Abstract
Glioma is the most common primary malignant brain tumor with high mortality and poor prognosis. Aerobic glycolysis is crucial for the malignant behavior of glioma by promoting their growth. Tripartite motif containing 65 (TRIM65) as an E3 ubiquitin ligase has been implicated in tumor progression, but its role and regulatory mechanism on aerobic glycolysis in glioma remains unclear. Here, it was demonstrated that TRIM65 was highly expressed in human glioma tissues and associated with poor prognosis. Moreover, TRIM65 knockdown inhibited the glioma cells proliferation in vitro and in vivo. RNA sequencing and biological verifications were performed to elucidate a novel mechanism underlying TRIM65 silencing attenuated glycolysis and enhanced OXPHOX to suppress the growth of glioma cells. Subsequently, we found that TRIM65 interacted with AMPK, a metabolic sensor, and mediated its K48-linkage ubiquitination and degradation though proteasomal pathway, thereby regulating HIF-1α-induced glycolysis. Importantly, the inhibitory effect of TRIM65 silencing on glycolysis was abrogated by AMPK knockdown or HIF-1α overexpression, indicating glucose metabolic reprogramming by TRIM65 is dependent on AMPK and HIF-1α pathway. These results reveal a new role for TRIM65/AMPK/HIF-1α axis in glioma cell proliferation and aerobic glycolysis, suggesting that TRIM65 may be a potential therapeutic target for intervention of glioma.
Similar content being viewed by others
Introduction
Glioma, the most prevalent primary tumor in central nervous system, accounts for 30% of brain tumors and 80% of malignant ones with poor prognosis and high mortality1. Based on the degree of malignancy, they are classified into WHO grades I-IV, with WHO Grade IV indicating the most malignant behavior2. Despite the standard regiments consisting of maximal safe surgical resection and subsequent radiochemotherapy, the median survival of diagnosed patients is approximately 15 months due to rapid tumor growth and aggressive invasion3,4. Inevitable recurrence remains a pivotal challenge. Therefore, the discovery of novel molecular pathogenesis and therapeutic targets for glioma is of clinical significance.
Aerobic glycolysis, a hallmark of glioma, is characterized by preferential dependence on glycolysis rather than oxidative phosphorylation (OXPHOS) for energy production to support tumor growth and metastasis regardless of oxygen sufficiency, also called Warburg effect5. High glucose uptake and increased lactate production were observed in glioma cells compared to normal cells6. Although the ATP yield per glucose is low, with a sufficiently high glycolytic flux, the Warburg effect is beneficial to bioenergetics and biosynthesis by increasing non-oxidative ATP generation and providing metabolic intermediates, respectively, and the percentage of ATP produced from glycolysis can be more than that produced by OXPHOS7,8. Previous studies showed that reversing the glycolytic phenotype to OXPHOS in cancer resulted in the inhibition of cell growth9,10. Based on glucose metabolic reprogramming, 18F-fluorodeoxyglucose PET is widely used for cancer diagnosis and staging11, and lactate is under clinical evaluation12. Targeting glycolysis as a promising therapeutic intervention for glioma remains attractive.
The AMP-activated protein kinase (AMPK) is a highly conserved Ser/Thr protein kinase complex that functions as a major sensor of metabolic energy homeostasis13. Its activity is tightly regulated by the AMP/ATP ratio, upstream kinases and post-translational modifications14, especially ubiquitination modifications. From a metabolic perspective, the fundamental role of AMPK is to promote ATP generation that is essential for multiple biological processes, such as lipid synthesis, glycolysis, mitochondrial biosynthesis and oxidative metabolism15,16. AMPK has been reported to be associated with tumorigenesis and tumor metabolism by modulating the expression and function of various factors, including HIF-1α17, mTOR18, c-Myc19 and SIRT120. Moreover, HIF-1α is a key mediator of metabolic transformation induced by AMPK loss21, suggesting a crucial role of AMPK and HIF-1α pathway in modulating glucose metabolic reprogramming.
TRIM65, a member of RBCC/TRIM (tripartite motif) protein family, is composed of an N-terminal RING domain, a B-box, a coiled-coil domain and a SPRY domain and is involved in various cellular processes including intracellular signaling, autophagy, innate immunity, differentiation and carcinogenesis22,23. In malignancies progression, TRIM65 acts as ubiquitin E3 ligase, targeting TPIT24, ARHGAP3525, TNRC6A26, ANXA227, vimentin28, p5329 and beta-catenin30 to promote proliferation and invasion of neuroendocrine tumors, colorectal cancer, lung cancer, bladder urothelial carcinoma, ovarian cancer, cervical cancer and hepatocellular carcinoma, respectively. A series of studies indicate that several TRIM proteins are linked to glucose metabolic reprogramming. For example, TRIM46 enhanced glycolysis of lung cancer by modifying PHLPP2 ubiquitylation31. Zhang et al. reported that TRIM23 was capable of modulating the glucose metabolism in lung adenocarcinoma32. However, the effect of TRIM65 on aerobic glycolysis in glioma and its underlying mechanism are not explored.
In the present study, we observed that TRIM65 expression was upregulated and associated with poor survival in glioma, and TRIM65 knockdown significantly inhibited glioma cell proliferation by attenuating glycolysis and enhancing OXPHOX. Moreover, AMPK was identified as a potential TRIM65-interacting protein by LC-MS/MS analysis. We further demonstrated that TRIM65 promoted K48 poly-ubiquitination and degradation of AMPK, leading to elevated HIF-1α expression, thereby enhancing glycolysis process. Collectively, we identified a novel TRIM65/AMPK/HIF-1α axis that results in a switch to glycolysis, suggesting TRIM65 might be a valuable therapeutic target for glioma.
Results
TRIM65 expression is upregulated in human glioma tissues and correlated with poor prognosis
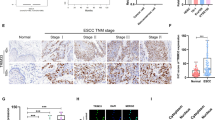
To investigate the potential role of TRIM65 in glioma, we queried the Cancer Genome Atlas (TCGA), Genotype-Tissue Expression (GTEx) datasets and Clinical Proteomic Tumor Analysis Consortium (CPTAC) datasets using the GEPIA website and UALCAN website, respectively. The results of analysis revealed that TRIM65 mRNA expression in low-grade glioma (LGG, grades II or III) and high-grade glioma (glioblastoma, GBM, grade IV) (Fig. 1A) and TRIM65 protein expression in GBM (Fig. 1B) were significantly elevated compared with normal tissues. Subsequently, we examined the protein expression of TRIM65 in glioma using tissue microarray comprised 149 glioma patients by IHC (Fig. 1C), and then the glioma tissues were divided into a high expression group and a low expression group based on the TRIM65 level. As shown in Fig. 1D, TRIM65 expression level was positively associated with the grades of glioma. Moreover, the patients with low TRIM65 expression exhibited tendentiously longer survival time than those with high TRIM65 expression (Fig. 1E). These results demonstrate that TRIM65 contributes to glioma progression and affects patient survival, and may regulate the tumorigenesis and development of glioma.
A TRIM65 mRNA levels in normal tissues, LGG tissues and GBM tissues from TCGA and GTEx datasets. TPM transcripts per kilobase of exon model per million mapped reads. B TRIM65 protein levels in normal tissues and GBM tissues from CPTAC data. C IHC staining of a representative glioma tissue microarray with an anti-TRIM65 antibody. D TRIM65 expression level correlates positively with the grades of glioma. E Kaplan-Meier survival curve of 149 patients in the glioma tissue microarray based on TRIM65 expression. *p < 0.05; **p < 0.01, ***p < 0.001.
TRIM65 promotes the proliferation of glioma cells in vitro and in vivo
To investigate the function of TRIM65 in the proliferation of glioma cells, TRIM65 was knocked down using specific siRNA or overexpressed in U251 and U87 cells, respectively (Fig. 2A). Then, we performed cell growth assays, and found that cell proliferation was remarkably decreased in two glioma cell lines with TRIM65 depletion, but increased in cells with TRIM65 overexpression (Fig. 2B). EdU incorporation assay also showed less EdU-positive cells were observed in si-TRIM65 group compared with si-Ctrl group, whereas TRIM65 overexpression significantly enhanced the proliferation rate of U251 and U87 (Fig. 2C, Supplementary Fig. 1a). Then, colony formation assay was performed and our data indicated that the expression level of TRIM65 is closely related to the colony-forming ability of glioma cells (Fig. 2D). In addition, the wound healing assay showed TRIM65 enhanced the migration of U251 cells (Supplementary Fig. 1b). To further detect the effects of TRIM65 on the tumorigenesis of glioma cells in vivo, we used lentiviral shRNA to knock down TRIM65 in U87 cells (Fig. 2E) and then performed xenograft tumor models by subcutaneously implanting U87 cells stably expressing sh-Ctrl or sh-TRIM65 into the flanks of NOD-SCID mice. A decrease in tumor growth, tumor weight and tumor volume was observed in sh-TIRM65 group compared with sh-Ctrl group (Fig. 2F–H). To confirm these results, the H&E staining and immunohistochemistry staining assay was conducted. Our results showed that TRIM65 silencing markedly reduced the number of Ki67-positive proliferation cells in xenografts (Fig. 2I). These data indicated that TRIM65 may function as an oncogene that promotes the growth and migration of glioma cells.
A TRIM65 knockdown or overexpression efficiency in U251 and U87 cells was detected by Western blotting. F Flag-tagged TRIM65, W wild-type TRIM65. B TRIM65 was knocked down using TRIM65 siRNA or overexpressed using pCMV-Flag-TRIM65 plasmid in U251 and U87 cell lines. 24 h later, cells were seeded in 12-well plates. At indicated time, cells were counted. C Estimation of cell proliferation by EdU assay. Scale bar = 50 μm. D Estimation of cell proliferation by colony formation assay. E U87 cells were infected with sh-TRIM65 lentivirus (a titer of 1 × 108TU/mL) at a multiplicity of infection (MOI) of 10 for 48 h. Western blot assay for TRIM65 expression level in U87 cells stably expressing sh-Ctrl or sh-TRIM65. F U87 cells stably expressing sh-Ctrl or sh-TRIM65 were injected subcutaneously into the flanks of NOD-SCID mice. Two weeks later, tumors were dissected out. Representative images of the xenograft tumors in each group. G Tumor weight was measured. H Tumor volume was measured. I H&E staining of tumor and IHC staining using anti-Ki67 and anti-TRIM65 antibodies at the indicated treatment. Scale bar = 20 μm. Data are presented as mean ± SD. The experiments were independently repeated at least three times and the data of one representative experiment was shown. *p < 0.05; **p < 0.01, ***p < 0.001.
TRIM65 enhances the glycolysis process and attenuates the OXPHOS process in glioma cells
To understand how TRIM65 modulates the cell proliferation in glioma, we performed RNA-seq analyses in si-Ctrl and si-TRIM65 cells, and found that there were 1039 DEGs including 481 upregulated and 558 downregulated DEGs in volcano plots (Fig. 3A). GSEA analysis demonstrated that DNA repair and metabolism of carbohydrates were enriched in TRIM65-silenced U251 cells (Fig. 3B). GO enrichment analysis results indicated the DEGs were involved in the metabolic process and ATP-dependent activity (Fig. 3C). Accordingly, we speculated that TRIM65 knockdown might affect glioma cell proliferation by regulating glucose metabolic reprogramming.
Aerobic glycolysis is a common hallmark of the glucose metabolism in cancer and shifting to OXPHOS can inhibit the growth of glioma cells33, thus we further investigated the effect of TRIM65 on aerobic glycolysis and OXPHOS. We measured the lactate production, the expression of glycolysis-related HK1, PDK1, Glut4, OXPHOS complexes, ATP level and MMP. As shown in Fig. 4A and Supplementary Fig. 2a, TRIM65 knockdown dramatically decreased lactate level compared with si-Ctrl cells in both U251 and U87 cells. Then, OXPHOS was inhibited by oligomycin to obtain lactate production at maximum glycolytic capacity as the previous reported34, which was found to be reduced by knockdown of TRIM65. Moreover, glycolytic reserve capacity was slightly suppressed in si-TRIM65 group compared to si-Ctrl group. In contrast, overexpression of TRIM65 increased the lactate production. Additionally, western blot analysis demonstrated that the expression levels of glycolysis-related HK1, PDK1 were down-regulated and OXPHOS complex V, III, II were up-regulated by TRIM65 depletion, while TRIM65 overexpression showed the opposite effect (Fig. 4B, C, Supplementary Figs. 2b and 3a). Moreover, TRIM65 elevated mRNA expression level of Glut4 (Supplementary Fig. 2c). The results from immunohistochemistry staining assay showed that TRIM65 silencing obviously reduced the expression of PDK1, while increasing the expression of IDH3B, OXPHOS-related protein (Fig. 4D). These results demonstrate that TRIM65 enhances the glycolysis process and attenuates the OXPHOS process in glioma cells.
A The effect of TRIM65 knockdown on lactate production in U251 cells treated with or without oligomycin (Left panel). Lactate production was measured after TRIM65 overexpression in U251 cells (Right panel). B, C Western blot assay for HK1, PDK1 expression and OXPHOS in U251 cells knocked down (Left panel) or overexpressed TRIM65 (Right panel) using anti-HK1, anti-PDK1 and OXPHOS monoclonal antibody cocktail. F Flag-tagged TRIM65, W wild-type TRIM65. D Representative IHC images of xenograft tumors with anti-TRIM65, anti-PDK1 and anti-IDH3B. Scale bar = 20 μm. E Mitochondrial and glycolytic ATP were measured after TRIM65 knockdown in U251 cells. F MMP was detected by TMRE staining at the indicated treatment. Scale bar = 100 μm. G Cell viability was detected in U251 cells overexpressed TRIM65 in the presence or absence of 2-DG. Data are presented as mean ± SD. The experiments were independently repeated at least three times and the data of one representative experiment was shown. *p < 0.05; **p < 0.01, ***p < 0.001.
Furthermore, the relative intracellular ATP level was measured in U251 and U87 cells. As illustrated in Fig. 4E and Supplementary Fig. 3b, TRIM65 knockdown significantly elevated the total ATP levels. Compared with si-Ctrl group, the decreased rate of glycolytic ATP production and the increased rate of mitochondrial ATP production were observed in si-TRIM65 group, suggesting TRIM65 knockdown shifts glycolysis to OXPHOS in glioma. MMP is the driving force for ATP production by OXPHOS35. Thus, TMRE staining was conducted to test MMP. TRIM65 silencing dramatically elevated MMP and TRIM65 overexpression reduced MMP, which further conformed that TRIM65 inhibited the OXPHOS process (Fig. 4F and Supplementary Fig. 3c). Then, we investigated if glucose metabolic reprogramming is necessary for the TRIM65-mediated glioma cell proliferation. The results showed that accelerated growth of U251 and U87 cells by TRIM65 was substantially alleviated by 2-DG, a glycolytic inhibitor (Fig. 4G and Supplementary Fig. 3d). Together, these findings suggested TRIM65 promotes cell proliferation by enhancing the glycolysis and attenuating the OXPHOS in glioma.
E3 ligase TRIM65 promotes K48-linkage ubiquitination and degradation of AMPK
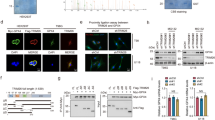
To explore the molecular mechanism of TRIM65 regulating glycolysis, we screened for its direct substrates by performing IP assay using IgG or Flag antibody in U251 cells expressing Flag-TRIM65 and LC-MS/MS analysis. 413 potential specific downstream substrates were found, and 16 proteins related to tumor glycolysis including AMPK were detected among the top 50% (210/413) proteins in abundance (Supplementary Fig. 4a). A large number of studies have shown that AMPK, as a cellular energy sensor coordinates metabolic activities, negatively regulates glycolysis36,37, we speculated that TRIM65 might affect glycolysis through ubiquitylation and degradation of AMPK. Firstly, TRIM65-AMPK interaction was confirmed by Co-IP (Fig. 5A, B). Moreover, the results from Western blot analysis showed that the expression levels of AMPK were negatively correlated with that of TRIM65 in U251 and U87 cells (Fig. 5C and Supplementary Fig. 4b). The results of qRT-PCR showed neither TRIM65 knockdown nor overexpression affected AMPK transcription (Supplementary Fig. 4c, d). Then, AMPK protein stability was examined. As presented in Fig. 5D, the half-life of AMPK protein was remarkably shortened by TRIM65 overexpression. In addition, decreased AMPK protein expression mediated by TRIM65-overexpression was rescued by MG132, a proteasome inhibitor (Fig. 5E), suggesting that AMPK was degraded by TRIM65 in a proteasome-dependent manner. Furthermore, we examined whether TRIM65, as an E3 ubiquitin ligase, facilitates AMPK protein degradation through ubiquitination modification. We performed the ubiquitin assay and found that TRIM65 overexpression significantly increased the ubiquitination of AMPK (Fig. 5F). Next, the types of ubiquitin chain linkage of AMPK induced by TRIM65 were analyzed. The results showed TRIM65 overexpression ubiquitinated AMPK through K48-linkage, and MG132 treatment enhanced this type of ubiquitination (Fig. 5G), but not the K63-linkage (Fig. 5H). Altogether, these data supported the model that TRIM65 promotes AMPK protein degradation via K48-linked ubiquitin-proteasome pathway.
The interaction between TRIM65 and AMPK was tested using anti-Flag (A) and anti-AMPK (B) antibodies for co-IP, followed by Western blot analysis. C Western blot analysis of AMPK protein expression in U251 cells with TRIM65 knockdown or overexpression. F Flag-tagged TRIM65, W wild-type TRIM65. D U251 cells were transfected with vector plasmid or Flag-TRIM65 and treated with 25 μg/mL CHX for different time points, followed by western blot analysis (Left panel). Related AMPK protein level was quantified at the indicated times (Right panel). E U251 cells were transfected with vector plasmid or Flag-TRIM65 in the presence or absence of MG132, followed by western blot analysis (Left panel). Related AMPK protein level was quantified (Right panel). F–H U251 cells were co-transfected with Flag-TRIM65 and His-Ub in the presence or absence of MG132. Proteins were immunoprecipitated with AMPK antibody. The ubiquitination (F), K48-linkage ubiquitination (G) and K63-linkage ubiquitination (H) were detected. Poly-Ub chains were indicated. F Flag-tagged TRIM65, W wild-type TRIM65. Data are presented as mean ± SD. The experiments were independently repeated at least three times and the data of one representative experiment was shown. *p < 0.05; **p < 0.01, ***p < 0.001.
The inhibitory effect of TRIM65 silencing on glycolysis is dependent on AMPK in glioma cells
To further elucidate if AMPK is indispensable for the TRIM65 silencing-mediated suppression of glycolysis, AMPKα1 knockdown was conducted in U251 cells using specific siRNAs, and siRNA#3 exhibiting the best knockdown efficiency was used for subsequent experiments (Fig. 6A). As shown in Fig. 6B–D, TRIM65 silencing decreased the cell viability and lactate production and increased ATP content in U251 and U87 cells, which was reversed by si-AMPK. In addition, si-AMPK abrogated TRIM65 silencing-mediated down-regulation of HK1 and PDK1 protein expression (Fig. 6E). These results indicate the inhibitory effect of TRIM65 knockdown on glycolysis is dependent on AMPK.
A AMPK knockdown efficiency was detected by Western blotting. Cell viability (B), lactate production (C) and ATP content (D) were measured in U251 and U87 cells knocked down TRIM65 in the presence or absence of si-AMPK. E Western blot analysis of HK1, PDK1, AMPK and TRIM65 protein expression in U251 (Left panel) and U87 cells (Right panel) treated as indicated, and then the related protein level was quantified. Data are presented as mean ± SD. The experiments were independently repeated at least three times and the data of one representative experiment was shown. *p < 0.05; **p < 0.01, ***p < 0.001.
HIF-1α is involved in the regulation of glycolysis process by TRIM65 via AMPK
It has been demonstrated that the transcription factor HIF-1α (hypoxia-inducible factor-1α) is a major mediator of AMPK-dependent effects on cellular metabolism38. Thus, we explored whether the HIF-1α pathway is involved in the regulation of glycolysis by TRIM65. It was found that TRIM65 knockdown decreased the protein expression of HIF-1α in U251 and U87 cells. The opposite results were observed in the cells with TRIM65 overexpression (Fig. 7A). To confirm these results, immunohistochemistry staining assay using anti-AMPK and anti-HIF-1α was performed. Our results demonstrated that TRIM65 deletion increased AMPK expression and decreased HIF-1α expression (Fig. 7B). Then, we generated HIF-1α overexpression in TRIM65 silencing glioma cells, and found the inhibition of lactate production and cell viability mediated by TRIM65 knockdown, which were significantly mitigated by HIF-1α overexpression (Fig. 7C, D), indicating TRIM65 silencing suppresses the glycolytic signature by regulating HIF-1α expression. Furthermore, we knocked down AMPK in TRIM65 silencing cells to explore whether TRIM65 depletion reduced HIF-1α expression via AMPK. The results showed that TRIM65 silencing did not obviously down-regulate the protein levels of HIF-1α when AMPK was knocked down (Fig. 7E), suggesting the TRIM65 depletion-mediated suppression of HIF-1α expression is dependent on AMPK. Collectively, these data suggest that TRIM65 knockdown attenuates the glycolysis process through enhancing AMPK expression and reducing HIF-1α expression.
A Western blot analysis of HIF-1α protein expression in U251 and U87 cells with TRIM65 knockdown or overexpression and then the related protein level was quantified. F Flag-tagged TRIM65, W wild-type TRIM65. B Representative IHC images of xenograft tumors with anti-TRIM65, anti-AMPK and anti-HIF-1α. Scale bar = 20 μm. Lactate production (C) and cell viability (D) were measured in U251 and U87 cells knocked down TRIM65 in the presence or absence of overexpressed HIF-1α. E Western blot analysis of HIF-1α protein expression in U251 and U87 cells treated as indicated. The related protein level was quantified. Data are presented as mean ± SD. The experiments were independently repeated at least three times and the data of one representative experiment was shown. *p < 0.05; **p < 0.01, ***p < 0.001.
Discussion
TRIM protein family, consisting of more than 80 family members, functions as an E3 ubiquitin ligase in a variety of biological processes including innate immunity, carcinogenesis, development, metabolic reprogramming and intracellular signaling29. E3 ligases were considered as the promising drug targets for tumor as crucial components of the ubiquitin-proteasome system39. Here, we found that TRIM65, a member of TRIM family proteins, is closely correlated with the poor prognosis of glioma patients. Moreover, our results demonstrated TRIM65 is essential for U251 and U87 cell proliferation in vitro and subcutaneous tumor growth in vivo, but the role of TRIM65 in patient-derived GBM cells and intracranial xenograft model of glioma still deserves further investigation. Consistent with our observations, Guozhang Hu et al. demonstrated that TRIM65 is involved in glioma cell proliferation and migration mediated by LncRNA LINC0185740. In fact, studies have indicated that TRIM65 is preferentially expressed in a wide range of cancers, such as human urothelial carcinoma of the bladder, hepatocellular carcinoma, lung, ovarian, cervical and colorectal cancer. This suggests that TRIM65 as an oncogene is involved in tumorigenesis and development of cancer, but different molecular mechanisms. The effect of TRIM65 on metabolic reprogramming in cancer cells has not been investigated.
Glucose metabolism has been recognized as one of the main metabolic changes in proliferating cells. Tumor cells usually favor glycolysis over OXPHOS for nutrients and energy production to meet their rapid growth and aggressive metastasis. This dependence on metabolic reprogramming remarkably distinguishes cancerous and normal cells41, making it an attractive therapeutic target for tumor clinically. Ubiquitination is one of the important post-translational modifications that is closely associated with glucose metabolism in cancer42. Recently, an increasing number of studies have shown that E3 ubiquitin ligases, especially RING-finger E3 proteins, control various cellular events, including DNA damage, cell apoptosis, metabolic pathways, signaling networks and glucose homeostasis43. In this study, RNA-seq analyses suggested that TRIM65 was closely related to glucose metabolic reprogramming in glioma. It was further found that TRIM65 depletion inhibited glioma cell proliferation by attenuating glycolysis and enhancing OXPHOS. Consistent with our results, it has been reported that the reversal of the Warburg effect by shifting glycolysis to OXPHOS is widely considered as a promising strategy for inhibiting tumor cell growth.
Currently, multiple studies have reported the metabolic control points and signal transduction pathways regulating the Warburg Effect and their importance to tumor tumorigenesis and progression44. AMPK as a metabolic gatekeeper mediates metabolic activities, energy homeostasis and lipid biosynthesis, and functions as a regulator of tumorigenesis45. Here, we identified AMPK as a major TRIM65-interating protein by IP-MS analysis. Our work further revealed that the E3 ligase TRIM65 promotes K48-linkage ubiquitination of AMPK, followed by recognition and degradation in the proteasome pathway. Moreover, the inhibitory effect of TRIM65 silencing on cell viability is dependent on AMPK in glioma. Numerous studies have demonstrated that AMPK activation was involved in regulating the inhibition of glioma cell growth46,47. In contrast to these results, AMPK induced the expression of PGC-1α as an important factor for glioma cell survival during nutrient scarcity48. Rishi Raj Chhipa et al. have also indicated that AMPK is required for viability of patient-derived GBM stem cell49. The role of AMPK as both a tumor suppressor and potential oncogene has been well debated. Many faces of AMPK possibly depend on cell types, metabolic status of the cell, the nature of damage, the intensity and duration of this metabolic sensor activation, and different microenvironmental contexts, suggesting a complex and non-univocal role for AMPK in glioma. As previously reported, AMPKα knockdown by itself had minimal effect on brain tumor initiating cells growth, but targeting AMPKα expression in the context of DRP1 inhibition rescued brain tumor initiating cell growth defect50. In this study, we mainly focused on the effect of AMPK on glioma proliferation in the context of TRIM65 knockdown. Furthermore, it was found that knockdown of AMPK effectively abrogated TRIM65 siRNA-mediated decrease of lactate production and down-regulation of HK1 and PDK1 expression level, suggesting the inhibitory effect of TRIM65 silencing on glycolysis is at least partially dependent on AMPK in glioma cells. Brandon Faubert et al. have indicated that AMPK negatively regulates the Warburg effect in cancer cells21. Similarly, AMPK activator SCT-1015 suppressed HIF-1α-mediated aerobic glycolysis in hepatocellular carcinoma38. This may further explain why AMPK played a key role in the inhibition of glycolysis mediated by TRIM65 depletion. However, whether other downstream targets of TRIM65 are involved in the regulation of glycolysis process remains to be determined.
HIF-1α, a hypoxia-inducible factor, drives glucose metabolism by promoting the genes encoding several glycolytic enzymes in cancer cells, and is associated with poor clinical prognosis51. Multiple studies have reported that AMPK deletion promoted the occurrence of HIF-1α-induced aerobic glycolysis and accelerated tumor progression10,21,38. Thus, it is worth further investigating whether TRIM65 enhances aerobic glycolysis through AMPK/HIF-1α signaling pathway. In this study, we found HIF-1α was involved in TRIM65-mediated glycolysis process. We also elucidated that TRIM65 deletion downregulated HIF-1α expression was dependent on AMPK level. These results further support the current view that HIF-1α is a key mediator of the metabolic reprogramming triggered by decreased AMPK activity in cancer cells.
In summary, our study demonstrated that TRIM65 silencing attenuated the glycolysis and enhanced the OXPHOS in glioma, thereby inhibiting glioma cell proliferation and tumor growth. Mechanistically, TRIM65 degraded AMPK through the K48-linked ubiquitination to enhance HIF-1α-induced glycolysis, revealing the regulation of the TRIM65/AMPK/ HIF-1α axis on metabolic reprogramming in glioma (Fig. 8), thus providing a theoretical basis for development of anti-cancer drugs targeting TRIM65.
Methods
Cell culture
Human glioma U251 and U87 cells were cultured in DMEM (BI, Israel) supplemented with 10% fetal bovine serum (FBS, BI, Israel) and 1% penicillin/streptomycin (Solarbio, China) at 37 °C in a 5% CO2 humidified atmosphere.
Tissue microarray
A total of 149 cases of human glioma tissues (HBraG149Su01) were obtained from Shanghai Outdo Biotech Company (China). Informed consent were obtained from participants. The study was approved by the Ethics Committee of Shanghai Outdo Biotech Company in agreement with the guidelines set forth by the Declaration of Helsinki.
Antibodies and reagents
Antibodies: the antibodies against the TRIM65 (Sigma, USA), OxPhos Human WB antibody cocktail (Thermo Fisher, USA), HK1, PDK1, HIF-1α, K48-linkage specific ubiquitin antibody, β-actin (all from ABclonal, USA), K63-linkage-specific polyubiquitin antibody (HUABIO, China), ubiquitin, AMPK rabbit mAb (Cell Signaling Technology, USA), AMPK mouse mAb (Santa Cruz, USA), Flag (OriGene, USA), goat anti-rabbit IgG, goat anti-mouse IgG (Abmart, China), Ki67, IDH3B (Servicebio, China) were purchased from the suppliers.
Reagents: TRIM65 siRNA (OriGene, USA), AMPK siRNA (RiboBio, China), lentiviral shRNA against TRIM65, puromycin (Genechem, China), oligomycin, 2-deoxyglucose (2-DG), cycloheximide(CHX), MG132 (all from MCE, China) were purchased from the suppliers.
Cell proliferation
The cell proliferation was tested by cell growth assay, colony formation and EdU incorporation assay according to the previously reported protocols52. For cell growth assay, 20000–40000 cells were seeded in 12-well plates, and each group had three replicates. The medium was changed every two days and cells were counted at the indicated time. For colony formation, 1000 cells were cultured in 6-well plates for 10 days. Then, colonies were fixed in 4% formaldehyde for 20 min at room temperature before staining with 1% crystal violet. For EdU assay, cells were seeded in 6-well plates and incubated with EdU (50 μΜ) for 2 h and washed with PBS before fixing in 4% paraformaldehyde. Subsequently, cells were permeabilized, washed and stained. The EdU-positive cells were observed under a fluorescence microscope (Olympus, Japan).
RNA-sequencing
The cells were cultured in 100 mm dish and transfected with si-Ctrl or si-TRIM65 using siRNA transfection reagent (OriGene, USA) once 60–70% confluency was reached. Three replicates of each group were performed. After 48 h, total RNA was extracted and RNA sequencing was carried out and analyzed by Metware Metabolic Biotechnology Co., Ltd. China. Differential expression analysis between si-Ctrl group and si-TRIM65 group was performed with the DESeq2 package. Benjamini & Hochberg correction was applied to p-value, controlling the false discovery rate (FDR). Genes with |log2 Fold Change | > 0.585 and FDR < 0.05 were considered significant differentially expressed genes (DEGs). Gene Ontology (GO) analysis was applied to DEGs, encompassing biological process (BP), cell component (CC), molecular function (MF). Gene set enrichment analysis (GSEA) was conducted using the clusterProfiler R packages. p-value < 0.05, FDR < 0.25 and |NES | > 1 indicated a significantly enriched term.
Measurement of lactate production, mitochondrial membrane potential (MMP) and ATP level
To detect lactate production, the cells were seed in 12-well plates and transfected with si-Ctrl or si-TRIM65. After 43 h, the cells were washed and treated with or without 1 μM oligomycin for 5 h. Subsequently, lactate production was measured using Lactate Assay Kit (Solarbio, China), following the manufacturer’s protocols.
For measurement of MMP, the cells were cultured in 35 mm dish and transfected as indicated. MMP was detected by using the tetramethylrhodamine ethyl ester (TMRE, Invitrogen, USA), which were performed as described previously53.
To examine ATP levels, the cells were transfected as indicated for 45 h. Then, the cells were treated with or without 2.5 μM oligomycin for 3 h and lysed for measuring the levels of ATP production using ATP Assay Kit (Beyotime, China), according to the manufacturer’s instructions. The intracellular ATP concentration was determined by the luminescence values and normalized with the protein content in each sample.
Immunoprecipitation (IP) and liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis
U251 cells at 80% confluence were transfected with Flag-TRIM65 for 48 h and then lysed with IP lysis buffer (Servicebio, China) and incubated with Flag or IgG antibody and protein A/G beads for overnight at 4 °C. Subsequently, the beads were washed, and the precipitates were subjected to SDS-PAGE and stained with Coomassie blue. The bound proteins were determined by LC-MS/MS (Biotree Biotech Co., Ltd., China).
Western blotting
We performed Western blotting assay as described previously54. Briefly, the cell extracts were subjected to SDS-PAGE and transferred to PVDF membranes (Millipore, USA), then blocked with 5% fat-free milk. Next, the membranes were incubated with indicated primary antibodies at 4 °C overnight, followed by incubation with secondary antibodies for 1 h at room temperature. ChemiDoc MP system (Bio Rad, USA) was used to visualize the protein bands.
Ubiquitination assay
The cells were transfected as indicated, and then treated with or without the MG132. The cell lysates were prepared in IP lysis buffer and reacted with AMPK antibody for 1 h, then co-incubated with protein A/G beads overnight at 4 °C. The immunoprecipitated complexes were washed and analyzed by Western blot analysis using ubiquitin, K48-linkage specific ubiquitin and K63-linkage specific ubiquitin antibody.
Tumor xenograft assay
NOD-SCID mice (6 weeks old) were purchased from SPF (Beijing) Biotechnology Co., Ltd. All animal experiments conducted in this study were approved by the Ethical Committee for Animal Experimentation of Jiangxi Provincial People’s Hospital in China, and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. 100 μL of U87 cells suspension (5 × 106 cells) stably expressing sh-Ctrl or sh-TRIM65 were injected subcutaneously into the flanks of mice. Two weeks later, the mice were euthanized. Then, tumors were dissected and weighted. The volumes of tumors were calculated as \(\pi /6\times ({\rm{large\; diameter}})\times {({\rm{smaller\; diameter}})}^{2}\).
Immunohistochemistry (IHC)
The IHC staining was performed as described previously. Briefly, tumor tissues were fixed in 4% paraformaldehyde. Then the tissue sections were stained with anti-Ki67, anti-PDK1, anti-IDH3B, anti-AMPK or anti-HIF-1α antibodies, and observed under light microscopy (Olympus, Japan).
Statistical analysis
Statistical analysis was performed with GraphPad Prism software. Statistical comparisons were conducted by Student’s t-test or one-way analysis of variance. Data from separate experiments at least three times are presented as mean ± SD. p-value < 0.05 was considered to be statistically significant.
Data availability
The data generated during the current study are included in this article and its Supplementary Information files. Raw data files are not publicly available due to privacy and security concerns, but are available from the corresponding author on reasonable request.
Code availability
No new algorithms were developed for this article. All code generated for analysis is available from the authors upon request.
References
Weller, M. et al. Glioma. Nat. Rev. Dis. Prim. 10, 33 (2024).
Louis, D. N. et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 131, 803–820 (2016).
Stupp, R. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352, 987–996 (2005).
Ghosh, D., Nandi, S. & Bhattacharjee, S. Combination therapy to checkmate Glioblastoma: clinical challenges and advances. Clin. Transl. Med. 7, 33 (2018).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Agnihotri, S. et al. PINK1 is a negative regulator of growth and the Warburg effect in glioblastoma. Cancer Res. 76, 4708–4719 (2016).
DeBerardinis, R. J., Lum, J. J., Hatzivassiliou, G. & Thompson, C. B. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 7, 11–20 (2008).
Greene, J., Segaran, A. & Lord, S. Targeting OXPHOS and the electron transport chain in cancer; molecular and therapeutic implications. Semin. Cancer Biol. 86, 851–859 (2022).
Liao, M. et al. Targeting the Warburg effect: a revisited perspective from molecular mechanisms to traditional and innovative therapeutic strategies in cancer. Acta Pharm. Sin. B 14, 953–1008 (2024).
Chen, Y. et al. TRPM7 silencing modulates glucose metabolic reprogramming to inhibit the growth of ovarian cancer by enhancing AMPK activation to promote HIF-1α degradation. J. Exp. Clin. Cancer Res.41, 44 (2022).
Confalonieri, S. et al. A PET-surrogate signature for the interrogation of the metabolic status of breast cancers. Adv. Sci. 11, e2308255 (2024).
Ippolito, L., Morandi, A., Giannoni, E. & Chiarugi, P. Lactate: a metabolic driver in the tumour landscape. Trends Biochem. Sci. 44, 153–166 (2019).
Langer, H. T., Rohm, M., Goncalves, M. D. & Sylow, L. AMPK as a mediator of tissue preservation: time for a shift in dogma?. Nat. Rev. Endocrinol 20, 526–540 (2024).
Ovens, A. J. et al. Post-translational modifications of the energy guardian AMP-activated protein kinase. Int. J. Mol. Sci. 22, 1229 (2021).
Keerthana, C. K. et al. The role of AMPK in cancer metabolism and its impact on the immunomodulation of the tumor microenvironment. Front. Immunol. 14, 1114582 (2023).
Hsu, C. C., Peng, D., Cai, Z. & Lin, H. K. AMPK signaling and its targeting in cancer progression and treatment. Semin. Cancer Biol. 85, 52–68 (2022).
Lee, S. H., Golinska, M. & Griffiths, J. R. HIF-1-independent mechanisms regulating metabolic adaptation in hypoxic cancer cells. Cells 10, 2371 (2021).
Zhao, M. et al. Icariin exerts anti-tumor activity by inducing autophagy via AMPK/mTOR/ULK1 pathway in triple-negative breast cancer. Cancer Cell Int. 24, 74 (2024).
Liu, C., Rokavec, M., Huang, Z. & Hermeking, H. Salicylate induces AMPK and inhibits c-MYC to activate a NRF2/ARE/miR-34a/b/c cascade resulting in suppression of colorectal cancer metastasis. Cell Death Dis. 14, 707 (2023).
Kung, M. L. et al. Deficiency of ADAR2 ameliorates metabolic-associated fatty liver disease via AMPK signaling pathways in obese mice. Commun. Biol. 7, 594 (2024).
Faubert, B. et al. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab. 17, 113–124 (2013).
Pan, X., Chen, Y., Shen, Y. & Tantai, J. Knockdown of TRIM65 inhibits autophagy and cisplatin resistance in A549/DDP cells by regulating miR-138-5p/ATG7. Cell Death Dis. 10, 429 (2019).
Lang, X. et al. TRIM65-catalized ubiquitination is essential for MDA5-mediated antiviral innate immunity. J. Exp. Med. 214, 459–473 (2017).
Yao, H. et al. TRIM65 determines the fate of a novel subtype of pituitary neuroendocrine tumors via ubiquitination and degradation of TPIT. Neuro-Oncol. 24, 1286–1297 (2022).
Chen, D. et al. Ubiquitin ligase TRIM65 promotes colorectal cancer metastasis by targeting ARHGAP35 for protein degradation. Oncogene 38, 6429–6444 (2019).
Li, S. et al. TRIM65 regulates microRNA activity by ubiquitination of TNRC6. Proc. Natl. Acad. Sci. USA 111, 6970–6975 (2014).
Wei, W. S. et al. TRIM65 supports bladder urothelial carcinoma cell aggressiveness by promoting ANXA2 ubiquitination and degradation. Cancer Lett. 435, 10–22 (2018).
Zhao, L., Zhang, P., Su, X. J. & Zhang, B. The ubiquitin ligase TRIM56 inhibits ovarian cancer progression by targeting vimentin. J. Cell Physiol. 233, 2420–2425 (2018).
Wang, X. Y. et al. TRIM65 promotes cervical cancer through selectively degrading p53-mediated inhibition of autophagy and apoptosis. Front. Oncol. 12, 853935 (2022).
Yang, Y. F., Zhang, M. F., Tian, Q. H. & Zhang, C. Z. TRIM65 triggers β-catenin signaling via ubiquitylation of Axin1 to promote hepatocellular carcinoma. J. Cell Sci. 130, 3108–3115 (2017).
Tantai, J., Pan, X., Chen, Y., Shen, Y. & Ji, C. TRIM46 activates AKT/HK2 signaling by modifying PHLPP2 ubiquitylation to promote glycolysis and chemoresistance of lung cancer cells. Cell Death Dis. 13, 285 (2022).
Zhang, Y. et al. Elevated TRIM23 expression predicts cisplatin resistance in lung adenocarcinoma. Cancer Sci. 111, 637–646 (2020).
Bonnet, S. et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell 11, 37–51 (2007).
Gu, H. et al. Liver-inspired polyetherketoneketone scaffolds simulate regenerative signals and mobilize anti-inflammatory reserves to reprogram macrophage metabolism for boosted osteoporotic osseointegration. Adv. Sci.10, e2302136 (2023).
Koranteng, J., Chung, K. F., Michaeloudes, C. & Bhavsar, P. The role of mitochondria in eosinophil function: implications for severe asthma pathogenesis. Front. Cell Dev. Biol. 12, 1360079 (2024).
Moldogazieva, N. T., Mokhosoev, I. M. & Terentiev, A. A. Metabolic heterogeneity of cancer cells: an interplay between HIF-1, GLUTs, and AMPK. Cancers 12, 862 (2020).
Kishton, R. J. et al. AMPK is essential to balance glycolysis and mitochondrial metabolism to control T-ALL cell stress and survival. Cell Metab. 23, 649–662 (2016).
Tseng, H. I. et al. A novel AMPK activator shows therapeutic potential in hepatocellular carcinoma by suppressing HIF1α-mediated aerobic glycolysis. Mol. Oncol. 16, 2274–2294 (2022).
Kaushik, A., Parashar, S., Ambasta, R. K. & Kumar, P. Ubiquitin E3 ligases assisted technologies in protein degradation: sharing pathways in neurodegenerative disorders and cancer. Ageing Res. Rev. 96, 102279 (2024).
Hu, G., Liu, N., Wang, H., Wang, Y. & Guo, Z. LncRNA LINC01857 promotes growth, migration, and invasion of glioma by modulating miR-1281/TRIM65 axis. J. Cell Physiol. 234, 22009–22016 (2019).
Shi, Z., Hu, C., Zheng, X., Sun, C. & Li, Q. Feedback loop between hypoxia and energy metabolic reprogramming aggravates the radioresistance of cancer cells. Exp. Hematol. Oncol. 13, 55 (2024).
Ni, X., Lu, C. P., Xu, G. Q. & Ma, J. J. Transcriptional regulation and post-translational modifications in the glycolytic pathway for targeted cancer therapy. Acta Pharmacol. Sin. 45, 1533–1555 (2024).
Wang, W. et al. RING-finger E3 ligases regulatory network in PI3K/AKT-mediated glucose metabolism. Cell Death Discov. 8, 372 (2022).
Jones, R. G. & Thompson, C. B. Tumor suppressors and cell metabolism: a recipe for cancer growth. Genes Dev. 23, 537–548 (2009).
Hardie, D. G. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 25, 1895–1908 (2011).
Zheng, X. et al. Sinomenine ester derivative inhibits glioblastoma by inducing mitochondria-dependent apoptosis and autophagy by PI3K/AKT/mTOR and AMPK/mTOR pathway. Acta Pharm. Sin. B 11, 3465–3480 (2021).
Zhang, S. et al. Fyn-phosphorylated PIKE-A binds and inhibits AMPK signaling, blocking its tumor suppressive activity. Cell Death Differ. 23, 52–63 (2016).
Sauer, B. et al. An AMP-activated protein kinase-PGC-1α axis mediates metabolic plasticity in glioblastoma. Clin. Transl. Med. 14, e70030 (2024).
Chhipa, R. R. et al. AMP kinase promotes glioblastoma bioenergetics and tumour growth. Nat. Cell Biol. 20, 823–835 (2018).
Xie, Q. et al. Mitochondrial control by DRP1 in brain tumor initiating cells. Nat. Neurosci. 18, 501–510 (2015).
Yun, H. J. et al. AMPK-HIF-1α signaling enhances glucose-derived de novo serine biosynthesis to promote glioblastoma growth. J. Exp. Clin. Cancer Res.42, 340 (2023).
Wang, T. et al. Deacetylation of glutaminase by HDAC4 contributes to lung cancer tumorigenesis. Int. J. Biol. Sci. 18, 4452–4465 (2022).
Peng, Z. Q. et al. Human amniotic mesenchymal stem cells-derived conditioned medium and exosomes alleviate oxidative stress-induced retinal degeneration by activating PI3K/Akt/FoxO3 pathway. Exp. Eye Res. 244, 109919 (2024).
Wang, X. Y. et al. Human amniotic stem cells-derived exosmal miR-181a-5p and miR-199a inhibit melanogenesis and promote melanosome degradation in skin hyperpigmentation, respectively. Stem Cell Res. Ther. 12, 501 (2021).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82260626 to X.-Y.W., 81472371 to X.-J.H., 82303229 to T.W., 82260173 to X.-H.G.), Jiangxi Provincial Natural Science Foundation, China (20224BAB216052 to X.-Y.W., 20224ACB206014 to X.-J.H., 20232BAB216078 to T.W., 20212BDH81020 to X.-H.G.), Jiangxi Provincial Traditional Chinese Medicine Science and Technology Plan Project, China (2022A346 to X.-Y.W.) and Jiangxi Provincial Health Commission Science and Technology Plan Project, China (202310128 to X.-Y.W., 2023ZD001 to X.-J.H.).
Author information
Authors and Affiliations
Contributions
X.Y.W. and X.J.H. were responsible for conception and design of the study, data analysis, manuscript writing and supervision. M.H.L. and T.W. were responsible for experiments, data collection and interpretation. X.H.G., Z.P.Y. and X.H.H. developed the methodology and analyzed the data. X.H.Q. and Z.P.C. illustrated the figures. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, MH., Wang, T., Guan, XH. et al. TRIM65 regulates glucose metabolic reprogramming to promote glioma cell proliferation via ubiquitination and degradation of AMPK. npj Precis. Onc. 9, 163 (2025). https://doi.org/10.1038/s41698-025-00964-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41698-025-00964-z