A., Sonksen, J. & Hodges, J. K. Penile vibratory stimulation in the marmoset monkey: a practical alternative to electro-ejaculation, yielding ejaculates of enhanced quality. J. Med. Primatol. 33, 98–104 (2004).
Toyoda, Y. & Yokoyama, M. The early history of the TYH medium for in vitro fertilization of mouse ova. J. Mamm. Ova Res. 33, 3–10 (2016).
Glanzner, W. G., Rissi, V. B. & Bordignon, V. Somatic cell nuclear transfer in pigs. Methods Mol. Biol. 2647, 197–210 (2023).
Van Thuan, N. et al. The histone deacetylase inhibitor scriptaid enhances nascent mRNA production and rescues full-term development in cloned inbred mice. Reproduction 138, 309–317 (2009).
Tomioka, I. et al. Generation of transgenic marmosets using a tetracyclin-inducible transgene expression system as a neurodegenerative disease model. Biol. Reprod. 97, 772–780 (2017).
Marshall, V. S., Kalishman, J. & Thomson, J. A. Nonsurgical embryo transfer in the common marmoset monkey. J. Med. Primatol. 26, 241–247 (1997).
Acknowledgements
This work was supported by the Canadian Institutes of Health Research (PJT-180573 to K.M.), Natural Sciences and Engineering Research Council of Canada (RGPIN/03395-2022 to K.M.), McGill University’s Healthy Brains, Healthy Lives Canada First Research Excellence Fund, Azrieli Centre for Autism Research, Fondation Courtois and Montreal General Hospital Foundation. We recognize the support and expert animal care from the laboratory animal professionals of the Animal Resources Division of the RI-MUHC.
Author information
Authors and Affiliations
Contributions
H.B., W.G.G., K.G., K.M. and V.B. designed this study. H.B., W.G.G., K.G., R.R., A.H.K.F., A.S., A.P., I.C. and L.C. contributed to LOPU activities. H.B., W.G.G., K.G., R.R., A.S., A.P. and I.C. performed embryo transfers. K.G. and W.G.G. performed IVM, IVF and SCNT experiments. I.C. collected marmoset sperm for IVF. H.B., W.G.G., K.G., R.R., K.M. and V.B. analyzed and interpreted the data. H.B., W.G.G., K.G., R.R., K.M. and V.B. wrote and edited the manuscript. All authors provided feedback and agreed on the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Lab Animal thanks Olena Eikel and Erika Sasaki for their contribution to this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Fig. 1 and Table 1.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Table 1
Statistical source data.
Source Data Table 2
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baldassarre, H., Glanzner, W.G., Gutierrez, K. et al. Efficient production of common marmoset embryos with in vitro fertilization and somatic cell nuclear transfer following optimized hormonal stimulation and laparoscopic oocyte collection. Lab Anim 55, 21–28 (2026). https://doi.org/10.1038/s41684-025-01652-y
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41684-025-01652-y