Abstract
Adipose tissue-derived mesenchymal stromal/stem cells (ADMSCs) represent a novel therapeutic intervention for Type 1 Diabetes (T1D). The attractiveness of ADMSCs is characterized by their immunomodulatory activities, regenerative properties, and relative ease of access. ADMSC therapies in animal models and clinical trials have revealed decreased insulin dependence, increased β cell mass, and improved islet graft acceptance. Despite their potential, challenges in quality control, small-scale investigations, functional heterogeneity, and standardization limit the application of these therapies. This review synthesizes the current knowledge and recent outcomes of ADMSC therapies in treating T1D and highlights areas that need further investigation.
Similar content being viewed by others
Introduction
Type 1 Diabetes (T1D) is an immune-related disease caused by the autoimmune destruction of the insulin-producing β cells, resulting in lifelong insulin dependence. The onset of T1D is a complex interplay between an individual’s genetic predisposition and environmental factors that trigger the immune system, causing hyperglycemia and related complications upon β cell destruction1. The global incidence of T1D has been estimated to increase to 13.5-–17.4 million people by 20402,3. The steady increase in T1D prevalence indicates the need for novel T1D treatments.
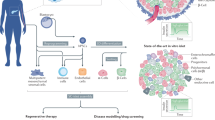
T1D pathogenesis can be divided into three stages based on hyperglycemia’s presence (or absence) and associated symptoms4. Stage 1 is defined by normoglycaemia accompanied by two or more islet-directed autoantibodies. There is an observed influx of dendritic cells (DCs) into islets, potentially triggered by impaired islet architecture remodeling via apoptosis, cross-presentation of endogenous peptides, or superantigen-driven immune responses5. Autoreactive CD4+ T cells stimulate APCs (antigen-presenting cells) and help CD8+ T cells attack β cells by releasing TNF-α, IFN-γ, Fas/FASL, and perforin/granzyme (Fig. 1). Released cytokines also stimulate macrophages to damage β cells, yielding a positive feedback loop. This triggers NF-κB (Nuclear factor-κB) enhancer and activates β cells, which is primarily pro-apoptotic6. IFN-γ upregulates the expression of FAS receptors (CD95) on β cells, making them susceptible to IL-1β-induced apoptosis. IFN-γ also increases TNF-α and IL-1β secretion, along with reactive oxygen species (ROS), amplifying immune responses and exacerbating β cell stress7. In addition to oxidative stress, ER (Endoplasmic Reticulum) stress plays a critical role in β cell dysfunction and apoptosis in T1D. Increased cytokine exposure and sustained inflammatory signaling disrupt protein folding in the ER, accumulating misfolded proteins. This triggers the unfolded protein response (UPR), which, if unresolved, activates pro-apoptotic pathways involving CHOP (C/EBP homologous protein) and JNK (c-Jun N-terminal kinase), further promoting β cell demise. Additionally, prolonged ER stress impairs insulin biosynthesis and secretion, exacerbating metabolic dysfunction in early T1D pathogenesis7.
T-lymphocyte-mediated insulitis, followed by the presence of one or more types of autoantibodies (AAbs) directed against insulin, glutamic acid decarboxylase (GAD), protein tyrosine phosphatase IA-2 or IA-2β, and zinc transporter 8 (ZnT8), is indicative of the immunological onset of T1D. Created in BioRender. Pociot, F. (2025) https://BioRender.com/c94c618.
Stage 2 is defined by two or more autoantibodies, β cell dysfunction, and progressive elimination of the β cell mass4. The inflammatory microenvironment created in the islets causes an increase in vascular permeability and the infiltration of CD8+ T cells, CD20+ B cells, CD4+ T cells, and CD68+ macrophages8,9. Stage 3 marks the clinical diagnosis of T1D and is usually the initiation of insulin therapy4,5. The rate and extent of β cell destruction can vary among patients as can the age of onset and the number and type of antibodies present at diagnosis, pointing to its heterogenous nature in presentation4. The recent regulatory approval of a T1D immunotherapy designed to slow the immune-mediated destruction of pancreatic β cells underscores the fundamental immunological basis of type 1 diabetes while establishing a precedent for future immune-modulating therapeutic approaches to combat this condition.
After insulin replacement, the most common treatment strategies for T1D are immunotherapies10 and cell replacement therapies11. These treatment strategies have shifted how therapeutic development is viewed for T1D, redirecting the focus on targeting the cause over treating the symptom. This change arises from the need to target islet-specific immune pathways while avoiding toxicities associated with conventional treatments10. Thus, novel therapeutics have shifted towards utilizing the regenerative and immunomodulatory properties of stem/stromal cells, specifically mesenchymal stem cells (MSCs). MSCs, non-hematopoietic, multipotent stem cells can differentiate into numerous cell types, have tissue regenerative and immunomodulatory properties, conferring substantial therapeutic potential for T1D intervention12,13 which release various growth and inflammatory factors like vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), fibroblast growth factor (FGF) and prostaglandin E2 (PGE2), which contribute to the repair of injured tissues14. MSCs can be isolated from various sources (bone, cartilage, adipose, endothelium, and muscle) and can differentiate into multiple lineages12. Preclinical trials using MSCs to treat rheumatoid arthritis, T1D, multiple sclerosis, systemic lupus erythematosus, inflammatory bowel disease, autoimmune liver disease, and Sjogren’s syndrome have exhibited the cells’ immunosuppressive and regenerative potential in treating autoimmune diseases15. Their low immunogenicity and low tumorigenic effects16 add to their therapeutic applications, which have been significant challenges associated with islet transplantation, along with 60% islet loss occurring in most cases due to hypoxia and nutrition deficiencies17. They also play an essential role in lowering fasting blood sugar levels, hemoglobin A1c and C peptide levels, and treating microvascular complications associated with T1D14,15. Due to their abundance, ease of isolation, applicability, and autologous nature, ADMSCs have become a focus in the clinical setting14. For this review, “ADMSCs” will refer to all forms of Adipose tissue-derived mesenchymal stromal/stem cells unless identified explicitly as ADSCs (Adipose tissue-derived stromal/stem cells). Here, we discuss the therapeutic benefits, progress, and prospects of ADMSC therapy for T1D.
Adipose-derived mesenchymal stromal/stem cells (ADMSCs)
ADMSCs represent an attractive source of MSCs for therapeutic applications. Adipose tissue yields a high content of MSCs and is easily accessible relative to harvesting other MSC sources, making it particularly attractive for research18. They are isolated from different adipocyte tissue depots, like visceral, subcutaneous, and preperitoneal19. Subcutaneous and visceral ADMSCs exhibit distinct biological profiles despite similar surface markers. Subcutaneous ADMSCs display classical mesenchymal morphology with directed motility, efficient focal adhesion turnover, and increased ciliated cells. Conversely, viscerally isolated ADMSCs demonstrate enhanced differentiation potential toward osteogenic and adipogenic lineages, higher stemness gene expression (c-MYC, KLF4, NANOG, SOX2), and increased inflammatory cytokine secretion (IL-6, IL-8, TNFα), reflecting their physiological role in metabolic regulation and inflammatory signaling19. ADMSCs are predominantly isolated from white subcutaneous adipose tissue from surgical waste like lipoaspirates18. Isolation typically employs enzymatic digestion with collagenase (0.05–0.15%) for 30–90 min at 37 °C to break down the extracellular matrix, followed by centrifugation to collect the stromal vascular fraction, red blood cell lysis, and filtration through 70–250 μm nylon mesh to remove debris. The resulting cells are cultured on plastic surfaces, with non-adherent cells removed by washing. Adipose tissue represents an exceptionally rich MSC source, yielding 2 × 105−5 × 104 cells/gram compared to bone marrow’s 6-60 × 103 cells/ml, though yields vary with donor characteristics, tissue collection methods, and culture conditions20.
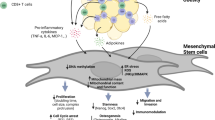
Stromal cells (adipocytes) retain the capacity for differentiation and self-renewal throughout an individual’s lifetime21. They also express α4 integrin that forms a heterodimer with CD29 to create very late activation antigen 4 (VLA-4) and mediate migration to inflammatory areas22. Further, ADMSCs have been shown to restore and preserve ß-cell mass effectively23, differentiate into insulin-producing cells (IPCs)24,25, and exert immunomodulatory effects (Fig. 2)26. ADMSCs have a higher differentiation potential into IPCs than bone marrow-derived MSCs (BMMSCs)27, more potent immunomodulatory effects, and higher cytokine secretion28. The International Society for Cell & Gene Therapy (ISCT) has defined the minimal criteria for characterizing ADMSCs: 1) adherence to plastic; 2) positive expression of CD73, CD90, CD105, CD13, CD29, and CD44 with negative expression of CD45, CD14, CD11b, CD79a, CD19, CD31, and CD235a; 3) ability to differentiate into pre-adipocytes, chondrocytes, and osteoblasts29. Beyond those included in the ISCT criteria, ADMSCs express other CD markers. Table 1 shows the positive cell markers expressed in ADMSCs and their biological and therapeutic functions.
ADMSCs modulate the immune system by shifting pro-inflammatory cells (M1 macrophages, Th1/Th17) to anti-inflammatory states (Tregs, M2 macrophages) while promoting insulin-producing β-cells regeneration via microRNAs. The dual effects of immunomodulation and regeneration aim to restore β-cell function and reduce autoimmune destruction. ADMSC, Adipose-Derived Mesenchymal Stem Cell; iPSCs, induced pluripotent stem cells; Th, T helper cell; Treg, regulatory T cell. Created in BioRender. Pociot, F. (2025) https://BioRender.com/o46i021.
Studies report that ADMSCs exhibit higher therapeutic capacity than other MSC sources30,31,32,33. When compared to BMMSCs, ADMSCs have been shown to exhibit higher proliferation rates34, adipogenic capacity30, and the ability to maintain morphology and cell activity up to the 15th passage29. A study by Yi et al. compared MSCs from different sources (adipose tissue, amniotic membrane, umbilical cord) and demonstrated that ADMSCs exhibit a more significant proportion of subpopulations associated with vascular regeneration, blood vessel development, and rates of angiogenesis35. Adipose tissue is highly vascularized to maintain body temperature and supply nutrients and oxygen, corresponding to its high angiogenic capabilities35. Additionally, a study by Liu et al. found that ADMSCs could help decrease body weight and adipose tissue in db/db mice. This result was not found by therapies using umbilical cord-derived mesenchymal stem cells (UCMSCs). Excess blood glucose can be stored as fat when not efficiently used or removed. While weight loss may signal improved glucose metabolism, it is not always reliable, as unintended weight loss can also occur in conditions like poorly managed diabetes. Although UCMSCs exhibited comparable regeneration of islet cells, they did not show any decrease in body weight or adipose tissue quantity36. Studies on BMMSCs have revealed compelling co-expression results of specific cell surface markers also present in ADMSCs. CD39 and CD73 work together in the catabolism of ATP, generating adenosine and phosphate from ATP/ADP. Adenosine is effective in the immunosuppression of T-cells via the adenosine A2A receptor. The suppression of T-cell proliferation has demonstrated solid anti-inflammatory effects, increased immunoregulation, and decreased tissue damage29. Since these CD markers are also present in ADMSCs, they may exhibit the same immunoregulatory effect, though no studies have been done in ADMSCs specifically.
The prevailing school of thought is that CD34 is negatively expressed in typical MSCs, which sets them apart from hematopoietic stem cells. However, CD34 can be positively expressed in ADMSCs37. However, this evidence for MSCs is based on cultured MSCs and not tissue-resident MSCs37. It has been shown that CD34 is variably expressed in ADMSCs cultured as a monolayer, and cells gradually lose expression across various passages. After 8-12 passages, most cultures have completely lost expression of CD3437,38. Although CD34 expression has been shown to enhance the proliferation and migration of progenitor cells, little is known regarding the biological function of CD34 in ADMSCs39. The functional role of CD34 in ADMSCs must be further researched to understand how its presence and absence affect the therapeutic effect of these cells. ADMSCs help suppress the overactive immune system and promote an anti-inflammatory response. Their immunotherapeutic properties allow them to target T1D development in the body by reducing the expression levels of proinflammatory cytokines, which attack the ß-cells. The immunomodulatory, regenerative, and trophic effects make them attractive for T1D therapy.
Immunomodulatory effect of ADMSCs
T1D is an autoimmune disease in which auto-reactive T cells are the primary attackers. Novel T1D treatments aim to target these lymphocytes. By evading CD8+ T cell activation, ADMSCs are less likely to be targeted and destroyed, allowing them to persist longer in allogeneic environments. ADMSC’s low MHC class I molecules and lack of MHC class II molecules do not effectively activate the CD4 + T cells, inhibiting T cell proliferation and reducing the overall alloimmune response40. Furthermore, their secretome has growth factors like i.e., granulocyte colony-stimulating factor (GCSF), granulocyte-macrophage colony-stimulating factor (GMCSF), nerve growth factor (NGF), keratinocyte growth factor (KGF), vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), or insulin-like growth factor 1 (IGF-1), antiapoptotic, antioxidative, and anti-inflammatory signaling molecules30. Studies indicate that ADMSCs can inhibit the self-reacting T-cell expansion, development of dendritic cells, and β cell proliferation by influencing the pancreatic microenvironment through immune modulation41,42. Ock et al. found that ADMSC were more potent immunomodulators and efficient in forming colonies due to their proliferative potential compared to bone marrow and dermal tissue derived MSCs43. Their interaction with innate and adaptive immune cells results in the downregulation of proinflammatory cytokines like IL-1β, TNFα, and IL-6 and the upregulation of anti-inflammatory cytokines such as IL-10, PGE2, or indoleamine 2, 3-dioxygenase (IDO)44. In addition, a negative feedback mechanism exists between the activated T cells that produce IFNγ and the ADMSCs. IFNγ secretion primes the ADMSCs against T cell proliferation, allowing the ADMSCs to evade detection by the immune system. Concurrently, this priming enhances their ability to maintain self-renewal and differentiation into multiple cell lineages, promoting effective allogenic tissue repair and regeneration45,46. Li et al. showed that ADMSCs decreased fasting blood glucose in STZ-induced T1D animals, increasing insulin expression46. Rahavi et al. showed in vitro ADMSCs inhibit splenocyte proliferation in a dose-dependent manner and preserve pancreatic islets’ viability and insulin secretion capabilities in the presence of reactive splenocytes47. Table 2 summarizes their immunomodulatory effects.
Treg regulation of ADMSCs
Individuals with T1D have reduced CD4+CD25+ regulatory T cell (Tregs) functionality48. These cells are essential in regulating the immune system, maintaining homeostasis, and tolerating self-antigens in T1D patients (Fig. 2)48.
The reduction of the Treg ratio by disrupting the B7/CD28 pathway has been shown to accelerate the onset of T1D in NOD mice49. At the same time, the expansion of Tregs in pancreatic lymph nodes was correlated with disease resistance50. Several experimental therapies for T1D have demonstrated a better outcome when there was an increase in the frequency of Tregs, especially CD4+CD25+FOXp3+ Tregs51,52,53. It has been demonstrated in NOD mice that ADMSC transplantation can induce the expansion and proliferation of CD4+CD25+Foxp3+ Tregs and reduce the Th1 immune response53, which can help improve blood glucose levels in early-onset T1D. In an experiment exploring the ADMSC therapy in T1D, Bassi et al. demonstrated that mice treated with ADMSCs had higher CD4 + FOXp3+Helios+ cells and a lower frequency of IFN-γ and TNF α in pancreatic lymph nodes54. These results helped demonstrate an efficient long-term immune regulatory effect of ADMSCs in T1D treatment.
Anti-inflammatory response of ADMSCs
Cytokines play a crucial role in orchestrating complex interactions between pancreatic β cells and immune cells in the development of T1D55. ADMSCs secrete high levels of anti-inflammatory cytokines, including IL-1Ra, IL-4, IL-10, TGF-β, and IL-1356. They are shown to induce the proliferation of a subset of CD5+ regulatory B cells that secrete immunosuppressive IL-10, which suppresses Th1-type cytokines (IL-2 and IFNγ)57. ADMSC transplantation has also been reported to induce M2 macrophage polarization; proliferation of CD4+ and CD8 + T-cells; inhibition of monocyte-derived dendritic cells; B cell and natural killer cell differentiation and maturation; and reduction of macrophage and neutrophil infiltration into inflammation sites46.
Immune checkpoint blockades released by ADMSCs
MSCs express different types of immune checkpoint blockade inhibitors and their ligands, which allows them to influence cells of the adaptive and innate immune system, thus playing an important role in immunomodulation, as illustrated in Table 3.
While PD-1/PD-L1 inhibitors remain prominent in treating non-small cell lung cancer, as discussed by Li et al.58 and Paz-Ares et al.56 researchers are increasingly exploring additional immune checkpoint pathways. These include: avelumab for Merkel cell carcinoma59, TIM3 as an emerging cancer target60, ICOS/ICOS-L costimulatory pathway61, A2aR antagonists as next-generation checkpoint therapy62, TIGIT for melanoma treatment63, BTLA in NSCLC therapy58,64, TNFR265, IDO inhibitors66,67, and CD47 for NSCLC and metastatic cancers as shown by Lau et al., and Lian et al.68,69, Hazrati et al. Address MSCs’ therapeutic and immunomodulatory potential through immune checkpoint-related molecules, representing a comprehensive examination of how MSCs expressing these molecules could be leveraged for treating various cancers, with NSCLC, melanoma, and Merkel cell carcinoma being the primary disease targets. Recent studies have been successful in showing why pre-treated MSCs in inflammatory conditions (treated by TNFα and IFN-γ) lead to increased immune checkpoints, ligand expression on the MSC surface, and, thus, an overall increase in the cells’ immunomodulatory potential70,71,72. The impacts of the proinflammatory environments on the production of anti-inflammatory cytokines and their effects on the signaling pathways lead to an increased expression of immune checkpoint blockades and ligands on MSC surfaces73. Negative immunological checkpoint receptors like cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 are key in giving inhibitory signals for potentially autoreactive T cells74. A study by Kawadi-Horitani et al. demonstrated that the systemic application of ADMSCs with an anti-PD-L1 monoclonal antibody (mAB) reduced the incidence of developing T1D in male NOD mice from PD-1/PD-L1 blockade-induced T1D from 64% to 19%75. Their systemic injections partially protected the pancreas from β cell loss and preserved insulin content76. Their results were in line with what has been shown in a study exploring the T lymphocyte infiltration in pancreatic islets of a patient who developed T1D after being administered with immune checkpoint inhibitors77. They showed a substantial increase in T cell positive area and accumulated CXCL9 positive macrophages78 in pancreatic islets when injected with anti-PD-L1 mAb induced T1D without ADMSCs. A separate study showed that CD8-positive T cells secrete IFN-γ in response to PD-1 blockade, which activates infiltrated monocyte-derived macrophages to accelerate the progression of T1D53,79.
Regenerative potential of ADMSCs
ADMSCs secrete bioactive growth factors, chemokines, and cytokines that help ameliorate metabolic abnormalities linked to T1D80. Possible mechanisms by which ADMSCs have been shown to improve hyperglycemia include islet β-cell regeneration, modulation of hepatic metabolism toward higher glucose utilization, reduction of inflammation, and amelioration of insulin resistance in peripheral tissues81. One key mechanism is their potential to differentiate into insulin-producing cells (IPCs), which has been demonstrated in vitro and in transplantation models (Fig. 2)81. However, these ADMSC-derived IPCs differ from primary β cells in their functionality. While they express key β cell markers such as PDX1, NKX6.1, and MAFA, their insulin secretion in response to glucose stimulation is often lower than that of native β cells. Compared to iPSC-derived β cells, ADMSC-derived IPCs exhibit lower insulin content and glucose responsiveness, highlighting the need for further optimization of differentiation protocols. Functional assessments of these IPCs have primarily been conducted in vitro through glucose-stimulated insulin secretion assays and in vivo transplantation studies in diabetic mouse models, showing partial glucose regulation and increased C-peptide levels. They also have the potential to facilitate endogenous β cell regeneration, preservation of residual β cell mass preservation, and improved islet graft acceptance82. Pre-clinical and clinical trials have shown effective β cell regeneration and preservation due to the paracrine release of trophic, immunomodulatory, and anti-inflammatory factors in the cells83,84. Furthermore, ADMSCs have been reported to promote β cell proliferation by enhancing the expression of pancreatic progenitor markers such as Ngn3 and NeuroD1, which are crucial for β cell neogenesis23. ADMSC-differentiated IPCs have been shown to induce insulin secretion and increase C-peptide, which is linked with alleviated hyperglycemia14. The regenerative and immunomodulatory properties of ADMSCs extend to β cell proliferation and restoration. The application of ADMSCs has shown increased activity of regulatory T cells and decreased autoreactive T cells, helping limit the autoimmune destruction of β cells41. Studies have also demonstrated increased pancreatic islet regeneration in T2D models85.
Genome engineering strategies have been explored to enhance ADMSC function for T1D therapy. Techniques such as viral and non-viral vectors and CRISPR/Cas9 have been employed to modify ADMSCs for improved immune evasion and therapeutic efficacy. Beyond the reported modifications involving IL-10 and CXCL4, additional studies have investigated overexpression of PDX1, NKX6.1, and MAFA to drive β cell-like differentiation and enhance insulin secretion86. However, the full potential of genome editing in ADMSCs remains underexplored. More studies are required to determine how genetic modifications impact ADMSC stability, differentiation potential, and long-term regenerative capacity. Notably, concerns remain regarding the unintended effects of genome editing on the epigenetic landscape of ADMSCs, which could influence their differentiation potential and safety profile. Addressing these challenges will be essential for optimizing genetically engineered ADMSCs for clinical applications.
Sen et al. demonstrated that delivering superoxide dismutase using ADMSCs as a gene delivery vehicle reduced inflammation, improved glucose tolerance, and enhanced homing in inflamed adipocyte pockets in vivo in mice86. These findings highlight the potential of ADMSCs as vehicles for targeted gene therapy in T1D. However, additional studies are needed to explore broader applications of genetic engineering in ADMSC-based therapies and assess long-term effects on cell function and stability.
Trophic activity of ADMSCs
The ability of ADMSCs to supply reparative cytokines and bioactive growth factors is superior in stimulating cell proliferation and differentiation87 compared to other MSCs. ADMSCs secrete growth factors like KDR, VEGF87, TGF-β, IL-8, HGF, KDR, and IGF-1. These factors promote angiogenesis, support the success of islet graft transplantation, and promote the production of IL-1Ra, IL-8, and HGF. The antiapoptotic and proangiogenic factors secreted by ADMSCs, such as VEGF88,89,90, IGF91,92, TGF-β86,93, and GM-F94, also add to their therapeutic benefits when used in islet transplantation for T1D14. However, culture conditions significantly affect ADMSC characteristics, including their growth capacity, surface marker expression, and therapeutic potential95. An increase in pancreatic duodenal homeobox (PDX-1) seen in multiple studies conducted in vitro in animal models23,30 is known to play a role in β cell differentiation by regulating normal pancreatic development and improving survival of graft transplants in STZ-induced diabetic mice96,97.
Understanding the effects of ADMSC on the bioenergetics of the target cells is also essential98, since cells affected by stress (e.g., diabetes condition) may have higher energetic demands, which can put cells under stress, impairing their repair and replication processes99. For instance, BMMSC treatment is shown to increase the bioenergetic capacity of the stressed cells through increased efficiency of oxidative phosphorylation and the TCA cycle100. It can be hypothesized that ADMSCs exhibit similar behaviors due to similar characteristics, but further research is necessary.
ADMSCs-derived extracellular vesicles (EVs)
Along with mediating intercellular communications, the molecular composition of EVs mirrors the effects of the parent cells, which makes them a valuable tool for diagnostic and therapeutic applications101. MSC-derived EVs contain bioactive cargos that provide therapeutic effects in Type 1 diabetes through dual mechanisms of immunomodulation and β-cell regeneration102. They carry functional miRNAs (miR-21, miR-106b-5p, miR-222-3p)103,104 and proteins like VEGFA that activate beneficial signaling pathways (PI3K/Akt/eNOS, GSK-3β)105 while inhibiting inflammatory ones (p38 MAPK, NF-κB)106. These molecular signals upregulate key pancreatic transcription factors (PDX1, PAX4, NeuroD) and survival proteins (Bcl-2, HIF-1α)107,108, collectively enhancing β-cell proliferation, insulin secretion, and islet survival—positioning MSC-EVs as promising therapeutic candidates for T1D treatment.
Bone marrow-derived MSC-EVs (BMMSC-EVs) and adipose-derived MSC-EVs (ADMSC-EVs) are the two most studied sources of MSC-derived EVs. While they share overlapping regenerative functions, they exhibit distinct molecular compositions and therapeutic effects. Studies comparing their regenerative capacity, immunomodulatory properties, and angiogenic potential have identified common signaling pathways, including VEGF109,110 and AKT-related pathways111,112. However, BMMSC-EVs tend to contain a broader range of protein types113, with higher levels of VEGFA, FGF-2, and PDGF-BB114, and they have been shown to enhance IL-10 secretion by 1.8-fold compared to ADMSC-EVs115, contributing to their potent immunosuppressive effects. In contrast, ADMSC-EVs exhibit higher levels of CD63 and phosphatidylserine116 and are enriched in hepatocyte growth factor (HGF), which supports tissue repair and anti-apoptotic functions. Their higher yield and accessibility make them attractive for scalable clinical applications.
Beyond EVs, MSCs release a wide array of bioactive molecules, collectively known as the MSC secretome, which plays a crucial role in their therapeutic potential. This secretome includes soluble proteins, cytokines, chemokines, growth factors, and metabolites that complement the effects of EVs. The MSC secretome contributes to β-cell regeneration by promoting proliferation, survival, and insulin secretion while modulating immune responses in T1D117. Among the key components, transforming growth factor-beta (TGF-β), IL-10, and prostaglandin E2 (PGE2) exert immunosuppressive effects, reducing autoreactive T-cell activation and fostering a tolerogenic microenvironment. Additionally, EV-contained cargo, such as miRNAs (e.g., miR-21, miR-146a, miR-155) and proteins (VEGFA, IGF-1, HGF), mediate islet protection, angiogenesis, and anti-inflammatory responses, ultimately enhancing β-cell function and islet survival118.
ADMSC-derived EVs exhibit the same immunoregulatory and multipotent properties as their parental cells, making them appealing as potential “mobile” drug delivery systems119. In 2018, Nojehdehi et al. demonstrated that in vivo intraperitoneal application of EVs derived from autologous ADMSCs ameliorated the autoimmune response in T1D mice120. Their study showed a significant increase in the levels of anti-inflammatory cytokines (TGF-β, IL-4, and IL-10) and a significant reduction in the production of pro-inflammatory cytokines (IL-17 and IFN-γ) without any significant changes in the stimulation index of tested mononuclear cells120. Another study conducted in 2021 by Gesmundo et al. demonstrated that ADMSC-derived EVs promoted β-cell proliferation and insulin secretion in INS-1E β cells and human pancreatic islets, even without cytokine exposure121. Similarly, Arzouni et al. reported improved glycemic control and islet function following administration of ADMSC-derived EVs in vivo, 28 days post-islet graft transplantation in mice122,123. Their findings also showed that these EVs improved insulin secretory function in both mouse and human islets in vitro124,125. By leveraging their unique cargo and immunomodulatory potential as summarized in Table 4, MSC-derived EVs—particularly those from ADMSCs and BMMSCs—offer a promising avenue for β-cell protection and regeneration in T1D treatment. However, further comparative studies are necessary to optimize their therapeutic potential for clinical applications.
Route of administration of ADMSCs
The route of administration significantly influences the therapeutic potential of ADMSC treatment119. Intravenous injection (IV) is the most examined route126. However, it is associated with MSC entrapment in the lungs and in the reticuloendothelial system (RES) organs, such as the spleen, liver, bone marrow, thymus, and skin127,128,129. The human body’s defense mechanism in circulation and tissues, and the RES cells play an essential role in the clearance of substances130. Thus, IV administration is associated with less therapeutic efficacy126,130,131 and some organ-specific complications. Lung accumulation causes pulmonary and hemodynamic alterations in lung vessels130,132 that hamper the ability of MSCs to reach the pancreas101 and other target organs133. This entrapment is also a result of interactions between the MSC adhesion molecules and the ligands in the endothelium103, causing nonspecific accumulation. Another reason for the microembolization is that the average size of the injected MSCs is greater than that of the pulmonary capillaries134.
Hashemi et al. conducted a study that investigated the effects of intraperitoneal (IP) and IV infusion of ADMSCs and MSC-Conditioned Medium (CM) on the C57B1/6 male mice130. They measured the blood urinary glucose, body weight, and percentages of CD4 + CD25 + FOXP3 + T cells, IFN-γ, TGF-β, IL-4, IL-17, and IL-1. Their study showed significant (p < 0.05) amelioration of hyperglycemia at 6 weeks after injection and a significant increase in the number of insulin-positive islets in the CM–IP. Their results also indicated that IP-injected MSCs had a more significant impact on splenocyte suppression than IV-injected MSCs and higher levels of anti-inflammatory cytokines than the ADMSC–IV group, which could result from mesenteric circulation absorption130.
Khatri et al. studied the MSCs in direct and indirect contact with pancreatic islets and evaluated the protective aspect of MSC administration through the intrapancreatic and IV routes135. In vitro, examination of STZ-damaged MIN6-cells showed superior protection to the cells from STZ through the AKT/ERK pathways involved in mitogenic signaling in the presence of MSCs. They also showed that the IPR route of administration in vivo resulted in a higher proliferation in pancreatic islets and balance in the Th1/Th2 response. In line with other experiments128,136,138,138, they demonstrated in vitro an upregulation of EGF and IL-10 and a downregulation of IL-1β and TNF-α; thus, the ADMSC secretome impeded the proapoptotic microenvironment. Additionally, Schröder et al. demonstrated that histone deacetylase inhibitors can enhance the differentiation potential of mesenchymal stem cells toward pancreatic endocrine lineages, with the broad-spectrum inhibitor LBH589 significantly upregulating key transcription factors Isl1 and Pax6 while reducing uncontrolled proliferation139. Another team also demonstrated that direct intra-arterial administration of ADMSCs into the pancreas could maintain glycemic regulation in an STZ-induced preclinical diabetic rat model140 better than when the same therapy was given via an IV route.
In an in vivo study, Yaochite et al. evaluated the long-term therapeutic efficacy and biodistribution of ADMSCs administered through intrasplenic and intrapancreatic routes141. They found that the intrasplenic route reversed hyperglycemia in 70% of diabetic mice, compared to 42% with the intrapancreatic route141. The intrapancreatic route was chosen to deliver ADMSCs directly to the pancreas. The intrasplenic route aimed to deliver therapeutics to the pancreas137 via the splenic artery and promote the modulation of splenocytes to reduce the immunogenic response to the β cells. This principle also aligns with a study that showed NOD mice treated with irradiated splenocytes that exhibited normoglycemia also exhibited the reappearance of pancreatic islets without invasive insulitis142, thus highlighting the role of the splenocytes in promoting β cell proliferation. Histological analysis of the pancreas 70 days after ADMSC administration showed that the β cell mass and insulin production from the intrasplenic route were significantly higher than the intrapancreatic route. There were also increased TGF-β levels in the pancreas in the group administered the ADMSCs through the intrasplenic route.
Clinical trials using ADMSCs
The success of ADMSCs in treating T1D in preclinical research has led to their application in the clinical setting, where their therapeutic potential is further investigated. ADMSCs’ regenerative properties have been tested for treating a range of conditions, including rheumatoid arthritis (NCT01663116, NCT03691909), tissue damage (NCT02298023, NCT02784964), and skin wounds (NCT02394873, NCT02092870), along with many others.
There are 409 registered clinical trials analyzing the potential of ADMSC-based therapies (search: adipose-derived stem cells https://clinicaltrials.gov/, accessed on 24 July 2024). Of these, only four studies are for T1D (results have been posted from three). These trials analyze the safety and efficacy of both autologous and allogeneic ADMSC treatment, administered IV, for decreasing insulin dependence in patients with T1D (Table 5). ADMSCs were collected from healthy adults in all trials, and IVs were injected into the patient’s arm. In NCT03920397, an oral dose of cholecalciferol 2000UI/day was also administered, leading to partial clinical remission in all patients receiving the combined treatment. This study defined partial clinical remission by an IDAA1c index < 9. Gabbay et al. studied combined treatments with cholecalciferol and insulin, finding patients in the combined treatment group to have higher levels of CCL2 serum and regulatory T cells. The increase in these levels may correspond to the delayed destruction of β cells. With ADMSCs, an oral vitamin dose has led to C peptide stability, preservation of T-cells, reduced insulin dependence, and lower HbA1c levels in patients143.
The ISCT has provided minimal criteria for defining ADMSCs, leading to variation in proliferation rates, cell quality, immunomodulatory effects, cellular composition, and CD marker expression144,145,146,147. This variability is a challenge for clinical application due to inconsistencies between studies. To accelerate the use of ADMSCs in the clinic, a set of quality control criteria must be implemented to define them, along with standardized assays and culturing methods147,148,149.
The use of MSCs in a clinical setting is also associated with ethical concerns. An article examining MSC-related complications like pulmonary embolism and tumor formation stressed the need to focus on the safety issues and complications associated with the clinical translation of MSCs150. This also extends to considerations for recruiting appropriate subjects, avoiding misconceptions regarding MSC therapeutic potency, and facilitating informed decisions regarding consent forms151.
Future prospects
ADMSCs have immense therapeutic potential152, but minimal protocol standardization and clinical understanding limit their applications. Before these treatments can be granted routine therapeutic approval, further research with more subjects and larger time frames must be completed, along with standardized ADMSC procedures. Moreover, several aspects require further investigation to optimize their therapeutic potential.
Different ADMSC collection methods and patient characteristics lead to varied results across cells, even when treated with similar procedures153. Not only for clinical applications but in pre-clinical research, variation in cell origins, culturing conditions, and obtainment procedures increase the difficulty of comparing across research outcomes or applying results to a clinical setting153. Thus, a standardized ADMSC collection, culture, and isolation protocol must be established before their application in the clinic. Further, understanding how donor characteristics (age, genetics, comorbidities, and general health) affect the function of collected cells is imperative to determine donor criteria154. Also, understanding how patient-specific factors influence ADMSC therapy outcomes is crucial for personalizing treatment approaches. Lipogems® is trying to redefine the use of adipose tissue by harnessing its regenerative powers with a new approach. Lipogems® obtains micro-fragmented adipose tissue through a minimally invasive procedure, yielding tissue with highly regenerative MSCs. After the tissue has been washed, it is emulsified, yielding adipose clusters between 0.3 and 0.8 mm that can be implanted into the body155. This technique has been clinically available since 2010 and suggests a possible route to continue studying the potential of ADMSCs to treat T1D. Although Lipogems® has been mainly used in cosmetic and orthopedic procedures, it is important to understand how their collection process may help improve ADMSC therapy availability.
Numerous studies suggest that altering the culture conditions of MSCs can enhance their therapeutic potential156,157,158. Cultivating MSCs in 3D cultures is reported to boost their immunomodulatory function159 and EV production160. Hazrati et al. showed that a multicellular spheroid 3D culture increases the angiogenic potential of the MSCs by inducing the production of CXCL12, HGF, VEGF, and FGF-2, improves MSC survival due to a higher binding rate than single-celled suspensions, and decreases expression of pro-inflammatory cytokines91,161. Patel et al. found that a lower seeding density of MSCs led to higher EV yield143. Hypoxia priming of MSCs is beneficial to their therapeutic use155. Studies show that using hypoxia158,162,163 and inflammatory conditions164 as a preconditioning tool can enhance MSC pro-survival markers and increase the release of growth factors and chemo-attractants involved in cell proliferation158,165. Specific studies analyzing these results in ADMSCs are imperative to understanding how these cells respond to the pre-treatments. These pre-treated ADMSCs in T1D therapeutics may increase their efficacy and decrease dosage requirements164. Thus, standardization and optimizing the ex vivo expansion and preparation of ADMSCs can significantly impact their therapeutic efficacy146,147.
While ADMSCs offer promising regenerative and immunomodulatory effects, concerns remain regarding their potential role in tumorigenesis. Their ability to secrete high growth factors, including VEGF, FGF, and HGF, which promote angiogenesis and cell proliferation, has raised concerns about their possible involvement in supporting tumor growth or therapy resistance. Some studies suggest that MSCs can contribute to tumor progression by interacting with the tumor microenvironment, enhancing cancer cell survival, and promoting metastasis in certain conditions57,166. For instance, MSCs have been shown to facilitate epithelial-to-mesenchymal transition (EMT) in some cancers, associated with increased invasiveness and resistance to therapy167. Although ADMSC-based therapies have not been directly linked to tumor formation in clinical trials, further long-term studies are necessary to ensure their safety. Future research should focus on identifying markers that distinguish pro-regenerative from pro-tumorigenic MSCs and evaluating strategies to mitigate any potential risks associated with ADMSC therapy. CD markers and secretory signatures might help establish more robust characterization criteria for ADMSCs. For instance, understanding CD34’s biological function might better guide researchers in understanding how passage numbers affect therapeutic potential165. Furthermore, identifying and selecting ADMSCs with specific surface markers that indicate their functional status might help their immunomodulatory and regenerative properties.
References
Dayan, C. M., Korah, M., Tatovic, D., Bundy, B. N. & Herold, K. C. Changing the Landscape for Type 1 Diabetes: The First Step to Prevention. Lancet 394, 1286–1296 (2019).
Gregory, A. et al. Global Incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modeling study. 10:741-760 (2022).
Beik, P. et al. Prevention of Type 1 Diabetes: Past Experiences and Future Opportunities. J. Clin. Med. 9, 2805 (2020).
Draznin, B. et al. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes. Diabetes Care 2022, S17–S38 (2020).
Herold, K.C., et al The immunology of type 1 diabetes. Nat Rev Immunol. 2. https://doi.org/10.1038/s41577-023-00985-4. (2024).
Colli, M. L., Szymczak, F. & Eizirik, D. L. Molecular Footprints of the Immune Assault on Pancreatic Beta Cells in Type 1 Diabetes. Front. Endocrinol. 11, 568446 (2020).
Lehuen, A., Diana, J., Zaccone, P. & Cooke, A. Immune cell crosstalk in type 1 diabetes. Nat Rev Immunol 10, 501–513 (2010).
Rodriguez-Calvo, T., Richardson, S. J. & Pugliese, A. Pancreas Pathology During the Natural History of Type 1 Diabetes. Curr Diab Rep. 18, 124 (2018).
Carré, A., Richardson, S. J., Larger, E. & Mallone, R. Presumption of guilt for T cells in type 1 diabetes: lead culprits or partners in crime depending on age of onset. Diabetologia. Jan 64, 15–25 (2021).
Warshauer, J. T., Bluestone, J. A. & Anderson, M. S. New Frontiers in the Treatment of Type 1 Diabetes. Cell Metab. 31, 46–61 (2020).
Shilleh, A. H. & Russ, H. A. Cell Replacement Therapy for Type 1 Diabetes Patients: Potential Mechanisms Leading to Stem-Cell-Derived Pancreatic β cell Loss upon Transplant. Cells 12, 698 (2023).
Erickson, G. R. et al. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochemical and Biophysical Research Communications 290, 763–769 (2002).
Watt, F. M. & Hogan, B. L. Out of Eden: stem cells and their niches. Science 287, 1427–1430 (2000).
Takahashi, H., Sakata, N., Yoshimatsu, G., Hasegawa, S. & Kodama, S. Regenerative and Transplantation Medicine: Cellular Therapy Using Adipose Tissue- Derived Mesenchymal Stromal Cells for Type 1 Diabetes Mellitus. J Clin Med. 8, 249 (2019).
Shen, Z. et al. Effects of Mesenchymal Stem Cell-Derived Exosomes on Autoimmune Diseases. Front Immunol. 12, 749192 (2021).
Jasim, S. A. et al. Shining the light on clinical application of mesenchymal stem cell therapy in autoimmune diseases. Stem Cell Res Ther 13, 101 (2022).
Biarnés, M. et al. Beta-cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes 51, 66–72 (2002).
Czerwiec, K. et al. Adipose-Derived Mesenchymal Stromal Cells in Basic Research and Clinical Applications. Int J Mol Sci. 2023 24, 3888 (2023).
Ritter, A. et al. Subcutaneous and Visceral Adipose-Derived Mesenchymal Stem Cells: Commonality and Diversity. Cells. 8, 1288 (2019).
Gimble, J. M., Katz, A. J. & Bunnell, B. A. Adipose-derived stem cells for regenerative medicine. Circ Res 100, 1249–1260 (2007).
Mushahary, D. et al. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A. 93, 19–31 (2018). 2018 Jan.
Zuk, P. A. et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 7, 211–228 (2001).
Dave, S.D., Vanikar, A.V., Trivedi, H.L. Ex vivo generation of glucose sensitive insulin secreting mesenchymal stem cells derived from human adipose tissue. Indian J Endocrinol Metab. :S65-S69. (2012).
Mikłosz, A. & Chabowski, A. Adipose-derived Mesenchymal Stem Cells Therapy as a new Treatment Option for Diabetes Mellitus. J Clin Endocrinol Metab 108, 1889–1897 (2023).
Lee, J. et al. Differentiation of human adipose tissue-derived stem cells into aggregates of insulin-producing cells through the overexpression of pancreatic and duodenal homeobox gene-1. Cell Transplant. 22, 1053–1060 (2013).
Jaber, H., Issa, K., Eid, A., Saleh, F.A..The therapeutic effects of adipose-derived mesenchymal stem cells on obesity and its associated diseases in diet-induced obese mice. Sci Rep. 2021 11:6291. doi: 10.1038/s41598-021-85917-9. Erratum in: Sci Rep. 2021 11(1):12482. doi: 10.1038/s41598-021-91860-6 (2021)
Karaoz, E. et al. Adipose tissue-derived mesenchymal stromal cells efficiently differentiate into insulin-producing cells in pancreatic islet microenvironment both in vitro and in vivo. Cytotherapy. 15, 557–570 (2013).
Melief, S. M., Zwaginga, J. J., Fibbe, W. E. & Roelofs, H. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl Med. 2, 455–463 (2012). 2013 Jun.
Philippe B., et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT), Cytotherapy, Volume 15, Issue 6, 2013, Pages 641-648, ISSN 1465-3249, https://doi.org/10.1016/j.jcyt.2013.02.006.
Mohamed-Ahmed, S. et al. Adipose-derived and bone marrow mesenchymal stem cells: A donor-matched comparison. Stem Cell Res. Ther 9, 168 (2018).
Dmitrieva, R. I. et al. Bone marrow- and subcutaneous adipose tissue-derived mesenchymal stem cells: Differences and similarities. Cell Cycle. 11, 377–383 (2012).
Puissant, B. et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: Comparison with bone marrow mesenchymal stem cells. Br. J. Haematol. 129, 118–129 (2005).
Valencia, J. et al. Comparative analysis of the immunomodulatory capacities of human bone marrow- and adipose tissue-derived mesenchymal stromal cells from the same donor. Cytotherapy. 18, 1297–1311 (2016).
Yoshimura, H. et al. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. Mar 327, 449–462 (2007).
Yi, N. et al. Functional variation among mesenchymal stem cells derived from different tissue sources. PeerJ 12, e17616 (2024).
Liu, G. Y. et al. Adipose-Derived Mesenchymal Stem Cells Ameliorate Lipid Metabolic Disturbance in Mice. Stem Cells Transl Med. 5, 1162–1170 (2016).
Lin, C. S., Ning, H., Lin, G. & Lue, T. F. Is CD34 truly a negative marker for mesenchymal stromal cells. Cytotherapy 14, 1159–1163 (2012).
Hermiston, M. L., Xu, Z. & Weiss, A. CD45: a critical regulator of signaling thresholds in immune cells. Annu Rev Immunol. 21, 107–137 (2001).
Sheng, M. et al. CD47-Mediated Hedgehog/SMO/GLI1 Signaling Promotes Mesenchymal Stem Cell Immunomodulation in Mouse Liver Inflammation. Hepatology. 74, 1560–1577 (2021).
Ceccarelli, S., Pontecorvi, P., Anastasiadou, E., Napoli, C. & Marchese, C. Immunomodulatory Effect of Adipose-Derived Stem Cells: The Cutting Edge of Clinical Application. Front Cell Dev Biol 8, 236 (2020).
Ezquer, F. et al. The antidiabetic effect of mesenchymal stem cells is unrelated to their transdifferentiation potential but to their capability to restore Th1/Th2 balance and to modify the pancreatic microenvironment. Stem Cells. 30, 1664–1674 (2012).
Nasef, A., Ashammakhi, N. & Fouillard, L. Immunomodulatory effect of mesenchymal stromal cells: possible mechanisms. Regen Med. 3, 531–546 (2008).
Ock, S. A. et al. Comparison of Immunomodulation Properties of Porcine Mesenchymal Stromal/Stem Cells Derived from the Bone Marrow, Adipose Tissue, and Dermal Skin Tissue. Stem Cells Int. 2016, 9581350 (2015).
Kocan, B., Maziarz, A., Tabarkiewicz, J., Ochiya, T. & Banaś-Ząbczyk, A. Trophic Activity and Phenotype of Adipose Tissue-Derived Mesenchymal Stem Cells as a Background of Their Regenerative Potential. Stem Cells Int 2017, 1653254 (2017).
Machado, C. D., da Silva Telles, P. D. & Nascimento, I. L. O. Immunological characteristics of mesenchymal stem cells. Rev. Bras. Hematol. Hemoter. 35, 62–67 (2013).
Li, Y. Y., Liu, H. H., Chen, H. L. & Li, Y. P. Adipose-derived mesenchymal stem cells ameliorate STZ-induced pancreas damage in type 1 diabetes. Biomed Mater Eng. 22, 97–103 (2012).
Rahavi, H. et al. Adipose tissue-derived mesenchymal stem cells exert in vitro immunomodulatory and beta cell protective functions in streptozotocin-induced diabetic mice model. J Diabetes Res. 2015, 878535 (2015).
Juedes, A. E. & von Herrath, M. G. Regulatory T-cells in type 1 diabetes. Diabetes Metab Res Rev. 20, 446–451 (2004).
Salomon, B. et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 12, 431–440 (2000). Apr.
Green, E. A., Gorelik, L., McGregor, C. M., Tran, E. H. & Flavell, R. A. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-beta-TGF-beta receptor interactions in type 1 diabetes. Proc Natl Acad Sci USA. 100, 10878–10883 (2003).
Gregori, S., Giarratana, N., Smiroldo, S., Uskokovic, M. & Adorini, L. A1alpha,25- dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes 51, 1367–1374 (2002).
Fousteri, G. et al. Subcutaneous insulin B:9-23/IFA immunisation induces Tregs that control late-stage prediabetes in NOD mice through IL-10 and IFNgamma. Diabetologia. 53, 1958–1970 (2010).
Grinberg-Bleyer, Y. et al. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med 207, 1871–1878 (2010).
Bassi, ÊJ. et al. Immune regulatory properties of allogeneic adipose-derived mesenchymal stem cells in the treatment of experimental autoimmune diabetes. Diabetes. 61, 2534–2545 (2012).
Lu, J., Liu, J., Li, L., Lan, Y. & Liang, Y. Cytokines in type 1 diabetes: mechanisms of action and immunotherapeutic targets. Clin Transl Immunology 9, e1122 (2020).
Banas, A. et al. IFATS collection: in vivo therapeutic potential of human adipose tissue mesenchymal stem cells after transplantation into mice with liver injury. Stem Cells 26, 2705–2712 (2008). no. 10, pp.2008.
Hervás-Salcedo, R. et al. Enhanced anti-inflammatory effects of mesenchymal stromal cells mediated by the transient ectopic expression of CXCR4 and IL10. Stem Cell Res Ther. 12, 124 (2021).
Li, J. X. et al. Current Clinical Progress of PD- 1/PD-L1 Immunotherapy and Potential Combination Treatment in Non-Small Cell Lung Cancer. Integr Cancer Ther. 18, 1534735419890020 (2019).
Shirley, M. Avelumab: A Review in Metastatic Merkel Cell Carcinoma. Target Oncol. 13, 409–416 (2018). 2018 Jun.
Friedlaender, A., Addeo, A., Banna, G. New emerging targets in cancer immunotherapy: the role of TIM3. ESMO Open. 2019 4(Suppl 3):e000497. (2019).
Solinas, C., Gu-Trantien, C. & Willard-Gallo, K. The rationale behind targeting the ICOS-ICOS ligand costimulatory pathway in cancer immunotherapy. ESMO Open. 5, e000544 (2020).
Leone, R. D., Lo, Y. C. & Powell, J. D. A2aR antagonists: Next generation checkpoint blockade for cancer immunotherapy. Comput Struct Biotechnol J. 13, 265–272 (2015).
Tang, W., Chen, J., Ji, T. & Cong, X. TIGIT, a novel immune checkpoint therapy for melanoma. Cell Death Dis. 14, 466 (2023).
Paulos, C. M. & June, C. H. Putting the brakes on BTLA in T cell-mediated cancer immunotherapy. J Clin Invest. 120, 76–80 (2010).
Yang, Y., Islam, M. S., Hu, Y. & Chen, X. TNFR2: Role in Cancer Immunology and Immunotherapy. Immunotargets Ther. 10, 103–122 (2021).
Fujiwara, Y. et al. Indoleamine 2,3-dioxygenase (IDO) inhibitors and cancer immunotherapy. Cancer Treat Rev. 110, 102461 (2022).
Meireson, A., Devos, M. & Brochez, L. IDO Expression in Cancer: Different Compartment, Different Functionality?. Front Immunol. 11, 531491 (2020).
Lau, A. P. Y., Khavkine, Binstock, S. S. & Thu, K. L. CD47: The Next Frontier in Immune Checkpoint Blockade for Non-Small Cell Lung Cancer. Cancers (Basel). 15, 5229 (2023).
Lian, S., Xie, X., Lu, Y. & Jia, L. Checkpoint CD47 Function On Tumor Metastasis And Immune Therapy. Onco Targets Ther. 12, 9105–9114 (2019).
Ivanova-Todorova, E. et al. Adipose tissue-derived mesenchymal stem cells are more potent suppressors of dendritic cells differentiation compared to bone marrow-derived mesenchymal stem cells. Immunol Lett. 126, 37–42 (2009).
Yi, T., Lee, D. S., Jeon, M. S., Kwon, S. W. & Song, S. U. Gene expression profile reveals that STAT2 is involved in the immunosuppressive function of human bone marrow-derived mesenchymal stem cells. Gene. 497, 131–139 (2012).
Saldanha-Araujo, F. et al. Mesenchymal stromal cells up-regulate CD39 and increase adenosine production to suppress activated T-lymphocytes. Stem Cell Res. 7, 66–74 (2011).
Cogdill, A. P., Andrews, M. C. & Wargo, J. A. Hallmarks of response to immune checkpoint blockade. Br J Cancer. 117, 1–7 (2017).
Murphy, K., Weaver, C., & Berg, L. Janeway's immunobiology (9th ed.). https://www.ncbi.nlm.nih.gov/books/NBK10757/ (Garland Science, 2016).
Kawada-Horitani, E. et al. Human adipose-derived mesenchymal stem cells prevent type 1 diabetes induced by immune checkpoint blockade. Diabetologia. 65, 1185–1197 (2022).
Gaber, T. et al. CTLA-4 mediates inhibitory function of mesenchymal stem/stromal cells. Int. J. Mol. Sci. 19, 2312 (2018).
Kubli, S. P., Berger, T., Araujo, D. V., Siu, L. L. & Mak, T. W. Beyond immune checkpoint blockade: emerging immunological strategies. Nat. Rev. Drug Discov. 20, 899–919 (2021).
Yoneda, S. et al. T-lymphocyte infiltration to islets in the pancreas of a patient who developed type 1 diabetes after administration of immune checkpoint inhibitors. Diabetes Care. 42, e116–e118 (2019).
Hu, H., Zakharov, P. N., Peterson, O. J. & Unanue, E. R. Cytocidal macrophages in symbiosis with CD4 and CD8 T cells cause acute diabetes following checkpoint blockade of PD-1 in NOD mice. Proc. Natl. Acad. Sci. USA117, 31319–31330 (2020).
El-Sawah, S. G. et al. AD-MSCs and BMMSCs ameliorating effects on the metabolic and hepato-renal abnormalities in type 1 diabetic rats. Saudi J. Biol. Sci. 29, 1053–1060 (2022).
Nam, J. S. et al. Transplantation of insulin-secreting cells differentiated from human adipose tissue-derived stem cells into type 2 diabetes mice. Biochem. Biophys. Res. Commun. 443, 775–781 (2014).
Silva, I. B. B., Kimura, C. H., Colantoni, V. P. & Sogayar, M. C. Stem cells differentiation into insulin-producing cells (IPCs): recent advances and current challenges. Stem Cell Res Ther. 13, 309 (2022).
Brinkhof, B. et al. ALCAM (CD166) as a gene expression marker for human mesenchymal stromal cell characterisation. Gene X. 5, 100031 (2020).
Chhabra, P., Brayman, K.L. Stem cell therapy to cure type 1 diabetes: from hype to hope. Stem Cells Transl. Med. 2, 328-336 (2013).
Su, W. et al. Diabetic microenvironment preconditioning of adipose tissue-derived mesenchymal stem cells enhances their anti- diabetic, anti-long-term complications, and anti-inflammatory effects in type 2 diabetic rats. Stem Cell Res. Ther. 13, 422 (2022).
Sen, S. et al. Genetic modification of human mesenchymal stem cells helps to reduce adiposity and improve glucose tolerance in an obese diabetic mouse model. Stem Cell Res. Ther. 6, 242 (2015).
Xue, B. et al. Mesenchymal stem cells modified by FGF21 and GLP1 ameliorate lipid metabolism while reducing blood glucose in type 2 diabetic mice. Stem Cell Res. Ther. 12, 133 (2021).
Kono, T. M. et al. Human adipose-derived stromal/stem cells protect against STZ-induced hyperglycemia: analysis of hASC-derived paracrine effectors. Stem Cells. 32, 1831–1842 (2014).
Badimon, L., Oñate, B. & Vilahur, G. Adipose-derived mesenchymal stem cells and their reparative potential in ischemic heart disease. Rev. Esp. Cardiol.68, 599–611 (2015). Jul.
Hoch, A. I., Binder, B. Y., Genetos, D. C. & Leach, J. K. Differentiation-dependent secretion of proangiogenic factors by mesenchymal stem cells. PLoS One 7, e35579 (2012).
Sadat, S. et al. The cardioprotective effect of mesenchymal stem cells is mediated by IGF-I and VEGF. Biochem. Biophys. Res. Commun 363, 674–679 (2007).
Zhu, X. Y. et al. Transplantation of adipose-derived stem cells overexpressing hHGF into cardiac tissue. Biochem. Biophys. Res. Commun 379, 1084–1090 (2009).
Nakagami, H. et al. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler. Thromb. Vasc. Biol. 25, 2542–2547 (2005).
Kandasamy, M. et al. TGF-beta signalling in the adult neurogenic niche promotes stem cell quiescence as well as generation of new neurons. J. Cell Mol. Med. 18, 1444–1459 (2014).
Tsuji, W., Rubin, J. P. & Marra, K. G. Adipose-derived stem cells: implications in tissue regeneration. World J. Stem Cells 6, 312–321 (2014).
Rehman, J. et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 109, 1292–1298 (2004).
Mfopou, J. K., Willems, E., Leyns, L. & Bouwens, L. Expression of regulatory genes for pancreas development during murine embryonic stem cell differentiation. Int. J. Dev. Biol. 49, 915–922 (2005).
Kodama, S. Islet regeneration during the reversal of autoimmune diabetes in NOD mice. Science. 302, 1223–1227 (2003).
Zhang, H., Shen, Y., Kim, I. M., Weintraub, N. L. & Tang, Y. The impaired bioenergetics of diabetic cardiac microvascular endothelial cells. Front. Endocrinol. 12, 642857 (2021).
Forte, D. et al. Bone marrow mesenchymal stem cells support acute myeloid leukemia bioenergetics and enhance antioxidant defense and escape from chemotherapy. Cell Metab. 32, 829–843.e9 (2020).
Kajiyama, H., Hamazaki, T. S., Tokuhara, M., Masui, S. & Okabayashi, K. Pdx1-transfected adipose tissue-derived stem cells differentiate into insulin-producing cells in vivo and reduce hyperglycemia in diabetic mice. Int. J. Dev. Biol. 54, 699–705 (2010).
Kumar, M. A. et al. Extracellular vesicles as tools and targets in therapy for diseases. Sig. Transduct. Target Ther. 9, 27 (2024).
Soltani, S. et al. Extracellular vesicle therapy for type 1 diabetes. Front. Immunol. 13, 865782 (2022).
Tsukita, S. et al. MicroRNAs 106b and 222 improve hyperglycemia in a mouse model of insulin-deficient diabetes via pancreatic β-cell proliferation. EBioMedicine 15, 163–172 (2017).
Chen, J. et al. Mesenchymal stem cell-derived exosomes protect beta cells against hypoxia-induced apoptosis via miR-21 by alleviating ER stress and inhibiting P38 MAPK phosphorylation. Stem Cell Res. Ther. 11, 97 (2020).
Cantaluppi, V. et al. Microvesicles derived from endothelial progenitor cells enhance neoangiogenesis of human pancreatic islets. Cell transplantation 21, 1305–1320 (2012).
Mohammadi, M. R. et al. Exosome loaded immunomodulatory biomaterials alleviate local immune response in immunocompetent diabetic mice post islet xenotransplantation. Commun. Biol. 4, 685 (2021).
Nie, W. et al. Human mesenchymal-stem-cells- derived exosomes are important in enhancing porcine islet resistance to hypoxia. Xenotransplantation 25, e12405 (2018).
Keshtkar, S. et al. Exosomes derived from human mesenchymal stem cells preserve mouse islet survival and insulin secretion function. EXCLI J 19, 1064–1080 (2020).
Zhang, L. et al. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res. Ther. 11, 1562–1569 (2020).
Zhu, Y., Jia, Y., Wang, Y., Xu, J. & Chai, Y. Impaired bone regenerative effect of exosomes derived from bone marrow mesenchymal stem cells in type 1 diabetes. Stem Cells Transl. Med. 8, 593–605 (2019).
Liang, B. et al. Dimethyloxaloylglycine-stimulated human bone marrow mesenchymal stem cell-derived exosomes enhance bone regeneration through angiogenesis by targeting the AKT/mTOR pathway. Stem Cell Res. Ther. 10, 1410 (2019).
Liu, W. et al. Melatoninstimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res. Ther 11, 1756 (2020).
Villatoro, A. J. et al. Comparative analysis and characterization of soluble factors and exosomes from cultured adipose tissue and bone marrow mesenchymal stem cells in canine species. Veterinary immunology and immunopathology 208, 6–15 (2019).
Hoang, D. H. et al. Differential wound healing capacity of mesenchymal stem cell- derived exosomes originated from bone marrow, adipose tissue and umbilical cord under serum- and xeno-free condition. Front. Mol. Biosci. 7, 119 (2020).
Bari, E. et al. Freeze-dried and GMP-compliant pharmaceuticals containing exosomes for acellular mesenchymal stromal cell immunomodulant therapy. Nanomedicine 14, 753–765 (2019).
Chance, T. C. et al. The effects of cell type and culture condition on the procoagulant activity of human mesenchymal stromal cell-derived extracellular vesicles. J. Trauma Acute Care Surg. 87, S74–S82 (2019).
Han, Y. et al. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Sig. Transduct. Target The.r 7, 92 (2022).
Bekeredjian-Ding, I. et al. Tumour-derived prostaglandin E and transforming growth factor-beta synergize to inhibit plasmacytoid dendritic cell-derived interferon-alpha. Immunology. 128, 439–450 (2009).
Horiguchi, M., Okada, Y., Turudome, Y. & Ushijima, K. Exosome degeneration in mesenchymal stem cells derived from patients with type 1 diabetes mellitus. Int. J. Mol. Sci. 22, 10906 (2021).
Kulaj, K. et al. Adipocyte-derived extracellular vesicles increase insulin secretion through transport of insulinotropic protein cargo. Nat Commun. 14, 709 (2023).
Gesmundo, I. et al. Adipocyte-derived extracellular vesicles regulate survival and function of pancreatic β cells. JCI Insight. 6, e141962 (2021).
Nojehdehi, S. et al. Immunomodulatory effects of mesenchymal stem cell-derived exosomes on experimental type-1 autoimmune diabetes. J. Cell Biochem. 119, 9433–9443 (2018).
Rahman, M. M. et al. CD13 promotes mesenchymal stem cell-mediated regeneration of ischemic muscle. Front Physiol. 4, 402 (2014).
Wu, C. C. et al. CD146+ mesenchymal stem cells display greater therapeutic potential than CD146– cells for treating collagen-induced arthritis in mice. Stem Cell Res. Ther. 7, 23 (2016).
Kurtz, A. Mesenchymal stem cell delivery routes and fate. Int. J. Stem Cells. 1, 1–7 (2008).
Dang, L. T. et al. Intravenous infusion of human adipose tissue-derived mesenchymal stem cells to treat type 1 diabetic mellitus in mice: an evaluation of grafted cell doses. Adv. Exp. Med. Biol. 1083, 145–156 (2017).
Barbash, I. M. et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 108, 863–868 (2003).
Rochefort, G. Y. et al. Influence of hypoxia on the domiciliation of mesenchymal stem cells after infusion into rats: possibilities of targeting pulmonary artery remodeling via cells therapies. Respir Res. 6, 125 (2005).
Baas, J., Senninger, N. & Elser, H. Das retikuloendotheliale System. Eine Ubersicht über Funktion, Pathologie und neuere Messmethoden [The reticuloendothelial system. An overview of function, pathology and recent methods of measurement]. Z Gastroenterol. 32, 117–123 (1994).
Hashemi, S. M., Hassan, Z. M., Hossein-Khannazer, N., Pourfathollah, A. A. & Soudi, S. Investigating the route of administration and efficacy of adipose tissue-derived mesenchymal stem cells and conditioned medium in type 1 diabetic mice. Inflammopharmacology. 28, 585–601 (2020).
Wang, M. et al. Intraperitoneal injection (IP), Intravenous injection (IV) or anal injection (AI)? Best way for mesenchymal stem cells transplantation for colitis. Sci. Rep. 6, 30696 (2016).
Arzouni, A. A. et al. Mesenchymal stromal cells improve human islet function through released products and extracellular matrix. Clin. Sci. 131, 2835–2845 (2017).
Leibacher, J. & Henschler, R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res. Ther. 7, 7 (2016).
Schrepfer, S. et al. Stem cell transplantation: the lung barrier. Transplant. Proc. 39, 573–576 (2007).
Schroder, C., Khatri, R., Petry, S.F., Linn, T. Class I and II histone deacetylase inhibitor LBH589 promotes endocrine differentiation in bone marrow derived human mesenchymal stem cells and suppresses uncontrolled proliferation. Exp. Clin. Endocrinol. Diabetes. 129, 357-364 (2020).
Wang, H. et al. Autologous mesenchymal stem cell and islet cotransplantation: safety and efficacy. Stem Cells Transl. Med. 7, 11–19 (2018).
Lee, R. H. et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 5, 54–63 (2009).
Khatri, R., Petry, S. F. & Linn, T. Intrapancreatic MSC transplantation facilitates pancreatic islet regeneration. Stem Cell Res. Ther. 12, 121 (2021).
Steingen, C. et al. Characterization of key mechanisms in transmigration and invasion of mesenchymal stem cells. J. Mol. Cell Cardiol. 44, 1072–1084 (2008).
Yarani, R. et al. Intra-arterial delivery of mesenchymal stromal cells maintains glycemic regulation in streptozotocin-treated rats. Cytotherapy 2024, 26 (2024).
Yaochite, J. N. et al. Therapeutic efficacy and biodistribution of allogeneic mesenchymal stem cells delivered by intrasplenic and intrapancreatic routes in streptozotocin-induced diabetic mice. Stem Cell Res. Ther. 6, 31 (2015).
Gabbay, M. A. et al. Effect of cholecalciferol as adjunctive therapy with insulin on protective immunologic profile and decline of residual β cell function in new-onset type 1 diabetes mellitus. Arch. Pediatr. Adolesc. Med. 166, 601–607 (2012).
Ministry of Food and Drug Safety, Republic of Korea. (Cell therapy products) ANTEROGEN. MFDS. Published February 15, 2017. Accessed August 12, 2024. https://www.mfds.go.kr/eng/brd/m_30/view.do?seq=71337
Scott, L. J. Darvadstrocel: a review in treatment-refractory complex perianal fistulas in Crohn's Disease. BioDrugs. 32, 627–634 (2018).
European Medicines Agency. Alofisel: EPAR—Product Information. Published May 31, 2018. Accessed August 12, 2024, https://www.ema.europa.eu/en/documents/product-information/alofisel-epar-product-information_en.pdf
Kostecka, A. et al. Adipose-derived mesenchymal stromal cells in clinical trials: Insights from single-cell studies. Life Sci. 351, 122761 (2024).
Wright, A., Arthaud-Day, M. L. & Weiss, M. L. Therapeutic use of mesenchymal stromal cells: The need for inclusive characterization guidelines to accommodate all tissue sources and species. Front. Cell Dev. Biol. 9, 632717 (2021).
Bourin, P. et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 15, 641–648 (2013).
Lan, T., Luo, M. & Wei, X. Mesenchymal stem/stromal cells in cancer therapy. J. Hematol. Oncol. 14, 195 (2021).
King, N. M. & Perrin, J. Ethical issues in stem cell research and therapy. Stem Cell Res. Ther. 5, 85 (2014).
Lappin, T. & Cheng, T. An urgent need for standardization of stem cells and stem cell-derived products toward clinical applications. Stem Cells Transl. Med. 10, S1–S3 (2021).
Firriolo, J. M., Condé-Green, A. & Pu, L. L. Q. Fat grafting as regenerative surgery: a current review. Plast. Reconstr. Surg. 150, 1340e–1347e (2022).
Fiorello, M. L., Treweeke, A. T., Macfarlane, D. P. & Megson, I. L. The impact of glucose exposure on bioenergetics and function in a cultured endothelial cell model and the implications for cardiovascular health in diabetes. Sci. Rep. 10, 19547 (2020).
Ahmed, L. & Al-Massri, K. New approaches for enhancement of the efficacy of mesenchymal stem cell-derived exosomes in cardiovascular diseases. Tissue Eng. Regen. Med. 19, 1129–1146 (2022).
Tremolada, C. et al. Adipose tissue and mesenchymal stem cells: state of the art and lipogems® technology development. Curr. Stem Cell Rep. 2, 304–312 (2016).
Chen, S., et al. (2022). Preconditioning and engineering strategies for improving the efficacy of mesenchymal stem cell-derived exosomes in cell-free therapy. Stem Cells Int. 2022, 1779346 (2022).
Alagesan, S. et al. Enhancement strategies for mesenchymal stem cells and related therapies. Stem Cell Res. Ther. 13, 75 (2022).
Hazrati, A. et al. Therapeutic and immunomodulatory potentials of mesenchymal stromal/stem cells and immune checkpoints related molecules. Biomark Res. 12, 35 (2024).
Magna, M. & Pisetsky, D. S. The role of HMGB1 in the pathogenesis of inflammatory and autoimmune diseases. Mol. Med. 20, 138–146 (2014).
Scherberich, A., Di Maggio, N. D. & McNagny, K. M. A familiar stranger: CD34 expression and putative functions in SVF cells of adipose tissue. World J. Stem Cells. 5, 1–8 (2013).
Deschepper, M. et al. Survival and function of mesenchymal stem cells (MSCs) depend on glucose to overcome exposure to long-term, severe and continuous hypoxia. J. Cell Mol. Med. 15, 1505–1514 (2011).
Tsai, C. C. et al. Benefits of hypoxic culture on bone marrow multipotent stromal cells. Am. J. Blood Res. 2, 148–159 (2012).
Park, W. S. et al. Strategies to enhance paracrine potency of transplanted mesenchymal stem cells in intractable neonatal disorders. Pediatr. Res. 83, 214–222 (2018).
Patel, D. B. et al. Impact of cell culture parameters on production and vascularization bioactivity of mesenchymal stem cell-derived extracellular vesicles. Bioeng. Transl. Med. 2, 170–179 (2017).
Huang, S. et al. Increased CD34 in pancreatic islet negatively predict islet β-cell decrease in type1 diabetes model. Front. Physiol. 13, 1032774 (2022).
Deaglio, S. et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 204, 1257–1265 (2007).
Sorgun, O. & Erbaş, O. Adipose-derived mesenchymal stem cells mitigate methotrexate-induced liver cirrhosis (fibrosis) model. Eur. Rev. Med. Pharmacol. Sci. 27, 11882–11889 (2023).
Yang, Y. et al. CD29 of human umbilical cord mesenchymal stem cells is required for expansion of CD34(+) cells. Cell Prolif. 47, 596–603 (2014).
Ode, A. et al. CD73 and CD29 concurrently mediate the mechanically induced decrease of migratory capacity of mesenchymal stromal cells. Eur Cell Mater. 22, 26–42 (2011).
Zhou, L. et al. Role of CD44 in increasing the potency of mesenchymal stem cell extracellular vesicles by hyaluronic acid in severe pneumonia. Stem Cell Res. Ther. 12, 293 (2021).
Li, C. et al. Adipose mesenchymal stem cell-derived exosomes promote wound healing through the WNT/β-catenin signaling pathway in dermal fibroblasts. Stem Cell Rev. Rep. 18, 2059–2073 (2022).
Monguió-Tortajada, M. et al. Mesenchymal stem cells induce expression of CD73 in human monocytes in vitro and in a swine model of myocardial infarction in vivo. Front. Immunol. 8, 1577 (2017).
Moraes, D. A. et al. A reduction in CD90 (THY-1) expression results in increased differentiation of mesenchymal stromal cells. Stem Cell Res. Ther. 9, 97 (2016).
Thitilertdecha, P. et al. Extensive characterization of mesenchymal stem cell marker expression on freshly isolated and in vitro expanded human adipose-derived stem cells from breast cancer patients. Stem Cells Int. 2020, 8237197 (2020).
Suga, H. et al. Functional implications of CD34 expression in human adipose-derived stem/progenitor cells. Stem Cells Dev. 18, 1201–1210 (2009).
Radu, P. et al. CD34—structure, functions and relationship with cancer stem cells. Medicina. 59, 938 (2023).
Sattler, C. et al. Inhibition of T-cell proliferation by murine multipotent mesenchymal stromal cells is mediated by CD39 expression and adenosine generation. Cell Transplant. 20, 1221–1230 (2011).
Shahbaz, S. et al. CD71+VISTA+ erythroid cells promote the development and function of regulatory T cells through TGF-β. PLoS Biol 16, e2006649 (2018).
Balazs, A. B., Fabian, A. J., Esmon, C. T. & Mulligan, R. C. Endothelial protein C receptor (CD201) explicitly identifies hematopoietic stem cells in murine bone marrow. Blood. 107, 2317–2321 (2006).
Kachroo, U., Ramasamy, B. & Vinod, E. Evaluation of CD49e as a distinguishing marker for human articular cartilage derived chondroprogenitors. Knee. 27, 833–837 (2020).
Mohammadi, A. et al. Immunomodulatory and protective effects of adipose tissue-derived mesenchymal stem cells in an allograft islet composite transplantation for experimental autoimmune type 1 diabetes. Immunol Lett. 188, 21–31 (2017). Aug.
Rolfo, C. et al. Immunotherapy in NSCLC: a promising and revolutionary weapon. Adv. Exp. Med. Biol. 995, 97–125 (2017).
Wu, G. Therapeutic effects of pembrolizumab combined with paclitaxel and cisplatin chemotherapy on advanced non-squamous non-small cell lung cancer and influencing factors. Indian J. Pharm. Sci. 83, 120–126 (2021).
Migden, M. R. et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N. Engl. J. Med. 379, 341–351 (2018).
Paz-Ares, L. et al. Outcomes with durvalumab by tumour PD-L1 expression in unresectable, stage III non-small-cell lung cancer in the PACIFIC trial. Ann. Oncol. 31, 798–806 (2018).
Li, X., Xu, Z., Cui, G., Yu, L. & Zhang, X. BTLA expression in stage I-III non- small-cell lung cancer and its correlation with PD-1/PD-L1 and clinical outcomes. Onco Targets Ther. 13, 215–224 (2020).
Peggs, K. S., Quezada, S. A. & Allison, J. P. Cell intrinsic mechanisms of T-cell inhibition and application to cancer therapy. Immunol. Rev. 224, 141–165 (2008).
Zhu, L.L., et al. Transplantation of adipose tissue-derived stem cell-derived exosomes ameliorates erectile function in diabetic rats. Andrologia. 2018. https://doi.org/10.1111/and.12871 (2017).
Acknowledgements
R.Y. is supported by the Lundbeck Foundation grant R303-2018-3148.
Author information
Authors and Affiliations
Contributions
V.S. and R.Y. conceptualized, conceived, and planned the review. V.S. and H.R. reviewed the literature and wrote the manuscript’s first draft. G.C.N., visualization, writing, review, and editing. R.P., review and editing. S.D., review and editing. A.S.T., review and editing. F.P., review, and editing. R.Y., project administration, supervision, writing, review and editing, visualization.
Corresponding author
Ethics declarations
Competing interests
AST is a cofounder and holds stock options for Teal Health and is on the Scientific Advisory Board, received grants, or is a consultant for RespondHealth Inc, Cellular Vehicles Inc, Nephrogen Inc, ReThink64 Inc, AlloTRx Inc, Inari Inc, and Genentech Inc. The other authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Jessie Barra and Tzuwen Hong for their contribution to the peer review of this work. Primary Handling Editor: Christina Karlsson Rosenthal. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sood, V., Ricioli, H., Njoku, G.C. et al. Adipose-derived mesenchymal stromal/stem cells in type 1 diabetes treatment. Commun Biol 8, 1094 (2025). https://doi.org/10.1038/s42003-025-08244-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-08244-z
This article is cited by
-
Preventing the onset of diabetes with precision delivery of mesenchymal stem cells to the pancreas in a preclinical model
Stem Cell Research & Therapy (2025)