Abstract
Digital health tools such as remote patient monitoring (RPM) and digital health coaching (DHC) offer promising strategies for type 2 diabetes (T2D) management, yet little is known about how underserved Black adults experience these technologies. Guided by the Consolidated Framework for Implementation Research (CFIR), we conducted semi-structured interviews with 34 Black adults with uncontrolled T2D in Alabama and Mississippi (mean age 55 years; 71% female; 44% with food insecurity; most with Area Deprivation Index ≥8). Participants described multilevel factors shaping engagement. RPM increased disease awareness, provided real-time clinical feedback from a care team, and allowed flexible blood glucose monitoring, though fingerstick burden and emotional resistance to high glucose readings reduced use. DHC provided personalized, relationship-based support that motivated behavior change, yet environmental constraints and competing life demands limited sustained impact. Engagement often evolved from skepticism to confidence when benefits were evident and supported by social networks. Sustained participation requires trusted, personalized digital tools responsive to structural barriers.
Similar content being viewed by others
Introduction
Type 2 diabetes (T2D) remains a major public health challenge in the United States, disproportionately affecting many racial and ethnic minority populations1,2. Black/African American communities—particularly those in the Deep South, a region in the southeastern United States with distinct historical, cultural, and demographic characteristics—experience some of the highest rates of T2D prevalence and complications1,2. For example, non-Hispanic Black adults have an approximately 60% higher prevalence of diagnosed diabetes than non-Hispanic White adults, face higher rates of diabetes-related complications1, and are nearly four times more likely to be hospitalized for uncontrolled diabetes1. These persistent disparities in T2D outcomes are largely attributed to socioeconomic and structural inequities that impede effective disease management3. Limited healthcare access, poverty, food insecurity, and adverse neighborhood environments disproportionately burden many Black Americans in the Deep South and other resource-limited settings, accounting for more than half of the excess risk and poor outcomes observed in these communities3. Given this context, interventions are needed that not only improve T2D control but also bridge underlying health equity gaps.
Telehealth interventions, including remote patient monitoring (RPM) of blood glucose and digital health coaching (DHC), have emerged as promising strategies to extend care beyond traditional clinical settings and support T2D self-management4,5,6,7,8. RPM leverages cellular-enabled glucometers to transmit real-time glucose data to care teams, enabling healthcare providers to monitor progress and intervene proactively in cases of critical alerts between visits. DHC provides personalized support through phone or video-based coaching sessions, where trained health coaches deliver tailored education, goal-setting, and encouragement to reinforce healthy behaviors. Together, these approaches can increase patient engagement, improve glycemic control, and help overcome access barriers4,8.
Meta-analyses and studies focused on minority populations, including Black and Hispanic adults with diabetes, demonstrate that telehealth interventions can significantly lower HbA1c levels9. However, despite growing evidence of clinical efficacy, critical gaps remain. Black adults are often underrepresented in telehealth studies9, and little is known about their lived experiences engaging with digital health tools, especially in low-resource settings where structural barriers may limit uptake. Prior work has predominantly focused on provider perspectives10, broader telehealth modalities11, rather than patient perspectives of RPM and DHC in Black/African American population12,13 or under-resourced community context.
To address these gaps, we conducted the first qualitative study of Black adults with T2D participating in a digital health intervention—including RPM and DHC—in the Deep South of the United States. Guided by the Consolidated Framework for Implementation Research (CFIR)14,15, we explored patient-reported barriers and facilitators to engagement with RPM and DHC among participants living in Alabama (AL) and Mississippi (MS). CFIR provided a structured lens to examine multilevel influences, including intervention characteristics, individual-level factors, and outer-setting conditions15.
The goal of this qualitative sub-study was to identify key barriers and facilitators to engagement with DHC and RPM and generate actionable insights for refining digital health interventions to better meet the needs of Black adults with T2D in resource-limited settings. We present both intervention-specific and cross-cutting themes, with an emphasis on illuminating the individual and social contextual nuances shaping digital health engagement.
Results
Participant characteristics
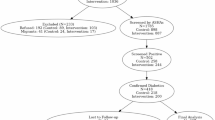
We conducted interviews with 34 participants enrolled in the FREEDOM study who received either DHC, RPM, or both. Of these, eight participants (23.5%) received DHC alone, 12 (35.3%) received RPM alone, and 14 (41.2%) received both. In total, 22 participants (64.7%) received DHC, and 26 (76.5%) received RPM. Participants were primarily recruited from UAB Medicine (73.5%), with smaller numbers from Cooper Green Mercy Health Services (17.6%) and the University of Mississippi Medical Center (8.8%).
All participants identified as Black or African American and not Hispanic or Latino. While individuals identifying as both Black and Hispanic/Latino were eligible, none enrolled in this qualitative sub-study. The mean age was 55.1 ± 10.0 years, with the majority being female (70.6%). The sample reflected a medically and socially underserved population: over half reported annual household incomes below $28,000, nearly three-quarters lived in areas with high deprivation (ADI ≥ 8), and 44.1% experienced low or very low food security. Educational attainment and insurance coverage were also limited; only 17.7% held a bachelor’s degree or higher, and 14.7% were uninsured (Table 1). Site-level differences were observed. Participants from Cooper Green Mercy Health Services were the most socioeconomically disadvantaged, with the lowest educational attainment and employment rates, the highest prevalence of food insecurity (75%), and the greatest proportion with annual household income <$28,000 (87.5%). UAB participants demonstrated relatively higher levels of education, private insurance coverage, and employment. UMMC participants fell between the two sites but had the highest rate of uninsured individuals (30%). All sites had a high proportion (73.5%) of participants residing in highly disadvantaged areas (ADI ≥ 8).
Tables 2 and 3 present our findings, identifying facilitators and barriers to engagement with RPM and DHC, respectively, using participant quotes organized by CFIR domains. Figures 1 provides an overview of cross-cutting and intervention-specific themes, while Fig. 2 illustrates the final coding tree linking CFIR domains, derived themes, and associated interventions.
RPM: facilitators and barriers
Within the Characteristics of Individuals domain, the codes identified were Self-Efficacy and Individual Stage of Change. The following themes describe participant-identified factors that either supported or hindered engagement with the RPM intervention, mapped to CFIR domains.
Facilitator: increased awareness of the disease process
Participants’ personal readiness and circumstances shaped their engagement with RPM. Many described an increased awareness of the disease process of how their daily behaviors impacted their blood glucose, which emerged as a key facilitator of engagement (CFIR construct: Individual Stage of Change). Seeing their readings in real-time helped participants recognize how their daily choices impacted their diabetes. By tracking their blood glucose levels, participants began to recognize cause-and-effect patterns in their diet and routine.
“I did not ever know that my blood sugar would go up and down depending on what I ate that day. Now I try to monitor myself more,” one participant reported (P898).
This heightened awareness often went hand-in-hand with participants' growing confidence in diabetes self-management as participants felt more knowledgeable and in control of their condition (CFIR construct: Self-Efficacy).
Transitioning to the Intervention Characteristics domain, the codes identified were Adaptability, Complexity, and Design Quality and Packaging.
Facilitator: ease and usability of the device and system
Another facilitator, falling under CFIR Intervention Characteristics (Design Quality & Packaging), was the design of a cellular-enabled glucometer. Patients described the glucometer as easy to learn and as highly user-friendly due to its simple setup and lack of the need for Wi-Fi or Bluetooth pairing (Design Quality & Packaging). The onboarding process, typically a guided phone call from the RPM team registered nurses, offered step-by-step instructions about the device, and any initial challenges with the technology were quickly overcome through clear instructions and support from the RPM team.
“Everything was laid out for me to understand how the system works… Transmitting everything went fine. The system is very informative,” said one individual, emphasizing that the program provided all the guidance needed to get started and make sense of the readings (P113).
Many also appreciated practical conveniences built into the intervention—for instance, test strips and supplies were automatically mailed to them with the device, and they received phone or text check-ins if they forgot to send readings, which removed logistical barriers to continued use. Although some participants experienced a short learning curve, most found the device easy to incorporate into daily routines. These characteristics—passive data transmission, minimal setup requirements, and team availability for troubleshooting—may be important for future device developers seeking to reduce barriers and enhance engagement, particularly in underserved or low-resource settings.
Facilitator: real-time feedback and proactive outreach from the care team
Participants also highly valued the design quality of the RPM intervention in terms of integration with clinical care – specifically, the real-time monitoring by providers and rapid feedback on their readings (CFIR construct: Design Quality and Packaging). Knowing that clinic staff were actively reviewing their glucose data made participants feel “someone was always watching,” which increased their sense of accountability and safety.
“By the time I got my phone to call them, they called me. And I thought that was awesome – it let me know they were checking everything,” one participant recounted, describing how impressed they were when a nurse proactively contacted them about a high reading before they even asked for help (P604).
This timely and proactive feedback from the care team reassured patients that they were not managing their diabetes alone and encouraged them to remain engaged with daily readings.
Facilitator: flexible blood glucose monitoring frequency
The adaptability of the RPM protocol was another facilitator noted by participants (CFIR construct: Adaptability). Rather than imposing a rigid testing schedule, the intervention allowed patients to adjust the frequency and timing of glucose checks to fit their needs and routines. Participants appreciated being able to find a sustainable monitoring rhythm.
“I was told that I could do as many [tests] as I would like in a day. But I kind of stuck with one and they were fine with that, as long as I checked,” said one participant (P557), highlighting the freedom to choose a comfortable testing frequency.
Others similarly reported that being able to reduce or space out fingersticks (for example, testing once daily on weekdays instead of multiple times per day) made the process feel less burdensome. Tailoring the monitoring regimen to individual preferences enabled patients to integrate RPM into their daily lives more easily and consistently.
Barrier: burden of repeated fingerstick monitoring and desire for continuous glucose monitoring
While the RPM glucometer was generally viewed as user-friendly and accessible, many participants expressed a clear preference for less invasive monitoring methods, specifically CGMs (CFIR construct: Complexity). Participants frequently described fingerstick testing as painful, irritating, and tedious. Several noted they had “hit all their fingers,” making it hard to continue testing daily and some struggled to draw enough blood or had to repeat the process multiple times, adding to frustration. One participant noted:
“It hurts. I’m not going to lie to you. And sometimes, I don’t get enough blood, and I have to do it again. That’s frustrating” (P898).
The routine of pricking fingers became disruptive, especially when participants were on the go or juggling other responsibilities. Some mentioned forgetting to test or avoiding testing altogether to escape discomfort. Those who had previous experience with CGMs, such as the FreeStyle Libre, highlighted the difference in comfort and convenience:
“I used to have a sensor on my arm. I loved that. No pricking, no pain. I wish I had that again” (P369).
DHC: facilitators and barriers
Following RPM, participants also described barriers and facilitators to DHC with themes beginning in the CFIR domain of Intervention Characteristics—specifically Design Quality and Packaging, Complexity, and Adaptability—and extending across other domains.
Facilitator: personalized, flexible, and compassionate coaching
A defining facilitator of engagement in the DHC arm was the highly personalized and supportive nature of the coaching interaction. This theme maps to the CFIR constructs, Adaptability and Design Quality & Packaging, in that the coaching was tailored and delivered in a patient-centered manner. Participants consistently described how the coaching experience was adapted to their individual needs, preferences, and circumstances. Coaches adjusted session lengths and content based on participants’ weekly needs and preferences.
“She [health coach] took the time that was necessary. Sometimes they were short sessions, sometimes long…depending on the concerns or questions I had” (P113), reflecting how the coach flexibly paced meetings.
Coaches also customized tools and strategies to match physical limitations or lifestyle barriers. For instance, one participant with hip pain recounted:
“We [my coach and I] came up with easy chair exercises” to accommodate her hip pain (P112).
Participants appreciated that coaches helped them set small, attainable goals, offering support that felt realistic and manageable.
In addition to content tailoring, the relational quality of the coaching was highlighted as exceptionally compassionate and encouraging. Many formed a trusting bond with their coach and described their coaches as empathetic, kind, and consistent.
One participant shared:
“Even when I was really sick… she [health coach] still checked on me when I went to the hospital. We just rescheduled. But she always made a point to check in and see how I was doing… She was very compassionate, very sensitive to others… she was like a therapist” (P419).
Others emphasized the importance of being listened to and feeling understood:
“She [health coach] was really kind, and she did listen. That’s very important… you’re explaining how you feel. So that really helped — to be heard” (P100).
This combination of personalization and emotional support was central to keeping participants engaged throughout the DHC program.
Facilitator: accessibility and reach of phone-based coaching
Another facilitator of DHC was the simple, accessible delivery format of the intervention (CFIR Construct: Design Quality & Packaging). Coaching was conducted via regular phone calls (and supplemented by texts or emails), which eliminated many logistical barriers that might otherwise hinder participation in a resource-limited setting. Participants appreciated not having to travel to appointments or manage video technology; instead, coaching conversations took place wherever was convenient for them.
“It was by phone, and I think I preferred the phone rather than Zoom,” one participant noted (P769).
The telephone format was particularly important in this resource-limited context, as some individuals had unreliable internet or limited transportation. Moreover, coaches made themselves readily reachable between formal sessions, creating an “open line” of communication (P268).
Facilitator: behavioral nudges and motivational reinforcement
Participants in DHC also highlighted the value of ongoing reminders and motivational messages that supplemented the coaching calls. These behavioral nudges (CFIR construct: Design Quality & Packaging) were built into the program’s design as weekly text messages, emails, or calendar alerts, and they served as another facilitator of engagement unique to the DHC intervention. The prompts kept diabetes management “on the radar” for participants between sessions, helping to maintain momentum. One participant noted,
“I continued to get weekly texts. And those are great reminders for me to stay focused,” underscoring how simple text prompts helped her stick to her goals (P304).
Another appreciated receiving follow-up emails with personalized “tidbits” of health information relevant to what had been discussed each week, which reinforced the coaching lessons:
“Every week I would get an email from my health coach with tidbits…health information that she thought would be beneficial to me based on what we discussed,” (P112).
These regular check-ins and encouragements made participants feel that someone cared about their progress and kept them accountable to themselves. By preventing them from “falling off the wagon” between calls, the nudges helped sustain engagement and behavior change. Even during the final three-month maintenance phase, when phone calls with health coaches shifted to an “as-needed” model, participants reported that ongoing digital educational nudges (via text or email) remained helpful for keeping them engaged, suggesting that low-touch behavioral nudges can continue to reinforce progress even as program intensity declines. In this phase, participants also continued to receive weekly “tiny step” prompts and retained the option to contact their coach as needed. Although not all participants completed each “tiny step” during this phase, they remained enrolled and received ongoing resources, allowing continued engagement with minimal burden.
Transitioning to the Outer Setting domain, the construct identified was Patient Needs and Resources.
Barrier: structural and environmental constraints to lifestyle change
Counterbalancing these facilitators, however, were persistent structural and environmental constraints (CFIR Construct: Patient Needs & Resources) that made lifestyle changes recommended in coaching more difficult to implement. Participants identified several community and neighborhood-level barriers that hindered their ability to act on goals set during the DHC. One commonly cited issue was the lack of safe or convenient spaces for exercise.
“My street does not have sidewalks… I used to walk in the street and had to carry a stick for the dogs…and run in the yard when cars were coming by. That’s the only thing I can think of that hindered my ability to exercise,” one participant explained (P769) highlighting how poor walkability and safety concerns (loose dogs, traffic) impeded her attempts to be physically active.
Beyond the physical environment, some participants noted a scarcity of local healthy food options or diabetes-friendly resources, which made it hard to follow nutrition advice from coaching sessions.
One participant lamented that “the actual community itself… it’s nonexistent [in terms of] helping. Or if it’s there, I don’t know what avenues to take to get to it,” expressing frustration at not knowing of any community support programs or resources for nutrition (P335).
Additionally, participants in crowded or multigenerational households sometimes had minimal privacy or quiet time for coaching calls and activities (for example, difficulty finding a private space to talk or do a mindfulness exercise). These outer-setting barriers did not reflect a lack of willingness on the participant’s part, but rather external limitations of their environment that made engagement an uphill battle at times. Coaches attempted to work around these issues (for instance, suggesting indoor exercises or discussing how to modify diets with available food options), but participants still felt that their progress was limited by what their environment would allow, underscoring the need to address broader social determinants in tandem with individual coaching efforts.
Cross-cutting themes
Beyond intervention-specific findings, participants also described cross-cutting themes that spanned both RPM and DHC. Within the Individual Characteristics domain, the codes identified were Knowledge and Beliefs, Self-Efficacy, and Individual Stage of Change.
Facilitator: growing confidence, from initial skepticism to empowerment
A shared journey that many participants underwent—cutting across both RPM and DHC—was an evolution in their readiness to engage, moving from initial skepticism or fear toward a sense of empowerment and increased self-efficacy over time in diabetes self-management (CFIR Constructs: Knowledge and Beliefs, Individual Stage of Change, and Self-Efficacy).
During initial interactions with the interventions, participants in both groups expressed hesitancy about fully engaging. In the RPM context, some individuals admitted to anxiety around monitoring. They were often afraid of seeing “bad” glucose numbers or being judged by providers based on their readings. One RPM participant even avoided transmitting readings on occasion if she anticipated a high value, saying,
“If it’s really high, I won’t send it in because I know they’re going to call… I know that it’s wrong” (P618).
Others were uneasy about the fingerstick process itself or what the data might reveal, leading to a cautious start with technology.
In the DHC arm, participants initially voiced skepticism about the coaching before becoming truly involved. Many were unsure whether talking to a health coach would make a meaningful difference, especially if they had managed their condition on their own for years.
“I was skeptical at first… I thought, ‘How is talking to someone every week going to help my diabetes?’” one DHC participant recalled (P335).
As participants engaged more consistently with their assigned interventions, many reported a shift in mindset–essentially a growing empowerment as they experienced progress. In the RPM group, repeated self-monitoring and timely provider feedback helped ease anxiety and normalize the process. One participant described overcoming her initial fear through daily practice:
“I used to take my sugar, I would get so scared… But now I’m much more comfortable with it. I got a lot better with it,” (P604).
That increased comfort and skill with self-monitoring contributed to a stronger sense of efficacy in managing T2D.
In DHC, participants credited their growing self-efficacy to the consistent encouragement and personalization provided by their health coach. As they achieved small goals—such as exercising regularly, losing weight, or lowering their HbA1c—confidence gradually replaced skepticism.
“I wasn’t really confident in the beginning… It’s skyrocketed because I’ve seen improvement – lowering my HbA1c and losing weight… I have great confidence in the program now” (P720).
While the catalysts for this change differed – objective feedback from RPM versus relational support and progress in DHC – the outcome was similar: participants in both groups became more psychologically ready and confident in managing T2D. This trajectory was often cited as a turning point that deepened engagement and enhanced their intrinsic motivation. Transitioning to the Outer Setting domain, the code identified was Patient Needs and Resources.
Barrier: competing responsibilities
A major cross-cutting barrier to engagement across both interventions was the strain of competing life demands (CFIR construct: Patient Needs and Resources). Participants in both the RPM and DHC arms frequently reported that work responsibilities, caregiving duties, and household tasks hindered their ability to consistently engage with the interventions. For example, one participant described how her daily routine made it easy to forget RPM readings:
“I normally get up early in the morning, and I start doing house chores… A lot of times I just forget. Then I go to take it and probably the telephone will ring or something… before I know it, it’s later on in the day” (P100).
Others similarly mentioned that between jobs, childcare, and caring for elderly relatives, they often “have to try to take care of myself more than I have been” because so much of their energy has gone into caring for others (as one caregiver of a 90-year-old mother reflected, P898).
These accounts indicate that, regardless of the intervention type, participants often struggled to prioritize the program when faced with pressing life obligations. Such competing life demands occasionally led to missed glucose checks or postponed coaching calls. Both interventions attempted to mitigate this barrier (RPM with automated reminders or data alerts, DHC with flexible scheduling and coach outreach), but the underlying challenge remained that participants needed to juggle self-care with many other duties in their lives.
Facilitator: social and familial support
In both RPM and DHC, having a strong support network emerged as an important facilitator of engagement (CFIR construct: Patient Needs & Resources). Participants who received encouragement, reminders, or practical help from family members, friends, or coworkers found it easier to stay committed to their monitoring and behavior change goals. This social support provided accountability and motivation beyond what the formal program offered.
In the DHC intervention, participants described how their loved ones joined them in adopting healthier habits, such as walking together or trying new recipes, which strengthened their motivation and accountability.
As one participant shared, “Now that I’m on a mission to eat better, [my wife] is as well – we are on the same page” (P720).
In the RPM arm, participants similarly credited family members with encouraging consistent self-monitoring.
“They reminded me… ‘Don’t forget your meter,’” one participant RPM recalled, underscoring how these small gestures had a big impact on adherence (P369).
These positive social influences often helped normalize new habits (such as daily testing or exercising) and integrate them into participants’ social lives – for instance, family members would join participants on walks or remind them to test their blood sugar. Conversely, those lacking support sometimes struggled more to remain active in the program (as family or work obligations could also compete, per the above theme), but the presence of supportive others clearly stood out as a cross-cutting facilitator that enhanced accountability and sustained participation.
Discussion
In this qualitative study of Black adults with uncontrolled T2D living in moderate to high-deprivation neighborhoods, we identified a range of patient-level and contextual factors that influenced engagement with RPM and DHC. Using the CFIR framework, our analysis revealed multi-level barriers and facilitators spanning individual characteristics, intervention design, and outer-setting domains. For RPM, participants emphasized several facilitators, including increased awareness of how daily behaviors affected their glucose levels—reinforced by real-time provider feedback and proactive outreach from the care team. They also appreciated the ease and convenience of the mailed cellular-enabled glucometer, which did not require Wi-Fi, the automatic delivery of test strips, and the flexibility of the monitoring schedule. However, the physical burden of repeated fingerstick testing and fear or avoidance of high readings emerged as key barriers. In contrast, DHC participants described high levels of satisfaction with the program’s personalized and compassionate coaching, as well as the flexibility of phone-based sessions. Still, environmental obstacles such as unsafe neighborhoods and limited access to healthy foods limited engagement. Across both interventions, participants experienced an evolving readiness for engagement, with many describing a shift from fear or doubt to growing self-efficacy and empowerment as the intervention progressed. Competing life demands—particularly work, caregiving, and household responsibilities—frequently disrupted adherence, while social and familial support played a key facilitating role by reinforcing positive habits and offering accountability. Ultimately, the usability and accessibility of digital platforms helped participants overcome some logistical barriers; however, engagement was still heavily influenced by their emotional readiness and the structural/environmental context. These insights highlight the need for intervention strategies that are not only accessible but also responsive to the emotional, social, and structural contexts shaping patient engagement.
Our findings both corroborate and extend existing literature on telehealth use in underserved populations. Technical access (internet or device availability) is often cited as a major barrier in low-income communities11. For example, Bazzano et al. (2024) studied telehealth for diabetes in Louisiana during COVID-19 and highlighted technology problems, such as outdated devices and internet connectivity as primary patient barriers11. In our study, many traditional technology obstacles were mitigated by the intervention design (provision of a cellular-enabled glucometer and phone-based coaching), yet engagement remained challenged by psychosocial and environmental factors. This aligns with recent evidence that even when >90% of Black patients have internet-capable devices, telehealth uptake can remain low (~39% in one sample) due to issues like lower trust in providers and greater neighborhood disadvantage16. The initial skepticism and hesitancy we observed echo these trust-related barriers, suggesting that discordant health beliefs and uncertainty about efficacy can lower engagement early on16. Encouragingly, our participants’ experiences of increased self-efficacy and diabetes control with continued engagement reinforce findings that supportive telehealth interventions can lead to tangible health improvements. For example, a meta-analysis of telehealth programs (mostly phone/text-based) in trials enrolling predominantly Black patients demonstrated significant HbA1c reductions8. This indicates that when engagement is achieved, telehealth can be effective in improving outcomes for high-risk populations. Our study adds qualitative depth to this evidence by illustrating how facilitators, such as real-time feedback, empathetic communication, and family encouragement, build the trust and motivation needed to achieve those outcomes. Likewise, the role of social support as a facilitator is consistent with prior research emphasizing family and peer involvement as motivators in diabetes self-care and monitoring17,18,19. In contrast, the structural barriers participants described (e.g., lack of safe spaces to exercise or healthy food access) align with existing literature noting safety concerns for exercise, and competing responsibilities in care management20,21,22,23 underscore a critical insight: even a well-designed telehealth program cannot fully overcome suboptimal social determinants. This is supported by other studies showing that neighborhood insecurity and resource deficits strongly limit diabetes-related behaviors23. Coaches in our study attempted to help patients adapt (for instance, suggesting chair exercises or indoor activities), but persistent environmental obstacles often capped participants’ ability to act on health recommendations. These comparisons suggest that our CFIR-guided themes map onto broader patterns identified in the literature – trust, usability, social support, and structural context20,24.
Implications for practice and policy stem directly from these findings. Health care providers and programs implementing telehealth for diabetes in resource-limited settings should proactively incorporate strategies to reduce barriers and strengthen facilitators identified in our study. Based on these findings, we developed a set of actionable recommendations to inform the refinement of digital health interventions for underserved populations (Figs. 3, 4). For example, to alleviate the burden of frequent fingerstick monitoring, programs could offer alternative glucose monitoring methods (e.g., continuous glucose monitors) or at least allow patient-tailored testing schedules that minimize discomfort25. To combat fear and anxiety around glucose readings, clinicians and digital health care team might provide counseling before beginning the intervention, and reframe feedback in an empowering, non-judgmental manner—emphasizing support and education over “compliance”—so that patients do not dread reporting high blood glucose values. Participants valued the regular proactive outreach from the care team, with several noting that the RPM experience gave them a sense that 'someone is always watching out' for them. Formalizing protocols to ensure that abnormal readings prompt timely, supportive outreach could help sustain this perceived benefit. Technical assistance should also be readily available. Although most users found the RPM glucometer easy to use, offering step-by-step training and a helpline can ensure that any usability issues are quickly resolved.
To better integrate DHC into patients’ busy lives, DHC services could offer more flexible scheduling options (e.g., evening sessions or brief check-ins) and asynchronous support (e.g., text reminders or app-based messaging) to accommodate those with work or caregiving responsibilities. Coaches should be trained in motivational interviewing and culturally tailored counseling, which can help build trust, address skepticism, and reinforce small victories – techniques that our participants found to boost their confidence. Importantly, leveraging the social environment can significantly amplify the impact of telehealth. In practice, this means involving family members or close peers in the intervention when appropriate, such as inviting a supportive family member to join occasional coaching calls or providing family-oriented educational materials to encourage lifestyle changes at home. Such engagement of patients’ natural support systems can create a reinforcing loop of accountability and encouragement beyond the clinical context.
This study has several strengths and limitations to consider, especially in the context of other research on Black adults with poorly controlled diabetes in disadvantaged areas. A key strength is the focus on a traditionally underserved population – all participants were Black Americans from moderate to high-ADI neighborhoods, a group often underrepresented in telehealth research8. By centering their voices, our analysis provides an understanding of barriers (like fear, competing demands, and structural hurdles) that generic telehealth studies might not capture. The use of the CFIR framework adds another strength: it allows us to systematically map patient-reported themes to established implementation constructs, enhancing the interpretation and potential transferability of our findings to similar settings. Moreover, our relatively large qualitative sample (N=34 across three different institutions in the Deep South) and inclusion of two telehealth modalities (RPM and DHC) increase the richness and applicability of the results. We were able to compare engagement factors across both an objective monitoring tool and a behavioral coaching service, which has not been done in tandem in previous research.
In terms of limitations, one is the potential selection bias: participants were drawn from those enrolled in the FREEDOM study, which means they had already consented to potentially be randomized to digital health interventions. Individuals who lack phone access or who were uncomfortable with technology or research may not be represented. Additionally, the structured trial context may differ from routine clinical settings, though it helped minimize hardware-related barriers and reveal more nuanced engagement challenges. Although this was a multisite study, recruitment was uneven across sites, with the majority of participants from UAB and only a small number from CGMHS and UMMC, which may limit the transferability of site-level comparisons. Another limitation is that our analysis did not capture perspectives from healthcare providers or program staff; such viewpoints might reveal additional “inner setting” factors (e.g., clinic workflow, provider training issues) that influence implementation but were outside the scope of our patient-focused interviews. Lastly, some participants (n=11, 32.4% of interviewed patients) also received two-monthly non-perishable food box deliveries as part of their randomization group in the parent study, which may have influenced their responses regarding ability to adhere to DHC recommendations, particularly around dietary behavior changes. Despite these limitations, this study contributes unique insights by focusing on a high-risk, under-resourced demographic and identifying concrete targets for improving telehealth engagement.
Overall, engaging Black patients with uncontrolled T2D in telehealth interventions requires a holistic approach that addresses individual, interpersonal, and contextual factors. Our CFIR-guided analysis illustrates that fear of monitoring, skepticism about unfamiliar programs, and day-to-day life stressors can significantly hinder participation, even when devices and connectivity are in place. Conversely, when interventions are flexible, user-friendly, and accompanied by empathetic, trusted human support, they can empower patients, leading to increased self-management confidence and improved health behaviors. The actionable strategies derived from our findings offer a roadmap for healthcare providers and policymakers: by reducing burdens (through simpler monitoring options and scheduling flexibility), enhancing support (through trust-building communication, education, and family involvement), and improving the fit of programs within patients’ lives, digital health engagement can be improved. Ultimately, tailoring digital health interventions to the lived realities of Black Americans in resource-limited communities—and coupling these efforts with broader policy initiatives to enhance community resources—will be essential for leveraging the full potential of digital and telehealth to reduce diabetes disparities. Future research should build on these insights by testing targeted implementation strategies and evaluating their impact on long-term engagement and glycemic control in underserved populations. This will help ensure that the rapid expansion of digital health equity reaches those who stand to benefit the most.
Methods
Study design
We performed a descriptive qualitative study guided by phenomenological principles to examine patient-reported barriers and facilitators to engaging with a digital health intervention for diabetes management. This study was embedded in the FREEDOM trial (ClinicalTrials.gov Identifier: NCT05288452), a 12-month hybrid optimization-implementation trial in AL and MS that evaluates a multilevel, multicomponent intervention targeting social determinants of health for improving T2D outcomes in Black adults. Our qualitative sub-study focused on the digital health elements (RPM and DHC). The study protocol, including all interview procedures, was approved by the Institutional Review Board of the University of Alabama at Birmingham as a central IRB for all institutions, and written informed consent was obtained from all participants in the parent FREEDOM trial prior to enrollment.
Setting
The research was conducted in two Deep South states: AL and MS. Participants were recruited from three healthcare settings involved in the FREEDOM trial: the University of Alabama at Birmingham Health System (UAB Medicine), an academic medical center in Birmingham, AL, Cooper Green Mercy Health Services (CGMHS), a safety-net health system in Birmingham Alabama/Community Partner for the FREEDOM study, and the University of Mississippi Medical Center (UMMC), an academic health center in Jackson, MS. All participants within this qualitative analysis received one or both of the two digital interventions offered in the trial–RPM or DHC–delivered in their home environment. No other digital health interventions were provided as part of the study.
Participants
Participants in this qualitative analysis were drawn from the pool of FREEDOM study participants using a purposeful sampling. Eligible individuals were adults (age 18 or older) who self-identified as Black or African American, had a confirmed diagnosis of T2D, lived in a neighborhood of area deprivation index of 5 and greater27, had an HbA1c ≥ 8%, proficient in spoken and written English, and were enrolled in the digital health intervention arm of the trial in UAB Medicine, CGMHS, UMMC. Exclusion criteria for the parent trial (and thus our analysis) included inability to provide informed consent, cognitive impairment, end-stage renal disease requiring dialysis, receipt of DHC or RPM within 60 days of screening, current pregnancy or plans for pregnancy within 12 months, and active enrollment in a structured lifestyle change program or intervention.
A total of 34 participants were recruited and completed the interview – 14 participants utilized both RPM and DHC, 8 participants utilized DHC only, and 12 had RPM only. These participants were sampled from the subset of trial participants who received one or both of the digital health interventions. They represent approximately 15% of the broader group exposed to RPM and/or DHC. This sample size was determined based on the principle of reaching thematic saturation, which we assessed using an iterative approach informed by Hennink et al. (2017)28. We monitored the emergence of new codes and insights through team debriefings and regular codebook updates, and saturation was considered achieved when no new themes emerged in the last final five interviews. Additional details on participant demographics and clinical characteristics are provided in the Results section. All participants received a $35 gift card to compensate for their time in the interview.
Interventions
The RPM component equipped participants with cellular-enabled glucometers (iGlucose® Blood Glucose Meter26) and test strips capable of transmitting real-time blood glucose data to the RPM care teams (nurses, nurse practitioners, and physicians) over a six-month intervention period. RPM implementation was led by site-specific teams at the University of Alabama at UAB Medicine UMMC, with the UAB team also providing support to CGMHS. Upon placement of an RPM referral through each site’s electronic medical record (EMR) system, devices and test strips were shipped directly to participants by the RPM team. The cellular functionality of the glucometer eliminated the need for Wi-Fi or Bluetooth connectivity.
Participants received a call from the RPM team and were onboarded within 10 days of enrollment. The onboarding process included instructions on device setup and use, as well as the collection of medical history via telephone and a review of the participant’s electronic medical record (EMR). Participants were encouraged to reach out to troubleshoot any technical issues, and advised to measure their blood glucose levels once to twice daily, with flexibility accomodated based on individual clinical needs and personal preference. Glucose readings were actively monitored by the RPM team during business hours (Monday through Friday, 8:00 AM to 5:00 PM). Adherence to monitoring was tracked throughout the intervention period. Participants with multiple missed readings within a given week were contacted by a registered nurse to identify and address potential barriers to adherence.
Automated critical alerts were generated for glucose values <70 mg/dL, 250–449 mg/dL, and >450 mg/dL. Based on the severity of the value, symptomatology, and temporal trends, participants received follow-up calls to assess the clinical context and provide guidance. Recommendations included medication adjustments, repeat glucose monitoring, review of dietary patterns and physical activity behaviors, or escalation to urgent or emergent medical care as clinically indicated. Standardized scripts were used to guide follow-up, beginning with assessment of medication adherence and followed by exploration of potential behavioral, dietary, or contextual contributors. Providers were notified of critical readings and clinical concerns through the EMR to facilitate timely clinical decision-making on potential medication changes and continuity of care.
The DHC intervention connected patients with trained diabetes health coaches who conducted regular one-on-one weekly sessions by phone for three months of the six-month intervention (phase 1). Each week included 1) a structured lesson to prepare for the one-on-one call with the health coach, 2) a weekly phone call from the health coach to the participant, and 3) followed by a collaboratively chosen “tiny step”—a small, achievable behavior aligned with the participant’s health goals sent from the health coach to the participant. Between calls, participants received up to three digital nudges (via text or email) tailored to their preferences. These nudges provided encouragement, reminders, or check-ins related to the lesson or tiny step.
Following the intensive phase, participants transitioned to a three-month maintenance phase characterized by a “low-touch” model (phase 2). During this phase, they no longer received scheduled coaching calls but continued to receive one weekly nudge prompting them to complete a tiny step, along with up to one additional nudge focused on motivation or reinforcement of previous goals. While coaching calls were no longer scheduled, participants retained open access to their health coach for support as needed. Completion of tiny steps was encouraged but not required, and participants remained enrolled regardless of response.
Data collection
Data was collected through one-on-one semi-structured in-depth interviews conducted between August 2024 and January 2025 by GG and SJ, senior medical students at UAB. GG was part of the Comprehensive Urban Underserved and Rural Experience (CU²RE) program—an enriched training initiative that prepares students for primary care careers in underserved communities. The program includes extensive clinical exposure to medically underserved populations, research ethics, behavioral health, and cultural competency. GG had direct engagement with the study population through clinical rotations and community-based health activities, which supported rapport-building and contextual familiarity during interviews. SJ also had extensive exposure to underserved and racially diverse populations through clinical training at a Federally Qualified Health Center, participation in Equal Access Birmingham, and volunteer work at a predominantly Black nursing home. Both interviewers received cultural sensitivity training as part of their medical education and had substantial experience caring for Black patients, particularly in the Southern U.S. context.
An interview guide was developed by AE and refined through discussions with a team member who had content and methodological expertise (LH), and was informed by CFIR 1.029. The guide included open-ended questions and prompts organized around key areas such as: participants’ experiences using the RPM devices (ease of use, technical issues, feelings about monitoring), interactions with the digital health coaches (helpfulness of coaching, communication quality), personal factors affecting engagement (motivation, health beliefs, confidence with technology), and external or environmental factors (support from family, work or home schedule constraints, community or clinic support for telehealth). Interviewers also asked follow-up probes to delve deeper into any mentioned facilitators or obstacles (Supplementary Text 1).
All interviews were conducted via telephone or secure video conference at times chosen by the participant. Each interview lasted approximately 30-45 minutes. At the start of each session, the interviewer reiterated the study purpose and assured participants of confidentiality. Verbal informed consent for participation in the interview and audio recording was obtained. Interviews were audio-recorded using an encrypted digital recorder and uploaded to an online and secure file-sharing platform (ShareFile), maintained by UAB Medicine.
In addition to the interviews, participants completed a brief survey capturing demographic and socioeconomic characteristics. Collected variables included age, sex, race/ethnicity, education level, employment status, marital status, household income, health insurance coverage, and living arrangement, as part of the baseline survey packet in the parent study. Food security status was assessed using the USDA Adult Food Security Survey Module, a validated 10-item instrument30. We also calculated each participant’s neighborhood-level Area Deprivation Index (ADI) based on their ZIP code, using publicly available national ADI rankings from the University of Wisconsin to classify neighborhood socioeconomic disadvantage31.
Transcription and data management
The interview audio recordings were professionally transcribed verbatim. AE reviewed each transcript alongside the recording to verify accuracy and assigned unique and anonymized study codes to each participant. The final de-identified transcripts were imported into NVivo 15 (QSR International, Melbourne, Australia) to facilitate systematic coding and organization of the data. Field notes and memos written by interviewers immediately after each interview (capturing initial impressions, context) were also used to complement the transcript data during analysis to contextualize the transcripts.
Data analysis
We utilized a framework analysis approach guided by the CFIR to analyze the qualitative data. Our analytic process involved both deductive coding (using predefined CFIR constructs as a starting framework) and inductive coding (allowing new themes to emerge from the data). The analysis unfolded in several stages:
Before coding began, AE developed a priori codebook based on the CFIR constructs most relevant to our digital health context. The team (AE, LH, TN, DH, TJ) conducted several meetings to align CFIR 1.0 domains, constructs, and codes. The team also conducted “test” quotes where several examples were given and each team member was asked to provide appropriate code(s), which was followed by discussions until consensus was reached. We drew upon the standard CFIR definitions for domains such as Intervention Characteristics (e.g., perceptions of the RPM/DHC intervention’s complexity, adaptability, and credibility), Outer Setting (e.g., influence of community resources, socioeconomic barriers, family support), Characteristics of Individuals (e.g., patients’ knowledge, self-efficacy, health literacy)15. This initial codebook served as a guide to ensure we systematically captured data corresponding to known implementation factors.
AE, DH, and TJ read and coded the transcripts, then met to reconcile coding differences and refine the codes. To establish consistency, AE, DH, and TJ first jointly coded four transcripts (approximately 10% of the sample). Data analysis consisted of reading through the transcripts and assigning segments of text to a priori codes, where appropriate. The team (AE, DH, TJ, TN) compared applications of the CFIR-based codes, with TN resolving any discrepancies, and LH guiding alignment with CFIR. Through these discussions, we refined code definitions and remained open to inductive coding of unanticipated responses. All codes were ultimately mapped to existing CFIR constructs. After achieving a high level of intercoder agreement on the pilot transcripts, the remaining transcripts were divided and coded independently with regular meetings to resolve questions. Coding queries in NVivo were used to track emerging ideas and ensure that important text segments were not overlooked.
Once coding was complete, we organized the coded data by CFIR domains and constructs to identify prominent themes. Key themes were defined as those that were salient (mentioned by many participants or emphasized with rich detail) and relevant to digital health engagement. We categorized each theme as primarily a barrier, a facilitator, or a dual role (acting as both, depending on context). We conducted peer debriefing sessions in which the qualitative team presented preliminary themes to LH and TN to get alternative interpretations.
Following this analytic process, we integrated the results to produce a narrative organized by the major CFIR domains and constructs. The Results section presents these themes along with illustrative quotations from participants. All quotations are labeled with participant IDs and CFIR domain. Qualitative reporting adhered to the Consolidated Criteria for Reporting Qualitative Research (COREQ) guidelines to ensure transparency and completeness (Supplementary Table 1).
Data availability
The qualitative datasets (interview transcripts) generated and analyzed during this study contain potentially identifying and sensitive patient information and are therefore not publicly available, in accordance with institutional IRB and participant consent restrictions. De-identified excerpts relevant to the study findings are included within the manuscript. Additional de-identified data may be requested from the corresponding author and will be shared upon reasonable request in line with institutional policies.
References
Office of Minority Health. Diabetes and Black/African Americans., <https://minorityhealth.hhs.gov/diabetes-and-blackafrican-americans> (2023).
Walker, A. F. et al. Interventions to address global inequity in diabetes: international progress. Lancet 402, 250–264 (2023).
Hill-Briggs, F. et al. Social determinants of health and diabetes: a scientific review. Diab. Care 44, 258–279 (2020).
Azelton, K. R. et al. Digital health coaching for type 2 diabetes: randomized controlled trial of healthy at home. Front Digit. Health 3, 764735 (2021).
Bollyky, J. B., Bravata, D., Yang, J., Williamson, M. & Schneider, J. Remote Lifestyle Coaching Plus a Connected Glucose Meter with Certified Diabetes Educator Support Improves Glucose and Weight Loss for People with Type 2 Diabetes. J. Diabetes Res. 2018, 3961730 (2018).
Davis, T. C., Hoover, K. W., Keller, S. & Replogle, W. H. Mississippi diabetes telehealth network: a collaborative approach to chronic care management. Telemed. J. E-Health 26, 184–189 (2020).
Seng, J. J. B., Nyanavoli, H., Decruz, G. M., Kwan, Y. H. & Low, L. L. Health coaching and its impact in the remote management of patients with type 2 diabetes mellitus: scoping review of the literature. J. Med. Internet Res. 27, e60703 (2025).
Ebekozien, O., Fantasia, K., Farrokhi, F., Sabharwal, A. & Kerr, D. Technology and health inequities in diabetes care: How do we widen access to underserved populations and utilize technology to improve outcomes for all. Diab. Obes. Metab. 26, 3–13 (2024).
Anderson, A., O'Connell, S. S., Thomas, C. & Chimmanamada, R. Telehealth interventions to improve diabetes management among black and hispanic patients: a systematic review and meta-analysis. J. Racial Ethn. Health Disparities 9, 2375–2386 (2022).
Borges do Nascimento, I. J. et al. Barriers and facilitators to utilizing digital health technologies by healthcare professionals. NPJ Digit Med 6, 161 (2023).
Bazzano, A. N., Patel, T., Nauman, E., Cernigliaro, D. & Shi, L. Optimizing telehealth for diabetes management in the deep south of the united states: qualitative study of barriers and facilitators on the patient and clinician journey. J. Med. Internet Res. 26, e43583 (2024).
Hailu, R., Sousa, J., Tang, M., Mehrotra, A. & Uscher-Pines, L. Challenges and facilitators in implementing remote patient monitoring programs in primary care. J. Gen. Intern. Med. 39, 2471–2477 (2024).
Joki, A., Ahola, A. J., Suojanen, L. U. & Pietilainen, K. H. Exploring successes, barriers, and enablers in the one-year digital healthy weight coaching. BMC Health Serv. Res. 24, 1367 (2024).
Kirk, M. A. et al. A systematic review of the use of the consolidated framework for implementation research. Implement Sci. 11, 72 (2016).
Reid, H. W. et al. Using the consolidated framework for implementation research to inform the design of the mobile inspeccion visual con acido acetico system: mixed methods case study. JMIR Form. Res. 6, e32577 (2022).
Rovner, B. W., Casten, R. J., Chang, A. M., Hollander, J. E. & Rising, K. Mistrust, neighborhood deprivation, and telehealth use in African Americans with diabetes. Popul Health Manag 24, 699–700 (2021).
Hasan, A. A., Ismail, A. & Noor, H. The Influence of social support on self-care behavior among T2DM patients. SAGE Open Nurs. 10, 23779608231219137 (2024).
Song, Y., Nam, S., Park, S., Shin, I. S. & Ku, B. J. The impact of social support on self-care of patients with diabetes: what is the effect of diabetes type? Systematic review and meta-analysis. Diab. Educ. 43, 396–412 (2017).
Rad, G. S., Bakht, L. A., Feizi, A. & Mohebi, S. Importance of social support in diabetes care. J. Educ. Health Promot 2, 62 (2013).
Bantham, A., Taverno Ross, S. E., Sebastiao, E. & Hall, G. Overcoming barriers to physical activity in underserved populations. Prog. Cardiovasc Dis. 64, 64–71 (2021).
Walker, R. E., Keane, C. R. & Burke, J. G. Disparities and access to healthy food in the United States: a review of food deserts literature. Health Place 16, 876–884 (2010).
Larson, N. I., Story, M. T. & Nelson, M. C. Neighborhood environments: disparities in access to healthy foods in the U.S. Am.J.Prev.Med. 36, 74–81 (2009).
Park, S., Zachary, W. W., Gittelsohn, J., Quinn, C. C. & Surkan, P. J. Neighborhood Influences on physical activity among low-income African American adults with type 2 diabetes mellitus. Diab. Educ. 46, 181–190 (2020).
Brown, O. The challenges of changing dietary behaviors of underserved. Am. J. Lifestyle Med. 7, 4–13 (2013).
Sergel-Stringer, O. T. et al. Acceptability and experiences of real-time continuous glucose monitoring in adults with type 2 diabetes using insulin: a qualitative study. J. Diab. Metab. Disord. 23, 1163–1171 (2024).
iGlucose. Smart Meter, available at https://consumerguide.diabetes.org/products/iglucose.
Maroko, A. R. et al. Integrating social determinants of health with treatment and prevention: a new tool to assess local area deprivation. Prev. Chronic Dis. 13, E128 (2016).
Hennink, M. M., Kaiser, B. N. & Marconi, V. C. Code saturation versus meaning saturation: how many interviews are enough? Qual. Health Res 27, 591–608 (2017).
CFIR Interview Guide Tool, https://cfirguide.org/guide/app/#/guide_select.
Economic Research Service (ERS), U.S., Department of Agriculture. Adult Food Security SurveyModule: three-stage design, with screeners., https://www.ers.usda.gov/topics/food-nutrition-assistance/food-security-in-the-u-s/survey-tools/ (2012).
Kind, A. J. H. & Buckingham, W. R. Making neighborhood-disadvantage metrics accessible - the neighborhood atlas. N. Engl. J. Med. 378, 2456–2458 (2018).
Acknowledgements
This research was supported by the National Institutes of Health (NIH), National Institute on Minority Health and Health Disparities (NIMHD), under award number 5P50MD017338-04. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Contributions
A.E. conceptualized the study, developed the interview guide and codebook, led the qualitative analysis, and wrote the manuscript. G.G. and S.J. conducted interviews. A.E., D.H., and T.J. coded the transcripts, with A.E. and L.H. providing methodological oversight and T.N. resolving discrepancies and assisting with analysis. L.H. provided feedback on study design and analysis. T.M. provided input on study design and manuscript revisions. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
El Zein, A., Hays, D., Glidden, G. et al. A CFIR-guided qualitative study of digital health engagement among Black adults with type 2 diabetes. npj Digit. Med. 8, 755 (2025). https://doi.org/10.1038/s41746-025-02107-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41746-025-02107-x