Abstract
Pediatric Central Nervous System (CNS) tumors are the leading cause of cancer-related death in children, yet effective treatment options remain limited. The tumor-associated antigen GD2 is a promising target for immunotherapeutic approaches aimed at treating pediatric CNS cancers; however, its heterogeneous expression within and between tumors can complicate the development of effective strategies. Here we review different aspects of GD2 biology, including its structure, synthesis pathway, and cellular and tissue expression, focusing on pediatric CNS tumors. We provide a detailed overview of the investigational and diagnostic methods for evaluating GD2 expression on freshly dissociated tumor samples, tissue sections, or tumor-derived cell lines, and as a circulating marker in liquid biopsy. Furthermore, we provide a comprehensive overview of GD2-based therapeutic strategies, such as monoclonal antibodies, CAR T-cells, aptamers, vaccines, and multimodal approaches, from preclinical studies to recent clinical applications, highlighting both the promise and challenges of targeting GD2 in these cancers.
Similar content being viewed by others
Introduction
GD2 is a tumor-associated antigen (TAA) that has emerged as a promising target for aggressive pediatric central nervous system (CNS) tumors. Highly expressed in cancers like neuroblastoma and gliomas, GD2’s selective expression on tumor cells and limited expression in normal tissues make it an ideal target for precision medicine. Other TAAs, such as B7-H3, HER2, IL-13Rα2, EphA2, and survivin, are being investigated for pediatric cancers, including pediatric CNS tumors. However, based on its tumor specificity, expression, and clinical relevance, GD2 remains a very promising and ideal target for precision therapies such as CAR T cells and antibody-based treatments.
Growing evidence supports the efficacy of GD2-targeted therapies to treat cancer. However, several hurdles remain, especially for pediatric CNS malignancies, including managing immune-related side effects, optimizing the delivery of therapies across the blood-brain barrier (BBB), and addressing the complex brain tumor microenvironment (TME).
Pediatric CNS tumors occur globally at about 1.8 cases per 100,000 children annually, with a 5-year survival rate of 73–77%, varying by tumor type. Despite conciderable advancement in the diagnosis and understanding of these cancers, treatment options are still limited. However, results from early-phase clinical trials indicate that immunotherapeutic approaches targeting GD2 may represent a promising strategy to treat these deadly tumor types.
In this review, we explore the future perspectives and emerging strategies for improving the efficacy of GD2-targeted therapies. These include the use of combination therapies, where GD2-targeting agents are combined with other treatment modalities. Additionally, the development of biomarker-driven approaches could help identify patients who would benefit most from GD2-targeted therapies, paving the way for more personalized and effective treatments.
This work provides an overview of GD2 biology in terms of synthesis pathway and expression, as well as a critical review of the GD2-based targeted therapies for pediatric CNS tumors. It highlights current progress and key challenges, aiming to guide future research and clinical trials to improve outcomes for pediatric CNS cancer patients.
GD2 structure and synthesis
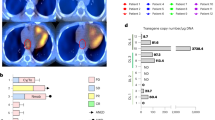
GD2 is a glycosphingolipid and a member of the ganglioside subfamily. In particular, GD2 is a disialoganglioside containing 2 sialic acids and 5 monosaccharides linked to a ceramide. The cellular synthesis of GD2 is a complex and well-regulated1. Its biosynthesis starts in the endoplasmic reticulum (ER) with the formation of the ceramide core, which is transported to the Golgi apparatus where the glycan chain is elongated and modified by glycosyltransferases (GT) and sialyltransferases (ST) (Fig. 1). The Beta-1,4 Galactosyltransferase (B4GALT6) will catalyze the synthesis of lactosyl-ceramide, which will be subsequently converted in the monosialoganglioside GM3 by the beta-galactoside alpha-2,3-sialyltransferase (ST3GAL5). Another ST, the alpha-N-acetyl-neuraminide alpha-2,8-sialyltransferase 1 (ST8SIA1), will then transfer a sialic acid molecule to GM3 to produce the disialoganglioside GD3, which will be finally converted by the Beta-1,4 N-acetylgalactosaminyltransferase 1 (B4GALNT1) in GD22. This last enzyme can also convert GM3 in the monosialoganglioside GM2.
GD2 can undergo further modifications by the beta 1,3-galactosyltransferase IV (B3GALT4), through the addition of a galactose molecule, leading to the synthesis of the GD1b ganglioside. In addition, the Sialyl O-Acetyl transferases (SOAT) can add an O-acetyl group to the outer sialic acid residue, leading to the production of an O-acetylated derivative of GD2, the O-Ac-GD2, which significantly alters GD2 immunological properties3,4 (Table 1).
Once the biosynthesis is complete, GD2 is transferred via vesicular transport to the cytoplasmic membrane5,6, where it will be anchored via the hydrophobic ceramide and then orient oligosaccharide chains towards the extracellular environment, allowing interaction with membrane molecules or extracellular ligands. In these interactions, gangliosides, including GD2, act as mediators and modulators of signal transduction pathways, as demonstrated for the epidermal growth factor receptor, the vascular endothelial growth factor receptor7,8,9,10 and for integrins11. It has also to be noted that GD2 can be shed from the cytoplasmic membrane as micelles or extracellular vesicles, which can be incorporated into GD2-negative neighboring cells, influencing their behavior12.
Gangliosides, with their amphiphilic nature, establish hydrophilic and hydrophobic interactions crucial for cell surface dynamics. Anchored securely to the plasma membrane via their hydrophobic ceramide tails, notably shared among ganglioside species, gangliosides like GD2 orient their oligosaccharide heads towards the extracellular environment, interacting through mild hydrophilic bonds with neighboring membrane molecules or extracellular ligands. This interaction serves to regulate the responsiveness of signaling molecules and enables gangliosides to function as mediators and modulators of signal transduction9. Moreover, extending their monosaccharide units into the extracellular space, gangliosides exhibit antigenic properties vital for cell–cell recognition and adhesion, contributing significantly to cellular communication and interactions7,9,10.
GD2 cellular and tissue expression
In contrast to other gangliosides present in numerous normal cells and human tissues, GD2 expression is limited to the surface of healthy cells from neuroectodermic origin, including the central nervous system (CNS), peripheral sensory nerve fibers and melanocytes13,14,15 GD2 is also expressed on mesenchymal stromal cells (MSCs) isolated from adipose tissue, but not on foreskin fibroblasts16. Moreover, its expression varies depending on the developmental stages. In CNS, GD2 expression initially constitutes 5–7% of the total brain gangliosides during gestation and gradually decreases to 2% in adult brain17,18, indicating a developmental-dependent restriction of GD2 expression19,20. Also, GD2 is potentially associated with neuronal differentiation. For instance, Jin and colleagues demonstrated that the inhibition of GD2 suppresses the neuronal differentiation of human umbilical cord blood-derived mesenchymal stem cells (UCB-MSCs) through the downregulation of neurogenic helix-loop-helix transcription factors21.
Besides its potential role in development and cell differentiation, GD2 stands out as an ideal target for anti-tumor therapy. Compared to other gangliosides, GD2 has limited expression in normal tissues, and its upregulation in tumor cells makes it an appealing tumor-associated antigen (TAA)7,22,23.
GD2 is found expressed mainly at the surface of tumor cells from the neuroectodermal origin, such as neuroblastoma, melanoma, and small-cell lung carcinoma, but also in cancer-associated stem cells (e.g., breast, bladder, and glioblastoma)14,24. Several reports indicate that GD2 may enhance proliferation, invasion (EMT), metastasis, as well as the motility and cell adhesion of cancer cells by interacting with the tumor microenvironment (TME) and regulating stem cell behavior through pathways associated with ganglioside biosynthesis and cellular signaling, such as ST8SIA125,26,27.
As mentioned, GD2 can be released from the cytoplasmic membrane through extracellular vesicles or micelles, and, thus, it can be found in the serum or be incorporated in neighboring cells. For example, GD2 has been identified in the serum of patients affected by neuroblastoma and retinoblastoma28,29,30. Sheeded GD2 from neuroblastoma cells is incorporated on the surface of T cells, enhancing T cell apoptosis and thereby downregulating the immune response31, suggesting that GD2 may contribute to tumor immune evasion and the shaping of TME. Moreover, one study reported GD2 expression on T cells, B cells, and dendritic cells in the lymph nodes of melanoma patients.
Such differences in GD2 expression levels across tissues not only highlight the complex regulatory networks governing its metabolism but also suggest that GD2 may have diverse functional roles depending on the specific cellular context.
The overexpression of GD2 in tumor cells is associated with the upregulation of enzymes such as ST8SIA1 and B4GALNT1 and the downregulation of B3GALT414,32,33,34,35. To date, none of these enzymes have yet been found mutated in cancer.
GD2 expression in tumor cells may be modulated by cell confluence in several tumors, such as osteosarcoma36, medulloblastoma37, and rhabdomyosarcoma cells38. Moreover, the variable expression of GD2 is finely modulated by epigenetic mechanism in Ewing sarcoma39.
In 2009, the U.S. National Cancer Institute ranked GD2 as the twelfth most promising target among 75 candidates for anti-cancer therapy, considering factors like therapeutic efficacy and immunogenicity40. Many tumors expressing GD2 are pediatric cancers, highlighting the need for targeting GD2 in pediatric patients.
GD2 in pediatric tumors of central nervous system
CNS tumors are the most prevalent solid neoplasms in childhood, representing the primary cause of cancer-related mortality. Notably, childhood CNS tumors exhibit distinct characteristics compared to adult brain tumors, including variations in their sites of origin, early dissemination, unique clinical presentation, as well as histological and biological features.
In the past decade, significant progress has been achieved in deconvoluting the molecular landscape of pediatric brain tumors. This enhanced understanding has translated into improved diagnostic methods, classification systems, and more precise prognostic assessments for many CNS tumors, thereby reshaping clinical practices. Despite these advancements, the prognosis for many young patients remains unfavorable41,42,43.
Historically, the classification of CNS tumors was exclusively based on histologic features44,45,46,47. More recently, novel tumor entities have been identified based on the integration of classical diagnostic criteria and key molecular alterations. This shift reflects the recognition of the importance of molecular features in refining disease classification48. Furthermore, methylation profiling has emerged as a powerful approach for the classification of CNS tumors.
In this review, we discuss various pediatric CNS tumors, including pediatric-type diffuse high-grade gliomas (PDHGG), pediatric-type low-grade gliomas (pLGG), ependymomas, and CNS embryonal tumors, such as medulloblastoma, with a particular focus on GD2 expression (Tables 2 and 3).
Pediatric-type diffuse high-grade gliomas
PDHGG is a large and heterogeneous family of aggressive tumors of the CNS. These comprise four main clinico-pathological entities: diffuse midline glioma, H3 K27-altered (DMG-H3K27-altered); diffuse hemispheric glioma, H3 G34-mutant (DHG-H3G34); diffuse pediatric-type high-grade glioma, H3-wildtype and IDH-wildtype (DHGG-H3, IDH1 WT); and infant-type hemispheric glioma (IHG)49.
DMG-H3K27-altered, a rare and aggressive tumor, primarily affects children and arises in the brain midline structures. It is commonly found in the pons, where it is known as diffuse intrinsic pontine glioma (DIPG)50. It also occurs in the thalamus, appearing bilaterally in children and monothalamically or in the spine in adolescents and young adults51,52,53. The prognosis is poor, with a 2-year survival rate lower than 10%54. Treatment options are limited to radiotherapy as the standard of care. Surgery is generally not feasible due to tumor location, and chemotherapy has shown limited benefit, but there is not yet a consensus on the management of these tumors with chemotherapeutic agents55.
In recent years, however, several investigational approaches have emerged, including the use of targeted agents such as ONC20156, which shows activity in H3K27-altered tumors, and epigenetic modulators like panobinostat57. Immunotherapeutic strategies, particularly GD2 and B7H3 targeted CAR T-cell therapy, have also demonstrated early promise in clinical trials, although challenges related to delivery and toxicity remain58,59,60. Additional approaches under investigation include peptide vaccines, immune checkpoint inhibitors, and convection-enhanced delivery of anticancer agents directly into the tumor.
Diffuse hemispheric glioma (DHG), H3G34 mutant, is a rare and aggressive brain tumor, primarily affecting adolescents and young adults, with a minor number of cases observed in children. Located in the cerebral hemisphere, these tumors exhibit a diffuse, infiltrative growth pattern that makes them difficult to completely resect through surgery. The defining feature is a missense mutation in the H3F3A gene, which leads to a substitution of glycine with arginine at position 34 of histone H3, along with other alterations. such as TP53 mutations, ATRX loss, which contribute to their aggressive nature, poor prognosis, and a tendency for early recurrence48,61,62. These features highlight the urgent need for novel, biology-driven therapeutic strategies. Liu and colleagues recently demonstrated that the interneuronal lineage origin of DHG-H3G34 tumors represents a therapeutic vulnerability, identifying CDK6 as a clinically actionable target whose inhibition can promote tumor cell differentiation, suppress growth, and extend survival63.
Other types of PDHGG, specifically H3-WT and IDH-WT, carry similar clinical characteristics but lack the typical histone mutations.
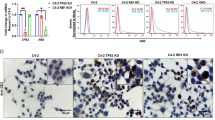
In PDHGG, GD2 expression has been strongly associated with the H3K27M mutation in DMG/PG64. A study from our group65 confirmed GD2 expression in DMG K27M and observed variable expression patterns across all the pDHGG subgroups, cytoplasmic and/or nuclear, focal or diffuse, and with intensities ranging from mild to strong. This heterogeneity appeared independent of tumor location and molecular subgroup. Differently from the results obtained by IHC on the tissue samples, flow cytometry analysis performed on primary patient-derived cell lines showed GD2 expression to be mainly restricted to the H3K27M-mutant cells. The authors hypothesized that TME in patient tissues may influence GD2 expression independently of histone mutation status65. Haydar and colleagues also investigated GD2 expression in various cell lines of HGG, n = 12, and DIPG, n = 8 using flow cytometry. Their study demonstrated similar patterns of GD2 expression across these tumor types. This research contributes to the understanding of GD2’s role in pediatric brain tumors, which will be further discussed in relation to the following specific tumor types66.
Pediatric-type low-grade gliomas (pLGGs)
Pediatric-type low-grade gliomas (pLGGs) represent approximately 30% of pediatric CNS tumors50. This diverse group includes glial, neuronal, and mixed glioneuronal tumors. These tumors grow slowly and generally have a favorable prognosis, especially when complete surgical resection is possible, with 10-year progression-free survival rates often exceeding 85%48. However, for patients with unresectable tumors, survival drops significantly, often necessitating additional therapies that may cause long-term side effects67.
Molecular profiling has advanced the classification and treatment of pLGGs, revealing that many pLGG harbor alterations in the RAS/MAPK signaling pathway, including BRAF and NF1 mutations, which play significant roles in tumor development68,69. Additional alterations include FGFR1/2/3, NTRK2, and ALK, as well as rarer mutations such as MYB and MYBL170,71. Despite advances in the biology and diagnosis of these tumors, biomarkers like GD2 have not been deeply investigated, as in other tumor types. Some studies have described the ganglioside profiles (including GD2) in low-grade glioma tissue samples using mass spectrometry72,73.
Ependymomas
Ependymomas, the third most common CNS tumor in children, account for 5–10% of cases and are classified into eight subtypes based on a combination of anatomical, histological, and molecular features. Despite some progress, treatment methods have remained largely unchanged in the past decades, with surgery and radiation representing primary therapies. The use of adjuvant chemotherapy is still controversial and not well-established50.
A recent study profiled potential chimeric antigen receptor (CAR)-T cell therapy targets, including GD2, in 49 pediatric brain tumor patient-derived orthotopic xenografts (PDOX), including 35 ependymomas. The study revealed heterogeneous antigen expression across tumor types, with GD2, along with B7-H3 as the most consistently expressed antigens, highlighting its potential as a target for immunotherapy66.
CNS embryonal tumors
CNS Embryonal tumors, particularly aggressive and driven by genetic events, primarily affect children74. Medulloblastoma (MB), the most common malignant embryonal brain tumor in children, accounts for about 20% of all pediatric CNS cancer. Originating from neuronal precursors in the posterior cranial fossa, MB is classified as a grade 4 tumor by the WHO. Prognosis varies across subgroups, some of them showing more favorable outcomes. Standard treatment involves a multimodal approach, including surgical resection, followed by adjuvant radiation therapy and systemic chemotherapy. However, these treatments often lead to significant side effects75,76.
In cases where standard therapies are inadequate, targeting GD2 has shown promise. Recently, Paret and colleagues characterized GD2 expression and related gene signatures (ST8SIA1 and B4GALNT1), which could help identify medulloblastoma (MB) subtypes likely to respond to GD2-targeted therapies, with higher GD2 expression found in SHH and group 4 MBs compared to group 3 and WNT subtypes66,77. Ciccone and colleagues further characterized GD2 expression by flow cytometry in 52 primary MB tumor biopsies freshly dissociated, finding GD2 expression in 82.68% of samples, with the highest levels in SHH and G3-G4 subtypes, and the lowest in the WNT subtype37.
Another CNS embryonal tumor, the Atypical Teratoid/Rhabdoid Tumor (AT/RT), is typically characterized by the inactivation of SMARCB1 (or SMARCA4) and includes three genetically, epigenetically, and clinically distinct molecular subgroups: ATRT-TYR, ATRT-SHH, and ATRT-MYC78. Haydar et al. examined GD2 cell surface expression across AT/RT subtypes using flow cytometry. They found that the proportion of GD2-positive cells ranged from 0% to 78% in ATRT-MYC tumors and from 12% to 75% in ATRT-SHH tumors66.
Retinoblastoma
Retinoblastoma (RB) is the most frequently occurring eye cancer in childhood, representing 3% of all pediatric cancers79. It may develop in one or both eyes and is typically linked to a mutation in the RB1 (retinoblastoma 1) gene located on chromosome 13q14 and essential for regulating the cell cycle. The loss of function of RB1 causes a proliferation of retinal cells during early ocular development, leading to tumor formation80.
The extent and severity of the disease determines the treatment of retinoblastoma. Current options include various forms of chemotherapy (intravenous, intra-arterial, and intravitreal), cryotherapy, radiotherapy, and surgery. Chemotherapy is essential for tumor control and preventing metastasis, with intra-arterial chemotherapy improving local control. Cryotherapy is often used alongside chemotherapy, while radiation therapy is considered a last option. Surgery is required for advanced cases to prevent metastasis. Despite these treatments, there remains an urgent need for new targeted therapies to enhance patient outcomes and preserve vision81,82. In recent years, GD2, among others, seems a promising target for RB83. GD2 is found to be expressed in vitro in cell lines, in the bone marrow of metastatic RB patients, as well as in the serum of RB patients. In fact, in the early 90 s, it had already been identified as a potential serum marker for RB since its serum level was found to be higher than in healthy individuals and its level decreased rapidly in successfully treated patients28. Based on this, recent efforts with GD2-CAR T-cell therapy and anti-GD2 mAbs Dinutuximab represent promising immunotherapeutic approaches for RB.
Pediatric CNS tumors vary widely in their clinical, histological, and molecular profiles, with GD2 emerging as a potential immunotherapeutic target across several tumor types. The table below outlines the main characteristics of each tumor type, including tumor subgroups, location, peculiar molecular alterations, age at diagnosis, and specific references on GD2 expression.
Investigational and diagnostic tools for GD2: advancements and perspectives
The heterogeneity of antigen expression may significantly challenge any effective GD2-based targeting strategy, complicating the efforts to leverage this ganglioside for accurate and reliable cancer diagnosis and treatment. For this reason, it is crucial to understand and standardize the detection of GD2 considering its diagnostic and prognostic value in the context of cancer. Investigations on GD2 are conducted both in vitro and in vivo. Samples of interest may include tissue sections, freshly dissociated cells, or cultured cells. Given the potential diagnostic and prognostic value of GD2, the challenge lies in establishing reliable and standardized detection assay protocols. One of the most common and well-standardized methods for the detection and quantification of GD2 is Flow Cytometry, allowing a precise identification and characterization of GD2-positive cells within cell suspensions from either cultured cells and/or freshly dissociated tissues, providing direct, valuable insights into disease progression, response to therapy, or even potential therapeutic targets24,84. Flow cytometry represents a fast and robust method to investigate GD2 expression with directly conjugated antibodies34,37,38,64,65. While very useful in assuring the preservation of the antigen, a disadvantage of this technique is the need to work with cell suspension, therefore missing the information on the antigen spatial localization in the tissue. Another issue may be the need to work with enough cells, which, for example, for tiny biopsy may not be feasible after tissue dissociation. In addition, it necessitates a fresh tumor sample and/or viable cryopreserved tumor cells, often requiring a dedicated sampling process as these are not part of standard practice.
Recently, scientists have described a GD2-specific blood test for neuroblastoma patients. The test, called Epitope Detection in Monocytes (EDIM), relies on the detection by flow cytometry of antigen expression by macrophages, which phagocytize fragments of neoplastic cells, extracellular vesicles, or circulating tumor cells85. In this study, the blood of 19 neuroblastoma patients and 22 healthy control patients was analysed, showing that 15/19 patients (79%) had positive EDIM-GD2 values, whereas none of the healthy individuals (0%) had a positive EDIM-GD2 level. This approach may represent a valuable liquid biopsy approach for the diagnosis or the follow-up of GD2-positive cancer types, though further research is needed before EDIM can be widely adopted as a standard diagnostic method.
Interestingly, in the 1990’s the first steps and initial protocols for detecting GD2 in tissue included a preliminary ganglioside extraction step, followed by chromatographic separation utilizing high-performance thin-layer chromatography and densitometric scanning28,29. Currently, advanced and improved techniques are being developed to detect circulating GD2 more simply and effectively, using high-performance liquid chromatography coupled with mass spectrometry, an approach that offers increased sensitivity and better separation of GD2 subspecies30. In a recent study, a liquid biopsy approach using a sensitive, cost-effective liquid chromatography-tandem mass spectrometry (LC-MS/MS) method demonstrated robust detection of different lipoforms of circulating GD2 in plasma samples, providing valuable diagnostic and prognostic insights for neuroblastoma patients and enabling non-invasive, longitudinal monitoring with minimal sample volume86. As a further novel approach, Galan and colleagues recently detected GD2 and GD3 in serum samples from ovarian cancer patients using the ELISA technique. The authors demonstrated the feasibility of such tests to diagnose ovarian cancer and showed that GD2 and GD3 can be used as biomarkers for ovarian cancer subtype classification and to track tumor progression87. In the same study, the authors used immunohistochemistry (IHC) to investigate the expression of GD2 and GD3 directly on tissue samples and were able to optimally score the staining in the different tissues analysed, establishing that GD2 and GD3 were expressed in all ovarian cancer subtypes and stages analysed, but not in the surrounding healthy or control tissues.
IHC is an important method generally considered the gold standard in the diagnostic practice for the detection of clinically relevant biomarkers, allowing the precise localization and quantification of the antigens of interest. However, in the case of GD2, this method is still investigational. In fact, GD2 detection on tumor biopsies is often challenging, due to its heterogenous expression across all solid tumors, its glycolipid structure, and membrane anchorage39,88. By employing a range of GD2-specific antibodies, researchers can assess GD2 levels in various tumor types, enabling accurate scoring of GD2 positivity. This scoring serves as a biomarker for patient selection, ensuring that those most likely to benefit from GD2-targeted therapies are identified.
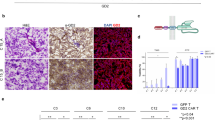
The expression of GD2 in DMG and DIPG was analyzed using IHC and immunofluorescence (IF), which facilitated the precise localization and characterization of GD2 expression patterns within tumor samples and patient-derived cell lines. This comprehensive approach allowed de Billy and colleagues to assess GD2 as a therapeutic target, supporting the development of GD2-CAR T-cell therapies in combination with selected inhibitors to enhance treatment effectiveness in pediatric high-grade gliomas65. Moreover, GD2 detection via IHC in other solid tumors, such as breast cancer, demonstrates its potential as a predictive biomarker, with its expression linked to favorable tumor characteristics, although it does not correlate with improved survival outcomes88.
The ability to accurately score GD2 expression through IHC holds significant implications for clinical trials, as this scoring system can identify and select patients with elevated GD2 expression who may benefit from targeted therapies. A recent study tackled the potential difficulty of detecting GD2 in formalin-fixed paraffin-embedded (FFPE) tissues by employing an immunofluorescence protocol that uses Tyramide for signal amplification to enhance sensitivity39. This approach seems to allow a reliable and robust detection of GD2, which could provide a solution to longstanding technical limitations in its assessment. A critical step for the detection of gangliosides on tissue sections, while preserving their structural integrity and ensuring a reliable staining, is the fixation. FFPE tissue sections are fixed in formalin, but then, during the dewaxing and rehydration steps, the use of ethanol may contribute to solubilizing the gangliosides. On the other side, frozen tissue sections may be fixed with acetone, which, used in its anhydrous form, has been shown to preserve ganglioside localization with minimal loss89.
While IHC and/or IF may provide precise localization and contextual information about GD2 expression within TME, ELISA offers a quantitative measurement of GD2 levels in biological samples87. These methods can complement each other, and it may be beneficial to consider using them in tandem to enhance our understanding of GD2 as a biomarker.
Alternatively, an indirect approach, such as the RT-qPCR for quantifying the ganglioside biosynthesis enzyme B4GALNT1, stands out for its high sensitivity compared to other techniques. This indirect method shows even greater sensitivity than the traditional immunohistochemistry approach90. Although it has been shown that B4GALNT1 is directly responsible for producing GD291, its gene expression level correlates with the expression of GD2. For example, in neuroblastoma cell lines, a stronger correlation between the expression of GD2 with ST8SIA1 compared to B4GALNT1 has been shown34. In DIPG primary patient-derived cell lines, it has been shown in general a good correlation between the expression of B4GALNT1 and GD2, except for a cell line that showed a high expression of GD2 and a low expression of B4GALNT164. Interestingly, in a recent study, where gene expression level in different cancer and normal tissues was analysed from public databases, it was found that a 2-gene signature given by ST8SIA1 + B4GALNT1 shows synergistic properties for the prediction of GD2-positive tissue phenotype32. Based on that, methods such as qRT-PCR, NanoString assay, and/or targeted RNA sequencing could be adopted and implemented in the diagnostic workflow and would be of particular impact for GD2-based clinical trials.
Many clinical trials based on GD2-directed therapies (see section “GD2-based therapeutic approaches” and Table 5) are established on previous in vitro and in vivo studies; therefore, it is important to have reliable assays and investigational tools that can be robustly translated to the clinic. Interestingly, recent studies have shown that the expression of GD2 in vitro is affected by cell confluence in 2D cell monolayers and 3D tumor-spheres in several tumor types, including osteosarcoma, medulloblastoma, and rhabdomyosarcoma36,37,38. The exact mechanism is unknown, but it may involve conditions such as hypoxia and oxidative stress driven by nutrient depletion in crowded cultures. Moreover, these environmental stressors might lead to changes in gene activation or silencing, revealing an intriguing connection between cell density and GD2 expression. These studies highlight how different cell environments affect GD2 expression and how important it is to model in vitro the patient disease to provide translatable results.
A variety of antibodies have been employed in GD2 research to enhance detection and characterization across different cancer types. These antibodies are used in a range of techniques, including flow cytometry, IHC, and more recent liquid biopsy approaches, allowing for a deeper understanding of GD2 expression and its role as a potential therapeutic target. The following table provides an overview of the main antibodies used in multiple studies, highlighting their specific applications and contributions to advancing GD2-related diagnostics and treatments.
Overall, significant advancements have been made for GD2 detection, each method offering distinct advantages and limitations (Table 4). Flow cytometry remains widely used due to its sensitivity, but is less suited for standard pathology workflows. IHC continues to provide valuable insights into tissue-based GD2 expression, aiding therapeutic decisions and clinical trial enrollment. However, the membrane-bound and glycolipid nature of GD2 can complicate consistent detection by IHC.
Emerging liquid biopsy/blood-based tests, such as EDIM-GD2, ELISA, and LC/MS, may represent promising non-invasive methods. To our knowledge, so far, only in two studies has GD2 been detected in cerebrospinal fluid of patients with retinoblastoma, either by an indirect method with an RT-PCR for GD2 synthase mRNA or by flow cytometry (Laurent V et al., 2013; Shen H et al., 2012). Further research into these liquid biopsy methods would be of particular interest for applications in the monitoring of GD2-positive CNS malignancies.
Despite innovations, challenges like sample requirements, detection consistency, and method validation still limit the widespread adoption of these techniques in investigational and clinical practice. Further research is needed for standardization to allow the integration of reliable and cost-effective methods into clinical practice. This will be helpful for enrollment into future clinical trials, but also necessary to establish the prognostic value of GD2 for CNS tumors.
GD2-based therapeutic approaches
Several innovative therapies are being explored for pediatric CNS tumors (Fig. 2), ranging from immunotherapeutic approaches, including GD2-specific antibodies and GD2-redirected CAR T-cell therapy, and multimodal strategies that harness the synergistic potential of different therapeutic modalities.
This evolving landscape reflects a strategic change towards more effective and personalized interventions with fewer adverse effects particularly important for the treatment of pediatric patients with CNS tumors. These efforts are being translated into several ongoing clinical trials targeting GD2 for the treatment of CNS tumors (Table 5).
Monoclonal antibodies targeting GD2
The first therapeutic strategy for targeting GD2 began with the development of mAbs, anti-GD2 mAbs, paving the way for additional immunotherapy approaches to broaden treatment options. Anti-GD2 mAbs can be produced using murine, chimeric, humanized, and fully human antibodies. The murine IgG3 mAb 3F8 was the first specific anti-GD2 antibody developed in 1985, which demonstrated killing of GD2-positive tumor cell92. In contrast to murine mAbs, the chimeric and the humanized anti-GD2 mAbs demonstrated reduced immunogenicity, prolonged half-lives, and enhanced effectiveness in promoting effector functions93,94.
MAbs targeting GD2 are thought to kill tumor cells expressing this ganglioside through three main mechanisms: (i) triggering macrophage-mediated phagocytosis and promoting antibody-dependent cell-mediated cytotoxicity (ADCC) by natural killer (NK) cells and granulocytes; (ii) inducing tumor cell lysis via complement-dependent cytotoxicity (CDC); and (iii) promoting apoptotic cell death by directly and specifically binding of the anti-GD2 mAbs to GD2 on tumor cells95,96.
Anti-GD2 mAbs mediated tumor cell death combine features reminiscent of both apoptosis and necrosis. The ADCC is dose-dependent, with the strongest anti-tumor effects observed in cells with high GD2 expression, indicating the specificity of the mechanism of action. Cell death appears to be mediated through the alteration of the mitochondrial membrane potential and the subsequent permeabilization of the plasma membrane, a key characteristic of apoptotic cell death induction95,97.
For brain tumors, Fleurence et al. demonstrated the anti-tumor efficacy of mAb8B6 anti-O-Ac-GD2 antibody mediated by an increase in apoptotic death of glioma cells, both in vitro and in vivo98. A phase II clinical trial demonstrated the safety and potential clinical utility of the murine mAb-antiGD2 (3F8) for the treatment of high-risk or recurrent medulloblastoma99.
The clinical benefit deriving from the use of anti-GD2 antibodies is highlighted by the FDA approval in 2015 of Dinutuximab for the treatment of high-risk neuroblastoma patients. Nevertheless, their clinical application is hampered by several limiting factors. Toxicity associated to the expression of GD2 by normal tissues and neurologic adverse effects of on-target/off-tumor is frequently observed in the clinic. Furthermore, resistance or relapse following anti-GD2 therapy is often observed in patients with GD2-positive cancer.
As for other antibody therapeutic strategies, other limitations are the insufficient affinity to the target, the limited penetration into the tumor mass, and, in the context of brain tumor, the low capability to cross the BBB. In this context, strategies to enhance anti-GD2 treatment involve either augmenting the dose of antibodies, constrained by side effects, or investigating alternative GD2-targeting immunotherapeutic strategies.
Aptamers
MAbs like dinutuximab, despite the promising therapeutic outcomes, have their limitations for the treatment of brain tumors, given their large size and subsequent difficulty in crossing the BBB. Aptamers offer a compelling alternative to antibodies. These single-stranded DNA or RNA molecules combine high specificity and affinity for their targets with structural adaptability100. Unlike antibodies, their small size allows for deep tissue penetration, which is crucial for reaching the CNS. Their low antigenicity reduces their immunogenicity, making them safer for repeated use. Chemically synthesized, aptamers enable scalable production at lower costs compared to biologics. Moreover, their versatility allows conjugation with agents like chemotherapeutics, siRNA, and nanoparticles for precise, targeted therapy101.
Preclinical studies have demonstrated the ability of GD2-specific aptamers to effectively bind GD2-positive cells and deliver drugs such as doxorubicin, showcasing their potential for both treatment and imaging. Moreover, aptamers offer a unique safety feature: complementary oligonucleotides can be used as antidotes to disrupt their function in vivo, mitigating potential off-target effects102. So far, two DNA-based GD2 aptamers have been developed for neuroblastoma that also incorporate doxorubicin into their structure: one is a pH-sensitive molecule (DB67) that more specifically targets and deliver the drug to the tumor cells based on the different pH between tumor and normal cells103 while the other one is more specifically directed against the MYCN amplified/overexpressed NB cells through a MYCN-siRNA (DB99)104. Despite their potential, further research is needed to optimize aptamer stability, enhance their pharmacokinetics through chemical modifications, and validate their efficacy in diverse preclinical and clinical settings. To the best of our knowledge, the development of GD2-aptamers for brain tumors has not been explored yet, despite their potential activity.
Vaccines
GD2 vaccines work by stimulating the immune system to target GD2-expressing tumor cells, often incorporating immune adjuvants or delivery systems to enhance their efficacy and prevent a recurrence105. These vaccines have shown particular promise as a therapeutic strategy for high-risk neuroblastoma. Cheung and colleagues demonstrated that the GD2/GD3 vaccine combined with β-glucan elicits strong antibody responses, even in patients with prior disease progression. A significant association was observed between elevated anti-GD2-IgG1 titers and improved progression-free and overall survival, highlighting the prognostic importance of antibody responses. These findings support the GD2/GD3 vaccine as a safe and promising option for enhancing long-term outcomes in patients106.
Another possible strategy consists of idiotypic vaccines, or anti-Id vaccines, designed to extend the immune response based on anti-idiotypic antibodies developed during previous anti-GD2 mAb therapies. This strategy uses the idiotype network theory (INT), which describes how antibodies can recognize not only antigens but also other antibodies, creating a regulatory network107.
The development of ganglidiomab, an anti-idiotype antibody targeting the anti-GD2 antibody family 14.18, has shown promise by inducing humoral responses in murine models and patients previously treated with anti-GD2 mAbs, with good tolerability and no significant side effects108,109. Similarly, ganglidiximab, another anti-idiotype antibody mimicking GD2, might offer a novel approach to enhance immune responses and refine GD2-targeted therapies110. Research is ongoing to determine the optimal design and application of these vaccines, as well as their potential combination with other treatments to enhance therapeutic outcomes. Although not yet applied for brain tumor malignancies, GD2 vaccines could open new therapeutic avenues for these high aggressive CNS cancers.
GD2-based CAR-T cell therapy
One of the most significant advancements in the onco-immunotherapy field involves the development of T cells engineered with CAR to target a specific antigen expressed at the surface of the tumor cells in a major histocompatibility complex (MHC)-independent manner. This is achieved by genetic modification of the T cells, either through viral vectors (retroviral or lentiviral transduction) or via non-viral methods, such as the Sleeping Beauty transposition, which is a synthetic transposon/transposase tool that enables stable genomic integration of the CAR constructs111. These approaches enable the expression of a chimeric receptor directed toward a specific tumor-associated antigen. The CAR is constituted by the variable portion of a monoclonal antibody fused to the signal transduction domain of the CD3z chain of the TCR. Several variations of the CAR have been subsequently implemented by adding one or two co-stimulatory molecules, such as CD28, 4-1BB, OX40, in order to improve the persistence and enhance the anti-tumor efficacy of CAR T-cells112.
The therapeutic benefit deriving from CAR T-cells is highlighted by the FDA approval of several CAR T-cell products, which include: Kymriah® (tisagenlecleucel), Tecartus® (brexucabtagene autoleucel), Breyanzi® (lisocabtagene maraleucel), and Yescarta® (axicabtagene ciloleucel). These therapies target the CD19 antigen for the treatment of blood cancers such as acute lymphoblastic leukemia (ALL) and non-Hodgkin lymphoma (NHL). In addition, Abecma® (idecabtagene vicleucel) and CARVYKTITM (ciltacabtagene autoleucel), which targets the B-cell maturation antigen (BCMA), have been approved for the treatment of relapsed and refractory multiple myeloma in adult patients113. Building on the successful experience in blood cancers, this therapeutic approach has been expanded to solid tumors, including neuroblastoma and brain cancers. GD2-CAR T cells have shown particular promise in neuroblastoma, a challenging pediatric cancer with high relapse rates and poor outcomes. Recent phase I/II clinical studies have demonstrated the safety, feasibility, and efficacy of GD2-CAR T cells, showing encouraging signs of sustained benefit in patients with relapsed and refractory neuroblastoma114.
Already some years ago, Louis et al. demonstrated a clinical benefit in the treatment of high-risk neuroblastoma using two different types of CAR-T cells, EBV-specific cytotoxic T lymphocytes (CAR-CTLs) and polyclonally activated T cells (CAR-ATCs), redirected against GD2. Among 19 patients treated, 3 out of 11 with active disease achieved complete remission. Prolonged persistence of CAR-ATCs (up to 192 weeks) and CAR-CTLs (up to 96 weeks) was associated with survival benefits and correlated with higher levels of CD4+ cells and central memory markers (CD45RO+ CD62L+) in the infused cells115. More recently, Che-Hsing and colleagues reported that one of the patients with active disease who achieved complete remission benefits from long-term survival over 18 years116.
More recently, Del Bufalo et al. reported on a GD2-directed CAR construct featuring two costimulatory domains, namely CD28 and 4-1BB. To mitigate potential neurotoxic effects linked to the utilization of these third-generation CAR T cells (GD2-CART01), they incorporated the gene for inducible caspase 9 (iC9) into the construct as a safety switch. This inclusion allows the adoptively transferred cells to be eliminated in the presence of life-threatening toxicities117,118. The study demonstrated impressive 3-year overall survival and event-free survival rates of 60% and 36%, respectively, in patients with relapsed or refractory high-risk neuroblastoma119. Even better results were obtained in patients with a limited tumor burden.
Quintarelli and colleagues have recently demonstrated that ALLO_GD2-CART01, an allogeneic GD2-specific CAR T cell therapy, exhibits robust antitumor activity, offering a novel treatment option for relapsed or refractory neuroblastoma. Unlike autologous CAR T cells, ALLO_GD2-CART01 uses T cells derived from healthy donors, making it accessible to patients with profound lymphopenia or insufficient T cell numbers for autologous production. ALLO_GD2-CART01 demonstrated significant expansion, persistence, and efficacy in patients who had exhausted other therapeutic options. This therapy may overcome challenges like lymphopenia or resistance to autologous CAR T therapies, but future studies are needed to further establish its potential and long-term safety in other GD2-expressing tumors like the CNS malignancies120.
In CNS tumors, GD2 has been found highly expressed in glioblastoma, medulloblastoma, and in DMG H3K27M-mutant, as well as hemispheric high-grade glioma37,65,66. In all these tumor types, GD2-CAR T-cells have been shown to be effective both in vitro and in vivo. Interestingly, Mount et al. found high expression of GD2 specifically in DMG cells harboring the H3K27M mutation. In the same study, the systemic administration of GD2-CAR T-cells in patient-derived orthotopic xenograft models of H3K27M-mutant DMG demonstrated potent antitumor efficacy. This work led to the opening in 2020 of the first GD2-CAR T-cell clinical trial (NCT04196413) for patients affected by DMG H3K27M-mutant64. Early clinical data have been reported for the first 4 DMG patients demonstrating improved outcome with signs of manageable and reversible tumor inflammation-associated neurotoxicity following intensive supportive care. The clinical trial is still ongoing to determine the optimal dose, schedule, and route, intravenous (IV) or intracerebroventricular (ICV), of GD2-CAR T cell administration, with the aim to improve anti-tumor efficacy and toxicity58. A recent update of this trial showed promising results, with significant tumor regression observed in many patients after initial IV infusions, while subsequent ICV treatments appeared to enhance therapeutic responses with lower toxicity profiles. This combined administration approach marks an important advance in the treatment of high-mortality brain cancers and suggests a strong potential for improved patient outcomes through optimized delivery methods and treatment protocols for targeted CAR T cell therapy121. Another trial (NCT04099797) started the same year for CNS pediatric cancer patients with histologically confirmed GD2 expression. Eleven patients have received either GD2-CAR T-cells or a modified GD2-CAR T-cells with a constitutively active IL-7 receptor, the latter demonstrated improved clinical benefit and radiographic tumor regression in some of the patients122.
In addition, a recent preclinical study demonstrated that CAR-T cells targeting the modified form of GD2, O-Ac-GD2, particularly Vδ2 T cells, effectively eliminated pHGG cells both in 2D and 3D models, highlighting its promise for an off-the-shelf allogeneic immunotherapeutic approach4. This is particularly promising as, compared to GD2, O-Ac-GD2 is largely absent in normal tissues, making it an interesting target, with reduced off-tumor toxicity123,124. However, its detection remains challenging, as standard anti-GD2 antibodies often fail to distinguish between the two forms.
GD2-based multimodal therapy
Despite the preclinical and clinical evidence on the feasibility and the tolerability of different anti-GD2-based therapeutic approaches, the anti-tumor efficacy is often partial and transient, resulting in tumor relapse. Several factors, including the antigen escape mechanisms122, the heterogeneous intra-tumor expression of GD265, as well as the cold TME of CNS tumors125, contribute to impairing the efficient anti-tumor efficacy. A multimodal approach consisting of combining different therapeutic agents will be more suitable to obtain a more efficacious and safe treatment.
The use of anti-GD2 antibodies in initial treatment protocols has markedly decreased relapse rates in neuroblastoma. Nevertheless, a notable proportion of patients still experience relapses, and the mechanisms underlying resistance, as well as the possibility of the occurrence of antigen escape to anti-GD2, remain poorly elucidated126,127,128. However, Mabe and colleagues identified preclinically a mechanism by which tumor cells regulate GD2 expression on their surface. They found that the expression of GD2 on neuroblastoma varies depending on the cell states, with a reduced expression associated with the mesenchymal state and correlates with resistance to anti-GD2 antibody. Moreover, they found that EZH2 inhibition triggers an epigenetic modification and re-establishes the efficacy of anti-GD2 therapy by restoring GD2 expression at the cell surface through the transcriptional upregulation of the GD3 synthase ST8SIA134.
Similarly, Ciccone et al. showed that inhibition of EZH2 with Tazemetostat in medulloblastoma cells re-establishes the expression of ST8SIA1 and consequently restores GD2 on the tumor cells, sensitizing low GD2 medulloblastoma cells to GD2-CAR T-cells37. The promising results of this experimental study support the ongoing phase I clinical trial (NCT05298995) currently evaluating the safety and therapeutic efficacy of CAR-GD2 therapy in relapsed and refractory MB and other pediatric CNS tumors. On the other hand, by increasing GD2 expression, Tazemetostat may not only increase tumor aggressiveness, but it is also responsible for some treatment-related adverse events, including asthenia, nausea, anemia, and vomiting in patients with advanced solid tumors129. Therefore, a multimodal therapeutic approach, including Tazemetostat, should be strictly controlled during all treatment courses.
In line with the regulation of ST8SIA1, Pilgrim et al. explored the role of YAP inhibition in augmenting anti-GD2 antibody responses in neuroblastoma. This study revealed that genetic inhibition of YAP sensitizes neuroblastoma cells to anti-GD2 antibody treatment both in vitro and in vivo. Furthermore, YAP transcriptionally suppresses ST8SIA1, indicating its potential as a therapeutic target to enhance patient responses to immunotherapeutic approaches targeting GD2. These findings suggest that YAP may serve as a mediator and potential biomarker of anti-GD2 antibody resistance in clinical settings130.
Recent findings by Rouaen and colleagues underscore the potential of integrating copper chelation to enhance anti-GD2 antibody therapy in neuroblastoma. Elevated copper levels within the TME have been linked to immune evasion mechanisms, including neutrophil dysfunction and immunosuppression. The study highlights that copper chelation, particularly with the FDA-approved agent TETA (Cuprior), remodels the TME, promoting pro-inflammatory cytokine activity and facilitating immune cell infiltration, especially neutrophils. This combination strategy not only improves ADCC but also prolongs animal survival in preclinical models. These results advocate for clinical trials to evaluate copper chelation as a safe, effective combination with anti-GD2 therapies to offer a promising avenue for the treatment of other solid cancers, including CNS tumors131.
More recently, Kasprowicz et al. performed a siRNA screen to identify inducers of O-Ac-GD2 expression and showed that inhibition of CERK, a kinase involved in glycosphingolipid metabolism, promotes the upregulation of O-Ac-GD2 expression level. The inhibition of CERK in combination with the chimeric c8B6 anti-O-Ac-GD2 antibody and in co-culture with NK-cells, increased the cytotoxicity in vitro against DIPG cells (DIPG13)33. Several combination strategies to boost the antitumor activity of GD2-targeting agents against tumor cells are being developed through the co-administration with agents such as MEK, PD-1, CTLA-4, and LAG-3 inhibitors. For example, antibodies targeting the immune checkpoint molecule PD1 have been used in combination with the GD2-targeting monoclonal antibody dinutuximab132 or with GD2-CAR T-cells, demonstrating synergistic anti-tumor effect in xenograft model, with, however, modest or partial responses in the clinic for refractory neuroblastoma patients.
In pediatric brain tumor and DIPG in particular, de Billy et al. have identified the small molecules and dual IGF1R/IR inhibitors BMS754807 and linsitinib as enhancers of GD2-CAR T-cells anti-tumor efficacy in preclinical models of DMG-H3K27M mutant. Moreover, they demonstrate that linsitinib exhibited selective toxicity towards DMG H3K27M cells while decreasing the induction of CAR T-cell exhaustion markers. The combination of GD2-CAR T-cells with linsitinib showed a synergistic reduction in DIPG cell invasion in ex vivo organotypic pontine brain slices and a sustained anti-tumor response in vivo. Importantly, this study showed that GD2 is not restricted to DMG-H3K27M mutant but, although heterogeneous, it is also expressed in other pediatric high-grade gliomas, suggesting potential broad applicability of GD2-CAR T cell therapy beyond histone-mutant DMG65.
Interestingly, when primary cell lines were derived from hemispheric high-grade glioma, both H3G34mutant and H3 wild-type/IDH1 wild-type tumors, GD2 expression was lost in culture. This highlights the complex and heterogeneous nature of GD2 expression within pediatric high-grade gliomas, underscoring the importance of comprehensive characterization for tailored therapeutic approaches.
GD2-based treatment-related toxicities
GD2-targeted therapies are associated with a spectrum of toxicities that vary depending on the therapeutic modality, delivery route, and tissue specificity. GD2-CAR T cell therapy, particularly in the context of DMGs, has shown promising efficacy but is challenged by significant toxicities. High-dose intravenous (IV) infusion can trigger dose-limiting cytokine release syndrome (CRS), a systemic inflammatory response that limits therapeutic dosing. In contrast, intracerebroventricular (ICV) delivery offers improved tolerability, even in the presence of elevated local cytokine levels, underscoring the importance of route optimization for safety133,134. Beyond CRS, several types of toxicities associated with CAR T therapies have been described. These include immune effector cell-associated neurotoxicity syndrome (ICANS), prolonged cytopenias, and syndromes like hemophagocytic lymphohistiocytosis.
In the CNS, tumor inflammation-associated neurotoxicity (TIAN) emerges as a distinct neurotoxicity observed during CAR T treatment in patients with brainstem tumors133. Within the TIAN spectrum, two main categories of neurotoxicity are described: Type 1 TIAN is driven by mechanical factors, such as increased intracranial pressure; Type 2 TIAN is primarily due to local neural dysfunction. In this case, local inflammation and clinical symptoms are driven by myeloid-related cytokines such as MCP1/CCL2, suggesting a key role for innate immune activation. These findings point to the delicate balance required to activate anti-tumor immunity in sensitive CNS structures without provoking excessive inflammation121,133.
Treatment options may include corticosteroids (e.g., dexamethasone), anti-edema measures such as mannitol or hypertonic saline, and anti-seizure prophylaxis. In cases of hydrocephalus or significant mass effect, neurosurgical intervention may be required133.
Outside the CNS, toxicity can result from low levels of GD2 expression in normal tissues, leading to on-target off-tumor effects. Peripheral nerves, for instance, express GD2 at low levels, and targeting this antigen can lead to acute neuropathic pain and, in some cases, neuroinflammation. Although monoclonal antibodies are more frequently associated with these effects, CAR T cells can also induce peripheral toxicity, particularly when high doses or potent lymphodepletion regimens are used. Prolonged cytopenias are another concern, especially in heavily pretreated patients, correlating with cumulative immunosuppression and the intensity of conditioning regimens. Intermediate-affinity CARs and incorporation of safety switches like suicide genes have helped mitigate the severity of these non-CNS toxicities, but dosing remains a key determinant of patient safety133,135.
GD2-targeting monoclonal antibodies, such as dinutuximab, are typically combined with other agents like IL-2, GM-CSF, and isotretinoin. While many patients complete the standard six-course regimen, toxicities are frequent and can be severe. Pain, often requiring opioid treatment, is a hallmark toxicity due to the GD2 expression on peripheral nerves. Fever, systemic inflammatory symptoms, and catheter-related infections are also common. These side effects can necessitate dose modifications or early cessation of therapy, highlighting the ongoing need for improved toxicity management strategies in GD2 antibody-based treatments135.
Conclusions
GD2 is a tumor-associated antigen with significant potential for the treatment of solid cancers. In particular, for many pediatric CNS tumors that are known to express GD2, new GD2-based therapeutic strategies are being studied at both preclinical and clinical levels, providing new hopes, especially for untreatable cancers, such as DMG and other aggressive high-grade brain tumors.
The detection of GD2 typically involves methods such as IHC, flow cytometry, or molecular imaging, which capitalize on its consistent overexpression in tumors. Accurate detection is essential for both diagnostic purposes and to guide targeted therapies, especially in pediatric patients, where precision and safety are critical.
Therapeutic strategies targeting GD2 open new opportunities beyond conventional treatments, which have often shown limited therapeutic benefits. Several approaches are being explored for pediatric CNS tumors. The first promising strategy includes mAbs, which not only bind specifically to tumor cells but also recruit immune effector cells, mediating ADCC. These therapies have demonstrated significant clinical efficacy and safety, though not yet in CNS tumors. On the other hand, GD2-CAR T-cell therapies have made significant advances in treating aggressive pediatric CNS tumors by engineering cells that recognize GD2 and exert direct cytotoxic effects on tumor cells58. Next-generation CAR designs will aim to improve persistence, reduce adverse effects, and overcome challenges such as the immunosuppressive TME.
Combining GD2-targeting agents with other tumor-targeting approaches shows promise. These multimodal strategies may include the use of CAR T cells alongside immune checkpoint inhibitors, epigenetic modulators, cytokines, or potentiate immune effectors, tailored to the unique biology of pediatric CNS tumors34,37,65.
Future research on GD2-targeted therapies must address key challenges to ensure they are safe, scalable, and cost-effective for broader clinical applications. The low incidence of CNS malignancies has made enrolling sufficient patients in clinical trials a significant barrier, slowing the progress of these treatments. Future research should aim to improve CAR T-cell therapies by reducing cell exhaustion, enhancing their persistence, and improving safety and efficacy. Similarly, optimizing antibody-based therapies by improving BBB penetration, minimizing off-tumor toxicity, and overcoming antigen heterogeneity is crucial for enhancing their efficacy. Through these innovations and strategies to improve trial feasibility, GD2-directed therapies hold the potential to transform the management of pediatric CNS tumors, offering new hope for improved outcomes in these challenging cases.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- ADCC:
-
Antibody-dependent cell-mediated cytotoxicity
- AT/RT:
-
Atypical Teratoid/Rhabdoid Tumor
- OPBG:
-
Bambino Gesù Children Hospital
- B3GALT4:
-
Beta 1,3-galactosyltransferase IV
- B4GALT6:
-
Beta-1,4 Galactosyltransferase
- B4GALNT1:
-
Beta-1,4 N-acetylgalactosaminyltransferase 1
- BBB:
-
Blood-brain barrier
- CNS:
-
Central nervous system
- CAR:
-
Chimeric Antigen Receptor
- CDC:
-
Complement-dependent cytotoxicity
- CRS:
-
Cytokine Release Syndrome
- DHG:
-
Diffuse hemispheric glioma
- DIPG:
-
Diffuse intrinsic pontine glioma
- DMGs:
-
Diffuse midline gliomas
- ELISA:
-
Enzyme-Linked Immunosorbent Assay
- ER:
-
Endoplasmic reticulum
- EDIM:
-
Epitope Detection in Monocytes
- FC:
-
Flow Cytometry
- GT:
-
Glycotransferases
- ICC:
-
Immunocytochemistry
- ICV:
-
Intracerebroventricular
- IF:
-
Immunofluorescence
- IHC-Fr:
-
Immunohistochemistry on Frozen Sections
- IHC:
-
Immunohistochemistry
- IC9:
-
Inducible caspase 9
- ICANS:
-
Immune Effector Cell-Associated Neurotoxicity Syndrome
- IHG:
-
Infant-type hemispheric glioma
- IV:
-
Intravenous
- MB:
-
Medulloblastoma
- MCP1/CCL2:
-
Monocyte Chemoattractant Protein-1 / C-C Motif Chemokine Ligand 2
- MSCs:
-
Mesenchymal stromal cells
- MICS:
-
MACSima Imaging Cyclic Staining
- MAbs:
-
Monoclonal antibodies
- GalNAc:
-
N-acetylgalactosamine
- NK:
-
Natural killer
- PDOXs:
-
Pediatric brain tumor patient-derived orthotopic xenografts
- PDHGG:
-
Pediatric type diffuse high-grade gliomas
- PLGGs:
-
Pediatric-type low-grade gliomas
- RB:
-
Retinoblastoma
- RB1:
-
Retinoblastoma 1 gene
- SB:
-
Sleeping Beauty
- SHH:
-
Sonic hedgehog
- SOAT:
-
Sialyl O-Acetyl transferases
- ST:
-
Sialytransferases
- ST3GAL5:
-
Beta-galactoside alpha-2,3-sialyltransferase (GM3 synthase)
- ST8SIA1:
-
ST8 Alpha-N-acetyl-neuraminide alpha-2,8-sialyltransferase 1 (GD3 synthase)
- TIAN:
-
Tumor Inflammation-Associated Neurotoxicity
- TLC:
-
Thin-Layer Chromatography
- TME:
-
Tumor microenvironment
- TAA:
-
Tumor-associated antigen
- UCB-MSCs:
-
Umbilical cord blood-derived mesenchymal stromal cells
- WB:
-
Western Blotting
- WNT:
-
Wingless
References
Sandhoff, R. Lipid structure matters in lysosomal storage disease. J. Lipid. Res. 64, 100476 (2023).
Nagata, Y. et al. Expression cloning of β1,4 N-acetylgalactosaminyltransferase cDNAs that determine the expression of GM2 and GD2 gangliosides. J. Biol. Chem. 267, 12082–12089 (1992).
Sjoberg, E. R., Manzi, A. E., Khoo, K. H., Dell, A. & Varki, A. Structural and immunological characterization of O-acetylated GD2: evidence that GD2 is an acceptor for ganglioside O-acetyltransferase in human melanoma cells. J. Biol. Chem. 267, 16200–16211 (1992).
Thomas, P. et al. Targeting pediatric High-Grade Gliomas with O AcGD2-CAR Vδ2 T cells. Preprint at https://doi.org/10.1101/2023.11.17.567375 (2023).
Tibbetts, R. et al. Anti-disialoganglioside antibody internalization by neuroblastoma cells as a mechanism of immunotherapy resistance. Cancer Immunol. Immunother. 71, 153–164 (2022).
Wargalla, U. C. & Reisfeld, R. A. Rate of internalization of an immunotoxin correlates with cytotoxic activity against human tumor cells. Proc. Natl. Acad. Sci. USA. 86, 5146–5150 (1989).
Krengel, U. & Bousquet, P. A. Molecular recognition of gangliosides and their potential for cancer immunotherapies. Front. Immunol. 5, 325 (2014).
Brodeur, G. M. Neuroblastoma: biological insights into a clinical enigma. Nat. Rev. Cancer 3, 203–216 (2003).
Lopez, P. H. H. & Schnaar, R. L. Gangliosides in cell recognition and membrane protein regulation. Curr. Opin. Struct. Biol. 19, 549–557 (2009).
Battula, V. L. et al. Ganglioside GD2 identifies breast cancer stem cells and promotes tumorigenesis. J. Clin. Investig. 122, 2066–2078 (2012).
Yesmin, F. et al. Ganglioside GD2 enhances the malignant phenotypes of melanoma cells by cooperating with integrins. Int. J. Mol. Sci. 23, 423 (2021).
Yesmin, F. et al. Extracellular vesicles released from ganglioside GD2-expressing melanoma cells enhance the malignant properties of GD2-negative melanomas. Sci. Rep. 13, 4987 (2023).
Cavdarli, S., Groux-Degroote, S. & Delannoy, P. Gangliosides: the double-edge sword of neuro-ectodermal derived tumors. Biomolecules 9, 311 (2019).
Yoshida, S. et al. Ganglioside G(D2) in small cell lung cancer cell lines: enhancement of cell proliferation and mediation of apoptosis. Cancer Res. 61, 4244–4252 (2001).
Navid, F., Santana, V. M. & Barfield, R. C. Anti-GD2 antibody therapy for GD2-expressing tumors. Curr. Cancer Drug Targets. 10, 200–9 (2010).
Martinez, C., Hofmann, T. J., Marino, R., Dominici, M. & Horwitz, E. M. Human bone marrow mesenchymal stromal cells express the neural ganglioside GD2: a novel surface marker for the identification of MSCs. Blood 109, 4245–4248 (2007).
Svennerholm, L., Boström, K., Helander, C. G. & Jungbjer, B. Membrane lipids in the aging human brain. J. Neurochem. 56, 2051–2059 (1991).
van den Bijgaart, R. J. E. et al. Combined sialic acid and histone deacetylase (HDAC) inhibitor treatment up-regulates the neuroblastoma antigen GD2. J. Biol. Chem. 294, 4437–4449 (2019).
Svennerholm, L. et al. Human brain gangliosides: developmental changes from early fetal stage to advanced age. Biochim. Biophys. Acta 1005, 109–117 (1989).
Sipione, S., Monyror, J., Galleguillos, D., Steinberg, N. & Kadam, V. Gangliosides in the brain: physiology, pathophysiology and therapeutic applications. Front. Neurosci. 14, 572965 (2020).
Jin, H. J. et al. GD2 expression is closely associated with neuronal differentiation of human umbilical cord blood-derived mesenchymal stem cells. Cell. Mol. Life Sci. 67, 1845–1858 (2010).
Yu, R. K., Tsai, Y.-T., Ariga, T. & Yanagisawa, M. Structures, biosynthesis, and functions of gangliosides–an overview. J. Oleo Sci. 60, 537–544 (2011).
Zhang, S. et al. Selection of tumor antigens as targets for immune attack using immunohistochemistry: I. Focus on gangliosides. Int. J. Cancer 73, 42–49 (1997).
Mujoo, K. et al. Functional properties and effect on growth suppression of human neuroblastoma tumors by isotype switch variants of monoclonal antiganglioside GD2 antibody 14.18. Cancer Res. 49, 2857–2861 (1989).
Berois, N. & Osinaga, E. Glycobiology of neuroblastoma: impact on tumor behavior, prognosis, and therapeutic strategies. Front. Oncol. 4, 114 (2014).
Hossain, D. M. S., Mohanty, S., Ray, P., Das, T. & Sa, G. Tumor gangliosides and T cells: a deadly encounter. Front. Biosci. 4, 502–519 (2012).
Potapenko, M., Shurin, G. V. & de León, J. Gangliosides as immunomodulators. Adv. Exp. Med. Biol. 601, 195–203 (2007).
Portoukalian, J., David, M. J., Gain, P. & Richard, M. Shedding of GD2 ganglioside in patients with retinoblastoma. Int. J. Cancer 53, 948–951 (1993).
Valentino, L. et al. Shed tumor gangliosides and progression of human neuroblastoma. Blood 75, 1564–1567 (1990).
Balis, F. M. et al. The ganglioside G(D2) as a circulating tumor biomarker for neuroblastoma. Pediatr. Blood Cancer 67, e28031 (2019).
Li, R., Gage, D., McKallip, R. & Ladisch, S. Structural characterization and in vivo immunosuppressive activity of neuroblastoma GD2. Glycoconj. J. 13, 385–389 (1996).
Sorokin, M. et al. RNA sequencing-based identification of ganglioside GD2-positive cancer phenotype. Biomedicines 8, 142 (2020).
Kasprowicz, A., Sophie, G.-D., Lagadec, C. & Delannoy, P. Role of GD3 synthase ST8Sia I in cancers. Cancers 14, 1299 (2022).
Mabe, N. W. et al. Transition to a mesenchymal state in neuroblastoma confers resistance to anti-GD2 antibody via reduced expression of ST8SIA1. Nat. Cancer 3, 976–993 (2022).
Sha, Y.-L. et al. B3GALT4 remodels the tumor microenvironment through GD2-mediated lipid raft formation and the c-met/AKT/mTOR/IRF-1 axis in neuroblastoma. J. Exp. Clin. Cancer Res. 41, 314 (2022).
Wiebel, M. et al. Surface expression of the immunotherapeutic target GD2 in osteosarcoma depends on cell confluency. Cancer Rep (Hoboken). 4, e1394 (2022).
Ciccone, R. et al. GD2-targeting CAR T-cell therapy for patients with GD2+ medulloblastoma. Clin. Cancer Res. 30, 2545–2557 (2024).
Pezzella, M. et al. Tumor-derived G-CSF induces an immunosuppressive microenvironment in an osteosarcoma model, reducing response to CAR.GD2 T-cells. J. Hematol. Oncol. 17, 127 (2024).
Kailayangiri, S. et al. Protocol for assessing GD2 on formalin-fixed paraffin-embedded tissue sections using immunofluorescence staining. STAR Protoc. 5, 103199 (2024).
Cheever, M. A. et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin. Cancer Res. 15, 5323–5337 (2009).
Liu, S. J. et al. Paediatric brain tumours in Singapore: a 15-year epidemiological and outcome study. J. Clin. Neurosci. 101, 154–161 (2022).
Subramanian, S. & Ahmad, T. Childhood brain tumors. in StatPearls (StatPearls Publishing, 2024).
Pollack, I. F., Agnihotri, S. & Broniscer, A. Childhood brain tumors: current management, biological insights, and future directions. J. Neurosurg. Pediatrics 23, 261–273 (2019).
Kleihues, P., Burger, P. C. & Scheithauer, B. W. The new WHO classification of brain tumours. Brain Pathol. 3, 255–268 (1993).
Kleihues, P. & Sobin, L. H. World Health Organization classification of tumors. Cancer 88, 2887 (2000).
Louis, D. N. et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 114, 97–109 (2007).
Zülch, K. J. Histological Typing of Tumours of the Central Nervous System (World Health Organization, 1979).
Louis, D. N. et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 131, 803–820 (2016).
Louis, D. N. et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 23, 1231–1251 (2021).
Ostrom, Q. T. et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2015–2019. Neuro Oncol. 24, v1–v95 (2022).
Hoffman, L. M. et al. Clinical, radiologic, pathologic, and molecular characteristics of long-term survivors of diffuse intrinsic pontine glioma (DIPG): a collaborative report from the International and European Society for Pediatric Oncology DIPG registries. J. Clin. Oncol. 36, 1963–1972 (2018).
Chai, R.-C. et al. The molecular characteristics of spinal cord gliomas with or without H3 K27M mutation. Acta Neuropathol. Commun. 8, 40 (2020).
Chai, R.-C. et al. Genomic profiling and prognostic factors of H3 K27M-mutant spinal cord diffuse glioma. Brain Pathol. 33, e13153 (2023).
Mackay, A. et al. Integrated molecular meta-analysis of 1000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell 32, 520–537.e5 (2017).
Szychot, E. et al. European standard clinical practice recommendations for paediatric high-grade gliomas. EJC Paediatr. Oncol. 5, 100210 (2025).
Purow, B. ONC201 and ONC206: metabolically ClipPing the wings of diffuse midline glioma. Neuro Oncol. 24, 1452–1453 (2022).
Monje, M. et al. Phase I trial of panobinostat in children with diffuse intrinsic pontine glioma: a report from the Pediatric Brain Tumor Consortium (PBTC-047). Neuro Oncol. 25, 2262–2272 (2023).
Majzner, R. G. et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 603, 934–941 (2022).
Vitanza, N. A. et al. Intraventricular B7-H3 CAR T cells for diffuse intrinsic pontine glioma: preliminary first-in-human bioactivity and safety. Cancer Discov. 13, 114–131 (2023).
Vitanza, N. A. et al. Intracerebroventricular B7-H3-targeting CAR T cells for diffuse intrinsic pontine glioma: a phase 1 trial. Nat. Med. 31, 861–868 (2025).
Picart, T. et al. Characteristics of diffuse hemispheric gliomas, H3 G34-mutant in adults. Neurooncol. Adv. 3, vdab061 (2021).
Chen, K. Y. et al. Reciprocal H3.3 gene editing identifies K27M and G34R mechanisms in pediatric glioma including NOTCH signaling. Commun. Biol. 3, 363 (2020).
Liu, I. et al. GABAergic neuronal lineage development determines clinically actionable targets in diffuse hemispheric glioma, H3G34-mutant. Cancer Cell 42, 1528–1548.e17 (2024).
Mount, C. W. et al. Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M+ diffuse midline gliomas letter. Nat. Med. 24, 572–579 (2018).
De Billy, E. et al. Dual IGF1R/IR inhibitors in combination with GD2-CAR T-cells display a potent anti-tumor activity in diffuse midline glioma H3K27M-mutant. Neuro Oncol. 24, 1150–1163 (2022).
Haydar, D. et al. Cell-surface antigen profiling of pediatric brain tumors: B7-H3 is consistently expressed and can be targeted via local or systemic CAR T-cell delivery. Neuro Oncol. 23, 999–1011 (2021).
Krishnatry, R. et al. Clinical and treatment factors determining long-term outcomes for adult survivors of childhood low-grade glioma: a population-based study. Cancer 122, 1261–1269 (2016).
Collins, V. P., Jones, D. T. W. & Giannini, C. Pilocytic astrocytoma: pathology, molecular mechanisms and markers. Acta Neuropathol. 129, 775–788 (2015).
Jones, D. T. W. et al. Genomic analysis of pilocytic astrocytomas at 0.97 Mb resolution shows an increasing tendency toward chromosomal copy number change with age. J. Neuropathol. Exp. Neurol. 65, 1049–1058 (2006).
Zhang, J. et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat. Genet. 45, 602–612 (2013).
Qaddoumi, I. et al. Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathol. 131, 833–845 (2016).
Wagener, R. et al. Ganglioside profiles in human gliomas: quantification by microbore high performance liquid chromatography and correlation to histomorphology and grading. Acta Neurochir. 141, 1339–1345 (1999).
Zamfir, A. D. et al. Profiling and sequence analysis of gangliosides in human astrocytoma by high-resolution mass spectrometry. Anal. Bioanal. Chem. 405, 7321–7335 (2013).
Sturm, D. et al. New brain tumor entities emerge from molecular classification of CNS-PNETs. Cell 164, 1060–1072 (2016).
Sursal, T. et al. Molecular stratification of medulloblastoma: clinical outcomes and therapeutic interventions. Anticancer Res. 42, 2225–2239 (2022).
Taylor, M. D. et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 123, 465–472 (2011).
Paret, C. et al. GD2 expression in medulloblastoma and neuroblastoma for personalized immunotherapy: a matter of subtype. Cancers 14, 1–22 (2022).
Ho, B. et al. Molecular subgrouping of atypical teratoid/rhabdoid tumors-a reinvestigation and current consensus. Neuro Oncol. 22, 613–624 (2020).
Alkatan, H. M., Al Marek, F. & Elkhamary, S. Demographics of pediatric orbital lesions: a tertiary eye center experience in Saudi Arabia. J. Epidemiol. Glob. Health 9, 3–10 (2019).
Yun, J., Li, Y., Xu, C.-T. & Pan, B.-R. Epidemiology and Rb1 gene of retinoblastoma. Int. J. Ophthalmol. 4, 103–109 (2011).
Martínez-Sánchez, M., Hernandez-Monge, J., Rangel, M. & Olivares-Illana, V. Retinoblastoma: from discovery to clinical management. FEBS J. 289, 4371–4382 (2021).
Ancona-Lezama, D., Dalvin, L. A. & Shields, C. L. Modern treatment of retinoblastoma: a 2020 review. Indian J. Ophthalmol. 68, 2356–2365 (2020).
Andersch, L. et al. CD171- and GD2-specific CAR-T cells potently target retinoblastoma cells in preclinical in vitro testing. BMC Cancer 19, 895 (2019).
Nazha, B., Inal, C. & Owonikoko, T. K. Disialoganglioside GD2 expression in solid tumors and role as a target for cancer therapy. Front. Oncol. 10, 1–15 (2020).
Stagno, M. J. et al. Epitope detection in monocytes (EDIM) for liquid biopsy including identification of GD2 in childhood neuroblastoma-a pilot study. Br. J. Cancer 127, 1324–1331 (2022).
Morini, M. et al. Detection of plasma circulating GD2 ganglioside in patients with neuroblastoma and age-matched healthy children. Diagnostic and prognostic evaluation. Oncologist 30, oyaf008 (2025).
Galan, A. et al. GD2 and GD3 gangliosides as diagnostic biomarkers for all stages and subtypes of epithelial ovarian cancer. Front. Oncol. 13, 1134763 (2023).
Zhong, E. et al. Expression analysis of GD2 by immunohistochemistry in invasive breast carcinoma: clinical and pathologic correlation. Appl. Immunohistochem. Mol. Morphol. 30, 113–118 (2022).
Petr, T. et al. Histochemical detection of GM1 ganglioside using cholera toxin- B subunit. Evaluation of critical factors optimal for in situ detection with special emphasis to acetone pre-extraction. Eur. J. Histochem. 54, 112–117 (2010).
Piccolo, M. S. L. o, Cheung, N. K. V. & Cheung, I. Y. GD2 synthase: a new molecular marker for detecting neuroblastoma. Cancer 92, 924–931 (2001).
Yamashiro, S. et al. Genetic and enzymatic basis for the differential expression of GM2 and GD2 gangliosides in human cancer cell lines. Cancer Res. 53, 5395–5400 (1993).
Cheung, N. K. et al. Ganglioside GD2 specific monoclonal antibody 3F8: a phase I study in patients with neuroblastoma and malignant melanoma. J. Clin. Oncol. 5, 1430–1440 (1987).
Cheung, N.-K. V. et al. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony-stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission. J. Clin. Oncol. 30, 3264–3270 (2012).
Soman, G. et al. Analytical characterization of ch14.18: a mouse-human chimeric disialoganglioside-specific therapeutic antibody. MAbs 4, 84–100 (2012).
Doronin, I. I. et al. Ganglioside GD2 in reception and transduction of cell death signal in tumor cells. BMC Cancer 14, 295 (2014).
Horta, Z. P., Goldberg, J. L. & Sondel, P. M. Anti-GD2 mAbs and next-generation mAb-based agents for cancer therapy. Immunotherapy 8, 1097–1117 (2016).
Kholodenko, I. V., Kalinovsky, D. V., Doronin Igor, I., Deyev, S. M. & Kholodenko, R. V. Neuroblastoma origin and therapeutic targets for immunotherapy. J. Immunol. Res. 2018, 7394268 (2018).
Fleurence, J. et al. Targeting and killing glioblastoma with monoclonal antibody to O-acetyl GD2 ganglioside. Oncotarget 7, 41172–41185 (2016).
Kramer, K. et al. A phase II study of radioimmunotherapy with intraventricular (131) I-3F8 for medulloblastoma. Pediatr. Blood Cancer 65, (2017).
Mirian, M. et al. Generation of HBsAg DNA aptamer using modified cell-based SELEX strategy. Mol. Biol. Rep. 48, 139–146 (2021).
Rangel, A. E., Hariri, A. A., Eisenstein, M. & Soh, H. T. Engineering aptamer switches for multifunctional stimulus-responsive nanosystems. Adv. Mater. 32, e2003704 (2020).
Sabbih, G. O. & Danquah, M. K. Neuroblastoma GD2 expression and computational analysis of aptamer-based bioaffinity targeting. Int. J. Mol. Sci. 22, 9101 (2021).
Zhang, L. et al. A novel pH-sensitive multifunctional DNA nanomedicine: an enhanced and harmless GD2 aptamer-mediated strategy for guiding neuroblastoma antitumor therapy. Int. J. Nanomed. 16, 3217–3240 (2021).
Zhang, L. et al. A GD2-aptamer-mediated, self-assembling nanomedicine for targeted multiple treatments in neuroblastoma theranostics. Mol. Ther. Nucleic Acids 26, 732–748 (2021).
Kushner, B. H. et al. Phase I trial of a bivalent gangliosides vaccine in combination with β-glucan for high-risk neuroblastoma in second or later remission. Clin. Cancer Res. 20, 1375–1382 (2014).
Cheung, I. Y. et al. Survival impact of anti-GD2 antibody response in a phase II ganglioside vaccine trial among patients with high-risk neuroblastoma with prior disease progression. J. Clin. Oncol. 39, 215–226 (2020).
Kieber-Emmons, T. et al. The promise of the anti-idiotype concept. Front. Oncol. 2, 196 (2012).
Klingel, L. et al. Immune response and outcome of high-risk neuroblastoma patients immunized with anti-idiotypic antibody ganglidiomab: results from compassionate-use treatments. Cancers 14, 5802 (2022).
Lode, H. N. et al. Vaccination with anti-idiotype antibody ganglidiomab mediates a GD(2)-specific anti-neuroblastoma immune response. Cancer Immunol. Immunother. 62, 999–1010 (2013).
Eger, C. et al. Generation and characterization of a human/mouse chimeric GD2-mimicking anti-idiotype antibody ganglidiximab for active immunotherapy against neuroblastoma. PLoS ONE 11, e0150479 (2016).
Kebriaei, P. et al. Phase I trials using Sleeping Beauty to generate CD19-specific CAR T cells. J. Clin. Investig. 126, 3363–3376 (2016).
Hong, M., Clubb, J. D. & Chen, Y. Y. Engineering CAR-T cells for next-generation cancer therapy. Cancer Cell 38, 473–488 (2020).
Ong, M. Z. et al. FDA-approved CAR T-cell therapy: a decade of progress and challenges. Curr. Pharm. Biotechnol. 25, 1377–1393 (2024).
Anderson, J., Barone, G. & Zehner, A. GD2 targeting CAR T cells for neuroblastoma. EJC Paediatr. Oncol. 4, (2024).
Louis, C. U. et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 118, 6050–6056 (2011).
Che-Hsing, L. et al. Eighteen-year survival after GD2-directed chimeric antigen-modified immune effector cell treatment for neuroblastoma. Res. Sq 3, rs-4232549 (2024).
Park, J. R. et al. Revisions to the international neuroblastoma response criteria: a consensus statement from the National Cancer Institute clinical trials planning meeting. J. Clin. Oncol. 35, 2580–2587 (2017).
Quintarelli, C. et al. Choice of costimulatory domains and of cytokines determines CAR T-cell activity in neuroblastoma. Oncoimmunology 7, e1433518 (2018).
Del Bufalo, F. et al. GD2-CART01 for relapsed or refractory high-risk neuroblastoma. N. Engl. J. Med. 388, 1284–1295 (2023).
Quintarelli, C. et al. Donor-derived GD2-specific CAR T cells in relapsed or refractory neuroblastoma. Nat. Med. 31, 849–860 (2025).
Monje, M. et al. Intravenous and intracranial GD2-CAR T cells for H3K27M+ diffuse midline gliomas. Nature https://doi.org/10.1038/s41586-024-08171-9 (2024).
Lin, H., Yang, X., Ye, S., Huang, L. & Mu, W. Antigen escape in CAR-T cell therapy: mechanisms and overcoming strategies. Biomed. Pharmacother. 178, 117252 (2024).
Ye, J. N. & Cheung, N.-K. V. A novel O-acetylated ganglioside detected by anti-GD2 monoclonal antibodies. Int. J. Cancer 50, 197–201 (1992).
Alvarez-Rueda, N., Desselle, A., Cochonneau, D., Chaumette, T. & Clemenceau, B. A Monoclonal Antibody to O-Acetyl-GD2 Ganglioside and Not to GD2 Shows Potent Anti-Tumor Activity without Peripheral Nervous System Cross-Reactivity. PLoS ONE 6, e25220 (2011).
Levine, A. B. et al. Immuno-oncologic profiling of pediatric brain tumors reveals major clinical significance of the tumor immune microenvironment. Nat. Commun. 15, 5790 (2024).
Yu, A. L. et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 363, 1324–1334 (2010).
Yu, A. L. et al. Long-term follow-up of a phase III study of ch14.18 (Dinutuximab) + cytokine immunotherapy in children with high-risk neuroblastoma: COG study ANBL0032. Clin. Cancer Res. 27, 2179–2189 (2021).
Cheung, N.-K. V., Guo, H., Hu, J., Tassev, D. V. & Cheung, I. Y. Humanizing murine IgG3 anti-GD2 antibody m3F8 substantially improves antibody-dependent cell-mediated cytotoxicity while retaining targeting in vivo. Oncoimmunology 1, 477–486 (2012).
Italiano, A. et al. Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study. Lancet Oncol. 19, 649–659 (2018).
Pilgrim, A. A. et al. The yes-associated protein (YAP) is associated with resistance to anti-GD2 immunotherapy in neuroblastoma through downregulation of ST8SIA1. Oncoimmunology 12, 2240678 (2023).
Rouaen, J. R. C. et al. Copper chelation redirects neutrophil function to enhance anti-GD2 antibody therapy in neuroblastoma. Nat. Commun. 15, 10462 (2024).
Ehlert, K. et al. Nivolumab and dinutuximab beta in two patients with refractory neuroblastoma. J. Immunother Cancer 8, e000540 (2020).
Mahdi, J. et al. Tumor inflammation-associated neurotoxicity. Nat. Med. 29, 803–810 (2023).
Monje, M. et al. Intravenous and intracranial GD2-CAR T cells for H3K27M+ diffuse midline gliomas. Nature 637, 708–715 (2025).
Rankin, A. W., Duncan, B. B., Allen, C., Silbert, S. K. & Shah, N. N. Evolving strategies for addressing CAR T-cell toxicities. Cancer Metastasis Rev. 44, 17 (2025).
Chang, F., Li, R., Noon, K., Gage, D. & Ladisch, S. Human Medulloblastoma Gangliosides. vol. 7 https://academic.oup.com/glycob/article/7/4/523/653473 (1997).
Orsi, G. et al. GD2 expression in breast cancer. Oncotarget 8, 31592–31600 (2017).
Aygün, Z. et al. Frequency of ALK and GD2 expression in neuroblastoma. Fetal Pediatr. Pathol. 38, 326–334 (2019).
Palacios, M. et al. Antitumor activity and carrier properties of novel hemocyanins coupled to a mimotope of GD2 ganglioside. Eur. J. Med. Chem. 150, 74–86 (2018).
Voeller, J. & Sondel, P. M. Advances in Anti-GD2 immunotherapy for treatment of high-risk neuroblastoma. J. Pediatr. Hematol. Oncol. 41, 163–169 (2019).
Acknowledgements
This work was funded by AIRC IG (27265) to MV. We also acknowledge the Italian Ministry of Health for the “Current Research funds”. Figures were created with Biorender (https://www.biorender.com/).
Author information
Authors and Affiliations
Contributions
AC.F.R. and M.V. conceived the manuscript; A.C.F.R. wrote the first draft of the manuscript; A.C.F.R., E.D.B., and M.V. wrote and reviewed and edited the manuscript; F.L. and A.M. provided intellectual input and critically reviewed the manuscript; G.D.B., B.D.A., S.R., F.D.B., C.Q., F.L., and A.M. reviewed and edited the manuscript. MV provided funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fernández-Rilo, A.C., de Billy, E., Del Baldo, G. et al. GD2: hopes and challenges for the treatment of pediatric patients with tumors of the central nervous system. npj Precis. Onc. 9, 295 (2025). https://doi.org/10.1038/s41698-025-01079-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41698-025-01079-1