Abstract
Multiple brain areas along the ventral pathway have been known to represent face images. Here, in a magnetoencephalography (MEG) experiment, we show dynamic representations of face-related eye movements in the ventral pathway in the absence of image perception. Participants followed a dot presented on a uniform background, the movement of which represented gaze tracks acquired previously during their free-viewing of face and house pictures. We found a dominant role of the ventral stream in representing face-related gaze tracks, starting from the orbitofrontal cortex (OFC) and anterior temporal lobe (ATL), and extending to the medial temporal and ventral occipitotemporal cortex. Our findings show that the ventral pathway represents the gaze tracks used to explore faces, by which top-down prediction of face category in OFC and ATL may guide, via the medial temporal cortex or directly, face perception in the ventral occipitotemporal cortex.

Similar content being viewed by others
Introduction
Ventral occipitotemporal cortex is well known for its role in high-level object perception1,2,3,4,5,6. In particular, the fusiform face area (FFA) is activated by face images7 and the parahippocampal place area (PPA) by house images8. In a previous study, however, it was shown that FFA and PPA exhibited distinct neural activation patterns to face- and house-related gaze tracks, elicited in the absence of face or house image perception9. In this study, participants followed a sequence of dots on a uniform background with eye movements, where the dot sequence replayed gaze tracks previously recorded during face or house viewing. The face- and house-related gaze tracks could be decoded by the activation patterns in the FFA and PPA, thus indicating category-specific representations of gaze tracks in areas that were known to be activated by the respective image categories. Furthermore, the category-selective activation patterns were more sensitive to self-generated gaze tracks than gaze tracks generated by other observers9, in line with known individual differences in looking at faces10.
Here, we asked what the function of these gaze-track representations in a high-level perceptual area such as the FFA might be. Multiple areas along the ventral pathway are dedicated to face processing. In addition to the already mentioned FFA, face-responsive patches along the rostrocaudal extent of the temporal lobes as well as in the orbitofrontal cortex have been found in both human and non-human primates11,12,13. Notably, all of these studies investigated the processing of face images, without recourse to eye movements. Recently, however, modulations of neural activity by eye movements have been reported in the orbitofrontal cortex, particularly involving face viewing14,15. Moreover, neuronal activity in the medial temporal lobes can be modulated by eye movements, and lesions in this area may lead to changes in eye movement patterns during active sampling of the environment16,17. Medial temporal structures also interact with PPA during scene exploration18. Taken together with the evidence of gaze-sequence representation in the FFA and PPA9, these findings may lead to the hypothesis that the ventral stream, from orbitofrontal cortex via the uncinate fascicle to anterior temporal cortex and, via medial temporal cortex or directly, further to ventral occipitotemporal cortex, might be involved in representing face-specific gaze tracks.
However, how might face-specific gaze track representations be processed along the ventral stream? A top-down hypothesis is that eye movement sequences, e.g., during face viewing, initially activate prefrontal cortex, creating a categorical prediction (in this example of the ‘face’ category) which is fed back via the ventral stream to guide recognition in posterior perceptual areas19,20,21. To test this hypothesis, we investigated the spatiotemporal gradient of the MEG signal changes during face-related (vs. house-related) gaze track following. We expected dynamic signal changes specific to face-related gaze tracks to occur along the ventral stream, from orbitofrontal via anterior temporal and medial temporal cortex to ventral occipitotemporal cortex. Considering the known interindividual differences in the eye movement patterns of faces10, we also expected that following self-generated gaze tracks would optimally stimulate face-specific neural representations9, leading to earlier and stronger MEG signal changes than other-generated gaze tracks.
Top-down processing in the prefrontal cortex has been particularly observed when there is a lack of rich and unambiguous visual information19,22, as is the case in our dot-following task. Specifically, the categorical prediction in the prefrontal cortex was activated by ambiguous visual information in early visual areas19. To test this constraint, we presented actual images of faces and houses in the MEG scanner as a control condition. In contrast to the dot-following task, here, we expected a dominance of feedforward processing along the ventral pathway.
Results
The study consisted of a behavioral experiment and an MEG experiment, with a one-week interval between the two experiments. In the behavioral experiment (Fig. 1a), gaze tracks of all participants were recorded while they were looking at images of faces and houses (see Table S1 in Supplementary Information for gaze parameters). In the MEG experiment, participants followed dot sequences of their own (self-face, SF; self-house, SH) or another participant’s gaze tracks (other-face, OF; other-house, OH) with eye movements (Gaze Session, Fig. 1b). After the Gaze Session, participants took part in an Image Session where they viewed images of faces and houses while maintaining the eyes on a central fixation point (Fig. 1c). The behavioral performance is presented in Behavioral results of Supplementary Information.
a An example trial sequence in the behavioral experiment. For anonymization, the face image shown here was complemented, binarized, and denoised using the functions of imcomplement, imbinarize, and bwareopen in MATLAB 2021b. b An example trial sequence in the Gaze Session of the MEG experiment. c An example block of the stimuli sequence in the Image Session of the MEG experiment. d An example face and house image overlaid with the fixations from one example participant (upper left panel), and the fixation patterns (lower left panel) collected from the same participant during the view of faces (blue star) and houses (green cross). These fixation patterns were used to train a classifier in discriminating face- and house-related fixation patterns. The trained classifier was then used to classify the fixation patterns collected during the following of gaze tracks from the current observer (SF vs. SH, upper right panel) and the fixation patterns during the following of gaze tracks from another participant (OF vs. OH, lower right panel, i.e., cross-experiment classification). e The prediction accuracies of the cross-experiment classification are shown as a function of the comparison (SF vs. SH and OF vs. OH) and the number of fixations included in the classifications. Chance-level accuracies were derived by permutating the labels of the categories, rendering a distribution of chance accuracies. The shaded areas indicate accuracies below the 95% percentile of the distribution of chance accuracies obtained from the permutation-based classification. **p < 0.01, *p < 0.05 for between self and other (Bonferroni-corrected).
Distinct patterns between face- and house-related gaze tracks
We first analyzed if the face- and house-related fixation sequences obtained in the behavioral experiment showed distinct patterns. A machine-learning classification analysis over the spatiotemporal parameters of the fixations (x, y coordinates, and fixation duration) showed a high prediction accuracy in discriminating the two categories of gaze sequences, 70.5 ± 11.4% (M ± SD), with above-chance significance (p < 0.001; permutation-based significance testing). The same analysis was also performed on the eye movements collected in the MEG-Gaze Session, yielding a high prediction accuracy in discriminating between SF and SH, 66.3 ± 10.2%, p < 0.001, and between OF and OH, 66.5 ± 9.7%, p < 0.001. Note that the significant classifications cannot be due to the different sizes of the two image categories (see Spatial dispersion of fixations in Supplementary Information).
We also performed cross-experiment classifications where the classifier was trained with the gaze patterns from the behavioral experiment and was used to predict the gaze patterns in the Gaze Session of the MEG experiment. The above-chance cross-experiment prediction accuracies confirmed that the distinct patterns of the online eye movement in the Gaze Session were related to the face vs. house categories in the behavioral experiment. Moreover, the cross-experiment prediction accuracies were higher for self-generated gaze tracks than for other-generated gaze tracks (Fig. 1e), indicating that participants followed their own gaze tracks better (see Cross-experiment classification of gaze patterns in Supplementary Information for the statistics).
Neural face-related gaze pattern representations
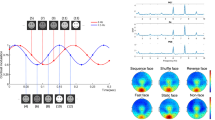
To reveal the face-related gaze representations, we compared the MEG signals of face-related gaze tracks with the MEG signals of house-related gaze tracks. Here, the house-related gaze tracks were taken as a control for face-related gaze tracks because there were eye movements and visual stimulation, but a lack of a structural pattern23 (for statistical evidence, see The structural pattern of face-related gaze tracks in Supplementary Information). For each condition (SF, SH, OF, OH) in the Gaze Session, the ERF signal from 0-1500 ms relative to the onset of the gaze track was calculated. The difference in the estimated cortical current maps was calculated between the following conditions: ‘SF – SH’, ‘OF – OH’, and ‘(SF – SH) – (OF – OH)’. These results would reveal the temporal development of the brain networks involved in the face-related gaze tracks, respectively areas that were sensitive to self-generated face-gaze tracks. The face-related gaze tracks elicited stronger ERF signals than the house-related gaze tracks in the orbitofrontal cortex (OFC) and the ventral anterior temporal lobe (ATL), extending to the medial temporal lobe (‘SF – SH’ and ‘OF – OH’ in Fig. 2a, Bonferroni-corrected for time and spatial clustering; see also Supplementary Video 1-4). Although small, there are also signal differences reaching back to the occipital cortex (OCC) (at 600 ms and 1400 ms, depending on the contrast). Importantly, the activities in the OFC and ATL emerged earlier than the activities in the medial temporal lobe and occipital cortex, suggesting a top-down prediction of the face category guided by the face-related gaze tracks.
Moreover, this network was more active during the following of self-generated gaze tracks than another observer’s gaze tracks, as revealed by the interaction contrast ‘(SF – SH) – (OF – OH)’ (Fig. 2a and Supplementary Video 5). The source reconstruction was dominantly localized in the ventral stream, with the notable exception of dorsal stream areas in frontal cortex, including the frontal eye field (FEF) and supplementary eye field (SEF), in the interaction contrast (Fig. 2b and Supplementary Video 6).
The ERF difference was displayed at the ventral (a) and dorsal (b) views in the source space. Two-tailed one-sample Chi2 test, n = 31, Bonferroni-corrected at p < 0.05. Upper row: the brain areas revealed by the contrast ‘Self-Face (SF) – Self-House (SH)’. Middle row: the brain areas revealed by the contrast ‘Other-Face (OF) – Other-House (OH)’. Lower row: the brain areas revealed by the interaction contrast ‘(SF – SH) – (OF – OH)’. The zero time points indicate the onsets of the gaze tracks. The anterior temporal lobe (ATL), medial temporal lobe (MTL), fusiform gyrus and parahippocampal gyrus, the pole of occipital cortex (OCC), and the orbitofrontal cortex (OFC) are marked in the 3D brain with the Destrieux Cortical ATLAS60.
The whole-brain source reconstruction was also performed based on the signal difference between Face and House in the Image Session. In contrast to the Gaze Session, here the strongest signal difference was localized in the posterior occipitotemporal areas, beginning already 200 ms post-stimulus onset (Fig. 3 and Supplementary Video 7-8).
Dynamic spatiotemporal patterns of the gaze-related representations in the brain
In order to provide statistical evidence for the temporal order of signal development observed in the event-related magnetic field analysis, we tested if there was an information flow from the anterior areas (e.g., OFC and ATL) to the posterior areas (e.g., FFA) during gaze following. We performed spatial gradient analysis, which quantified how the MEG signals gradually changed along a specific dimension (e.g., anterior-posterior) in the brain space24. Here, the top-down hypothesis of the gaze-track representations predicted information flow from the anterior areas to the posterior areas along the ventral pathway. This can be probed with the gradually decreased activity from the anterior areas to the posterior areas, in particular, how the gradient became face-selective during gaze following. As an area or neural network with stronger signals would be faster to exceed the neural threshold of maintaining sensory selectivity or perceptual preference25, the stronger signal changes in the anterior areas indicated that the face-selective representation of the gaze tracks emerged earlier than the posterior areas.
To test our hypothesis, here the spatial gradient was analyzed based on the ERF signal differences (e.g., ‘SF – SH’, ‘OF – OH’) to show how the signal changed at the anterior-posterior dimension. The analysis was performed at each time point during gaze following to show how the gradient pattern became face-selective over time. To provide a complete gradient pattern at the whole-brain level, the analysis was also performed for the dorsal-ventral and left-right dimensions. For each of the three dimensions (x, y, z, hence left-right, anterior-posterior, dorsal-ventral dimension), we modeled the coordinates with the ERF difference at each time point24. Note that we included the ERF difference as the fixed factor and the spatial coordinates as the model estimations because the ERF difference at each time point was fixed across the three dimensions. The R2 of the model was calculated to assess the accounted variance. The first-order derivatives of the estimated model were calculated to test if the spatial gradient increased or decreased monotonically along a specific dimension.
As shown in Fig. 4a (left), the ERF signal difference between SF and SH showed a significant gradient pattern along the y and z dimensions, cluster-based permutation correction at p < 0.05 (see Fig. 4a for the significant time ranges), whereas the x dimension did not reach significance (no time ranges reached significance). The significant gradient pattern that emerged over time during the gaze following (i.e., significantly higher R2 than baseline) indicated that the gradient pattern was not due to the general intrinsic brain dynamics but rather to the face-specific gaze following. Along both the y and z dimensions, the estimated model showed a monotonic characteristic, with 97.5% of the derivative values > 0 at the time point with the strongest gradient pattern along the y dimension and 98.9% of the derivative values < 0 along the z dimension (Supplementary Fig. S2). The signal difference between OF and OH showed a similar pattern, with 98.7% of the derivative values > 0 along the y dimension and 97.7% of the derivative values < 0 along the z dimension (Fig. 5a right, Supplementary Fig. S2). The significant results at the y dimension indicated that the signal difference between SF and SH, and between OF and OH, decreased along the anterior-to-posterior axis, as shown by the detailed pattern in Fig. 4b, c. The peak time points (marked by triangles in Fig. 4a) denote that the changes of the MEG signal difference as a function of the spatial coordinates at a specific dimension became the strongest. Specifically, at the time point where the gradient pattern reached its peak at the y dimension (910 ms for ‘SF – SH’, 1485 ms for ‘OF – OH’), the values of the y coordinates increased as a function of the amplitudes of the MEG signal difference (marked in magenta). These results indicated strongest neural representation of face-related gaze tracks in the anterior part of the brain, which decreased along the anterior-posterior axis. Importantly, this gradient pattern became more evident from the onset of the gaze tracks (i.e., the zero time point) to the 25%, 50%, 75% and 100% peak time points, indicating that the gradient pattern emerged as gaze following. The significant results at the z dimension indicated that the signal difference between SF and SH, and between OF and OH, decreased along the ventral-to-dorsal axis of the brain, as shown by the detailed pattern in Fig. 4d, e. Specifically, at the time point where the gradient pattern reached its peak at the z dimension (670 ms for ‘SF – SH’, 650 ms for ‘OF – OH’), the values of the z coordinates decreased as a function of the amplitudes of the MEG signal difference (marked in magenta). Such a pattern became more evident from the onset of the gaze tracks (i.e., the zero time point) to the 25%, 50%, 75% and 100% peak time points, indicating that the gradient pattern emerged as gaze following. However, there was no difference between the left and right hemispheres, as shown by the symmetry-like pattern of the spatial gradient at the x dimension (Fig. 4b–e).
The R2 of the spatial gradient model is shown as a function of the spatial dimensions (x, y, z) and time in the Gaze Session (a) and the Image Session (f). The horizontal lines at the bottom of each panel indicate the time ranges where the R2 were significantly higher than the baseline (multiple comparisons corrected with cluster-based permutation at p < 0.05). The small triangles indicate the peak of the R2 along a specific dimension. The spatial coordinates of each dimension (x, y, z) are shown as a function of the amplitudes of the MEG signal difference (Z-scored) at the time point where the gradient pattern (in terms of R2 values) reached to peak at the y dimension (b: ‘SF – SH’, c: ‘OF – OH’, g: ‘Face – House’), and at the time point where the gradient pattern reached to peak at the z dimension (d: ‘SF – SH’, e: ‘OF – OH’, h: ‘Face – House’).
a The counts that the spatial gradient pattern for ‘SF – SH’ emerged earlier than the spatial gradient pattern for ‘OF – OH’ along the y and z dimensions. The latency difference between the two R2 time courses was estimated with a cross-correlation method and was tested using a bootstrapping method (see Methods). Dashed lines indicate the 95% confidence interval. b The predicted coordinates sorted by the ERF amplitudes in the Image Session are shown as a function of the predicted coordinates in the Gaze Session along the y (left column) and the z (right column) dimensions. Upper row for ‘SF – SH’ and lower row for ‘OF – OH’.
Summarizing the results at the anterior-posterior and the ventral-dorsal dimensions, these findings provided statistical evidence for the temporal sequence observed in the event-related magnetic field analysis, showing that the representations of face-related gaze tracks dynamically progressed along the ventral pathway, starting from the ventral anterior areas (e.g., OFC and ATL) via medial temporal lobe (MTL) to ventral occipitotemporal cortex.
The spatial patterns at the time point where the gradient reached its peak are shown in Fig. 4a (left: ‘SF – SH’, R2 peaked at 910 ms along the y dimension and at 670 ms along the z dimension; right: ‘OF – OH’, R2 peaked at 1485 ms along the y dimension and at 650 ms along the z dimension). Importantly, the spatial gradient emerged earlier for ‘SF – SH’ than the spatial gradient for ‘OF – OH’ along the y dimension (i.e., the posterior-to-anterior dimension), mean latency difference = −310 ms, 95% CI = [−460 ms, −165 ms], but not along the z dimension mean latency difference = 20 ms, 95% CI = [−360 ms, 450 ms] (Fig. 5a). Together with the cross-experiment classifications of gaze patterns, these results indicated that self-generated gaze tracks were more sensitive than other-generated gaze tracks to activate the face-selective neural representations.
In the Image Session, the ERF signal difference between Face and House also showed a significant gradient pattern along the y and z dimensions, cluster-based permutation at p < 0.05, whereas the x dimension did not reach significance (Fig. 4f). Along both the y and z dimensions, the estimated model showed a monotonic characteristic, with 99.4% of the derivative values < 0 along the y dimension and 82.3% of the derivative values < 0 along the z dimension (Supplementary Fig. S2). The spatial patterns at the time point where the gradient reached its peak are shown in Fig. 4g, h (R2 peaked at 260 ms along the y dimension and at 710 ms along the z dimension). While the gradient pattern along the z dimension was consistent with the Gaze Session, with signal difference decreasing from the ventral to the dorsal part of the brain (Fig. 4h), the gradient pattern along the y dimension was reversed, with signal difference decreased from the posterior to the anterior part of the brain (Fig. 4g). The opposite anterior-posterior pattern between the Gaze Session and the Image Session can be seen from the positive function in the Gaze Session (y coordinates, marked in magenta in Fig. 4b, c) and the negative function in the Image Session (y coordinates, marked in magenta in Fig. 4g). Importantly, the reversed gradient pattern along the y dimension between the Gaze Session and the Image Session again indicated that the observed gradient pattern was not due to the general intrinsic brain dynamics along the ventral pathway, but rather reflected the feedback-dominant vs. feedforward-dominant processing specific to the current task (i.e., gaze following vs. image processing).
The reversed pattern along the anterior-posterior direction between the Gaze Session and the Image Session was further confirmed by the statistical evidence that the spatial gradient along the y dimension showed a negative correlation between the two sessions, r = −0.99, 95%CI = [−0.994, −0.957], p < 0.001 at the peak time point for ‘SF – SH’, and r = −0.98, 95%CI = [−0.991, −0.934], p < 0.001 at the peak time point for ‘OF – OH’ (Fig. 5b, left). By contrast, the spatial gradient along the z dimension showed a positive correlation between the two sessions, r = 0.90, 95%CI = [0.863, 0.951], p < 0.001 at the peak time point for ‘SF – SH’, and r = 0.92, 95%CI = [0.856, 0.953], p < 0.001 at the peak time point for ‘OF – OH’ (Fig. 5b, right). Collectively, the reversed pattern along the anterior-posterior direction between the Gaze Session and the Image Session suggested a combination of feedback and feedforward processing in natural face perception (i.e., when we look at a face using eye movements).
Classification-based whole-brain source-reconstruction of the categorical gaze tracks
A classification analysis was also performed to show how the different categories of gaze tracks could be distinguished by the multivariate MEG signals of different channels. The classification was performed for ‘SF vs. SH’ and ‘OF vs. OH’, respectively, using all 306 channels as features. The whole-brain source reconstruction was conducted based on the coefficients of the linear regression between the predicted category and the features of MEG signals. For ‘SF vs. SH’, as shown in Fig. 6a, c, five spatio-temporal clusters were indentified as significantly above chance, with the earliest cluster at 200–265 ms (p = 0.024) in right OFC, and later clusters at 495–545 ms (p = 0.024) in left OCC, 495–540 ms (p = 0.024) in right MTL and OCC, 505-575 ms (p = 0.033) in right OCC, and 700–790 ms (p = 0.021) in right MTL. For ‘OF vs. OH’, as shown in Fig. 6b, d, five spatio-temporal clusters were indentified as significantly above chance, with the earliest cluster at 575–705 ms (p = 0.004) in right OFC, followed by two immediate clusters at 645–740 ms (p = 0.018) in left ATL and MTL, 675–805 ms (p = 0.001) in left OCC, a later cluster at 820–870 ms (p = 0.020) in left OCC, and a final cluster at 1350–1400 ms (p = 0.011) in right OFC and ATL. These results are consistent with the above ERF-based results, indicating earlier involvement of OFC than ATL, MTL, and OCC in distinguishing the categorical gaze tracks. Note that, in contrast to the ERF-based results, here OFC and ATL seemed to be more pronounced in the classification-based results. This might be due to that the ERF-based results revealed the neural patterns that were stronger for face-related gaze tracks than house-related gaze tracks, which relied on the interaction between the top-down prediction in OFC and ATL and the perceptual representation of faces in ventral occipitotemporal cortex. However, the classification-based results revealed the neural patterns that distinguished the two categories of gaze tracks, which relied more on the top-down prediction of the object categories.
The coefficients of the linear regression performed to predict the gaze-track category with the channel features of MEG are shown as a function of time for ‘SF vs. SH’ (a) and ‘OF vs. OH’ (b). The shaded bars indicate significant spatio-temporal clusters after multiple comparisons (overlapped bars indicate different spatial clusters within the same time range). The ventral (upper row) and dorsal (lower row) views of the significant spatial clusters correspond to the significant temporal clusters for ‘SF vs. SH’ (c) and ‘OF vs. OH’ (d).
The classification-based results in the Image Session showed a similar pattern to the ERF-based results (Fig. 7). The distinct neural activities between Face and House were strongest in the posterior occipitotemporal areas, peaking around 200 ms post-stimulus onset.
The coefficients of the linear regression performed to predict the image category with the channel features of MEG are shown as a function of time for ‘Face vs. House’ (left). The ventral (upper row) and dorsal (lower row) views of the significant spatial clusters correspond to the significant temporal clusters (right).
Discussion
We have shown that face-related gaze tracks were dynamically represented along the ventral pathway, from OFC via ATL, to MTL and ventral occipitotemporal cortex. During the gaze following, there was a gradient pattern along the ventral stream, with face-selective activity progressing from OFC to the occipitotemporal cortex. However, when actual images of faces and houses were presented, the reverse gradient was observed, face-selective activity progressing from the ventral posterior occipitotemporal cortex to the prefrontal cortex. Taken together, our findings show that the ventral pathway represents aspects of the eye-movement program used to explore faces. The fixation sequences may help to form a top-down prediction of face category in OFC and ATL to guide, via the MTL or directly, the perceptual representation of faces in ventral occipitotemporal cortex, particularly under demanding viewing conditions.
The brain areas that we found representing categorical face-related gaze tracks, from OFC via ATL to ventral occipitotemporal cortex, have previously been found to support face perception, both in human and non-human primates11,12,13. Importantly, we found these areas to represent face-specific gaze patterns in the absence of face or house images, indicating that not only visual features, but also information about category-specific gaze sequences are represented, like fixation locations and their temporal sequence. In addition, the representation of face-related gaze sequences was particularly early and strong when gaze sequences that were followed were generated by the same participant during actual face viewing. This pattern is in line with the stable interindividual differences in gaze patterns that can be found across different viewing conditions10.
Face-specific activity was observed earliest in OFC and ATL, spreading backwards along the ventral stream. Although we do not know any previous reports of eye movement processing in human orbitofrontal cortex, modulation of orbitofrontal activity by eye movements has recently been reported, particularly during looking at faces14,15. OFC and ATL, connected via the uncinate fasciculus, are known to be vital for social interaction, with lesions in this network leading to the behavioral variant of frontotemporal dementia24,25. Given the importance of face perception - including perception of facial expressions - for social interaction, it is not astonishing to find representations of face-specific gaze sequences in these areas. The early occurrence of face-specific gaze representation in OFC and ATL in the ventral face processing stream may suggest that the processing of face-specific gaze patterns is vital for social interaction. It may thus be worthwhile to investigate if face-specific gaze sequences break down in degenerative diseases affecting OFC and ATL, like frontotemporal dementia.
Here, the suggestion that the representation of the gaze sequences in OFC and ATL may play an important role in social interaction should be differentiated from the social processing pathway suggested by recent studies26,27. The social pathway, starting from the early visual cortex to the superior temporal sulcus (STS) via the middle temporal area (MT), was suggested to be dedicated to processing the social information conveyed by a face stimulus, such as emotion or voice. However, it should be noted that this pathway, in particular STS, is well-known to be activated by dynamic changes such as facial expression or gaze shift of the perceived face. This is different from the eye movements in the present study, which were carried out to explore static and neural (non-emotional) faces. We thus speculate that the third pathway may be involved in representing the gaze tracks used to explore emotional faces.
One may note that there were differences in low-level visual information between the face and the house images in the Image Session, such as the image contrast and size. However, the observed MEG results cannot be simply due to the low-level visual information for the following reasons. First, the neural contrast was calculated based on the two categories, where the individual images within a category were collapsed. Although there was common low-level visual information among the face images, such as the high spatial frequency information, there was a large variance of the low-level information among the house images, suggesting that there was hardly systematic difference at the category level. Second, the potential systematic difference in image sizes should have elicited early neural activities in the visual cortex. For instance, it has been shown that the visual saliency map, which reflects the local contrast of the visual information, was calculated as early as 50 ms in the primary visual cortex after the visual stimulation28. However, as shown in Fig. 3 and Fig. 7, the neural activities emerged around 200 ms after the image onset, which cannot be simply due to the low-level visual information.
Face-related gaze sequence activation spread further to the medial temporal lobe, which has previously been shown to be vital for the exploration of the environment with eye movements, particularly, but not only, in memory-guided vision29,30,31. Moreover, during face viewing, activation in the hippocampal as well as in the fusiform face area was modulated by the number of fixations32 and during scene viewing, functional connectivity between the hippocampus and the PPA was enhanced during free viewing (versus forced central fixation)18. The MTL is connected to the orbitofrontal and temporopolar cortex via the perirhinal cortex33. Thus, in the context of face viewing, information from OFC and ATL about the highly structured (T-shaped) fixation pattern may elicit a memory trace of the ‘face’-category in the hippocampus. This, however, needs further investigation.
If OFC and ATL are the first to represent face-specific gaze patterns, how does the information about fixation patterns arrive at these areas in the first place? In normal looking behaviour, this may be answered by our finding that during the presentation of actual face images, faces and houses can be discriminated early and most strongly in the occipitotemporal cortex, spreading fast to anterior brain areas. Thus, during free viewing of a face, there will be an interaction of feedforward and feedback signals supporting perception. Our data, in line with previous reports, suggest that information about face-specific gaze sequences may aid face perception, particularly in the absence of rich and unambiguous visual information19,20,22. A recent MEG study also showed that the ventral prefrontal cortex guides the construction of low-dimensional categorical prediction from the high-dimensional visual information in the occipitotemporal cortex21. Taken together, following the gaze tracks may activate category-predictive processes in OFC and ATL, sending feedback signals via the MTL or directly to ventral occipitotemporal face patches, including the FFA, to facilitate face perception.
Recently, it has been emphasized that categorical object representation in the brain may be linked to the object’s behavioral relevance34, in line with models of perception-action coupling35,36,37. Given that eye movements are mostly generated fast and without conscious control38, representations of category-specific gaze sequences as part of an object’s neural representation may be a natural case of object representation, including relevant behavior, at least for object categories with structured gaze sequences, such as for faces. This fits with the observations that the category-specific activations were both stronger and emerged earlier for the observer’s own gaze tracks than another observer’s gaze sequences. While different observers could have a similar spatial distribution of the fixations during face viewing, the order of the fixations had a large variation among individuals, as shown by the interindividual differences of the first fixation location10. As the recognition of visual stimuli can be facilitated by the eye movements that are carried out during the encoding of the stimuli39, the neural representations were more sensitive to the gaze tracks that were used to encode the images (i.e., the observer’s own gaze sequences).
While the finding that the neural contrast between faces and houses reached peak firstly around 200 ms was consistent with previous MEG/EEG studies on decoding face category, identity, and familiarity40,41,42,43, the neural contrast between face- and house-related gaze tracks became significant around 300 ms after the gaze onset. This difference of time course could be due to that all visual information was immediately available at the image onset in the Image Session, whereas the information in the Gaze Session was accumulated with the fixation sequence in the way that around 200 ms would pass until the second dot was presented. As two fixations are typically needed to recognize a face44,45, this may explain the delay in the gaze condition.
It may seem puzzling that we found gaze-specific activation patterns for faces mainly in areas of the ventral stream, whereas the dorsal stream’s importance for eye movement control is well known46,47,48,49. We also could discriminate face versus house-related gaze following in dorsal brain areas, particularly in the frontal eye fields and the superior frontal cortex, known to support attentional control50, as well as in the left frontopolar cortex, known to support exploratory attentional resource allocation51. Interestingly, this was only the case for self-generated dot following, ruling out that these activation patterns were simply due to differences in basic eye movement parameters like saccade amplitudes. Nevertheless, dorsal stream activation differences for face versus house-related gaze following were much less than in ventral stream areas. This may be due to the nature of our contrasts, which asked for a categorical (‘face – house’) distinction between the associated gaze sequences. This categorical distinction may be more associated with the known capabilities of the ventral stream in object categorization than with the visuomotor control functions associated with the dorsal stream52.
A ventral network of brain areas known to support face processing, reaching from orbitofrontal and anterior temporal cortex via medial temporal to ventral occipitotemporal cortex, was found to represent face-related gaze sequences. During gaze following, activation in this network followed an anterior-to-posterior gradient, indicating feedback from anterior areas to ventral occipitotemporal perceptual areas, possibly using gaze patterns to support face perception. Our findings add an ideomotor perspective to the ventral stream function and the reconsideration of both ventral and dorsal streams in representing eye movements. The face-related eye movement representations in OFC and ATL suggest the role of the frontotemporal network in gaze control during social interaction.
Methods
Participants
The sample size was decided with G-Power 3.053 based on a previous study that examined the capabilities of MEG signals in decoding eye movement patterns54. The bivariate normal model of correlation was included as the test family, and the required sample size (i.e., type of power analysis: a priori) was computed given the alpha error probability, the statistical power (i.e., 1-beta error probability), and the effect size of the correlation reported in the previous study54. Given a correlation coefficient between eye movement and MEG patterns = 0.86 (two-tailed), alpha = 0.0001 reported in this study, a sample size of 27 is required to achieve an expected power of 99%. Following this criterion while considering potential exclusion, 32 university students (19 females, 13 males, mean age 20.8 years old) were recruited in the present study. All participants reported normal or corrected to normal vision. Informed written consent was obtained prior to the experiment. One participant was excluded due to his drop-out from the MEG experiment, resulting in 31 participants (19 females, 12 males, mean age 20.6 years old). This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the School of Psychological and Cognitive Sciences, Peking University (#2020-10-01). All ethical regulations relevant to human research participants were followed.
Design and procedure
Each participant went through a behavioral experiment (Fig. 1a) and an MEG experiment, with a one-week interval between the two experiments. In the behavioral experiment, we collected the gaze patterns of all participants while they were looking at faces and houses. These gaze patterns were then presented in the Gaze Session of the MEG experiment, and participants had to follow the gaze patterns with eye movements.
In the behavioral experiment, stimuli were images of faces and houses presented on a grey background of a computer screen. For each participant, the images of faces and houses presented during the experiment were randomly chosen from an image set (20 male faces, 20 female faces, and 40 houses). The size of the face images was fixed at a width of 14.4° × height of 16.7° of visual angle, with an eye-to-mouth distance of 7°. Due to the varying structures, the size of houses was not constant, with a mean width of 19.3° ± 1.0°, and a height of 13.1° ± 2.4°. Participants were required to complete a memorization-and-detection task while looking at each of the pictures9. At the beginning of each trial, a green dot (0.2° of visual angle in diameter) was presented at one of the four corners (15° from the center of the screen) to attract eye fixation. The green dot was presented for a varying interval of 1200–2000 ms. In 20% of the trials, a small black dot (0.05° in diameter) was presented at the center of the green dot for 100 ms. Participants were asked to detect the black dot by pressing the ‘z’ button using the left index finger on a standard keyboard. The onset of this small black dot was randomly chosen from the time point during the presentation of the green dot. After the offset of the green dot, a face or house picture was presented at the center of the screen and remained on the screen for 1500 ms. Participants were instructed to look at the images with free eye movements. There were 14 blocks of trials in the experiment. In the first block, a set of 6 face images (3 males and 3 females) and 6 house images were presented, one per trial, in a random order. In each of the following 13 blocks, 1–3 new images (either face or house) were added into the original 12 images. Participants were asked to memorize the images in the first block, and detect if a new image was presented in the following 13 blocks by pressing the ‘m’ button using the right index finger.
In the MEG experiment, stimuli were presented through an LCD projector onto a rear screen located in front of the scanner. There were two sessions in the MEG experiment: a Gaze Session and an Image Session.
In the Gaze Session, each participant was asked to follow dots that represented his/her own gaze patterns as well as dots that represented another participant’s gaze patterns. Each trial started with a red dot on a grey background, which remained at the center of the screen for 1400-2000ms. After a blank screen of a jittered interval (450 – 650 ms), the gaze track was presented on the screen, in the form of a sequence of green dots. The sequence of green dots represented the gaze pattern for a specific picture obtained from the behavioral experiment, and each dot represented a fixation of the gaze pattern. Given that the gaze patterns were collected during the 1500 ms-time range of the picture presentation, the dot sequence lasted ~1500 ms on the screen. In 20% of the trials, a small black dot (0.05° in diameter) was presented at the center of the central red dot or the moving green dot (with equal probabilities). This small black dot was presented for 100 ms, and participants were asked to detect the black dot by pressing the button using the right index finger. According to our design, four categories of gaze tracks were presented: face-related gaze tracks from the current observer (self-face, SF), house-related gaze tracks from the current observer (self-house, SH), face-related gaze tracks from another participant (other-face, OF), and house-related gaze tracks from another participant (other-house, OH). There were 10 blocks of gaze-tracks, with 40 trials (10 trials per condition) in each block. Trials from the 4 conditions were mixed and presented in a random order. At the end of each block, a feedback screen was presented to inform the participants’ performance in detecting the small black dots. The correspondence of fixations and the dot presentation (Supplementary Information) demonstrated that the dot was well followed by participants’ eye movements. Note that given the experimental design, the dot was not followed by smooth pursuit eye movements, but rather saccadic eye movements to fixate a dot that rapidly appeared at different locations on the screen (Supplementary Fig. S3).
In the Image Session, participants were asked to view successively presented images in a one-back task. Images were grouped into 10 blocks of faces and 10 blocks of houses. The two block types were presented in a random order. Each block started with a central fixation (a green cross) at a varying interval of 1000–1600 ms. Then images of the same category (face or house) were successively presented (each lasted for 1000 ms), with a jittered interval of 300–600 ms between each two images. The Image Session was included as a control condition for the Gaze session. To ensure a fair comparison such that the observed brain activity was not confounded by free eye movements, the central fixation was presented throughout the block. Participants were informed that their eye movements were monitored and were required to maintain their eyes on the central fixation. In each block, 10–11 images were presented with one or two images that were repeated immediately after their first presentation. Participants were asked to detect the immediate repetition of the image by button press. Apart from the immediate repetition, there were no other repetitions of images in each block. In total, each participant viewed 100–110 images of each category. There was a ~ 30 s break between each two blocks.
The images of faces and houses in the behavioral experiment and the Image Session were from the same stimulus set (20 female faces, 20 male faces, 40 houses), which was not familiar to the participants. Note that, for each participant and each session, the presented images were randomly selected from the stimulus set. Thus, the images in the two sessions may have partial overlap, but cannot be the same. Moreover, there was a 1-week interval between the two sessions. Therefore, the results cannot be due to familiar effects of the images.
For both the behavioral experiment and the MEG experiment, eye-movement data were recorded during the experiment with an EyeLink 1000 plus system (SR-Research, Canada), at an online sampling rate of 1000 Hz. A standard procedure of nine-point calibration and validation was performed at the beginning of the experiment, with a maximum error of 1.0° as the threshold. A drift check was performed at the beginning of each block, and the calibration and validation were performed if the error of the drift check exceeded the threshold (i.e., > 1.0°). To minimize eye movements in the Image Session, we also performed a central fixation check at the beginning of each block. The image presentation of each block did not start unless the eye fixation was less than 1.0° away from the central fixation. The online eye-tracking data showed that no participants made systematic eye movements.
MEG data acquisition and preprocessing
Neuromagnetic signals were recorded using a whole-head MEG system, with 204 planar gradiometers and 102 magnetometers (Eleka Neuromag TRIUX) in a magnetically shielded room. Four head position indication (HPI) coils were placed in each participant’s head to estimate head position during recording, with two coils in the left and right mastoids and two on the forehead. The raw MEG signals were online sampled at 1000 Hz and were band-pass filtered between 0.1 and 330 Hz. The structural MRI of each participant was obtained using a 3 T Siemens Prisma MR scanner. The MRI scanning was conducted on a different day after the MEG experiment.
Head shapes were quantified using the Probe Position Identification system (Polhemus), and three anatomical landmarks (nasion, left, and right pre-auricular points) were used to co-register the MEG data with MRI coordinates. Max-filter was used to reduce external noise and compensate for head movements (temporal signal space separation method, tSSS55). The offline pre-processing analysis of MEG data was performed using Brainstorm56. The continuous MEG data was first down-sampled to 200 Hz. Then the MEG data was band-pass filtered (0.1 Hz to 60 Hz, zero phase shift FIR filter) and notch filtered at 50 Hz. Independent component analysis (ICA) was used to detect and discard artifacts related to eye blinks, head movements, and heartbeats. The data were then epoched with the time interval of −500 to 1500 ms relative to the onset of the first fixation in the Gaze Session and with the time interval of −200 to 1000 ms relative to the onset of the image in the Image Session.
Analysis of eye-movement data
In the behavioral experiment, eye-movement data were extracted from the 1.5-s image presentation. Data were preprocessed using the cili module, a python-based tool for detecting and correcting eye blinks. Eye blinks were firstly removed, and fixations were identified based on the velocity threshold of 30 °/s and the acceleration threshold of 8000°/s2. Trials without any valid fixation events and trials with fixation localized beyond the region of the picture were also excluded. To prepare the gaze tracks in the MEG experiment, following the previous study9, a fixation was identified as a gaze event if its duration was longer than or equal to 100 ms, while identified as a non-gaze event if its duration was shorter than 100 ms. This non-gaze event was represented by a blank screen in the Gaze Session of the MEG experiment. Then, trials with less than two gazes were excluded. The gaze coordinates were proportionally transformed and co-registered with the screen resolution in the MEG scanner. In the Gaze Session of the MEG experiment, the online fixation events were also identified and analyzed.
Multivariate classifications were performed on the gaze features to show the distinct patterns between categories. The classification analysis was performed using the scikit-learn package (http://github.com/scikit-learn). Three features were included: the x and y coordinates, and the duration of each gaze. The fixation data was parsed in the way that 80% of the data was included as the training set and 20% of the data as the test set. A linear support vector (SVM) classifier was trained and cross-validated based on the fixational features of the two categories (Face vs. House). The classification was performed for each participant, rendering both individual-level prediction accuracies and the group mean of the accuracies. Permutation-based testing was conducted to assess the statistical significance. For each participant, the classifier was trained with randomly shuffled labels of the two categories, and a permuted accuracy was calculated. This procedure was repeated 100 times, rendering a set of 100 chance accuracies for each participant. For group-level statistical testing, one chance accuracy was selected from each participant and the individual chance accuracies were averaged into a group chance accuracy. This procedure was repeated 105 times, resulting in a set of 105 group chance accuracies. Significance testing was performed by calculating the probability of the unpermuted group mean accuracy across participants in the distribution of the group chance accuracies (one-tailed). The classification was performed both for the fixation data in the behavioral experiment (Face vs. House) and the fixation data in the Gaze Session of the MEG experiment (SF vs. SH, OF vs. OH).
Note that the SF vs. SH and OF vs. OH classifications in the Gaze Session were not strictly specific to the Face vs. House distinction because no face or house images were presented. To show the specificity, cross-experiment classifications were performed where the fixation patterns in the behavioral experiment were used to train the classifier (Face vs. House), which was then used to predict the fixation categories in the Gaze Session (SF vs. SH, OF vs. OH). Importantly, to assess the sensitivity of the distinct fixation patterns, the cross-experiment classification was performed by varying the number of fixations (i.e., the first fixation, the 1–2 fixations, the 1–3 fixations, and the 1–4 fixations). Multiple comparisons were corrected with the Bonferroni method.
Representational distance4 was calculated to assess if the fixation data had a consistent structure specific to the visual category in the Gaze Session. Specifically, the representational distance was quantified by the Euclidean distance between the fixations within each category, assuming that a lower distance indicates a higher fixation structure23. We also calculated the representational distance between the categories as the control. The fixation pattern for a specific category was identified as structural if the representational distance within that category was significantly lower than the between-category representational distance.
Event-related magnetic field (ERF) analysis of MEG data
After the pre-processing, the epoched data were averaged over the trials for each condition and each participant. Individual T1-weighted MRIs were segmented with the FreeSurfer software package57 (http://surfer.nmr.mgh.harvard.edu) and then imported to the Brainstorm (https://neuroimage.usc.edu/brainstorm) for further source-level analysis. The white-gray matter boundary segmented by the FreeSurfer was used as a source space for activity estimation in the cortex. After co-registration between the individual anatomy and MEG sensors, the cortical currents were estimated using a distributed model consisting of 15,002 current dipoles from the averaged epochs (evoked activities) using a linear inverse estimator (minimum norm current estimation). The density map was standardized using a Z-score transformation with respect to a noise matrix which was calculated with a 2-minute empty-room recording of the MEG signal. The dipole orientation was constrained to the orthogonality of the white-gray matter boundary of the individual MRIs.
The difference in the estimated cortical current maps was calculated between the following conditions: ‘SF – SH’, ‘OF – OH’, ‘(SF – SH) – (OF – OH)’. Then the source maps were filtered with a low-pass filter (30 Hz), standardized through a z-score baseline normalization (−450 to 0 ms relative to the gaze onset as the baseline, with the first 50 ms of the baseline period being excluded to avoid the edge effect resulted from the low-pass filter), and rectified to retain only absolute values. The source maps were then projected on a standard brain (ICBM152) and spatially smoothed (Full Width at Half Maximum, FWHM = 3 mm) before group statistical analysis. A two-tailed one-sample Chi2 test was used for group statistical analysis for each time point and each vertex with the null hypothesis that the difference in variances of the cortical activities between the two conditions was equal to zero58. Bonferroni correction was used to solve the multiple comparison problems. The significance threshold was set at p < 0.05 after corrections.
To show the brain areas that were involved in the Image Session, the whole-brain source reconstruction was also performed by comparing the ERF signals during face viewing and the ERF signals during house viewing (‘Face – House’).
Modeling the gradient of MEG signal in source space
To quantify the spatial patterns of MEG signals during the gaze-track following, a five-order polynomial function was used to approximate the data along each of the three spatial dimensions (x, y, and z coordinates for the data in the source space)24. For each time point, we employed a polynomial function p(v) = p0 + p1v + p2v2 + p3v3 + p4v4 + p5v5 (polyfit, MATLAB 2022a) to estimate the coordinates along each spatial dimension with the MEG signal difference between conditions (e.g., ‘SF – SH’, ‘OF – OH’). The amplitude of MEG signal was normalized to z-scores across vertices to avoid an ill-conditioned Vandermonde matrix in model fitting. For each spatial dimension, the model fitted the signal difference in Z-scored amplitudes of each vertex v to its spatial coordinate (MNI coordinates) across vertices. The quality of the model was quantified by the adjusted R2, which determined the proportion of variance explained by the model. R2 was adjusted by the number of coefficients. To assess the dynamic spatial gradient of the MEG signal difference, a Jackknife method was used to fit the model and calculate R2 for each of the 3 dimensions. Specifically, one of the participants was excluded and the source-reconstructed MEG signals of the remaining participants were averaged to fit the model. This procedure was iterated across participants. A one-sample t test (one-tailed) was used to test if R2 at each time point was higher than the baseline, the time interval of −500 to 0 ms relative to the stimulus onset. Cluster-based permutation was used to resolve the multiple-comparison problem across time points. We also calculated the first-order derivatives of the estimated model to test if the spatial gradient had a monotonic increasing or decreasing pattern along a specific dimension. The calculation was performed on the model at the time point with peak R2, and the evaluation of the derivatives was based on the signal range between the minimum and the maximum value of the MEG amplitude. The spatial gradient was identified as monotonically increasing given the derivative values > 0 and monotonically decreasing given the derivative values < 0.
To test if the spatial gradient of ‘SF – SH’ emerged earlier than the spatial gradient of ‘OF – OH’, cross correlation (xcorr, MATLAB 2022a, ‘unbiased’, maxlag = 200) was performed on the two R2 time courses to calculate the latency difference. The latency difference was defined as the temporal lag with which the R2 time courses showed maximum correlation between the two time courses across participants. The Bootstrapping method (iteration number = 1000) was used to estimate the 95% confidence interval of the latency difference.
The same analysis was also performed on the MEG signal difference between Face and House in the Image Session to show the spatial gradient. The analysis was performed on the 0–1000 ms interval during the image presentation (0 denotes the image onset), with the −200–0 ms interval as the baseline.
To assess the similarity/dissimilarity of the spatial gradient between the Gaze Session and the Image Session, we performed correlation analyses between the two sessions. Specifically, we performed the model fitting with the group average of source-reconstructed MEG signal difference between conditions (‘SF – SH’ and ‘OF – OH’ for the Gaze Session and ‘Face – House’ for the Image Session). A Bootstrapping method (iteration number = 1000) was used to estimate the variance of R2 time courses that were calculated between the model and the group average of the MEG data. At the peak time point of R2 time courses (y and z, respectively), we projected the fitted function p(v) in the three-dimensional space with the polynomial function. The predicted coordinates of the function p(v) were sorted according to the amplitude of the MEG signal. Then we calculated the Pearson coefficients of the sorted coordinates between the two sessions.
Classification analysis of gaze-related MEG data
Multivariate classifications were performed on the MEG data in the Gaze Session to show how the face- and house-related gaze tacks could be distinguished by the MEG signals. For ‘SF vs. SH’ and ‘OF vs. OH’ separately, the MEG signals of the 306 channels were included as features. For each time point in each participant, a ‘leave-one-fold-out’ classification was performed, with 80% of the data included as the training set and 20% of the data included as the test set (number of folds = 5). A logistic regression classifier was trained and cross-validated based on the channel features of the two categories (‘SF vs. SH’, ‘OF vs. OH’). The regression coefficients of all channels were extracted from the classifier and normalized to describe the contribution of the neural activities from each channel. For each time point in each participant, the labels of the two categories were randomly shuffled with a permutation method (n = 200) to generate the null distribution of the coefficients. The normalized coefficients and classification accuracies were calculated as Z-scores relative to this null distribution. The normalized coefficients were then projected into source space using minimum current estimation implemented in the MNE toolbox59. At the group-level, a one-sample t test was conducted to assess if the coefficients were significantly above chance level. To correct for multiple comparisons across spatial vertices and time points, a cluster-based permutation test was applied, with a vertex-level threshold of p < 0.01 and a cluster-level threshold of p < 0.05. The same analysis was also performed in the Image Session to show distinct neural activities between Face and House images.
Statistics and reproducibility
The analyses were carried out with MATLAB and Python. A permutation-based testing was used to assess the statistical significance for classification analysis of eye movement data. At the individual level, 100 permutations were performed to generate a chance-level distribution of accuracies. For the group-level null distribution, 105 samples were drawn from each individual distribution, and the averages were obtained to render the group-level chance distribution. The observed group mean accuracy was then compared against this null distribution using a one-tailed test, with Bonferroni corrections applied for multiple comparisons. A one-sample two-tailed Chi2 test was used to test if there was a significant difference of ERF signals between the two conditions, with Bonferroni corrections for multiple comparisons across time points and spatial vertices. The Bootstrapping method (iteration number = 1000) was used to test if there was a significant latency difference of the spatial gradient patterns between self- and other-generated gaze-track conditions. For the classification analysis of the MEG data in the Gaze Session, a permutation method (iteration number = 200) was employed to normalize the coefficient estimated by the classifier, and a one-sample t-test was used to test if the normalized coefficient was > 0, with cluster-based permutations for correcting multiple-comparisons.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data have been deposited at OSF, accession code: osf.io/rg4az. All other data are available from the corresponding author on reasonable request.
Code availability
Codes have been deposited at OSF, accession code: osf.io/rg4az.
References
Ungerleider, L. G. & Mishkin, M. Two cortical visual systems. in Analysis of visual behavior (eds. Ingle, D. J., Goodale, M. A. & Mansfield, R. J. W.) 549–586 (MIT Press, 1982).
Desimone, R., Albright, T. D., Gross, C. G. & Bruce, C. Stimulus-selective properties of inferior temporal neurons in the macaque. J. Neurosci. 4, 2051–2062 (1984).
Kiani, R., Esteky, H., Mirpour, K. & Tanaka, K. Object category structure in response patterns of neuronal population in monkey inferior temporal cortex. J. Neurophysiol. 97, 4296–4309 (2007).
Kriegeskorte, N. et al. Matching categorical object representations in inferior temporal cortex of man and monkey. Neuron 60, 1126–1141 (2008).
Kravitz, D. J., Saleem, K. S., Baker, C. I., Ungerleider, L. G. & Mishkin, M. The ventral visual pathway: An expanded neural framework for the processing of object quality. Trends Cogn. Sci. 17, 26–49 (2013).
Majaj, N. J., Hong, H., Solomon, E. A. & DiCarlo, J. J. Simple learned weighted sums of inferior temporal neuronal firing rates accurately predict human core object recognition performance. J. Neurosci. 35, 13402–13418 (2015).
Kanwisher, N., McDermott, J. & Chun, M. M. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J. Neurosci. 17, 4302–4311 (1997).
Epstein, R. & Kanwisher, N. A cortical representation of the local visual environment. Nature 392, 598–601 (1998).
Wang, L., Baumgartner, F., Kaule, F. R., Hanke, M. & Pollmann, S. Individual face- and house-related eye movement patterns distinctively activate FFA and PPA. Nat. Commun. 10, 5532 (2019).
Peterson, M. F. & Eckstein, M. P. Individual differences in eye movements during face identification reflect observer-specific optimal points of fixation. Physiol. Sci. 27, 1216–1225 (2013).
Tsao, D. Y., Moeller, S. & Freiwald, W. A. Comparing face patch systems in macaques and humans. Proc. Natl. Acad. Sci. Usa. 105, 19514–19519 (2008).
Tsao, D. Y., Schweers, N., Moeller, S. & Freiwald, W. A. Patches of face-selective cortex in the macaque frontal lobe. Nat. Neurosci. 11, 877–879 (2008).
Landi, S. M. & Freiwald, W. A. Two areas for familiar face recognition in the primate brain. Science 357, 591–595 (2017).
Dal Monte, O. et al. Widespread implementations of interactive social gaze neurons in the primate prefrontal-amygdala networks. Neuron 110, 2183–2197.e7 (2022).
Fan, S., Dal Monte, O., Nair, A. R., Fagan, N. A. & Chang, S. W. C. Closed-loop microstimulations of the orbitofrontal cortex during real-life gaze interaction enhance dynamic social attention. Neuron 112, 2631–2644.e6 (2024).
Voss, J. L. et al. Spontaneous revisitation during visual exploration as a link among strategic behavior, learning, and the hippocampus. Proc. Natl. Acad. Sci. USA 108, 402–409 (2011).
Ryan, J. D., Shen, K. & Liu, Z. X. The intersection between the oculomotor and hippocampal memory systems: empirical developments and clinical implications. Ann. N. Y. Acad. Sci. 1464, 115–141 (2020).
Liu, Z.-X., Rosenbaum, R. S. & Ryan, J. D. Restricting visual exploration directly impedes neural activity, functional connectivity, and memory. Cereb. Cortex Commun. 1, tgaa054 (2020).
Bar, M. et al. Top-down facilitation of visual recognition. Proc. Natl. Acad. Sci. USA 103, 449–454 (2006).
Summerfield, C. et al. Predictive codes for forthcoming perception in the frontal cortex. Science 314, 1311–1314 (2006).
Duan, Y., Zhan, J., Gross, J., Ince, R. A. A. & Schyns, P. G. Pre-frontal cortex guides dimension-reducing transformations in the occipito-ventral pathway for categorization behaviors. Curr. Biol. 34, 3392–3404.e5 (2024).
Freedman, D. J., Riesenhuber, M., Poggio, T. & Miller, E. K. Categorical representation of visual stimuli in the primate prefrontal cortex. Science 291, 312–316 (2001).
Wang, Z., Meghanathan, R. N. & Pollmann, S. & Wang, L. Common structure of saccades and microsaccades in visual perception. J. Vis. 24, 1–13 (2024).
Neary, D., Snowden, J. S., Northen, B. & Goulding, P. Dementia of frontal lobe type. J. Neurol. Neurosurg. Psychiatry 51, 353–361 (1988).
Rouse, M. A., Binney, R. J., Patterson, K., Rowe, J. B. & Lambon Ralph, M. A. A neuroanatomical and cognitive model of impaired social behaviour in frontotemporal dementia. Brain 147, 1953–1966 (2024).
Pitcher, D. & Ungerleider, L. G. Evidence for a Third Visual Pathway Specialized for Social Perception. Trends Cogn. Sci. 25, 100–110 (2021).
Yan, Y. et al. The brain computes dynamic facial movements for emotion categorization using a third pathway. Proc. Natl. Acad. Sci. USA 122, e2423560122 (2025).
Zhang, X., Li, Z., Zhou, T. & Fang, F. Article neural activities in V1 create a bottom-up saliency map. Neuron 73, 183–192 (2012).
Hannula, D. E., Ryan, J. D., Tranel, D. & Cohen, N. J. Rapid onset relational memory effects are evident in eye movement behavior, but not in hippocampal amnesia. J. Cogn. Neurosci. 19, 1690–1705 (2007).
Ryals, A. J., Wang, J. X., Polnaszek, K. L. & Voss, J. L. Hippocampal contribution to implicit configuration memory expressed via eye movements during scene exploration. Hippocampus 25, 1028–1041 (2015).
Pollmann, S. & Schneider, W. X. Working memory and active sampling of the environment: Medial temporal contributions. in Handbook of Clinical Neurology (eds. Miceli, G., Bartolomeo, P. & Navarro, V.) 187, 339–357 (Elsevier, 2022).
Liu, Z. X., Shen, K., Olsen, R. K. & Ryan, J. D. Visual sampling predicts hippocampal activity. J. Neurosci. 37, 599–609 (2017).
Ranganath, C. & Ritchey, M. Two cortical systems for memory-guided behaviour. Nat. Rev. Neurosci. 13, 713–726 (2012).
Contier, O., Baker, C. I. & Hebart, M. N. Distributed representations of behaviour-derived object dimensions in the human visual system. Nat. Hum. Behav. 8, 2179–2193 (2024).
Prinz, W. A Common Coding Approach to Perception and Action. in Relationships Between Perception and Action: Current Approaches 167–201 (Springer-Verlag Berlin Heidelberg, 1990).
Olivers, C. N. L. & Roelfsema, P. R. Attention for action in visual working memory. Cortex 131, 179–194 (2020).
Van Ede, F. & Nobre, A. C. Turning attention inside out: how working memory serves behavior. Annu. Rev. Psychol. 74, 137–165 (2023).
Land, M. & Tatler, B. Looking and Acting: Vision and eye movements in natural behaviour. (Oxford University Press, 2009).
Damiano, C. & Walther, D. B. Distinct roles of eye movements during memory encoding and retrieval. Cognition 184, 119–129 (2019).
Nemrodov, D. & Itier, R. J. Is the rapid adaptation paradigm too rapid? Implications for face and object processing. Neuroimage 61, 812–822 (2012).
Vida, M. D., Nestor, A., Plaut, D. C. & Behrmann, M. Spatiotemporal dynamics of similarity-based neural representations of facial identity. Proc. Natl. Acad. Sci. USA 114, 388–393 (2017).
Ambrus, G. G., Kaiser, D., Cichy, R. M. & Kovács, G. The neural dynamics of familiar face recognition. Cereb. Cortex 29, 4775–4784 (2019).
Dobs, K., Isik, L., Pantazis, D. & Kanwisher, N. How face perception unfolds over time. Nat. Commun. 10, 1258 (2019).
Hsiao, J. H. & Cottrell, G. Two fixations suffice in face recognition. Psychol. Sci. 19, 998–1006 (2008).
Liu, M., Zhan, J. & Wang, L. Specified functions of the first two fixations in face recognition: Sampling the general-to-specific facial information. iScience 27, 110686 (2024).
Bruce, C. J. & Goldberg, M. E. Physiology of the frontal eye fields. Trends Neurosci. 7, 436–441 (1984).
Andersen, R. A., Brotchie, P. R. & Mazzoni, P. Evidence for the lateral intraparietal area as the parietal eye field. Curr. Opin. Neurobiol. 2, 840–846 (1992).
Paus, T. Location and function of the human frontal eye-field: a selective review. Neuropsychologia 34, 475–483 (1996).
Coiner, B. et al. Functional neuroanatomy of the human eye movement network: a review and atlas. Brain Struct. Funct. 224, 2603–2617 (2019).
Hopfinger, J. B., Buonocore, M. H. & Mangun, G. R. The neural mechanisms of top-down attentional control. Nat. Neurosci. 3, 284–291 (2000).
Pollmann, S. Frontopolar resource allocation in human and nonhuman primates. Trends Cogn. Sci. 20, 84–86 (2016).
Gallivan, J. P. & Goodale, M. A. The dorsal “action” pathway. in Handbook of Clinical Neurology (eds. Vallar, G. & Coslett, H. B.) 151, 449–466 (2018).
Faul, F., Erdfelder, E., Lang, A. G. & Buchner, A. G. Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191 (2007).
Quax, S. C., Dijkstra, N., van Staveren, M. J., Bosch, S. E. & van Gerven, M. A. J. Eye movements explain decodability during perception and cued attention in MEG. Neuroimage 195, 444–453 (2019).
Taulu, S. & Simola, J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys. Med. Biol. 51, 1759–1768 (2006).
Tadel, F., Baillet, S., Mosher, J. C., Pantazis, D. & Leahy, R. M. Brainstorm: a user-friendly application for MEG/EEG analysis. Comput. Intell. Neurosci. 2011, 879716 (2011).
Fischl, B. FreeSurfer. Neuroimage 62, 774–781 (2012).
Sandberg, K. et al. Distinct MEG correlates of conscious experience, perceptual reversals and stabilization during binocular rivalry. Neuroimage 100, 161–175 (2014).
Gramfort, A. et al. MEG and EEG data analysis with MNE-Python. Front. Neurosci. 7, 267 (2013).
Destrieux, C., Fischl, B., Dale, A. & Halgren, E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage 53, 1–15 (2010).
Acknowledgements
We thank Dr. Jiayu Zhan and Dr. Liyu Cao for their suggestions on the design of the MEG experiment. This study was supported by the National Natural Science Foundation of China (32271086), a Shanghai Sailing Program (20YF1422100), a Mercator Fellowship of the Deutsche Forschungsgemeinschaft (DFG, 450600965) to LW, and a DFG grant (PO548/18-1) to SP.
Author information
Authors and Affiliations
Contributions
Conceptualization, L.W., S.P., Z.S.; Methodology, Z.S., L.W.; Investigation, Z.S., L.W.; Formal Analysis, Z.S., L.W.; Visualization, Z.S.; Writing—Original Draft, S.P., L.W., Z.S.; Writing—Review and Editing, S.P., L.W., Z.S., X.Z.; Supervision, L.W., X.Z.; Funding Acquisition, L.W., X.Z., S.P.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Gyula Kovács, Anthony Atkinson and the other anonymous reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Christian Beste and Benjamin Bessieres. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Su, Z., Zhou, X., Pollmann, S. et al. Dynamic face-related eye movement representations in the human ventral pathway. Commun Biol 8, 1652 (2025). https://doi.org/10.1038/s42003-025-09039-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-09039-y