Abstract
Exercise is an effective intervention to promote bone mass by regulating bone homeostasis, but the specific mechanism is ambiguous. Here this study shows that high-intensity interval training (HIIT) was more effective than low-intensity continuous training (LICT) in promoting bone mineral density (BMD). Lactate, a major by-product of HIIT, emerges as a focal point in our investigation of bone homeostasis regulation. In vitro experiments revealed that lactate promotes osteoblast differentiation while inhibiting osteoclast differentiation. However, this effect is markedly suppressed in Gpr81-deficient osteoblasts and osteoclasts. We next found lactate inhibits osteoclast differentiation via the activation of the Gpr81-TAK1-p65 signaling pathway, while promoting osteoblast differentiation through activation of the Gpr81-Wnt/β-catenin pathway. Subsequently, HIIT interventions were performed using ovariectomized (OVX) mice with osteoporosis. It was found that HIIT not only attenuated bone resorption but also promoted bone formation, thereby partially rescuing the low bone mass phenotype of OVX mice. Furthermore, administering lactate in a mouse model of osteoporosis induced by ovariectomy effectively prevented bone loss. Our results provided a potential drug target for osteoporosis treatment, the activation of Gpr81 holds promise as a potential strategy for preventing osteoporosis.

Similar content being viewed by others
Introduction
Exercise is widely recognized as an effective non-pharmacological intervention to increase peak bone mass and combat osteoporosis1. The results of recent studies indicate that high-intensity exercise has a greater impact on bone mineral density (BMD) than moderate or lower-intensity exercise2,3. However, the specific mechanisms by which high-intensity exercise influences bone remodeling remain unclear. High-intensity interval training (HIIT) is regarded as a secure and efficacious exercise regimen for enhancing bone mass4. HIIT has been shown to be more effective than continuous exercise or resistance training in reducing abdominal and visceral fat in postmenopausal women5. Lactate, a major byproduct of high-intensity interval training (HIIT), has been shown to function as a signaling molecule in recent studies6. It can activate multiple pathways, including those regulating angiogenesis, inflammation, and metabolic processes7. G-protein-coupled receptor 81 (Gpr81) is an endogenous receptor for lactate8. Nevertheless, the function of lactate as a regulator of bone homeostasis through the activation of Gpr81 on the membranes of osteoblasts and osteoclasts remains largely uninvestigated9,10. Our previous research has demonstrated that GPCRs play a pivotal role in bone development, bone remodeling, and the etiology of bone diseases11,12. However, the precise mechanism by which lactate-Gpr81 signaling regulates bone homeostasis remains unclear. Accordingly, the objective of this study was to evaluate the impact of HIIT on bone mass enhancement by comparing the effects of HIIT and LICT on bone homeostasis. Our objective was to elucidate the mechanism by which HIIT regulates bone homeostasis, focusing on the lactate-Gpr81 signaling pathway.
The Wnt/β-catenin signaling pathway plays a key role in the development of osteoporosis. OVX mice had downregulated β-catenin expression, which also could inhibit the expression of osteoblast differentiation-related genes such as Runx213. Mouse genetics have confirmed the importance of classical Wnt signaling in regulating bone homeostasis. Activation of this signaling pathway leads to increased bone mass, while inhibition leads to decreased bone mass14. Moreover, it has been demonstrated that lactate activates Gpr81 and mediates the Wnt3/β-catenin pathway in intestinal stem cell15. Furthermore, lactate stimulates Gpr81 on retinal Müller cells, promoting the secretion of angiogenic factors such as Wnt3 and Wnt7, thereby enhancing retinal angiogenesis and alleviating ischemic symptoms16. However, it remains unknown whether Gpr81 is involved in the OVX-mediated alterations of the Wnt pathway in osteoblasts. In this study, we found that Gpr81 is an upstream regulator of Wnt/β-catenin in osteoblast function. Osteoclasts derive from bone marrow macrophages (BMM), and research has demonstrated that lactate can impede the phosphorylation of NF-κB via the Gpr81-mediated signaling pathway, which in-turn suppresses the inflammatory response of macrophages to lipopolysaccharide (LPS) stimulation17. This suggests a potential role for lactate in inhibiting osteoclast differentiation through the Gpr81-NF-κB signaling pathway.
Postmenopausal osteoporosis (PMOP) is a prevalent bone disease that poses a significant risk for fractures and disability18. Ovariectomy (OVX) is a surgical procedure that removes both ovaries to simulate estrogen deficiency and to study conditions such as postmenopausal osteoporosis19. The OVX mouse model is widely used for PMOP mechanism studies and preclinical drug evaluation19. Estrogen replacement therapy has historically been used to treat PMOP but carries significant inherent risks20. HIIT is considered an effective intervention against bone mass loss. However, the potential of its metabolite lactate to alleviate bone loss in OVX mice via Gpr81 remains unclear.
Our data showed lactate generated during HIIT could enhance bone mass in OVX mice by activating Gpr81 located on the membrane of osteoblasts and osteoclasts, which may be manner for treating OVX-induced bone loss. For molecular mechanism, our results showed Gpr81 deletion diminishes bone formation while increasing bone resorption. Furthermore, we demonstrate that lactate, acting as an activating ligand of Gpr81, played a crucial regulating role with respect to the differentiation and function both of osteoblasts and osteoclasts. This was achieved by stimulating the Wnt/β-catenin and TAK1-p65 signaling pathways, which were identified as a principal downstream signal of Gpr81 in osteoblasts and osteoclasts. This also provided a deeper insight into the functions of Gpr81 in bone.
Results
HIIT was superior to LICT for bone mass enhancement in mice by regulating bone homeostasis
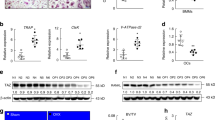
The graphical overview of the experimental design is presented in Fig. 1A. To investigate the impact of HIIT on bone mass regulation, we assessed the femoral skeleton of mice subjected to LICT, HIIT, and sedentary conditions. Our results of microcomputed tomography (Micro-CT) indicated that both LICT and HIIT resulted in enhanced bone mineral density (BMD), trabecular bone volume (BV/TV), and the quantity of trabeculae (Tb.N) in both female and male mice. Notably, the thickness of trabeculae (Tb.Th) was elevated exclusively within the HIIT group (Fig. 1B–D). The histological examination also indicated that HIIT group exhibited a substantially greater trabecular area compared with the LICT group (Fig. 1E–G). To compare the effects of HIIT and LICT on bone mechanical properties, we conducted a three-point bending test. The results showed that HIIT significantly enhanced tibial stiffness (Fig. 1H, I). Analysis of cortical bone indicated that only HIIT resulted in increased cortical thickness and BMD (Fig. 1J–L). The above results indicate that HIIT is more effective than LICT in increasing bone mass in both male and female.
A Graphical overview of the experimental design. Twenty-four male mice 8 weeks old were randomly divided into three groups with eight mice in each group. All mice were acclimatized and fed for one week; the LICT and HIIT groups underwent 1 week of adaptation training, followed by 8 weeks of treadmill exercise at 12 m/min in the LICT group and 10 rounds of treadmill exercise alternating between 80–90% of maximal speed and 40–50% of maximal speed in the HIIT group. This figure was originally created by the authors using Microsoft PowerPoint. B The proximal femur (top) and trabecular bone of the femoral metaphysis (bottom) are shown in representative micro-CT images of the femur of mice. Scale bar: 1 mm (all panels). C Quantitative analysis of trabecular bone parameters by micro-CT in the femurs of female mice. BMD bone mineral density, BV/TV bone volume/tissue volume ratio, Tb.N trabecular number, Tb.Sp trabecular separation, Tb.Th trabecular thickness. n = 6, *p < 0.05, **p < 0.01, ***p < 0.001. D Quantitative analysis of trabecular bone parameters by micro-CT in the femurs of male mice. n = 6, *p < 0.05, **p < 0.01, ***p < 0.001. E Representational figures of HE staining of trabecular bone in the femurs of female and male mice. Scale bars, 200 μm. F Quantification of trabecular bone area in the femurs of female mice. All data are presented as mean ± SD, n = 6, **p < 0.01, ***p < 0.001. G Quantification of trabecular bone area in the femurs of male mice. All data are presented as mean ± SD, n = 6, **p < 0.01, ***p < 0.001. H 3-point bending testing for evaluating femurs whole-bone mechanical properties in female mice. n = 6, *p < 0.05, **p < 0.01. I 3-point bending testing for evaluating femurs whole-bone mechanical properties in male mice. n = 6, **p < 0.01, ***p < 0.001. J Representational micro-CT images showing the cortical bone of the femur in mice. Scale bar: 1 mm. K Quantitative analysis of cortical bone parameters by micro-CT in the femurs of female mice. n = 6, **p < 0.01, ***p < 0.001. L Quantitative analysis of cortical bone parameters by micro-CT in the femurs of male mice. n = 6, **p < 0.01, ***p < 0.001.
To further investigate the potential mechanisms by which different intensities of exercise improve bone mass, we examined bone formation and bone resorption capacity in mice after HIIT and LICT. To determine whether the differences in bone mass were caused by bone formation rate (BFR) and osteogenesis processes, BFR and mineral apposition rate (MAR) were performed from the third lumbar vertebrae of male mice. The BFR and MAR was dramatically increased in HIIT mice compared with Ctrl or LICT groups (Fig. 2A, B). To further investigate osteoblast function, we performed Goldner’s staining on mouse lumbar vertebrae and found that HIIT effectively increased the number of osteoblasts in vivo and promoted osteoid formation (Fig. 2C, D). We detected osteocalcin (OCN) expression in non-load-bearing skull bones using immunohistochemistry and found that HIIT promoted OCN expression (Fig. 2E, F). These results indicate that HIIT can promote bone formation independently of mechanical stimulation.
A Representative images of calcein double-labelled mouse vertebral trabeculae. Scale bars, 10 μm. B Mouse spine sections for bone formation parameters. BFR/BS bone formation rate per bone surface area, MAR mineral apposition rate. ***p < 0.001, n = 6. C HIIT had a greater regulatory effect on osteoblast differentiation and osteoid formation than the LICT group mice. Goldner’s staining of mouse vertebral trabecular bone. Scale bars, 50 μm. D Histomorphometry parameters of vertebral bone. Ob.S/BS osteoblast surface per bone surface, OS/BS osteoid per bone surface. *p < 0.05, n = 3. E Representative OCN immunohistochemical staining in the calvarias of female mice in the control group, LICT group, and HIIT group. Scale bars = 100 μm F Percentage of OCN-positive areas in the calvarias of the samples shown in (C). The data are presented as the means ± SD. **p < 0.01, n = 3. G Representative Trap staining images of mouse femur. Scale bars, 200 μm. H Femur bone histomorphometry parameters of Trap staining. N.OC/B number of osteoclasts per bone surface, Oc.S/BS osteoclasts surface per bone surface, ES/BS eroded surface per bone surface. *p < 0.05, **p < 0.01, ***p < 0.001, n = 6. I Representative IF images for Trap protein expression in femur trabecular bone of mice. Scale bar, 200 μm. (J) Percentage of Trap positive area in the femur of the sample in (I). All data are presented as mean ± SD, n = 6; **p < 0.01, ***p < 0.001. K Representative image of TRAP staining of the calvarias of female mice in the control group, LICT group, and HIIT group. Scale bars = 100 μm. L Percentage of TRAP-positive areas in the calvarias of the samples shown in (K). The data are presented as the means ± SD. ***p < 0.001, n = 3. A two-way ANOVA followed by the Dunnett’s test was used for the multiple comparison test.
To examine bone resorption capacity, we stained for the mature osteoclast marker tartrate-resistant acid phosphatase (TRAP). Our results revealed that osteoclast number, osteoclast surface, and eroded surface also were remarkably decreased in LICT and HIIT groups compared to Ctrl group. However, HIIT had a greater inhibitory effect on osteoclast activity than LICT (Fig. 2G, H). To verify whether HIIT’s regulation of bone resorption involves increased expression of TRAP protein, we examined marker protein levels related to bone resorption by immunofluorescence. Our results indicated that HIIT was more efficacious than LICT in inhibiting TRAP protein expression (Fig. 2I, J). We performed Trap staining on the calvaria and found that LICT was not effective in reducing Trap enzyme activity, while HIIT significantly reduced Trap enzyme activity (Fig. 2K, L). These results indicate that HIIT was more effective than LICT in inhibiting bone resorption.
Lactate inhibits osteoclasts and promotes osteoblast differentiation via TAK1-p65 and Wnt/β-catenin in a Gpr81-dependent manner
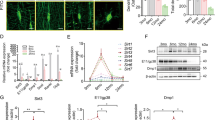
As a consequence of the considerable production of lactate by means of glycolysis during HIIT21, we examined the blood lactate levels across different groups of mice. Our results revealed a marked increase in lactate levels post-HIIT compared to controls, whereas LICT did not induce significant lactate elevation (Fig. 3A). These findings indicate that lactate may be a pivotal factor in maintaining bone homeostasis during HIIT. It is noteworthy that lactate is a naturally occurring endogenous ligand for Gpr818. Therefore, we examined Gpr81 expression on bone trabeculae surfaces among different groups, revealing a significant upregulation following HIIT (Supplementary Fig. 3A, B). To gain further insight into the function of lactate-Gpr81 in the regulation of bone homeostasis, we generated Gpr81 knockout mice using the CRISPR/Cas9 system (Supplementary Fig. 1A–D). The bone mass of Gpr81−/− mice was observed to be significantly lower than that of their wild-type littermates (Fig. 2B, C). However, there was little difference in cortical bone mineral density (Supplementary Fig. 4A, B). To investigate whether lactate modulates bone resorption via Gpr81 in osteoclasts, BMM was isolated from Gpr81−/− and WT mice, and osteoclast differentiation was induced with M-CSF and RANKL (Fig. 3D). Our results demonstrated the knockout of Gpr81 markedly promoted osteoclastgenesis, while lactate can inhibit osteoclastgenesis in WT mice. Notably, there was little inhibitory effect of lactate on osteoclast differentiation in the absency of Gpr81 (Fig. 3E), as demonstrated by a reduction in the expression of osteoclast-specific markers including Trap, Nfatc1, and Ctsk (Fig. 3F, G). To confirm whether lactate regulates bone formation via the activation of Gpr81 on osteoblasts, we isolated BMSC with tri-lineage differentiation potential (Supplementary Fig. 5A). BMSC were isolated and the differentiation and mineralization of osteoblasts were then evaluated. The findings demonstrated that lactate enhanced the differentiation and mineralization of osteoblasts. However, the role of lactate in promoting osteoblast differentiation was abolished in Gpr81 knockout mice (Fig. 3H, I). Moreover, RNA was extracted from osteoblasts to assess the mRNA expression levels of specific osteoblast marker genes, including Runx2, Alp, and Osterix. These marker genes for osteoblast differentiation were significantly upregulated after lactate stimulation via Gpr81 (Fig. 3J). The above results suggest that lactate promotes osteoblast differentiation and inhibits osteoclast differentiation through Gpr81.
A Measuring blood lactate concentration after blood collection from the tail vein. Bars represent mean ± SD. ***p < 0.001. n = 6 per time point. B Representative micro-CT images of the femurs of 8-week-old WT and Gpr81−/− mice showing the distal femur (top; scale bars = 1 mm) and trabeculae (bottom; scale bars = 1 mm). C Quantitative micro-CT analysis of the trabecular bone parameters of the femurs shown in (B). *p < 0.05, **p < 0.01, n = 6. D Schematic illustration of grouping of BMM extracts: extracts of WT and Gpr81−/− mice with BMM induced differentiation towards osteoclasts were stimulated by the lactate or PBS, and relevant assays were performed after 7 days. E Representative trap staining images of osteoclast. Scale bars, 200 μm. F Quantifying osteoclast number and Trap-positive area in (E). n = 6. **p < 0.01, ***p < 0.001. For multiple comparisons, experiments were replicated three times independently. G Analysis of Trap, Nfatc1 and CTSK mRNA levels by RT-qPCR in wild-type, Gpr81−/−, wild-type and Gpr81−/− stimulated with lactate (10 mM) for 7 days. All data are expressed as mean ± SD, n = 6. **p < 0.01, ***p < 0.001. Two-way ANOVA followed by Dunnett’s test was used for multiple comparative tests with experiments independently repeated three times. H BMSC were isolated and subjected to ALP staining (day 7), Von Kossa staining (day 14) and Alizarin red staining (day 21) from 1-month old Gpr81−/− mice and WT littermates. I Representative ALP staining, Alizarin Red S staining and Von Kossa staining of osteoblasts from WT littermates and Gpr81-deficient mice. J RT-qPCR analysis of Runx2, ALP and Osterix mRNA levels in WT and Gpr81−/− osteoblasts stimulated with lactate (10 mM) for 14 days. All data are expressed as mean ± SD; *p < 0.05, **p < 0.01, ***p < 0.001. Two-way ANOVA followed by Dunnett’s test was used for multiple comparative tests with experiments repeated independently three times.
TAK1 is a pivotal regulator of the NF-κB signaling pathway22. The results of our research demonstrated that a TAK1 inhibitor (NG-25) was capable of preventing the inhibiting effect of lactate from affecting the differentiation of osteoclasts (Fig. 4A, B). qRT-PCR analysis of RANKL-induced BMM revealed a significant reduction in osteolytic markers, such as Trap, Nfatc1, and CTSK, following lactate treatment. However, this effect was no longer observed after NG-25 treatment (Fig. 4C). To further demonstrate whether Gpr81 inhibits osteoclast differentiation through NF-κB, we used NF-κB inhibitor BAY 11-7082 to evaluate the correlation between Gpr81 and osteoclast differentiation, our results showed inhibiting NF-κB indeed could block lactate induced osteoclast decline (Supplementary Fig. 6A, B), indicating that Gpr81 inhibits osteoclast differentiation through the TAK1-NF-κB signaling pathway. Furthermore, our findings indicate that Gpr81 knockout resulted in elevated TAK1 and phosphorylated p65 expression in osteoclasts following RANKL stimulation. Notably, lactate reduced phosphorylated p65 and TAK1 in a Gpr81-dependent manner (Fig. 4D, E). The collective findings illustrate that lactate can inhibit RANKL-induced osteoclastogenesis via the Gpr81-TAK1-p65 signaling cascade.
A The BMM of mice from the vehicle and NG-25-treated groups were taken to induce osteoclast differentiation for 6 days with or without 10 mM lactate intervention, followed by Trap staining. Scale bars, 200 μm. B Quantifying osteoclast number and Trap-positive area in (B). n = 6. ***p < 0.001. Two-way ANOVA was performed followed by Dunnett’s test for multiple comparisons with experiments repeated independently three times. C RT-qPCR analysis of Trap, Nfatc1 and CTSK mRNA levels, *p < 0.05, **p < 0.01, ***p < 0.001. n = 4. D BMM of mice from the vehicle and NG-25-treated cells were taken to induce osteoclast differentiation for 6 days with or without 10 mM lactate intervention, and Western blots analysis of p65, p-p65, TAK1 and Gpr81 proteins levels in osteoclasts. E Quantification of TAK1, Gpr81 and p-p65 protein expression levels. *p < 0.05, **p < 0.01. n = 6. F Lactate promotes osteogenic differentiation and mineralization, and BMSC differentiation and mineralization are inhibited in iCRT(Wnt inhibitor) treated mice. The BMSC were differentiated for 7, 14 and 21 days, respectively, and then stained for ALP, von Kossa’s and Alizarine Red in the presence or absence of 10 mM lactate. G BMSC of mice from the vehicle and iCRT3-treated groups were taken to induce osteogenic differentiation for 14 days with or without 10 mM lactate intervention, respectively, and RT-qPCR analysis of Runx2, ALP, and Osterix mRNA levels in osteoblasts. All data are presented as mean ± SD. n = 6. **p < 0.01., ***p < 0.001. H BMSC of mice from the vehicle and iCRT3-treated groups were taken to induce osteogenic differentiation for 14 days with or without 10 mM lactic acid intervention, and Western blots analysis of Wnt3, β-catenin, and OCN proteins levels in osteoblasts. I Quantification of Wnt3, β-catenin and OCN protein expression levels. n = 6. *p < 0.05, **p < 0.01, ***p < 0.001.
The Wnt/β-catenin signaling pathway has been identified as a pivotal regulator of bone formation through its influence over osteoblast proliferation and differentiation13. Prior research has demonstrated that 5 mM lactate can stimulate the Wnt/β -catenin signaling cascade via Gpr81, thereby facilitating the proliferation and differentiation of intestinal stem cells15. The objective of our study was to ascertain whether lactate-Gpr81 can also activate the Wnt/β-catenin pathway in osteoblasts, thereby promoting bone formation. The results indicate lactate could promote osteoblast differentiation and mineralization, whereas inhibiting the Wnt/β-catenin signaling by iCRT3 could block those increasing induced by lactate (Fig. 4F), besides, the expression levels of osteoblast marker genes, including Runx2, ALP, and Osterix also showed the same phenomenon (Fig. 4G), those results demonstrated Gpr81 promotes osteoblast differentiation through the Wnt/β-catenin pathway. Furthermore, Western blot results showed that knockout of Gpr81 resulted in a significant reduction of Wnt3, β-catenin and OCN expression in osteoblasts. Furthermore, we observed that lactate upregulated Wnt3, β -catenin and OCN expression, but the observed effect was dependent on the presence of Gpr81 (Fig. 4H, I). Therefore, lactate-Gpr81 plays a regulatory role in osteoblast differentiation and function, acting to activate the Wnt/β -catenin pathway.
HIIT improves bone mass of OVX mice partly via Gpr81
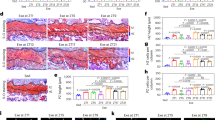
To investigate the effect of the Gpr81 on bone metabolism in pathological states, we established OVX mouse model of WT and Gpr81−/− to simulate PMOP and underwent 8 weeks of HIIT (Fig. 5A). The cancellous and cortical bone density of OVX mice was significantly lower than that of sham group, suggesting that the OVX mice were successfully modelled (Supplementary Fig. 7A–C). To ensure that high-intensity exercise was consistently maintained at 80–90% of maximum running speed, we conducted a maximum running speed test every 2 weeks. There was no significant difference in maximum running speed between WT and Gpr81−/− mice (Supplementary Fig. 8A). HIIT exercise significantly increased blood lactate levels in both WT and Gpr81−/− mice, but 8 weeks of OVX did not affect blood lactate levels in WT and Gpr81−/− mice (Supplementary Fig. 8B). To investigate whether the improvements in bone mass induced by HIIT in OVX mice depend on Gpr81, we used micro-CT analysis to show that HIIT increases bone mass in both WT and Gpr81−/− mice, but does not increase Tb.N or Tb.Th in Gpr81−/− mice (Fig. 5B, C). While HIIT increased BMD and BV/TV in Gpr81−/− mice, the magnitude of the increase was significantly weaker than in WT mice (Fig. 5D). HIIT increased cortical thickness and BMD in WT mice, but there were little significant differences in cortical bone parameters after HIIT in Gpr81−/− mice (Fig. 5E). In order to reduce the impact of mechanical stimulation on bone, we performed non-decalcified histomorphometry analyses of lumbar vertebrae in HIIT and sedentary WT and Gpr81−/− mice (Fig. 5F). It was found that lumbar cortical bone thickness was increased by HIIT in WT mice only, with no such effect on either parameter in Gpr81−/− mice (Fig. 5G, H). The results of this study indicated that Gpr81 plays a pivotal role in improving bone mass during HIIT.
A Graphical overview of the experimental design. All mice were subjected to OVX surgery. WT and Gpr81−/− mice were divided into sedentary and HIIT groups. the mice in the HIIT group were subjected to treadmill exercise for a period of 8 weeks, and the running protocol was kept consistent with that of mice. They were sacrificed two days after the end of the last exercise. B Micro-CT representative images of the femur from OVX WT and Gpr81−/− mice showing the proximal femur (top) and the trabecular (middle) and cortical bone (bottom) of the femoral metaphysis. Scale bar: 1 mm (all panels). C Quantitative micro-CT analysis of trabecular bone parameters of femurs from OVX WT and Gpr81−/− mice. *p < 0.05, **p < 0.01, ***p < 0.001. n = 6. D Differences in BMD and BV/TV after HIIT in cancellous bone of WT and Gpr81−/− mice *p < 0.05, ***p < 0.001. n = 6. E Micro-CT quantitative analysis of cortical bone parameters in femurs of OVX WT and Gpr81−/− mice. *p < 0.05, **p < 0.01. n = 6. F Von Kossa staining of vertebral sections from WT and Gpr81−/− mice. Scale bars, 500 μm. n = 6. G Quantitative Von Kossa staining of cortical bone thickness of lumbar sections from WT and Gpr81−/− mice. **p < 0.01. n = 6. H Quantitative Von Kossa staining of trabecular bone parameters of lumbar sections from WT and Gpr81−/− mice. **p < 0.01, ***p < 0.001. n = 6.
HIIT promotes bone formation and inhibits bone resorption in OVX mice partly via Gpr81
In order to further evaluate the role of Gpr81 in the regulation of bone homeostasis by HIIT in OVX mice, we first assessed bone formation in OVX Gpr81−/− mice and control littermates. The calcein double labelling assay revealed a notable increase in bone formation and mineralization rates in HIIT-treated WT mice. Conversely, in Gpr81−/− mice, the rate of bone formation and mineralization exhibited an upward trend, although this did not reach statistical significance (Fig. 6A, B). Additionally, Goldner’s staining for osteoblast number and osteoid area illustrated a twofold increase in osteoblast area and osteoid mass in HIIT-treated WT mice, contrasting with a roughly 1.2-fold increase in Gpr81−/− mice (Fig. 6C, D).
A In Gpr81−/− OVX mice, the effect of HIIT on bone formation rate was reduced. Images representative of calcein double labelling of spinal trabecular bone from OVX mice. Scale bars, 10 μm. B Bone formation parameters in vertebral sections of WT and Gpr81−/− OVX mice (bottom). *p < 0.05, **p < 0.01, ***p < 0.001, n = 6. C The regulatory effects of HIIT on osteoblast differentiation and osteoid formation are decreased in Gpr81−/− OVX mice. Goldner’s staining of mouse vertebral trabecular bone. Scale bars, 50 μm. D Histomorphometry parameters of vertebral bone. Ob.S/BS osteoblast surface per bone surface, OS/BS osteoid per bone surface. *p < 0.05, **p < 0.01, ***p < 0.001, n = 6. E The inhibitory effects of HIIT on osteoclast activity are decreased in Gpr81−/− mice. Trap staining representative images of mouse femoral trabecular bone. Scale bars, 200 μm. F Femur bone histomorphometry parameters of Trap staining. *p < 0.05, **p < 0.01, ***p < 0.001, n = 6. G Representative image of TRAP staining of the calvarias of OVX mice. Scale bars = 1 mm. H Percentage of TRAP-positive areas in the calvarias of the samples shown in (G). The data are presented as the means ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, n = 6.
To ascertain whether the gain in bone mass subsequent to HIIT is attributable to a diminution in bone resorption, we undertook an examination of the activity of the osteoclast secretory protein-TRAP. Reductions in the number and area of osteoclasts were observed in the HIIT group in the trabecular bone of the femur (Fig. 6E, F) and on the cranial surface (Fig. 6G, H), whereas no significant difference was observed in Gpr81−/− mice after HIIT. These findings suggested that HIIT increased bone mass in OVX mice in a manner that promoted bone formation and inhibited bone resorption via Gpr81, while knockout of Gpr81 resulted in decreased bone mass.
Lactate rescues low bone mass phenotype of OVX mice in vivo via Gpr81
To further confirm whether lactate could rescue OVX induced osteoporosis, we intraperitoneally injected WT and Gpr81−/− mice daily with 1 g/kg lactate (Fig. 7A). Femoral 3D reconstruction using micro-CT demonstrated lactate rescue for trabecular BMD, BV/TV, TB.N and Tb.Th in WT mice (Fig. 7B, C). Lactate also promoted cortical bone thickness as well as BMD in WT mice, but the enhancing effects of lactate on trabecular and cortical bone were dependent on Gpr81 (Fig. 7D, E). To study the possible role of lactate-Gpr81 in rescuing mineralized vertebral bone in OVX mice, we performed non-decalcified histomorphometry analyses of the spine in Gpr81−/− mice and control littermates. Our results suggest that lactate injection leads to an increase in BV/TV in WT mice whereas it does not in Gpr81−/− mice. This effect was attributed to an increase in trabecular number, an increase in Tb.Th, and a decrease in Tb.Sp compared to the saline group (Fig. 7F, H). Concurrently, lactate increased the thickness of the lumbar cortical bone in mice depending on the Gpr81 (Fig. 7G). Furthermore, we found that lactate can promote bone formation and osteoblast function (Supplementary Fig. 9A–D) while inhibiting bone resorption in vivo (Supplementary Fig. 9E, F). Therefore, our data indicated that lactate can effectively rescue the bone mass phenotype in OVX mice via Gpr81.
A Schematic diagram illustrating the experimental design of lactate treatment in WT mice: 2 weeks after OVX surgery, mice were subjected to intraperitoneal injections of lactate five times a week for a total of 8 weeks. This figure was originally created by the authors using Microsoft PowerPoint. B Micro-CT representative images of the femur from OVX WT and Gpr81−/− mice show the proximal femur (top) and the trabecular (bottom) of the femoral metaphysis. Scale bar: 1 mm (all panels). C Micro-CT quantitative analysis of trabecular bone parameters in femurs of OVX WT and Gpr81−/− mice. *p < 0.05, **p < 0.01, ***p < 0.001. n = 6. D Micro-CT representative images of the femur from OVX WT and Gpr81−/− mice showing the cortical bone (bottom) of the femoral metaphysis. Scale bar: 1 mm. E Quantitative micro-CT analysis of cortical bone parameters of femurs from OVX WT and Gpr81−/− mice. *p < 0.05. n = 6. F Von Kossa staining of vertebral sections from OVX WT and Gpr81−/− mice. Scale bars, 500 μm. n = 6. G Quantitative Von Kossa staining of cortical bone parameters of lumbar sections from WT and Gpr81−/− mice. **p < 0.01. n = 6. H Quantitative Von Kossa staining of trabecular bone parameters of vertebral sections from OVX WT and Gpr81−/− mice. *p < 0.05, **p < 0.01, ***p < 0.001. n = 6.
Discussion
A previous study revealed that HIIT modulates bone mass superior to low-intensity exercise2. However, the mechanism underlying this effect remains unclear. Here, we demonstrated that HIIT effectively increases bone formation and reduces bone resorption compared to LICT. HIIT also elevates blood lactate levels and upregulates Gpr81 expression on bone trabecular surfaces. In terms of molecular mechanisms, lactate inhibits osteoclast differentiation through activation of the Gpr81-TAK1-p65 signaling pathway, while promoting osteoblast differentiation via activation of the Gpr81-Wnt/β-catenin pathway. Further studies showed that HIIT could rescue ovariectomy-induced bone loss, whereas Gpr81 deficiency in mice attenuated the bone-preserving effects of HIIT in OVX mice. It can therefore be concluded that the effects of HIIT on bone formation and bone resorption were mediated by Gpr81 in OVX mice.
HIIT has demonstrated superior efficacy in preventing and treating metabolic disorders compared to continuous aerobic training23. Here we found that HIIT had a greater effect on bone mass than LICT. One major difference between HIIT and LICT is that HIIT mainly relies on anaerobic glycolysis for energy, with lactate as its main by-product. Recent studies have indicated that lactate produced by HIIT can regulate hippocampal mitochondrial function6 and promote angiogenesis24 via Gpr81. However, the mechanism by which HIIT modulates bone homeostasis remains unclear. Lactate is considered a metabolite that can reduce inflammatory responses25. Research has found that lactate can reprogram inflammatory T cells into regulatory T cells26. Importantly, HIIT has shown greater potential than continuous aerobic training in regulating anti-inflammatory responses in aging rats27,28. Considering that osteoclast overactivation is a primary contributor to PMOP, with osteoclast activity positively associated with inflammation29. Our research has demonstrated that lactate can inhibit osteoclastogenesis by activating Gpr81. Given that the EC₅₀ for lactate-mediated Gpr81 activation is ~5 mmol/L30, and that physiological lactate levels (even during low-intensity, aerobic exercise) are insufficient to activate this receptor31,32, this metabolic threshold may explain why HIIT outperforms LICT in enhancing bone mass. In addition, we found that HIIT promotes osteoblast differentiation and inhibits osteoclast differentiation more effectively than LICT (Supplementary Fig. 2A–C). Lactate can serve as a substrate and, under the action of histone lactyl transferase, the lactate group is covalently attached to specific amino acid residues of histones, thereby achieving histone lactylation modification29. This suggests that lactate produced during exercise may regulate osteoblast and osteoclast differentiation through epigenetic modifications; however, the specific mechanisms require further investigation.
Although studies have reported that HIIT can increase bone mass in OVX mice, it is important to note that in the intensity of HIIT in different exercise programme, which have different effects on the organs33. Considering that lactate threshold increases progressively with exercise training, we conducted fortnightly maximal running speed tests. We maintained the mice at 80–90% of their maximum running speed during high-intensity training. Blood lactate levels may be one of the main reasons why previous studies have found no significant effect of HIIT on bone resorption in OVX mice34. Existing research has primarily focused on the effects of lactate on bone formation, while its impact on bone resorption remains unclear35. Due to PMOP is a disease of osteoclast overactivation, and the lack of estrogen promotes RANKL production which in turn leads to bone loss27. Therefore, investigating lactate’s mechanism in regulating bone resorption in OVX mice holds clinical significance.
Gpr81 is an endogenous receptor specifically recognizing lactate8, and its specific agonists have been widely used in other diseases36. For instance, agonists targeting HCAR1 have demonstrated neuroprotective effects against progressive retinal ganglion cell dysfunction37, while in adipocytes, Gpr81 activation suppresses lipolysis, suggesting potential therapeutic implications in dyslipidemia and related conditions38. In this study, we found that Gpr81 is an upstream signaling molecule for the NF-κB and Wnt/β-catenin pathways. Activation of the NF-κB pathway typically promotes osteoclast differentiation and the release of pro-inflammatory factors, thereby exacerbating bone resorption. Previous studies have shown that lactate activates Gpr81 to inhibit the production of pro-inflammatory cytokines in macrophages, thereby disrupting the interaction between YAP and NF-κB and their nuclear translocation in macrophages17. However, the immune-suppressive effects induced by lactate are unrelated to cAMP signaling. Studies have shown that the cytoplasmic signaling molecule β-arrestin 2 is essential for these immunosuppressive effects, and there is a direct interaction between β-arrestin2 and GPR81. β-arrestin2 signaling is known to antagonize the TLR and NLRP3 pathways, which may explain how GPR81 inhibits the NF-κB signaling pathway via β-arrestin rather than Gαi39. The Wnt/β-catenin pathway is a core driver of osteoblast differentiation and bone matrix synthesis. Studies have shown that cAMP-PKA enhances Wnt signaling by inhibiting GSK-3β, suggesting that Gαi inhibition of cAMP-PKA may reversely inhibit the Wnt pathway. However, in bone cells, GPR81 indirectly promotes the osteogenic effects of the Wnt pathway through the Gαi-RhoA axis40. As an upstream regulator of both pathways, Gpr81 may act as a “bidirectional switch” in bone metabolism. The core pathological feature of osteoporosis is that bone resorption exceeds bone formation. In current treatment regimens, long-term use of bisphosphonates may increase the risk of osteonecrosis, while parathyroid hormone-like drugs require daily injections and are associated with a risk of fractures41. Therefore, the combination of Gpr81 agonist with HIIT represents a promising and safe therapeutic strategy for PMOP.
There are some limitations to this study as well. We did not generate conditional Gpr81 knockout mice in osteoblasts and osteoclasts, lactate can still regulate bone mass by activating other cells, such as Gpr81 on adipocytes42. Although it has been reported that Gpr81 downstream is coupled with Gαi and β-arrestin, these small G proteins are believed to be associated with the classical Wnt and NF-κB signaling pathways, but whether they are the primary effector molecules downstream of Gpr81 in osteoblasts and osteoclasts remains to be further investigated. Additionally, the efficacy and mechanisms of HIIT in patients with osteoporosis require further investigation.
Methods
Animals
The experiments involving mice were reviewed and approval was granted by the East China Normal University (ECNU) Animal Care and Use Committee (m20201103). Gpr81 knockout mice (Gpr81−/−) were generated by the Animal Center at East China Normal University using the CRISPR/Cas9 system in the C57BL/6 J mouse strain. A 720-base pair segment was excised from the Gpr81 gene and confirmed through PCR. Wild-type and Gpr81−/− mice used in this study were sacrificed at 8 weeks of age.
Mice were euthanized using a validated carbon dioxide (CO2) asphyxiation system in accordance with institutional animal welfare guidelines. Briefly, animals were individually transferred into a well-ventilated euthanasia chamber pre-calibrated to maintain proper gas flow dynamics. High-purity CO2 was introduced at an initial flow rate of 20–30% chamber volume per minute to rapidly displace ambient air, followed by a maintenance flow rate of 10–15% chamber volume per minute to sustain a euthanogenic environment. Animals were monitored continuously until complete cessation of spontaneous respiration and corneal reflex loss, typically achieved within 2–3 min. Post-euthanasia, absence of heartbeat was confirmed by thoracic palpation, and euthanasia timepoints were recorded for each subject. Then, separate the mouse skull, remove excess tissue such as the brain and skeletal muscle, and keep only the cranium. Then, separate the mouse femur from the acetabulum and remove the skeletal muscle surrounding the bone. Take the mouse’s lumbar vertebrae, remove the excess tissue on the ventral side, and make the vertebral bodies clearly visible. Place the prepared bone tissue in 4% paraformaldehyde for 48 h for subsequent testing.
Gpr81 knockout strategy
CRISPR/Cas9 technology was used to manipulate the Gpr81 gene (Gene ID: 243270) in C57BL/6 strain mice. After bioinformatics analysis, there is only one exon within the structure of one protein-coding transcript (201 transcript) at this gene locus, so two target sites spaced 727 bp apart were selected on its exon1 sgRNA was constructed and Cas9 mRNA was prepared (HCAR1-KO-sgRNA1: TGGAGATATCGCCTGTCGCCTGG; HCAR1-KO-sgRNA2: GGAACGATTTGC C ATCGATGG), and the target site with a higher score was screened by off-target rate prediction, sgRNA was constructed and Cas9 mRNA was prepared. The two were mixed at a certain concentration and injected into the cytoplasmic portion of fertilized eggs of mice through microinjection to construct specific embryonic cells, and the surviving fertilized eggs were transplanted into the oviducts of pseudo-pregnant females after 1–2 h of in vitro cultivation; the resulting offspring mice were extracted from the genome DNA to carry out genotypic identification, and the F1 generation of the knockout mice was obtained by crossing the knockout mice with the wild-type mice and re-identified. A 727 bp segment was deleted in exon 1 (Supplementary Fig. 1C). To confirm successful knockout, we further performed genotyping of Gpr81 knockout mice using PCR (Supplementary Fig. 1D).
Ovariectomized mice
Twenty-four 8-week-old female mice were acclimatized and fed normally for 2 week and injected intraperitoneally with a 0.02 ml/g dose of tribromoethanol. The hairs near the ovaries were removed with forceps, the ovaries were removed bilaterally, and the paraspinal musculature was excised approximately 1 cm above the knee joint. After surgery, the rats were kept at body temperature on a 37° roasting table and released back into their cages when they awoke.
Lactate intervention
Ten weeks-old female mice underwent ovariectomy (OVX) surgery. Two weeks later, lactate intervention was initiated. A 200 mg/ml solution of L-lactate sodium (≥99.0%, Aldrich, 71718) was prepared using physiological saline as the solvent. The pH was adjusted to 7.4, and lactate was administered via intraperitoneal injection at a dose of 1 g/kg, five times per week, for a total of 8 weeks.
Micro-CT
A small animal CT machine (Skyscan 1076, Bruker) was used for scanning at a precision of 9 μm. A region of interest of the distal femur was selected below the growth plate from 0.215 mm (1200 image slices) to 1.72 mm (1300 image slices), where the growth plate slice was defined as 0 mm. The micro-CT system uses a source to emit a beam of X-rays, which is directed onto the sample. The intensity of the X-rays is attenuated on the basis of the sample density and composition, so the attenuated signal recorded by the detector provides information about the sample structure. Three-dimensional reconstruction was subsequently performed using specialized software, followed by analysis of the parameters related to bone volume and bone mineral density. The bone mineral density of the sample can be determined by measuring the sample attenuation coefficient and then comparing this attenuation value with those of known reference samples (usually standard bone density materials). Thus, the bone density of samples can be indirectly calculated.
Exercise protocol
For healthy mice, a one-week adaptation training period is conducted prior to formal training. For the low-intensity continuous exercise group, the speed on the first day is 4 m/min for 10 min, 6 m/min for 20 min on the second and third days, and 8 m/min for 40 min on the fourth and fifth days. Formal training is conducted on a treadmill with a 0° incline at a speed of 10 m/min for 60 min, 5 days a week. The HIIT group’s adaptive training programme comprised a 10-min warm-up session with a pace of 8 m per min, followed by a HIIT exercise session with a pace of 18 m per minute and a resting pace of 10 m per minute. The number of cycles increased in sequence. The formal training protocol was modified with reference to the Morland et al.24 study protocol: The mice underwent a regimen of HIIT on a treadmill with a 0° incline, 5 days per week, for a total of 8 weeks. A 10-mine warm-up at a pace of 10 m/min was conducted, followed by 10 sessions of alternating interval training at 80–90% of speedmax for 4 min per exercise during high-intensity exercise and 40–50% of speedmax for 2 min per exercise during the active recovery period (low-intensity exercise). For OVX mice, exercise began two weeks after surgery. The HIIT exercise programme was consistent with that of healthy mice.
For all mice undergoing HIIT. After acclimatization, the mice were tested for maximal exercise capacity at weeks 2, 4 and 6. During training, running speeds were adjusted to match anticipated blood lactate levels and maximal oxygen uptake. Mouse maximal running speed test method: first let the mice warm up in the treadmill for 10 min (speed of 10 m/min), then increase the running speed by 2 m/min every 2 min until the mice exhausted, at this time close to the maximal oxygen uptake, and record the maximal running speed of the mice.
Histomorphometry
Calcein double labeling was performed by injecting calcein (30 mg/kg) into mice twice. Vertebral bone was fixed with 4% PFA for 2 days and embedded in methyl methacrylate. Histomorphometry was performed on plastic-embedded using standard protocols12. For Goldner’s staining, the prepared Weigert iron hematoxylin was added and dyed for 15–20 min. After washing with running water for 1 min, Acid Ponceau staining solution was added for 5 min. The slices were washed with weak acid working solution for 15–30 s. Orange G staining solution for staining was added until Ponceau staining solution was removed, which generally took 3–10 min. The slices were rinsed with the prepared weak acid working solution for 15–30 s. The slices were added into bright green staining solution for dyeing for 5 min and rinsed with the prepared weak acid working solution for 3 times, 15 s each time. Finally, the slices were rinsed with distilled water, sucked or air dried, dehydrated with absolute ethanol, sealed with neutral gum, and observed under microscope. Bone dynamic histomorphometry analyses for BFR/BS (bone formation rate per bone surface) and MAR (mineral apposition rate) as well as bone static histomorphometry analyses for N.OB/B (the number of osteoclasts/bone), OS/BS (osteoid per bone surface) were quantified using the Osteomeasure Histomorphometry System (OsteoMetrics, Decatur, GA, USA). Bone dynamic histomorphometry analyses for BFR/BS (bone formation rate per bone surface) and MAR (mineral apposition rate) as well as bone static histomorphometry analyses for N.OB/B (the number of osteoclasts/bone), OS/BS (osteoid per bone surface) were quantified using the Osteomeasure Histomorphometry System (OsteoMetrics, Decatur, GA, USA).
Three-point bending test
The three-point bending test was employed to assess the humeral bone strength using a small animal bone strength testing device (YLS-16A, Yanyi Jinan Corp., P.R. China). Following dissection, the freshly excised femurs was promptly analyzed via this test, which utilized two supporting points at the ends and a single loading point at the center. Biomechanical data were obtained from the resultant load-deformation curves, with the maximum load (expressed in Newtons) being documented.
Immunofluorescence
Paraffin sections underwent a 20-min fixation in 4% paraformaldehyde (Servicebio, G1101) following a dewaxing recovery water process. Subsequently, the sections were placed in citric acid antigen repair solution at 100 °C for 5–15 min. Subsequently, the tissues were subjected to permeabilization with a solution of 0.2% TritonX100 and PBS (Servicebio, G1204) over a 20-min period until the temperature reached room level. The tissues were then incubated in 3% bovine serum albumin (Sigma, A4781) for a period of 60 min. The sections were then incubated with an anti-tartrate ATPase (Trap, 1:200, Proteintech, 11594-1-AP) antibody at 4 °C. The sections were then washed three times with PBS and incubated with a Cy3 conjugated Goat Anti-Rabbit IgG (H + L) (Servicebio, GB21303) for one hour, protected from light. The cell nuclei were visualized using 1 mg/mL 4,6-diamidino-2-phenylindole (Servicebio, GB21303).
Immunohistochemistry
Paraffin sections underwent a gradient dehydration process, followed by fixation in 4% polyformaldehyde (PFA) for 30 min. Subsequently, the tissues were permeabilized with a solution containing 0.1% Triton X-100 and 0.1% phosphate-buffered saline tween (PBST) for 30 min. Antigen retrieval was carried out using 20 μg/mL proteinase K for 20 min. To quench endogenous peroxidase activity, the sections were treated with 3% H₂O₂. Blocking was then performed with 2% bovine serum albumin (BSA) for 60 min. The sections were incubated overnight at 4 °C with the Gpr81 antibody (Proteintech, Catalog No. 20146-1-AP, diluted at 1:300). After thorough washing, the sections were incubated with corresponding secondary antibodies and visualized using diaminobenzidine (DAB; Servicebio, Product Code G1212). Hematoxylin counterstaining was applied, and images were captured using an Olympus microsystems microscope (Olympus Corporation, Tokyo, Japan). Image analysis and quantification were conducted using ImageJ software.
Inducing BMSC differentiation to osteoblasts
To evaluate BMSC osteogenic differentiation potential, we isolated bone marrow-derived cells from male mice and retained only the adherent cell population, which predominantly consists of BMSCs12. We used α-Minimum Essential Medium (Hyclone, SH30265.01) with 10% FBS (FBS; Gibco, 10099-1633101) and 1% penicillin-streptomycin (HyClone, SH40003-12). Bone marrow-derived mesenchymal stem cells (BMSC) were inoculated at 8 × 104 cells per well in 24-well plates. The osteogenic differentiation medium consisted of α-MEM supplemented with 10% fetal bovine serum (FBS), 50 μg/ml l-ascorbic acid (Sigma, A4034), 0.1 μM dexamethasone (Sigma, D4902), 10 mM β-glycerophosphate (Sigma, G6376), and 1% penicillin-streptomycin (HyClone, SH40003-12). The medium was changed every 2 days to maintain optimal culture conditions. For osteogenic induction, BMSC were seeded at a density of 1 × 105 cells per well in 24-well plates and cultured in a humidified incubator at 37 °C with 5% CO2.
ALP, Alizarin red S and Von Kossa staining
BMSC were plated at 8 × 10⁴ cells per well in 24-well plates and expanded for 3 days. Osteoblasts were differentiated by culturing them in a differentiation medium. On days 7, 14 and 21, the cells were fixed for 25 min. The cells were stained using the Alkaline Phosphatase Assay Kit (Beyotime, C3206). Alkaline phosphatase, alizarin red S (Sigma, A5533) and von Kossa staining (Energy Chemical, 7761-88-8) were conducted using 2.5% silver nitrate and 1% alizarin red S, respectively. Von Kossa staining requires the cells to be transferred to UV light for 30 min. Alizarin red S was incubated at room temperature for 20 min. After which the non-specific staining was washed away using distilled water and photographed on a light box.
Inducing BMM differentiation to osteoclast
Bone marrow cells from mice aged 4–8 weeks were resuspended in 6 cm dishes. Samples were incubated at 37 °C with 5% CO₂ for 12 h. The medium was α-MEM with 10% FBS (Excell, FSD500) and 1% P/S (HyClone, SH40003-12). Bone marrow cells were extracted and resuspended in 6 cm dishes. The next day, the supernatant was resuspended by centrifugation at 800 rpm for 5 min. In total, 10 ng/ml of M-CSF (R&D, 416-mL-050), was added and the cells were cultured for 24 h. They were then inoculated in 96-well plates at 1 × 104 cells per well with 50 ng/ml of RANKL(R&D, 462-TEC). The differentiation medium was replaced every 2 days for 6 days.
TRAP staining
For the mature osteoclasts cultured in 96-well plates, the medium was first aspirated, and the cells were then rinsed three times with phosphate-buffered saline (PBS). Subsequently, the cells were fixed with 4% paraformaldehyde (PFA) for 15 min, followed by a 10-min permeabilization step using 0.1% Triton X-100. After that, the cells were stained for tartrate-resistant acid phosphatase (TRAP) using a commercial staining kit (Sigma 387 A) and incubated at 37 °C for 2 h.
The paraffin sections were fixed in a solution containing 4% PFA and 0.1% Triton X-100 for 10 min, followed by incubation with the TRAP staining kit (sigma 387 A) at 37 °C for 2 h. the osteoclast surface/bone surface (OC. S/BS), the number of osteoclasts/bone perimeter (N. OC/B.Pm), and the ES/BS 0.2–2 mm below the growth plate were analyzed using the OsteoMeasure Analysis System (Osteometrics, Decatur, GA) and ImageJ software (NIH) according to the manufacturers’ protocols.
For calvarial bone staining, TRAP staining was performed for 2 h, and the osteoclast area was quantified using ImageJ software.
Paraffin embedded sections
Dehydration: The femurs of different groups were put into the embedding box and rinsed under running water for 20–25 min, then put into 50% ethanol for 1 h, 75%, 85% and 95% ethanol for 1 h, and 100% ethanol overnight. Transparent: Transfer the tissue in 100% ethanol to ethanol: xylene = 1:1 solution for 35 min, then transfer to xylene I solution for 35 min, and finally in xylene II for 35 min, the composition is transparent. Wax dipping: the paraffin cylinder was heated in advance in a water bath at 55°, the tissues were immersed in xylene II transferred to a solution of xylene: paraffin = 1:1 for 3 h, then transferred to paraffin I overnight, and on the next day, the tissues were transferred from paraffin I to paraffin II solution for 35 min, and the composition was transparent. The next day, the tissue was transferred from paraffin I to paraffin II solution for 3 h, which completed the process of tissue dipping. Embedding: take out a femur and put it into the embedding apparatus, fill it with paraffin wax and cover the embedding box, then freeze it quickly to solidify the paraffin wax. Slicing: Prepare the wrapped wax blocks, trim them to the appropriate size with a slicer, place them on a slicer and adjust the thickness of the slices to 5 μm.
RNA extraction and real-time quantitative PCR
RNA was extracted using TRIzol reagent-phenol chloroform (Vazyme Biotech, Nanjing, China) and cDNA was synthesised from RNA using the 5×HiScript II qRT SuperMix (Vazyme, Nanjing, China). Quantitative real-time PCR was conducted using the ChamQ Universal SYBR qPCR Master Mix. The 18S gene was used to quantify relative expression levels. Fold changes were calculated using the 2−ΔΔCt method (Table 1).
Western blotting
The cells were homogenized and ground using RIPA lysis buffer, which contained protease and phosphatase inhibitors, and lysed on ice for 30 min. The insoluble material was separated from the lysate by centrifugation (12,000 rpm, 10 min, 4 °C) and the supernatant was collected. Protein concentration was determined using a bicinchoninic acid assay (Thermo Fisher Scientific). The membranes were blocked with BSA and incubated with specific antibodies, including an osteocalcin antibody (Bioss, bs-0470R, 1:1000) and Wnt3 antibody (Proteintech, 28156-1-A, 1:1000). Incubation of HRP-coupled secondary antibody with β-catenin (Proteintech, 66379-1-Ig, 1:1500) and GAPDH (CST, USA, 2118). p-p65 (Proteintech, 2983, 1:1500) and p65 (Proteintech). 10745-1-AP, 1:1500; TAK1 (Proteintech, 12330-2-AP, 1:1500); Gpr81 (Proteintech, 20146-1-AP, 1:1500).
Plasma lactate measurements
For the measurement of plasma lactate levels, blood samples were collected from the tail vein immediately after high-intensity interval training. Blood was collected into 0.5-ml mini-collection tubes containing 2.5 mg of sodium fluoride (NaF) and 2.0 mg of potassium oxalate (KOx) per milliliter, to prevent post-exercise metabolism (lactate production) and clotting (respectively). Blood samples were centrifuged, and the resulting plasma samples were frozen in liquid nitrogen and stored at −80 °C. This was achieved through the utilization of an L-lactate assay kit (Abcam, ab65331), which was employed in accordance with the instructions outlined by the manufacturer.
Statistical analysis
The data are presented as means with standard deviations indicated on the vertical axes of the figures as absolute values. The statistical analyses were conducted with the assistance of the Prism 10.0 software package (GraphPad Prism). The statistical significance of the difference between the two groups was evaluated through the application of a two-tailed Student’s t-test, while a two-way ANOVA with Dunnett’s test was used when multiple comparisons were involved. A p-value of less than 0.05 was deemed to indicate statistical significance.
Ethics statement
All aspects of the protocol and experimental procedures were conducted in accordance with the relevant institutional guidelines and following the approval of the East China Normal University Laboratory Animal Ethics Committee (m20201103).
Data availability
All data supporting the findings of this study are available within the main paper and its Supplementary Information. The source data behind the graphs in the paper are provided as Supplementary Data 1. Original blots are provided in the Supplementary Figs. Further details related to this study can be obtained upon reasonable request from the corresponding author, Peng Sun (email: psun@tyxx.ecnu.edu.cn).
References
Behan, F. P. et al. Developing an exercise intervention to minimise hip bone mineral density loss following traumatic lower limb amputation: a delphi study. Br. J. Sports Med. 58, 1251–1257 (2024).
Kast, S. et al. Effects of different exercise intensity on bone mineral density in adults: a comparative systematic review and meta-analysis. Osteoporos. Int. 33, 1643–1657 (2022).
Kistler-Fischbacher, M., Weeks, B. K. & Beck, B. R. The effect of exercise intensity on bone in postmenopausal women (part 1): a systematic review. Bone 143, 115696 (2021).
Julian, V. et al. Bone response to high-intensity interval training versus moderate-intensity continuous training in adolescents with obesity. Obes. Facts 15, 46–54 (2022).
Dupuit, M. et al. Moderate-intensity continuous training or high-intensity interval training with or without resistance training for altering body composition in postmenopausal women. Med. Sci. Sports Exerc. 52, 736–745 (2020).
Shang, Q. et al. Lactate mediates high-intensity interval training-induced promotion of hippocampal mitochondrial function through the GPR81-ERK1/2 pathway. Antioxidants 12, 2087 (2023).
Li, X. et al. Lactate metabolism in human health and disease. Signal Transduct. Target. Ther. 7, 305 (2022).
Davidsson, O. et al. Identification of novel GPR81 agonist lead series for target biology evaluation. Bioorg. Med. Chem. Lett. 30, 126953 (2020).
Huang, Z., Zhang, Y., Zhou, R., Yang, L. & Pan, H. Lactate as potential mediators for exercise-induced positive effects on neuroplasticity and cerebrovascular plasticity. Front. Physiol. 12, 656455 (2021).
Goodwin, M. L., Harris, J. E., Hernandez, A. & Gladden, L. B. Blood lactate measurements and analysis during exercise: a guide for clinicians. J. Diabetes Sci. Technol. 1, 558–569 (2007).
Luo, J., Sun, P., Siwko, S., Liu, M. & Xiao, J. The role of GPCRs in bone diseases and dysfunctions. Bone Res. 7, 19 (2019).
Sun, P. et al. Regulation of body length and bone mass by gpr126/adgrg6. Sci. Adv. 6, eaaz0368 (2020).
Xiong, L. et al. ATP6AP2, a regulator of LRP6/beta-catenin protein trafficking, promotes wnt/beta-catenin signaling and bone formation in a cell type dependent manner. Bone Res. 12, 33 (2024).
Baron, R. & Kneissel, M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat. Med. 19, 179–192 (2013).
Lee, Y. S. et al. Microbiota-derived lactate accelerates intestinal stem-cell-mediated epithelial development. Cell Host Microbe 24, 833–846 (2018).
Madaan, A. et al. Muller cell-localized g-protein-coupled receptor 81 (hydroxycarboxylic acid receptor 1) regulates inner retinal vasculature via norrin/wnt pathways. Am. J. Pathol. 189, 1878–1896 (2019).
Yang, K. et al. Lactate suppresses macrophage pro-inflammatory response to LPS stimulation by inhibition of YAP and NF-kappab activation via GPR81-mediated signaling. Front. Immunol. 11, 587913 (2020).
Black, D. M. & Rosen, C. J. Clinical practice. Postmenopausal osteoporosis. N. Engl. J. Med. 374, 254–262 (2016).
You, Y. et al. GnIH secreted by green light exposure, regulates bone mass through the activation of gpr147. Bone Res. 13, 13 (2025).
Rossouw, J. E. et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women’s health initiative randomized controlled trial. J. Am. Med. Assoc. 288, 321–333 (2002).
Kitagawa, T., Hiraya, K., Denda, T. & Yamamoto, S. A comparison of different exercise intensities for improving bone mineral density in postmenopausal women with osteoporosis: a systematic review and meta-analysis. Bone Rep. 17, 101631 (2022).
Yang, W. et al. TAZ inhibits osteoclastogenesis by attenuating TAK1/NF-kappab signaling. Bone Res. 9, 33 (2021).
Atakan, M. M. et al. Effects of high-intensity interval training (HIIT) and sprint interval training (SIT) on fat oxidation during exercise: a systematic review and meta-analysis. Br. J. Sports Med. 56, 988–996 (2022).
Morland, C. et al. Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat. Commun. 8, 15557 (2017).
Hu, J. et al. The roles of GRP81 as a metabolic sensor and inflammatory mediator. J. Cell. Physiol. 235, 8938–8950 (2020).
Lopez, K. A. et al. Lactate induces metabolic and epigenetic reprogramming of pro-inflammatory th17 cells. EMBO Rep. 23, e54685 (2022).
Fischer, V. & Haffner-Luntzer, M. Interaction between bone and immune cells: implications for postmenopausal osteoporosis. Semin. Cell Dev. Biol. 123, 14–21 (2022).
Sun, L. et al. Effects of high-intensity interval training on adipose tissue lipolysis, inflammation, and metabolomics in aged rats. Pflug. Arch. 472, 245–258 (2020).
Zhang, X. et al. Lactate drives epithelial-mesenchymal transition in diabetic kidney disease via the h3k14la/KLF5 pathway. Redox Biol. 75, 103246 (2024).
Jones, N. K. et al. Endothelin-1 mediates the systemic and renal hemodynamic effects of GPR81 activation. Hypertension 75, 1213–1222 (2020).
Coxon, J. P. et al. GABA concentration in sensorimotor cortex following high-intensity exercise and relationship to lactate levels. J. Physiol. 596, 691–702 (2018).
Liu, C. et al. Lactate inhibits lipolysis in fat cells through activation of an orphan g-protein-coupled receptor, GPR81. J. Biol. Chem. 284, 2811–2822 (2009).
Moholdt, T., Madssen, E., Rognmo, O. & Aamot, I. L. The higher the better? Interval training intensity in coronary heart disease. J. Sci. Med. Sport 17, 506–510 (2014).
Zhu, Z. et al. Lactate mediates the bone anabolic effect of high-intensity interval training by inducing osteoblast differentiation. J. Bone Jt. Surg. Am. 105, 369–379 (2023).
Wu, J. et al. Endothelial cell-derived lactate triggers bone mesenchymal stem cell histone lactylation to attenuate osteoporosis. Adv. Sci. 10, e2301300 (2023).
Guo, S. et al. Potentiated effects of lactate receptor GPR81 on immune microenvironment in breast cancer. Mol. Carcinog. 62, 1369–1377 (2023).
Vohra, R. et al. Prevention of cell death by activation of hydroxycarboxylic acid receptor 1 (GPR81) in retinal explants. Cells 11, 2098 (2022).
Wallenius, K. et al. Involvement of the metabolic sensor GPR81 in cardiovascular control. JCI Insight 2, e92564 (2017).
Hoque, R., Farooq, A., Ghani, A., Gorelick, F. & Mehal, W. Z. Lactate reduces liver and pancreatic injury in toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology 146, 1763–1774 (2014).
Suzuki, A. et al. PTH/cAMP/PKA signaling facilitates canonical wnt signaling via inactivation of glycogen synthase kinase-3beta in osteoblastic saos-2 cells. J. Cell. Biochem. 104, 304–317 (2008).
Palacios, S. Medical treatment of osteoporosis. Climacteric 25, 43–49 (2022).
Yao, Z. et al. A combination of exercise and yogurt intake protects mice against obesity by synergistic promotion of adipose browning. J. Agric. Food. Chem. 72, 13906–13917 (2024).
Acknowledgements
We are grateful to all members of the Peng Sun’s lab for their technical help and discussion. This work was supported by grants from the National Natural Science Foundation of China (32571318, 32171130 to P.S., 82302778 to L.H.), and East China Normal University Interdisciplinary Advancement Project (04-222351 to P.S.).
Author information
Authors and Affiliations
Contributions
P.S. and L.H. conceived and designed the study. Y.G. performed most experiments and data analyses. A.X. performed exercise interventions in mice and other data analyses. Y.S., R.L., M.Z., F.Y., P.G., Y.Z., X.L. and L.T. performed some experiments and provided technical assistance. Y.L. reviewed the data and provided significant intellectual input. W.L. provided the Gpr81-knockout mice. Y.G. wrote the paper with input from all other authors. P.S. provided scientific direction, established collaborations, and allocated funding for this study.
Corresponding authors
Ethics declarations
Declarations
The animal experimental procedures were approved by the Institutional Animal Use and Care Committee at the East China Normal University (Shanghai, China m20201103).
Competing interests
The authors declared no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
Peer review
Peer review information
Communications Biology thanks Husam Bensreti and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Aline Bozec and Joao Valente. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Guo, Y., He, L., Xue, A. et al. Exercise increases bone mass by lactate/Gpr81 signaling pathway. Commun Biol 8, 1548 (2025). https://doi.org/10.1038/s42003-025-08957-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-08957-1
This article is cited by
-
The role of lactate on arthritis-associated cells: physiology, pathology, and therapeutic strategies
Cellular and Molecular Life Sciences (2026)