Abstract
Human epidermal growth factor receptor 2 (HER2) overexpression and gene amplification are established biomarkers in breast and gastric cancers. However, their role in cervical squamous cell carcinoma (CSCC) remains unclear. We evaluated 548 untreated primary CSCC lesions (313 biopsies and 235 hysterectomies). Initial immunohistochemistry (IHC) results were: 0 (56.9%), 1+ (26.3%), 2+ (equivocal, 14.1%), and 3+ (positive, 2.7%). Fluorescence in situ hybridization confirmed HER2 gene amplification in all 3+ cases and 5.2% of 2+ cases. Based on reclassification, HER2 status was categorized as HER2-null (24.4%), HER2-ultralow (32.5%), HER2-low (39.6%), and HER2-positive (3.5%). HER2 discordance rates were observed in 48.3% of paired biopsies and hysterectomy specimens, 52.2% of post-neoadjuvant residual lesions, and 50.8% of metastatic sites. Temporal analysis showed conversion rates of 50.0% for synchronous metastases, 30.8% within 12 months, and 63.6% beyond 12 months. We identified a high prevalence of HER2-low and HER2-ultralow subtypes, suggesting potential eligibility for ADC therapies.
Similar content being viewed by others
Introduction
Cervical squamous cell carcinoma (CSCC) remains a leading cause of cancer-related morbidity and mortality among women globally, particularly in regions with limited access to screening and early intervention1. Despite preventive strategies, such as human papillomavirus (HPV) vaccination and cytological screening, the prognosis for patients with advanced or recurrent CSCC remains poor owing to the limited efficacy of current treatments. Existing therapeutic options—including surgery, chemotherapy, and radiation therapy—often fail to effectively treat metastatic or recurrent disease, highlighting the need for novel, targeted therapies1.
Human epidermal growth factor receptor 2 (HER2), a member of the ErbB family of receptor tyrosine kinases, plays a critical role in regulating cell proliferation, survival, and differentiation2,3,4. The approval of HER2-targeted monoclonal antibodies, such as trastuzumab and pertuzumab, marked a significant advancement in treating HER2-positive breast cancer, thereby improving patient outcomes and establishing a foundation for targeted therapy. Encouraged by these successes, HER2-targeted monoclonal antibodies have demonstrated promising efficacy in other solid tumors, including gastric, urothelial, and endometrial cancers5,6. This clinical success has sparked interest in investigating HER2 as a therapeutic target in CSCC.
Antibody–drug conjugates (ADCs), combining HER2-targeting antibodies with potent cytotoxic payloads, have revitalized the therapeutic targeting of HER2 in cancers exhibiting low-level expression7. This resurgence is largely driven by the marked efficacy demonstrated by ADCs, particularly trastuzumab deruxtecan (T-DXd), in HER2-low tumors. Consequently, HER2-low status has been redefined as a predictive biomarker for ADC response in breast cancer8. The pivotal DESTINY-Breast06 trial (N = 866)9 further extends this paradigm by demonstrating significant clinical benefits of T-DXd not only in the HER2-low metastatic breast cancer cohort (n = 713) but also in patients with HER2-ultralow disease (n = 153). HER2-ultralow was methodologically defined within this trial as immunohistochemistry (IHC) 0 with membrane staining (defined here as IHC > 0 and <1+, characterized by faint, incomplete membrane staining in ≤10% of tumor cells).
Recent data from the 2024 European Society of Gynecological Oncology (ESGO) RC48-C018 trial (NCT04965519) reported an overall response rate (ORR) of 36.4% in 22 patients with cervical cancer treated with disitamab vedotin, along with a median duration of response of 5.52 months, median progression-free survival (PFS) of 4.37 months, and a 12-month overall survival rate of 66%10. These findings suggest that low HER2 expression may represent a viable target for ADC therapy in CSCC, potentially offering a new therapeutic avenue for this challenging malignancy.
Although HER2 overexpression and gene amplification are well-validated biomarkers in breast and gastric carcinomas, their biological and clinical significance in CSCC remains poorly understood. In this dual-focus study, we systematically evaluated HER2 expression in 548 CSCC cases using the 2023 American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines11 and assessed spatiotemporal heterogeneity through a multidimensional analysis of histopathological specimen types (biopsies vs. hysterectomy specimens), treatment phases (pre-treatment biopsies vs. post-neoadjuvant therapy [NAT] residual lesions), and disease progression stages (primary cervical lesions vs. matched metastatic foci). Furthermore, a comparative analysis of HER2 expression between breast cancer11 and the 2017 CAP/ASCP/ASCO criteria for gastric cancer was performed12.
Results
HER2 expression, gene amplification, and reassessment in 548 patients with cervical cancer
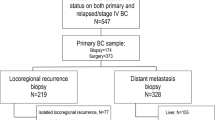
The study cohort comprised 694 specimens from 548 patients, including the core analysis set of 548 primary cervical cancer lesions without neoadjuvant therapy (NAT), along with 60 hysterectomy specimens matched to pretreatment biopsies, 23 hysterectomy specimens post-NAT, and 63 metastatic lesions (Fig. S1). Two pathologists (W.L. and D.H.) independently assessed HER2 expression for all cases. Initial scoring yielded concordant results in 625 cases (90.1%), with discordance observed in 69 cases (9.9%). These discordant cases underwent joint review using a multi-headed microscope, achieving consensus for 46 cases. The remaining 23 cases without consensus were adjudicated by a third senior pathologist (Y.C.), with the majority diagnosis adopted as the final result.
The core analysis set comprised 548 primary cancer lesions without NAT, including 313 cervical biopsies and 235 hysterectomies. The median age at diagnosis was 57 (range: 27–87) years. Initial immunohistochemical evaluation revealed HER2 scores of 0 in 312 cases (56.9%), 1+ in 144 (26.3%), equivocal (2+) in 77 (14.1%), and positive (3+) in 15 (2.7%) (Fig. 1A). FISH analysis was successfully performed in all 77 tumors scored as 2+, revealing gene amplification in four cases (5.2%). All 15 tumors with a score of 3+ showed gene amplification by FISH (Fig. 1B).
A HER2 testing process and results: HER2-null accounted for 24.4%, HER2-ultralow for 32.5%, HER2-low for 39.6%, and HER2-positive for 3.5%. B HER2 2+ cases showed positive FISH testing. C HER2 immunohistochemistry: HER2-null, HER2-ultralow, HER2 1+, HER2 2+, and HER2 3+. D HER2 expression was significantly higher in poorly differentiated cervical squamous cell carcinoma (CSCC) compared to well-to-moderately differentiated cases (p = 0.02).
Re-evaluation of HER2 status yielded the following results: after reassessment of 312 HER2-0 cases, HER2-ultralow accounted for 32.5% (178/548), whereas HER2-null accounted for 24.4% (134/548). In total, 217 cases (39.6%) were HER2-low (144 cases with a score of 1+ and 73 cases with a score of 2+ but lacking gene amplification); and 19 cases (3.5%) were HER2-positive (15 with a score of 3+ and 4 with a score of 2+ along with gene amplification) (Fig. 1C).
HER2 expression was significantly higher in poorly differentiated CSCC than in well-differentiated SCC (p = 0.02). No significant associations were observed between HER2 expression and clinicopathological parameters, including patient age, International Federation of Gynecology and Obstetrics (FIGO) stage, and HPV subtype (Fig. 1D and Table S1).
Intratumoral heterogeneity of HER2 expression
Tumor cells with varying staining intensities often coexisted in intermingled patterns within the same tumor, reflecting diverse distribution profiles. Four distinct HER2 expression patterns were identified: (1) diffuse homogeneous pattern‒‒intense, uniform HER2 membranous staining across the entire tumor cell population; (2) geographic clustering pattern—spatially demarcated clusters of cells showing varied HER2 staining intensities (e.g., 0/1+/2+/3+) resembling topographic maps; (3) mosaic pattern—randomly intermixed HER2-positive and HER2-negative/heterogeneous cells without spatial organization, occurring in variable proportions; and (4) scattered pattern—presence of isolated HER2-positive cells within regions of HER2-0 or HER2-low (IHC 1+/2+) expression (Fig. 2). Specifically, the diffuse homogeneous pattern and geographic clustering pattern were predominant in HER2 3+ cases, whereas in, the mosaic and scattered patterns are frequently observed in HER2 2+ cases.
A Homogeneous pattern: HER2-3+ case with nearly 100% tumor cells exhibiting diffuse strong positivity. B Clustering pattern: clear boundary between HER2-3+ and HER2-0 regions. C Mosaic pattern: clusters of HER2-3+ tumor cells in a HER2-2+ background. D Mosaic pattern: clusters of HER2-2+ tumor cells in a HER2-0 background. E Scattered pattern: isolated HER2-3+ tumor cells in a HER2-2+ background. F Scattered pattern: isolated HER2-3+ tumor cells in a HER2-0 background.
Additionally, the proportion of stained tumor cells varied among cases. Among the 15 cases with HER2 3+ overexpression, the tumor area demonstrating 3+ staining intensity (strong staining) ranged from 30%–100% (median: 75%). In HER2 2+ cases, 2+ staining intensity (moderate staining) occupied 10%–85% of the tumor area (median: 25%), whereas 1+ cases showed 1+ staining intensity (weak staining) in 10%–55% of tumor regions (median: 20%) (Fig. 3).
In HER2-3+ cases, it is observed that HER3+ coexists with HER-2+ and HER2-1+ staining intensities (left). Stained tumor cell proportions varied: HER2 3+ cases showed 30%–100% (median 75%) tumor area with 3+ intensity; 2+ cases covered 10%–85% (median 25%) with 2+ intensity; 1+ cases had 10%–55% (median 20%) 1+ intensity (right).
In one HER2 2+ case, intense complete staining was observed in <10% of the tumor cells, whereas the remaining cells were HER2-negative. FISH analysis revealed clustered HER2 gene amplification (Fig. 4).
HER2 alterations in paired cervical biopsy and subsequent hysterectomy specimens without prior NAT
Within this cohort of 60 patients, concordant HER2 status between cervical biopsy and total hysterectomy specimens was observed in 31 cases (51.7%), while the discordance occurred in 29 cases (48.3%). Specific status changes included: among 11 cases originally HER2-null, three transitioned to HER2-ultralow and four to HER2-low; of 16 cases initially HER2-ultralow, one reverted to HER2-null and nine progressed to HER-low; among 30 HER2-low cases, four became HER2-null, six converted to HER2-ultralow, and one advanced to HER2-positive; finally, among three HER2-positive cases, one downgraded to HER2-ultralow whereas the other two remained unchanged (Fig. 5 and Table S2).
HER2 evolution from baseline biopsy to residual disease after NAT
Among 23 patients who underwent NAT, matched cervical biopsies and residual tumors were compared. No pathological complete response was observed. The concordant HER2 status between cervical biopsy and residual tumors was observed in 11 cases (47.8%). The overall discordance rate was 52.2% (12/23). Specifically, among seven patients with HER2-null at baseline biopsy, one converted to HER2-ultralow and four to HER2-low. Of the six patients with HER2-ultralow at baseline, two converted to HER2-null and two to HER2-low. Additionally, among patients with HER2-low at baseline, two converted to HER2-null and one to HER2-ultralow. Remarkable HER2 status instabilities were observed between primary and residual tumors (Fig. 6 and Table S3).
Changes in HER2 expression between primary cervical lesions and metastatic lesions
During the study period, 63 matched pairs of primary and metastatic CSCC with available HER2 testing were analyzed. In primary tumors, HER2-null, HER2-ultralow, HER2-low, and HER2-positive phenotypes accounted for 19.0% (n = 12), 25.4% (n = 16), 50.8% (n = 32), and 4.8% (n = 3), respectively. In metastatic lesions, the corresponding distributions were 27.0% (n = 17), 27.0% (n = 17), 41.2% (n = 26), and 4.8% (n = 3). The concordant HER2 status between primary cervical lesions and metastatic lesions was observed in 31 cases (49.2%). Discordant HER2 status occurred in 32 cases (50.8%), with the following specific changes compared to the primary lesions: among 12 cases originally HER2-null, nine cases showed upregulation in metastases (two to HER2-ultralow, seven to HER2-low). Of 16 cases initially HER2-ultralow, eight reverted to HER2-null, and one progressed to HER2-low. Among 32 HER2-low cases, 14 exhibited downgrading (six to HER2-null, eight to HER2-ultralow). All three HER2-positive cases remained unchanged. This discordance reached statistical significance (p = 0.010) (Fig. 7 and Table S4).
The most frequent metastatic sites were cervical lymph nodes (25/63, 39.7%) and lungs (15/63, 23.8%). Other metastatic locations, in descending frequency, included the inguinal lymph nodes (9/63, 14.3%), pelvic lymph nodes (7/63, 11.1%), and liver (4/63, 6.3%). Additionally, isolated cases of metastasis to the bladder, colon, and brain were observed. HER2 discordance rates varied significantly across metastatic sites: lung demonstrated the highest rate (66.7%, 10/15), followed by pelvic lymph nodes (57.1%, 4/7), inguinal lymph nodes (55.6%, 5/9), cervical lymph nodes (40.0%, 10/25), and liver (25.0%, 1/4).
The interval between CSCC diagnosis and metastatic lesion detection ranged from 0 to 60 months. Among the 63 analyzed cases, 28 (44.5%) presented with synchronous metastasis at the time of primary diagnosis, with an HER2 conversion rate of 50% (14/28). Metastases developed within 12 months of primary diagnosis in 13 cases (20.6%), with an HER2 conversion rate of 30.8% (4/13). In 22 cases (34.9%) with delayed metastases (>12 months), the HER2 conversion rate was highest at 63.6% (14/22) (Fig. 7). These findings highlight significant HER2 status instability throughout disease progression, with the most significant instability observed in the delayed metastasis cohorts. HER2 phenotypic shifts in metastatic lesions may reveal actionable ADC targets in clinically relevant patient subsets.
Comparative analysis of HER2 expression between breast cancer and gastric cancer scoring criteria
A comparative analysis of HER2 expression patterns between breast and gastric cancer scoring criteria revealed the following: utilizing the 2017 CAP/ASCP/ASCO guidelines for gastric cancer evaluation, 6 of 144 initially categorized 1+ cases (4.2%) demonstrated weak-to-moderate incomplete membrane staining (>10% tumor cells with basal/lateral “U-shaped” pattern), warranting upgrade to 2+ status. Notably, FISH testing confirmed the absence of HER2 amplification in these reclassified cases. The original scores for the 77 cases rated as 2+ and 15 cases rated as 3+ remained unchanged. The final HER2 scoring was as follows: 0 in 312 (56.9%), 1+ in 138 (25.2%), equivocal (2+) in 83 (15.2%), and 3+ in 15 (2.7%). Upon reassessment, the HER2 status distribution revealed 312 tumors (56.9%) as HER2-0, 217 cases (39.6%) as HER2-low (including 138 with 1+ score and 79 with 2+ score without gene amplification), and 19 cases (3.5%) as HER2-positive (15 with 3+ score and 4 with 2+ score accompanied by gene amplification), indicating no major discrepancy compared to the original breast cancer-based scoring system.
Discussion
The development of ADCs represents a paradigm shift in cancer therapeutics, driving groundbreaking advancements in recent years13. Clinical evidence has demonstrated that HER2-targeted ADCs have expanded therapeutic indications across various HER2-positive malignancies. Notably, beyond traditional HER2-positive populations, specific ADC agents have shown clinically meaningful antitumor efficacy in HER2-low-expressing tumors (defined as HER2 1+ or 2+ without gene amplification)7. At the 2023 ASCO Annual Meeting, the DESTINY-PanTumor0214 trial demonstrated that T-DXd treatment yielded an encouraging ORR and durable clinical benefit across multiple tumor types. Its efficacy was particularly notable in patients with HER2 3+ expression, among whom those with gynecological malignancies exhibited the most significant benefit. The ORRs for endometrial, cervical, and ovarian cancers were 57.5%, 50%, and 45%, respectively. Additionally, the phase Ⅱ PRaG3.0 (NCT05115500) clinical study15,16 showed that single-agent disitamab vedotin achieved an ORR of 66.7% in gynecological tumors, including cervical cancer, epithelial ovarian cancer, fallopian tube cancer, and primary peritoneal cancer. Importantly, patients with HER2 1+ expression demonstrated response rates comparable to those with 2+ and 3+, with ORRs of 43.8% and 30.0%, respectively. The safety profile was favorable, and no significant pulmonary or ocular adverse events were observed. In the present study, the proportion of HER2 low cases was 39.6%. These findings suggest that this patient subgroup may benefit from ADC therapy, offering a novel therapeutic avenue with the potential to improve outcomes in gynecological malignancies. Consequently, HER2 testing and accurate assessment of protein expression have emerged as critical diagnostic imperatives. In our cohort of 548 treatment-naïve CSCC specimens, HER2 immunostaining revealed 3+ expression in 2.7%, 2+ in 14.1%, 1+ in 26.3%, and HER2-0 in 56.9% of patients. These results align with the meta-analysis by Itkin et al.17, which reported an HER2 3+ rate of 5.7% (95% CI: 1.5‒11.7%) in cervical cancer, although our rate (2.7%) falls at the lower boundary of this interval. Gene amplification occurred in 5.2% (4/77) of 2+ cases. Following reclassification, the HER2 expression categories were as follows: HER2-null (0 with no membrane staining; 24.4%), ultralow (0 with faint membrane staining; 32.5%), low (1+/non-amplified 2+; 39.6%), and positive (3+/amplified 2+; 3.5%) staining. Notably, HER2-low/ultralow phenotypes collectively comprised 72.1% of cases, suggesting the potential applicability of ADCs in most patients with CSCC.
In the HER2-low cohort of DESTINY-Breast06 trial9, T-DXd significantly prolonged median PFS compared to the physician’s choice of chemotherapy (13.2 vs. 8.1 months; HR 0.62, 95% CI 0.51–0.74; p < 0.0001), with consistent results observed in the intention-to-treat population, which included patients with both low and ultra-low HER2 expression (modified PFS [mPFS] 13.2 vs. 8.1 months; HR 0.63, 95% CI 0.53–0.75; p < 0.0001). While the HER2-ultralow subgroup did not reach statistical significance (mPFS 13.2 vs. 8.3 months; HR 0.78, 95% CI 0.50–1.21), the clinically meaningful improvement prompted FDA approval in January 2025 for HR+/HER2-low/ultralow metastatic breast cancer following progression on endocrine therapy. The central laboratory reassessment revealed 40% of local IHC 0 cases upgraded to HER2-ultralow and 24% to HER2-low9. Our cohort further demonstrated a 57.1% (178/312) diagnostic reclassification from HER2-0 to HER2-ultralow, underscoring the critical need for standardized HER2 quantification to optimize ADC-targeted therapy selection. These findings redefine therapeutic paradigms by validating continuous HER2 expression models over traditional binary classifications, highlighting the clinical implications of intratumoral heterogeneity and assay standardization challenges.
Our study further identified pronounced intratumoral heterogeneity of HER2 protein expression in CSCC tissues. Tumor cells with variable staining intensities coexisted within individual tumors, displaying four characteristic spatial distribution patterns: (1) diffuse homogeneous, (2) geographic clustering, (3) mosaic heterogeneity, and (4) scattered isolated patterns. These morphological patterns align with classical descriptions of HER2 heterogeneity in breast cancer, as reported by Marchiò et al.18. Furthermore, the proportion of HER2-positive tumor cells varied markedly across IHC categories. Among 15 HER2 3+ cases, strongly positive tumor areas (3+ intensity) ranged from 30% to 100% (median, 75%). HER2 2+ specimens showed moderately positive regions (2+ intensity) occupying 10%–85% (median 25%), in contrast with HER2 1+ cases, where weakly positive areas (1+ intensity) spanned 10%–55% (median 20%). Given the potential impact of this intratumoral heterogeneity on anti-HER2 therapy response, we propose reporting the percentage of HER2-positive tumor cells in pathological assessments. This quantitative parameter may serve as a critical reference for personalizing treatment strategies and predicting clinical outcomes.
The observed discordance in HER2 status between paired cervical biopsy and hysterectomy specimens (48.3%%, 29/60) highlights the critical challenges in HER2 assessment for cervical cancer. While 51.7% of cases (31/60) maintained stable HER2 profiles, most discordant cases (27/29 cases, 93.1%) involved shifts between HER2-null, HER2-ultralow, and HER2-low categories. This trend aligns with emerging evidence from other solid tumors, where spatial and temporal HER2 heterogeneity can result in diagnostic variability, particularly in low-expressing tumors. Interestingly, bidirectional shifts were common—13 cases were upgraded from HER2-null and ultralow to HER2-low, while 10 cases were downgraded from HER2-low to HER2-null and ultralow, suggesting that single-site biopsy sampling may not fully capture the full spectrum of HER2 expression in heterogeneous tumors. The two cases with more dramatic shifts (from 3+ to ultralow and HER2-low to 3+) may reflect significant intratumoral heterogeneity.
This study compared HER2 protein expression in 23 patients before and after NAT, revealing an overall HER2 discordance rate of 52.2% (12/23), exclusively involving transitions between HER2-null, ultralow, and low status. These findings align with Katayama et al.’s report19 of a 22% conversion from HER2-positive to HER2-negative status following treatment in breast cancer and with a separate retrospective analysis showing 13% HER2-negativity in residual tumors, which was associated with poorer prognosis20. The dynamic instability of HER2-low status between primary and residual tumors may result from (1) sampling limitations in core needle biopsies combined with treatment-induced HER2 modulation, (2) selective suppression of HER2-positive clones in multifocal tumors, and (3) the survival of treatment-resistant subclones with distinct biological profiles, driven by tumor heterogeneity and differential therapy responses. These mechanisms underscore the critical need for HER2 re-evaluation after NAT in the era of anti-HER2 ADC therapies.
Chen et al.21 analyzed 390 paired primary and metastatic breast cancer samples and found that HER2 status conversion from positive to negative (29%) occurred more frequently than conversion from negative to positive (8%), with a 14% discordance rate among distant metastases. Notably, one patient exhibited intralesional heterogeneity: a HER2-positive primary tumor metastasized to the liver, producing one HER2-negative and one HER2-positive lesion. This case suggests that HER2 discordance can occur even within metastases to the same organ. Similarly, Miglietta et al.22 reported an overall HER2 discordance rate of 38%, with bidirectional shifts between HER2 0 and 1+ occurring at comparable frequencies (47% vs. 40%). HER2-positive (3+) tumors demonstrated high stability in both primary and recurrent settings, consistent with our observation that all three HER2 3+ cases showed no status change. In our study, heterogeneous HER2 status was observed in 32 cases (50.8%). The metastatic sites also exhibited notable conversion, particularly in the lung demonstrated the highest rate (66.7%, 10/15), followed by pelvic lymph nodes (57.1%, 4/7), and inguinal lymph nodes (55.6%, 5/9). A temporal analysis revealed a marked disparity in HER2 conversion between metastases emerging >12 months after diagnosis (63.6%) and those developing within 12 months (20.6%). The dynamic nature of HER2 expression, especially between HER2-null, HER2-ultralow, and HER2-low subtypes during tumor progression, underscores the necessity of HER2 status re-evaluation in recurrent/metastatic CSCC cases.
Standardization of HER2 assessment in CSCC remains controversial, with no consensus on whether breast or gastric cancer criteria should be adopted. While both guidelines employ a 10% staining threshold, they differ in morphological interpretation: breast cancer typically shows nested cell arrangements with complete circumferential HER2 membrane staining, whereas gastric cancer displays glandular structures with partial “U-shaped” basal and lateral membrane staining and weak or absent staining of apical regions. In this study, the application of the 2017 CAP/ASCP/ASCO gastric cancer HER2 guideline led to the reclassification of 6 out of 144 cases, initially 1+ cases, to 2+, based on >10% of tumor cells showing weak-to-moderate U-shaped staining. However, all six cases tested negative on FISH. Notably, applying gastric cancer criteria demonstrated no significant discrepancy compared to the original breast cancer HER2 scoring criteria. One unique case in our study involved <10% of tumor cells with strong HER2 positivity—classified as 2+ with clustered FISH positivity under breast criteria but unclassifiable under gastric standards‒highlighting interpretive ambiguities. Overall, HER2 expression in CSCC more closely resembled the breast cancer pattern: 93.9% of 2+ and 3+ cases exhibited complete membrane staining, while U-shaped patterns were seen in only 6.1% of cases (all FISH-negative). This finding supports the potential suitability of breast cancer criteria for CSCC, pending further validation. Conversely, other gynecological malignancies (e.g., cervical adenocarcinoma, endometrial/ovarian cancers) with gastric-like glandular morphology may exhibit higher U-shaped staining frequency, suggesting gastric criteria as a more appropriate choice, subject to confirmatory studies.
Although our study benefited from a large sample size and comprehensive HER2 expression analysis, it had some limitations. First, this study is a single-center investigation, and the retrospective design posed inherent risks of selection bias and incomplete clinical data. Second, inter- and intra-observer variability in tumors characterized by a low proportion of tumor cells exhibiting weak HER2 expression may also contribute to discrepancies in distinguishing between HER2-null, HER2-ultralow, and low categories; future integration of artificial intelligence may help enhance scoring precision, particularly for these low-expression cases, thereby reducing observer-related variability. Third, the lack of prospective treatment response data limited our ability to correlate HER2 expression patterns with clinical outcomes, warranting future studies to correlate HER2 status with ADC treatment outcomes.
In conclusion, this study identified a clinically significant prevalence of HER2-low and HER2-ultralow subtypes in CSCC, encompassing most cases potentially eligible for ADC therapy. HER2 expression demonstrated considerable spatiotemporal heterogeneity during tumor progression, with substantial discordance between primary and metastatic lesions and dynamic shifts influenced by treatment and time. These findings underscore the need for repeated HER2 reassessment through sequential biopsies to optimize therapeutic stratification, especially for emerging therapies targeting low HER2-expressing tumors. Applying gastric cancer criteria showed no significant discrepancy compared to breast scoring criteria, further supporting the potential utility of breast cancer HER2 guidelines in CSCC.
Methods
The study protocol was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Fujian Cancer Hospital (Approval No. K2024-051-01; Approval Date: January 23, 2024). All patients provided written informed consent for the use and publication of their medical data at their first hospital visit.
Study population and design
In this retrospective cohort study, we included 548 consecutive patients diagnosed with CSCC who underwent initial surgical hysterectomy or cervical biopsy at Fujian Cancer Hospital between 2020 and 2024. None of the patients received NAT—including chemotherapy, radiotherapy, targeted therapy, or immunotherapy—prior to specimen collection. Clinical information, including age, clinical progression, laboratory test results, imaging findings, and treatment details, was retrieved from electronic health records. Follow-up data were obtained from medical records and phone interviews.
To investigate spatiotemporal heterogeneity, multidimensional comparisons were conducted as follows: (1) HER2 protein detection analysis of 60 paired cervical biopsy and hysterectomy specimens (from patients who did not receive NAT), (2) parallel HER2 protein assessment in 23 paired cervical biopsy and post-neoadjuvant therapeutic hysterectomy specimens, and (3) HER2 protein expression concordance analysis in 63 matched pairs of primary cervical lesions and corresponding metastatic lesions.
Immunohistochemical staining
Four-micrometer-thick tissue sections were cut from 10% formalin-fixed, paraffin-embedded (FFPE) tissue blocks and stained for HER2 using a rabbit monoclonal primary antibody (clone 4B5, Roche, Switzerland) on the VENTANA BenchMark ULTRA (Roche) autostainer. Antigen retrieval was performed with cell conditioning solution (CC1, pH 8.5) at 100 °C for 64 min. Endogenous peroxidase activity was blocked using 3% H₂O₂ for 8 min, followed by application of the prediluted HER2 (4B5) primary antibody and incubation at 36 °C for 36 min. DAB chromogen was then applied for 8 min for visualization. Counterstaining was performed with Hematoxylin II (8 min) and a bluing reagent (4 min), followed by slide dehydration, clearing, and mounting. HER2-positive and -negative tissue samples were included as quality controls to validate the protocol.
Fluorescence in situ hybridization (FISH)
FISH was performed on all IHC 2+ and 3+ tumors. The HER2 gene amplification detection kit (No. 20183400001, Health Care Biotechnology Co., Ltd., China) was used to identify HER2 in paraffin-embedded tissue sections following the manufacturer’s instructions. This HER2/CEP17 dual-color probe labels HER2 (red) and the centromere of chromosome 17 (CEP17, green). The HER2 FISH assay began with 4-micrometer-thick FFPE tumor sections, baked at 65 °C for 2 h. After deparaffinization with xylene and hydration through a graded ethanol series, nucleic acids were denatured in a 95 °C water bath for 30 min, followed by protease K digestion at 37 °C for 10 min. Subsequently, the dual HER2/CEP17 probes were co-denatured at 83 °C for 5 min and hybridized at 42 °C for 16 h under controlled conditions to ensure specific binding. Post-hybridization washes removed unbound probes, and nuclei were counterstained with DAPI for clear visualization. According to the 2023 ASCO/CAP guidelines10, HER2-positive amplification is defined by a HER2/CEP17 ratio of ≥2.0 with an average HER2 copy number of ≥4.0 signals/cell, while HER2-negative status requires both a HER2/CEP17 ratio of <2.0 and HER2 showing <4.0 signals/cell. For cases where the HER2/CEP17 ratio is ≥2.0 but the average HER2 signals per cell are <4.0, or if a case has an average HER2 signals per tumor cell of ≥4.0 and <6.0 with HER2/CEP17 ratio <2.0, the diagnostic pathway specifies the following: if the IHC result is 3+, the diagnosis is HER2-positive; if IHC is 2+, an additional observer recounts ISH by counting at least 20 cells from invasive cancer areas with IHC 2+ staining, blinded to previous ISH results—if reviewing the count alters the ISH category, adjudicate per internal procedures; if unchanged, diagnosis is HER2-negative with a comment; for IHC 0/1+, diagnosis is HER2-negative with a comment. Similarly, for cases with average HER2 signals of ≥6.0 per cell and ratio of <2.0, the pathway is: HER2-positive if IHC 3+; for IHC 2+, perform an equivalent blinded recount—adjudicate if the category changes, HER2-positive if unchanged; HER2-negative with a comment for IHC 0/1+.
HER2 reassessment
Two senior pathologists (W.L. and D.H.) independently reviewed all cases, confirmed diagnoses, performed histological assessments, and conducted IHC HER2 scoring. Subsequently, cases with discordant HER2 scores underwent joint reassessment using a multi-view microscope, and for instances where consensus remained unresolved, a third pathologist (Y.C.) was consulted, with the majority opinion adopted as the definitive assessment. HER2 IHC scoring and FISH testing were conducted according to the 2023 ASCO/CAP breast cancer guidelines11. HER2 IHC scores were defined as follows: 3+ (positive): strong, continuous, and complete circumferential membrane staining in >10% of tumor cells; 2+ (equivocal): weak-to-moderate complete circumferential membrane staining in >10% of tumor cells, and if strong circumferential membrane staining is observed in <10% of cells, the case is provisionally classified as 2+ (equivocal) and requires HER2 IHC testing on additional samples and FISH testing for confirmation; 1+: faint/incomplete membrane staining in >10% of cells; 0 (Negative): no membrane staining or faint/barely perceptible incomplete staining in ≤10% of tumor cells. Cases scored as 2+ required mandatory reflex testing using FISH to confirm HER2 amplification status.
Tumors with IHC scores of 1+ or 2+ without FISH-confirmed gene amplification were classified as HER2-low, while those with IHC 3+ or 2+ with gene amplification were designated as HER2-positive. According to the DESTINY-Breast 069 trial stratification protocol, HER2-0 cases were subclassified as HER2-ultralow (HER2-0 with membrane staining) and HER2-null (HER-0 with absent membrane staining).
Comparative analysis of HER2 expression between breast cancer and gastric cancer scoring criteria
This study compared HER2 scoring outcomes in 548 CSCC cases using two distinct evaluation systems: the updated 2023 ASCO/CAP guidelines for breast cancer11 and the 2017 CAP/ASCP/ASCO criteria for gastric cancer12. HER2 expression levels were stratified into four categories (3+, 2+, 1+, 0), with particular emphasis on identifying statistical differences in HER2 expression distribution between the two scoring systems.
Statistical analysis
All statistical analyses were performed using SPSS version 21.0 (IBM Corp., Armonk, NY, USA). Medians were reported for continuous data not following a normal distribution, while between-group comparisons were performed using the Mann–Whitney U test. Categorical variables were compared using the chi-squared test (χ2). The concordance rates of HER2 status between cervical biopsies and hysterectomy specimens, as well as between primary cervical cancer, metastases, and post-NAT residual disease, were assessed using the Kappa test. Kappa values were interpreted as follows: <0.2 (poor agreement), 0.2–0.4 (fair agreement), 0.4–0.6 (moderate agreement), and >0.6 (reasonable agreement). Sankey diagrams were used to visualize categorical changes in HER2 expression. A p value of <0.05 was considered statistically significant.
Data availability
All data generated or analyzed during the current study are included in this published article.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Kovacs, E. et al. A structural perspective on the regulation of the epidermal growth factor receptor. Annu. Rev. Biochem. 84, 739–764 (2015).
Yarden, Y. & Pines, G. The ERBB network: at last, cancer therapy meets systems biology. Nat. Rev. Cancer 12, 553–563 (2012).
Koopman, T. et al. HER2 immunohistochemistry in endometrial and ovarian clear cell carcinoma: discordance between antibodies and with in-situ hybridisation. Histopathology 73, 852–863 (2018).
Meric-Bernstam, F. et al. Advances in HER2-targeted therapy: novel agents and opportunities beyond breast and gastric cancer. Clin. Cancer Res. 25, 2033–2041 (2019).
Erickson, B. K. et al. Human epidermal growth factor 2 (HER2) in early stage uterine serous carcinoma: a multi-institutional cohort study. Gynecol. Oncol. 159, 17–22 (2020).
Ogitani, Y. et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin. Cancer Res. 22, 5097–5108 (2016).
Dhritlahre, R. K. & Saneja, A. Recent advances in HER2-targeted delivery for cancer therapy. Drug Discov. Today 26, 1319–1329 (2021).
Bardia, A. et al. Trastuzumab deruxtecan after endocrine therapy in metastatic breast cancer. N. Engl J. Med. 391, 2110–2122 (2024).
Yuan, G. et al. Evaluation of the effectiveness and safety of disitamab vedotin in HER2-expressing 2L recurrent or metastatic cervical cancer (r/mCC): interim results of RC48-C018. J. Clin. Oncol. 42, 5528 (2024).
Wolff, A. C. et al. Human epidermal growth factor receptor 2 testing in breast cancer: ASCO-College of American Pathologists Guideline Update. J. Clin. Oncol. 41, 3867–3872 (2023).
Bartley, A. N. et al. HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: guideline from the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J. Clin. Oncol. 35, 446–464 (2017).
Anastasio, M. K., Shuey, S. & Davidson, B. A. Antibody-drug conjugates in gynecologic cancers. Curr. Treat. Options Oncol. 25, 1–19 (2024).
Meric-Bernstam, F. et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: primary results from the DESTINY-PanTumor02 Phase II Trial. J. Clin. Oncol. 42, 47–58 (2024).
Xu, M. et al. Early data of a phase II study of PRaG3.0 regimen for remedy of HER2-expressing advanced pan-cancer. Radiother. Oncol. 182, S1990 (2023).
Xu, M. L. et al. A multicenter, phase II trial of RC48-ADC combined with radiotherapy, PD-1/PD-L1 inhibitor, GM-CSF, and sequential IL-2 (PRaG3.0 regimen) for salvage therapy in patients with HER2-expressing advanced solid tumors. J. Clin. Oncol. 41, e14614 (2023).
Itkin, B. et al. Prevalence of HER2 overexpression and amplification in cervical cancer: a systematic review and meta-analysis. PLoS ONE 16, e0257976 (2021).
Marchiò, C. et al. Evolving concepts in HER2 evaluation in breast cancer: heterogeneity, HER2-low carcinomas and beyond. Semin. Cancer Biol. 72, 123–135 (2021).
Katayama, A. et al. Predictors of pathological complete response to neoadjuvant treatment and changes to post-neoadjuvant HER2 status in HER2-positive invasive breast cancer. Mod. Pathol. 34, 1271–1281 (2021).
Branco, F. P. et al. Loss of HER2 and disease prognosis after neoadjuvant treatment of HER2+ breast cancer. Am. J. Transl. Res. 11, 6110–6116 (2019).
Chen, R., Qarmali, M., Siegal, G. P. & Wei, S. Receptor conversion in metastatic breast cancer: analysis of 390 cases from a single institution. Mod. Pathol. 33, 2499–2506 (2020).
Miglietta, F. et al. Evolution of HER2-low expression from primary to recurrent breast cancer. NPJ Breast Cancer 7, 137 (2021).
Acknowledgements
This work was supported by the Natural Science Foundation of Fujian Province (2025J01215), Joint Funds for the Innovation of Science and Technology, Fujian province (2023Y9436, 2024Y9614, 2024Y9625), the Fujian Provincial Health Technology Project (2023CXA037), and the High-level Talents Training Program of Fujian Cancer Hospital (2022YNG16).
Author information
Authors and Affiliations
Contributions
W.L., X.W., Y.C. and D.H. performed study concept and design; W.L., X.W., Y.C. and D.H. performed development of methodology and writing, review, and revision of the paper; X.W., Y.C., X.L., X.W., Wc.S., J.L. and Q.X. provided acquisition, analysis, and interpretation of data, and statistical analysis; L.Z., W.M., J.Y., K.S., T.H. and Wq.L. provided technical and material support. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, W., Wang, X., Cui, Y. et al. HER2 expression in cervical squamous cell carcinoma: high prevalence of HER2-low/ultralow and spatiotemporal heterogeneity across tumor evolution. npj Precis. Onc. 9, 410 (2025). https://doi.org/10.1038/s41698-025-01189-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41698-025-01189-w