Abstract
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a chronic disease with multiple etiologies, stemming from the interplay between local and systemic genetic, diet, and gene-environment interactions. To understand the progression of MASLD in a controlled setting, we utilized a human liver microphysiological system (MPS) to establish a physiologically relevant metabolic baseline and probe how primary human hepatocytes respond to perturbations in insulin, glucose, and free fatty acids (FFAs). Replicate liver MPS were maintained in media with either 200 pM or 800 pM insulin for up to 3 weeks alone and in combination with standard glucose (5.5 mM), hyperglycemia (11 mM glucose), normal (20 µM) and elevated FFA (100 µM). Together, hyperinsulinemia along with elevated glucose and FFAs, induces the release of pro-inflammatory chemokines, accumulation of triglycerides, and predisposes hepatocytes to insulin resistance. Treatment with the thyroid receptor β agonist resmetirom normalizes hepatic fat content and partially rescues insulin sensitivity, but paradoxically induces higher CXCL1 and IL8 expression in male and female donors. In aggregate, our enhanced in vitro MPS model establishes a metabolic baseline and perturbed condition that recapitulates a spectrum of phenotypes observed in MASLD, offering improved quantification and insight into disease progression with relevance to human physiology.
Similar content being viewed by others
Introduction
Type 2 diabetes (T2D) and metabolic dysfunction-associated steatotic liver disease (MASLD) are chronic diseases affecting millions of individuals worldwide1. Over 70% of T2D patients also suffer from MASLD with high incidence of metabolic dysfunction-associated steatohepatitis (MASH)1. In addition to excess fat accumulation in the liver, a pathophysiological hallmark of MASLD, insulin resistance in liver, muscle, and adipose tissue are retained and augmented by chronic inflammation as MASLD progresses into MASH and cirrhosis1,2. Underlying drivers of both diseases are multifaceted, involving interplay between genetics, diet, exercise, and gene-environment interactions arising from various stressors (e.g., infection, environmental exposure)3. MASLD is a heterogeneous disease with a spectrum of patient phenotypes, suggesting subgroups of patients may ultimately require different therapeutic interventions3. Translation of findings from preclinical animal models to therapeutic efficacy in humans remains poor4, thus motivating new approaches to measure salient phenomena in patients, and more sophisticated humanized in vitro models5,6.
Direct actions of insulin in healthy liver lead to inhibition of gluconeogenesis, activation of glycogen synthesis, and upregulation of lipogenic gene expression4. Insulin also acts indirectly on the liver by decreasing lipolysis in adipose tissue, resulting in reduction of circulating free fatty acids (FFA) and glycerol, further decreasing hepatic gluconeogenesis4. Chronically elevated levels of insulin and loss of receptor stimulation may enhance internalization/degradation, further altering acute metabolic activity and driving hepatic de novo lipogenesis7. This is compounded through indirect FFA and carbohydrate flux, influencing both acute activity and chronic expression of metabolic genes3,4,8.
Although insights into mechanistic underpinnings of MASLD in humans continue to emerge from well-crafted clinical studies9,10,11,12, new experimental models are needed to parse the multi-variate contributions in different patient groups, and to better translate model predictions into therapeutic efficacy. The emergence of microphysiological systems (MPS), combining advances in 3D culture, microfabrication, and mesofluidics, has driven the development of diverse approaches to extend functional performance of primary hepatocytes alone or in co-culture with non-parenchymal cells (NPCs), allowing extended and mature culture periods. Several of these MPS technologies have been deployed to model facets of insulin action, MASH, and T2D13,14,15. These models achieve a metabolically reactive disease state through modulation of glucose, FFA, and insulin concentrations. However, among in vitro models, both the physiological and disease state is defined idiosyncratically, especially with respect to insulin concentrations. Insulin, glucose, and FFA media concentrations, when reported at all, can range up to 1000 (or more)-fold greater than typical values in human plasma16.
To address this, we parse the contributions of insulin and nutrient concentrations on the development and reversibility of insulin resistance in a primary human hepatocyte model, while concurrently capturing other key phenotypes of MASLD. We implemented a liver MPS platform, the CNBio LiverChipTM, that features microperfusion of cells cultured in a 3D scaffold, as this platform supports sustained human liver cell function under physiological conditions17 and has been used for modeling human liver pharmacokinetics18, metabolic diseases15,19, and inflammatory conditions20,21. Perfusion in this MPS generates a pressure drop across the scaffold, in turn establishing an approximate oxygen concentration and range similar to what is found across the hepatic acinus22,23. The utility of this platform for long-term retention of select human liver functions, compared to other culture methods, was recently described by the FDA24. Of interest, the pharmacokinetic capability of this platform enables ready analysis of hepatocellular metabolic flux; insulin uptake/removal from media, glucose production, bile acid and chemokine production, which are altered similarly in MASLD patients and within our MPS model8. Finally, leveraging these insights, we employ this model to independently evaluate two intervention strategies: a dietary approach involving a return to physiologically healthy media, and therapeutically via stimulation with the thyroid receptor β 1 agonist resmetirom25.
Results
Hyperinsulinemia induced insulin resistance in vitro
Insulin resistance is the cornerstone underlying pathophysiology found within obesity, T2D, dyslipidemia, and MASLD7. To assay the effect of insulin concentrations in driving insulin resistance in vitro, primary human hepatocytes were cultured for 7–15 days in the liver MPS in either baseline media (Condition 1; 200 pM insulin, physiological nutrients) or in Condition 1 + Ins (800 pM insulin, physiological nutrients), reflecting the reported human portal vein insulin concentrations in healthy and T2D patients26,27. Insulin uptake and albumin production rates were monitored between regular 48-h feeding periods, and hepatocyte glucose production (HGP) was evaluated over 24 h in glucose-free media with insulin dose-response (0–100 nM) assayed from days 7–8 and 14–15 of the experiment (see “Methods”). Supporting the notion that hepatocytes cultured in our device are molecularly “mature” and in agreement with previous findings15,19, hepatocytes exhibit stable albumin secretion rates over 15 days of culture following an initial transient increase, with no differences in magnitude among baseline and high insulin media conditions or between LiverChipTM MPS models (Supplementary Fig. 1).
Insulin uptake as a fraction of total insulin present in the media was comparable in both 200 and 800 pM insulin-treated hepatocytes for the first week of culture (Fig. 1A), suggesting a first-order clearance rate consistent with the reported 190 pM Kd for high-affinity insulin binding to the insulin receptor28. Reactors seeded at lower cell numbers (400,000, 200,000, or 100,000) show roughly proportional reductions in uptake compared to the standard seeding density of 600,000 cells per reactor (Supplementary Fig. 2). The per-cell removal rate at the nominal 200 and 800 pM is between 350 and 1500 molecules/min, well within a feasible range for trafficking of the insulin receptor29, additionally validating our choice of insulin concentration. Cells maintained in baseline conditions retain their ability to clear insulin at close to initial rates throughout the 14-day culture period, with a modest decline from 80% to 77% (Fig. 1A). In contrast, hepatocytes maintained in 800 pM insulin lose almost half their initial insulin removal rate by day 14, starting on day 9 (Fig. 1A). Further, when cultured in 800 pM insulin, hepatocytes lose their ability to initiate downstream signaling upon stimulation with insulin, as assessed by phosphorylation of the downstream insulin receptor target, AKT (Fig. 1B). Similarly, cells maintained in 800 pM insulin displayed increased basal gluconeogenesis and reduced insulin sensitivity by days 8 and 15, as measured by insulin-induced suppression of HGP, compared to cells maintained in 200 pM insulin. This impairment was observable at only the lowest insulin doses used in the HGP assay on day 8, but widely observed in all insulin doses by day 15 in hepatocytes maintained in hyperinsulinemic conditions (Fig. 1C). Supporting the replicability of these assays, congruent results are obtained from experiments performed using hepatocytes obtained from a separate donor (Supplementary Fig. 3A, B) and in slightly different bioreactors (Supplementary Fig. 1C). In sum, hepatocytes cultured in physiologically relevant hyperinsulinemic conditions within our in vitro MPS model develop reduced insulin uptake, which alters downstream AKT signaling cascades and impairs HGP.
A Cells in hyperinsulinemic conditions (Condition 1 + Ins; 800 pM insulin) gradually lose the ability to clear insulin over 15 days in culture compared to (Condition 1; 200 pM insulin). Two-way ANOVA interaction term p < 0.001, and asterisks indicate significance in post-hoc Sidak test between 800 and 200 pM maintenance media (* p < 0.05, ** p < 0.01, *** p < 0.001). B Cells in the hyperinsulinemic condition have reduced responsiveness to insulin stimulation, as assessed by activation of the downstream signaling target AKT, using phosphorylation as a surrogate for activation. Two-way ANOVA was significant for maintenance media term (p < 0.0001) and post-hoc Sidak’s test between 800 and 200 pM maintenance media (*** p < 0.001, ** p < 0.01). C Cells maintained in hyperinsulinemia show decreased suppression of hepatic glucose output upon 24-h insulin stimulation. At day 8, two-way ANOVA was significant for insulin dose-response ($$$ p < 0.001 for row factor) and maintenance media (## p < 0.01 for column factor). At day 15, glucose output was significantly higher in 800 pM insulin maintenance media than 200 pM insulin maintenance media at all insulin dose responses (interaction term p < 0.01, post-hoc Sidak *** p < 0.001, ** p < 0.01, * p < 0.05). All data acquired from male SMC donor.
Hyperglycemia- and hyperlipidemia-induced insulin resistance
Having established that hyperinsulinemia alone induced several features of the insulin-resistant phenotype in our in vitro platform, we then assessed hepatocellular responsiveness following exposure to other elevated substrates observed in MASLD; FFAs, glucose, alone or in combination with hyperinsulinemic conditions. Condition 1 (200 pM insulin, 5.5 mM glucose, 20 µM FFA), Condition 1 + FFA (100 µM), Condition 1 + G (glucose: 11 mM) + FFA (100 µM), and Condition 2 (800 pM insulin, 11 mM glucose, 100 µM FFA) media formulations were used to assay hepatic insulin uptake and HGP through 19 days of culture. By day 19, FFA stimulation alone (Condition 1 + FFA) leads to the highest IC50 (0.052) value of the three metabolically stimulated conditions (Fig. 2A). However, only hepatocytes treated with Condition 2 display a significantly heightened glucose production across all doses and all time points (12 d: IC50Condition 1 = 0.0076 vs IC50Condition 2 = 0.027, 19 d: IC50Condition 1 = 0.0087 vs IC50Condition 2 = 0.028) (Fig. 2A). In congruence with this data, RT-qPCR of two gluconeogenic genes, PCK1 and G6PC30, revealed hampered transcriptional repression in hepatocytes maintained in either Condition 1 + G + FFA or Condition 2 compared to cells in Condition 1 following a 24-hour incubation with 0.1 and 1.0 nM insulin (Fig. 2B). Thus, even in the absence of hyperinsulinemia, high glucose/FFA results in protein and transcriptional impairment of the hepatocellular response to insulin. Conversely, insulin uptake by hepatocytes maintained in the other nutrient agonists is reduced only if hyperinsulinemia is concomitantly present (12 d: IC50Condition 1 = 0.0087 vs IC50Condition 2 = 0.028, 19 d: IC50Condition 1 = 0.012 vs IC50Condition 2 = 0.023) (Fig. 2C), suggesting high insulin as the primary driver of metabolic impairment within our culture model.
A Glucose output is significantly increased in both Condition 1 + FFA and Condition 2 over the baseline condition on days 12 and day 19. ### p < 0.001 for the dose variable, and * p < 0.05, ** p < 0.01, *** p < 0.001 via two-way ANOVA. B Condition 2 has impaired PCK1 responsiveness to insulin at 0.1 and 10 nM compared with Condition 1. G6PC expression is similar across all doses and sample sets. * p < 0.05, ** p < 0.01 *** p < 0.001 via two-way ANOVA. C Insulin uptake is significantly different between Condition 2 and all other conditions at 9 and 16 days incubation. All data acquired from male SMC donor. Two-way ANOVA; * p < 0.05, *** p < 0.001. n = 4; all samples.
Individual high-nutrient conditions further exacerbate hyperinsulinemia-induced insulin resistance
Leveraging these insights, we individually assayed which metabolic substrate, glucose or FFA, further influenced the various insulin resistance metrics in addition to hyperinsulinemic conditions. Insulin uptake was evaluated during each 48-h media exchange period, and HGP was monitored between days 7–8 and days 14–15. Consistent with Figs. 1 and 2, adding either high glucose (Condition 1 + Ins + G), or both high glucose/FFA to the high insulin media (Condition 2) does not reduce insulin uptake compared to high insulin alone (Fig. 3A). Interestingly however, it is the addition of both high glucose and high FFA that impairs insulin sensitivity the most to transcriptional and protein-level HGP suppression beyond that for hyperinsulinemia alone (8 d/15 d: IC50 Condition 1+Ins = 0.025/0.090 vs IC50 Condition 1+Ins+G = 0.041/0.065 vs IC50Condition 2 = 0.051/0.065)(Fig. 3B, C). Thus, hepatocytes in our model phenotypically recapitulate insulin resistance most aptly through joint incubation with high insulin, glucose, and FFAs; Condition 2.
A Insulin uptake decreases over time in culture in all groups, but there is no additive effect of high glucose or high FFA on insulin uptake. B Both Condition 1 + Ins + G, and Condition 2 have significantly increased glucose output over Condition 1 + Ins hepatocytes at days 8 and 15 via two-way ANOVA ($$$ p < 0.001 for dose variable and ### p < 0.001 for maintenance media variable). C All groups show decreased gluconeogenic gene expression with increasing insulin dose; however, Condition 2 cells have significantly higher expression of PCK1 after incubation with 0.01, 0.1, and 1.0 nM insulin than Condition 1 + Ins alone (p < 0.001). No difference in G6PC expression was noticed amongst groups; two-way ANOVA. All data acquired from male SMC donor.
Insulin and nutrient overload induce the phenotypic spectrum of MASLD in vitro
However, while insulin resistance is frequently implicated in metabolic dysfunction, multiple other pathophysiologies are observed in MASLD. Importantly, Condition 2 hepatocytes recapitulate some of these features, including significantly higher intracellular triglyceride content in comparison with Condition 1 (Fig. 4A). Further, metabolic analyses identified a significant increase in taurine-conjugated cholic acid in Condition 2-treated hepatocytes compared to baseline after 9 and 18 days in our bioreactor. Glycine-conjugated cholic acid was only elevated at day 9 in Condition 2 hepatocytes. (Fig. 4B). There were no significant differences in chenodeoxycholic acid or its conjugated forms (data not shown). This phenotype suggests a role for cholesterol catabolism in response to hyperinsulinemic/nutrient overload31.
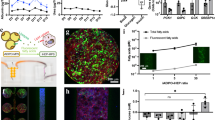
A Male (donor SMC) and female (donor HU8406) intracellular triglycerides, normalized to total protein abundance, are significantly elevated in Condition 2 hepatocytes compared with Condition 1. B Abundance of primary conjugated bile acids is significantly increased at day 9 in Condition 2 vs. Condition 1. After 18 days in culture, taurocholic acid is still significantly elevated in Condition 2 over the Condition 1 stimulated male hepatocytes. C CCL2, IL8, CXCL1, and CXCL10 secretion at days 3, 9, 14, and 19 was assessed in Condition 1 and Condition 2 male and D female hepatocytes within our MPS model, revealing a pro-inflammatory state in hepatocytes stimulated with physiologically high amounts of insulin, glucose, and FFA. E Multiphoton label-free imaging of male Condition 1 and Condition 2 hepatocytes using third harmonic generation (THG; Lipids), three-photon autofluorescence (3PAF; NAD(P)H), and two-photon autofluorescence (2PAF; FAD). F Quantitation of label-free imaging revealed increased intracellular lipid content in Condition 2 vs Condition 1 hepatocytes. * p < 0.05, ** p < 0.01 *** p < 0.001, **** p < 0.0001; Student’s t-test or two-way ANOVA. Scale bar: 50 µm.
As inflammation is definitive in the transition from MASLD to MASH, we assayed four well-known secreted chemokines across multiple days in male and female hepatocytes treated with Condition 1 or 2 media32. This experiment captured a hepatic pro-inflammatory signature that is temporally regulated (Fig. 4C, D). Specifically, our male Condition 2 hepatocytes display modest, but significant, increases in CCL2, IL8, CXCL1, and CXCL10 in the first ten days of culture, which taper for each analyte except CXCL1 by day 19. Female Condition 2 hepatocytes have a less robust chemokine response compared withmale Condition 2 hepatocytes (Fig. 4D). This finding is consistent with known sexual dimorphisms in metabolic disease and their pathomechanisms of liver fibrosis33,34. Further, our male and female donors have a large variance in BMI (#SMC, Male, 29.9 vs #HU8406, Female, 20.6), possibly adding to the observed discordance in immunological secretions35.
Multiphoton label-free imaging was employed to visualize metabolic activity (flavin adenine dinucleotide (FAD), nicotinamide adenine dinucleotide phosphate (NAD(P)H), and lipids) in both hepatocyte groups (Fig. 4E)36,37. Quantitative analysis reveals a significant increase in lipids within our Condition 2 male hepatocytes (Fig. 4F), corroborating our biochemical measurements of elevated intracellular triglycerides (Fig. 4A). Additionally, assays of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) revealed no significant differences in Condition 1 vs Condition 2 hepatocytes (Supplementary Fig. 4). Elevation of ALT, AST, and LDH is observed at day 3, likely due to post-thaw stress38,39, but tapers significantly by day 7 onwards. Together, these data demonstrate that Condition 2 hepatocytes replicate phenotypic features observed in MASLD, including insulin resistance, steatosis, expansion of the total bile acid pool, and pro-inflammatory chemokine expression.
Transcriptome analysis of an MPS model of MASLD
RNA sequencing analysis of hepatocytes incubated in Condition 1 and Condition 2 media formulations for 15 days identified 914 differentially expressed genes (DEGs; padj < 0.05)(GEO#: GSE313774), with condition-specific separation by principal component analysis (PCA; Fig. 5A). Of the 914 DEGs, 38 and 27 had a Log2FoldChange > 1 or < −1, respectively (Fig. 5B). Significant downregulation of metabolic mediators; FOXQ1, IGFBP1, SERPINE1, and ADM, and upregulation of pro-inflammatory cues; GFAP, BMF, and FGF1, indicate a reactive hepatocellular cell state following two weeks in Condition 2 media (Fig. 5B). Expanding on this, DEG enrichment utilizing Enrichr40 inferred significant overlap with primary human liver tissue according to the Human Gene Atlas41, in addition to shared genes in GO and KEGG terms; cellular metabolic process, glucose import, and bile secretion (Fig. 5C; top 3 panels)42,43. Providing additional confidence in our approach, there is also significant homology between Condition 2 DEGs and multiple disease terms such as Acquired Metabolic Disease, Cholestasis, and T2D, and enrichment of transcription factors FOXA2, HNF-4α, and LXR (Fig. 5C; bottom 3 panels)44,45,46. Indeed, Condition 2 hepatocytes have expected significant metabolic dysfunction, as shown by transcriptional alterations of CYP3A4, RXRA, and PTEN, and concomitantly increased transport/synthesis of bile acid genes; ABCB4 (MDR3), AKR1D1, and CYP7A1 (Fig. 5D). There is also significant basal upregulation of chemokine CXCL10 and other immune cell mediators, in agreement with our cytokine data (Fig. 4C, D).
A Principal component analysis (PCA) separates samples by exposure to 15 days culture in Condition 1 (n = 7) and Condition 2 (n = 9). B Volcano plot of log2 fold change and p-values between Condition 1 and Condition 2 transcripts. Red dots and blue dots indicate transcripts that had both a padj < 0.05 and log2 fold change > 1 or < −1, respectively. Gray dots are transcripts that remain relatively unaffected between samples. C Transcriptional enrichment of Condition 2 MASLD hepatocytes compared with the Human Gene Atlas, GO Biological Processes, KEGG, Jensen Diseases, DisGeNET, and ChEA databases were confirmed with EnrichR (* padj < 0.05). D Select differentially expressed genes (DEGs; * padj < 0.05) log2 fold changes were plotted in pathways regulating metabolic, bile acid, and immune response. E Translatable component (TC1) and extra latent variable (Extra LV1) separation of human MASLD & MASH data, adapted from Cadavid & Meimetis et al. 47 F Comparison of transcriptomic data from Condition 1 and 2 incubated hepatocytes against a fibrotic MPS model of MASH with TGF-β treated hepatocytes uncover significant separation in Extra LV1. All data acquired from male SMC donor. * p < 0.05, **** p < 0.0001; two-sided unpaired Wilcoxon test.
We additionally employed a machine-learning framework called Latent in vitro to in vivo Translation (LIV2TRANS)47 to ascertain how our MPS transcriptomic data align with the direction of molecular variance of an MPS model of fibrosis in MASH48 and human patient MASLD & MASH data49. Briefly, using paired histologic-transcriptomic MASLD & MASH data, LIV2TRANS is a mathematically driven framework to identify experimental conditions that would more accurately capture the in vivo physiologic paradigm. Graphical representation, via the mathematical space already defined by LIV2TRANS, of human MASLD and MASH transcriptomic data outlines a qualitative trend between fibrosis and MASLD progression (Fig. 5E). The X-axis; Translatable components (TC1), is defined to capture the most relevant information of the MPS model of fibrosis in MASH, whereas the Y-axis; extra latent variables (Extra LV1), outlines missing information in the fibrotic MPS and correlates with MASLD activity score. As expected with the compared MPS model aimed at experimental fibrosis48, TGF-β induces a clear separation co-observed in human TC1, which is not present in our media. Notably, however, Condition 2 media drives a translatable separation in Extra LV1 between Condition 1 (Fig. 5F), representing MASLD activity score, that is not captured in the other MPS model. These data highlight our Condition 2 media as one that uncovers a novel missing in vitro component of MASLD progression. In sum, hepatocytes exposed to insulin/nutrient overload are pro-inflammatory and have perturbed metabolic signaling cascades, encompassing a significant portion of the phenotypic spectrum observed in human MASLD, and likely inducing the observed attenuated response to insulin (Figs. 1–3).
To further parse this hypothesis, that our diseased hepatocytes are transcriptionally poised to an impaired insulin response, we exposed hepatocytes incubated in 14 days of Condition 1 and Condition 2 media to 1 nM of insulin for 24 h and performed RNA sequencing on their cell lysates (Fig. 6A, B). Both 15-day hepatocyte cultures separated via PCA following insulin treatment (Supplementary Fig. 5A, B). Surprisingly, both had similar levels of DEGs, 1060 and 1079 in Condition 1 and Condition 2 after insulin treatment, respectively. To identify the genes that are differentially insulin-responsive between each condition, we plotted the DEGs (padj in either insulin-stimulated Condition 1 or Condition 2 cells) of both groups according to their response similar index (RSI) (Fig. 6C), which colors DEGs by their signed probability of joint differential expression, where values near 1 indicate a high probability of concordance, and near −1 are likely discordant50. DEGs were additionally separated by magnitude (|Log2FCCondition 1 vs Condition 1 + Insulin 1nM - Log2FCCondition 2 vs Condition 2 + Insulin 1nM|) (Supplementary Fig. 5C) to produce a focused list of 422 DEGs that are differentially concordant (RSI > 0, Mag > 0.25) between insulin-stimulated Condition 1 and Condition 2 hepatocytes (Supplementary Data 1). Interestingly, Gene Ontology (GO) enrichment of the focused DEGs identified bile acid and bile salt transport as the most differentially regulated biological process dividing the Condition 1 and Condition 2 hepatocellular response to insulin (Fig. 6D). Within this pathway, major bile acid biosynthesis/transport genes are differentially upregulated between our two groups following insulin treatment; CYP7A1, ABCC4 (MRP4), SLC51A (OSTα), and ABCB11 (BSEP) (Fig. 6E), aptly modeling the strong correlation found between elevated cholestatic markers and patients with MASLD, and coinciding with our metabolite data (Fig. 4B)51,52. Immune system/metabolic mediators AXL, GAS6, and downstream AKT3 were also among our list of concordant DEGs (Fig. 6E), suggesting a possible role for AXL-GAS6 in mediating insulin receptor trafficking53. Of importance, and within our differential concordant list of DEGs, GK and G6PC both had a shunted transcriptional response to insulin in Condition 2 hepatocytes (Fig. 6E), amongst other key glucose homeostasis genes. Subsequent mitochondrial (MT) reprogramming factors, PPARGC-1α (PGC-1α), and CPT1A/B, also had a significantly impaired response to insulin in Condition 2 hepatocytes compared to those in Condition 1 (Fig. 6E). In sum, these data outline a hepatocellular state modeling several aspects of insulin resistance in the metabolic syndrome and serve as a starting point for any therapeutics considered in this approach.
A, B Volcano plot of log2 fold change and p values between Condition 1 (n = 7) and Condition 2 (n = 9) transcripts following exposure to 1 nM insulin. Red dots and blue dots indicate transcripts that had both a padj < 0.05 and log2 fold change > 1 or < −1, respectively. Gray dots are transcripts that remain relatively unaffected between samples following insulin exposure. C Response similarity index outlining transcriptional concordance (values near 1, purple) and discordance (values near −1, green) between differentially expressed genes (DEGs) in Condition 1 and Condition 2 following incubation with 1 nM insulin. D Concordant DEGs were enriched in multiple metabolic pathways following biological process Gene Ontology Enrichment. E Select differentially expressed concordant genes (DEGs [* padj < 0.05] with RSI > 0, magnitude > 0.25) log2 fold changes were plotted in pathways regulating bile acid, immune, mitochondrial (MT), and glucose homeostatic response. All data acquired from male SMC donor.
Paradoxical effect of thyroid receptor β agonist resmetirom
Dietary and exercise modification are typical first-pass interventions to help reduce MASLD progression into MASH54,55. As such, to assess if Condition 2 hepatocytes were capable of returning to a baseline metabolic status, hepatocytes were initially stimulated with either Condition 1 or Condition 2 media for 12 days, followed by treatment with a 50% supplement of Condition 1 (recovery media) for days 12–19. Condition 2 hepatocytes cultured in the recovery condition for days 12–19 displayed HGP (Supplementary Fig. 6A) and insulin uptake (Supplementary Fig. 6B) that were nearly identical to cells maintained in Condition 1 media for the entirety of the 19-day experiment, regardless of which MASLD-mimicking media in which they were initially cultured. Conversely, hepatocyte intracellular triglycerides are persistently elevated in Condition 2, even after supplementation with 12 days of recovery media, indicating an incomplete reversal of MASLD within our system, and the need for longer time points or additional treatment options (Supplementary Fig. 6C).
Thus, we saw this as an opportunity to test if our model of MASLD would be more responsive to a targeted therapeutic, rather than a diet-based intervention. We postulated that utilizing resmetirom, a selective thyroid receptor β 1 agonist known to reduce hepatic steatosis56 and the only FDA-approved compound for non-cirrhotic MASH25, would ameliorate some of the phenotypes observed in our model. Accordingly, healthy and MASLD hepatocytes were established following 10 days of culture in Condition 1 or 2 media, respectively, with 2 µM resmetirom (reconstituted in DMSO) or DMSO supplementation every 48-hour for an additional 10 days (Fig. 7A). This administration schedule doubles the clinical human Cmax of 1 µM at a 100 mg oral dose, to consistently expose our cells to a therapeutically relevant concentration across the 48-hour exchange period25,57. Condition 2 hepatocytes treated with the thyromimetic became significantly more sensitized to insulin, in comparison with Condition 2 cells without treatment, but did not normalize to levels observed in Condition 1 or in Condition 1 + resmetirom, as measured through insulin uptake and HGP (Fig. 7B, C). Transcriptionally, gluconeogenic PCK1 and G6PC repression is normalized in treated Condition 2 hepatocytes and further downregulated in treated Condition 1 hepatocytes compared with their unstimulated counterparts (Fig. 7D). Importantly, and in agreement with previous findings, we confirm a significant reduction in hepatic steatosis within resmetirom treated cells, normalizing our MASLD hepatocytes to levels seen in Condition 1 (Fig. 7E)58,59. We also confirm significant activation of target gene DIO1 (Fig. 7F)60. CPT1A, another target gene of resmetirom, is up in males and unaffected in females (Supplementary Fig. 7). No effect is noticed in the resmetirom transporter gene, SLCO1B1, following drug treatment in male or female hepatocytes (Supplementary Fig. 7).
A Schematic illustration of the experimental pipeline utilized to assay C1 and C2 hepatocellular response to resmetirom treatment. B Male (donor SMC) and female (donor HU8406) hepatocytes were incubated in Condition 1 & 2 media for 10 days, followed by supplementation with 2 µM resmetirom for an additional 10 days, ameliorating insulin uptake in Condition 2 treated cells. C At day 19, Condition 2 + Resmetirom hepatocytes were more insulin responsive compared with untreated Condition 2 cells, as assessed through HGP (D) and PCK1 and G6PC repression at 1 nM insulin stimulation. E Intracellular triglyceride content at day 19 is significantly higher in Condition 2 compared with Condition 1, but normalized in resmetirom-treated male and female C1 and C2 hepatocytes. F Thyroid receptor β agonist target gene DIO1 is significantly upregulated following treatment with resmetirom in C1 and C2 hepatocytes. G IL8, CCL2, CXCL1, and CXCL10 cytokine secretion was assayed 4-(D14) and 9(D19)-days post-treatment. H Multiphoton label-free imaging of male Condition 1, Condition 1 + Resmetirom, Condition 2, and Condition 2 + Resmetirom (I) revealed normalization of lipids after drug treatment and an increase in NAD(P)H in Condition 2 + Resmetirom cells. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 via two-way ANOVA. Scale bar: 25 µm. Schematic was made with BioRender.com under a purchased license agreement.
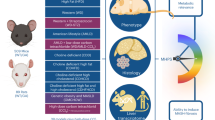
Paradoxically, however, following 10 days of stimulation with resmetirom, Condition 1 & 2 hepatocytes develop an inflammatory shift within our MPS, increasing from day 14 onward and more so in Condition 2 (Fig. 7G). Male and female hepatocytes displayed similar expression patterns and were concordant in CXCL1 and IL8 secretion following incubation with the drug. Of the cytokines analyzed, only CXCL10 was reduced in both male and female donor cells after treatment. To delineate this further, we performed label-free multiphoton imaging of all four groups, which corroborated our biochemical analysis showing normalization of intracellular triglycerides in resmetirom-treated cells (Fig. 7H, I). Wide-field images of label-free images acquired from all four groups can be found in the supplement (Supplementary Fig. 8). Interestingly, there is also a significant increase in NAD(P)H within Condition 2 + resmetirom cells. This is in alignment with an observed upregulation of MT reprogramming/stress genes PGC-1a and SOD2 in treated male hepatocytes (Supplementary Fig. 7). PGC-1a and SOD2 trended upward in treated female hepatocytes, but were not significant. As resmetirom is known to stimulate MT activity57,61,62, RT-qPCR and label-free data implicate MT stress as a possible mediator of the reactive hepatic state following treatment in our MPS model of MASLD (Fig. 8; schematic). Further mechanistic exploration into the observed paradoxical response to the drug is warranted.
On the left is a hepatocyte treated with physiological levels of insulin, glucose, and FFAs, mirrored by Condition 2 hepatocytes incubated with elevated levels of insulin and nutrients. Condition 2 hepatocytes are pro-inflammatory, contain increased intracellular triglycerides, and have elevated bile acid expression, recapitulating a spectrum of phenotypes within MASLD. Treatment with the thyroid receptor β agonist resmetirom reduced steatosis and ameliorated insulin resistance, but leads to increased inflammation in Condition 2 hepatocytes, possibly through an overactive mitochondrial response. This figure was made with BioRender.com under a purchased license agreement.
Discussion
Here, we used an established liver MPS model15,18,19,20,21,24 to parse the contributions of hyperinsulinemia and elevated nutrients on the development of the MASLD phenotype in primary human hepatocytes. Of note, primary human hepatocytes cultured in a physiological medium (Condition 1; 200 pM insulin, 5.5 mM glucose, 20 µM FFA) remain insulin sensitive over almost 3 weeks in culture, as demonstrated by insulin uptake, dose-dependent AKT phosphorylation, and dose-dependent suppression of HGP by insulin (Figs. 1, 2, and 6). Second, a modest increase in insulin from 200 to 800 pM induces an insulin-resistant phenotype over 7–10 days in culture (Figs. 1 and 3), jointly validated with an additional donor set (Supplementary Fig. 3). Hepatocytes cultured in hyperinsulinemic conditions have exacerbated phenotypes in the presence of hyperglycemia and elevated FFAs (Figs. 2 and 3). Most prominently, our high insulin, glucose, and FFA (Condition 2) hepatocytes display steatosis, produce significantly more bile acids, and are pro-inflammatory (Fig. 4). We corroborate these findings transcriptionally, identifying significant metabolic and immune perturbations, comparable to those identified in MASLD progression (Fig. 5), that poise our Condition 2 hepatocytes to an impaired insulin response (Fig. 6). Finally, we assess the therapeutic applicability of our MASLD model, applying independently a nutrient- (Fig. S6) and drug-based intervention to the system, revealing an unexpected response to the drug resmetirom in male and female donors (Fig. 7). The results from such a succinct array of analyses indicate that the MASLD hepatocytes within our established bioreactor fundamentally model multiple aspects of what is seen in the human condition (Fig. 8; schematic).
Our physiologically relevant healthy and diseased media formulation is among the first instance of its implementation to in vitro primary human hepatocytes in a microfluidic MPS. Insulin concentrations of 200 pM for healthy and 800 pM for disease approximate portal vein values for these states integrated over fasting/fed excursions in vivo in healthy and metabolic diseased states26,27. Corroborating this choice, by elevating insulin concentrations alone to the values expected in T2D subjects, we recapitulated several features of the hepatic insulin-resistant phenotype (Fig. 1). The finding of reduced uptake/removal in our models’ disease state is in accordance with multiple human studies reporting a decrease in insulin clearance in T2D subjects and isolated cells from T2D livers63,64. In comparison with reported in vitro hepatic disease models, most use supraphysiological insulin for both healthy- and disease-states65,66, pair a physiological healthy value of nutrients (glucose/FFA) with supraphysiological for disease66,67 or do not report insulin/nutrient values at all19,48. Further, insulin uptake is not typically reported, perhaps because cell concentrations are either much lower66,68, higher65, or certain in vitro models lack pharmacokinetic capability18,69. A more comprehensive picture of insulin, nutrient, and steroid values will likely emerge as studies standardize protocols for metabolic exposure, including the complex interactions with serum proteins.
Nonetheless, significant strides have been made in developing apt models for pharmacokinetic performance70 and innovative approaches for MASLD MPS modeling6,71. Noteworthy comparators to our model include: the CN-Bio “Nash-in-a-box”, which outlined undefined concentrations of media additives that would drive MASH within a multicellular LiverChipTM48, the “MASLD Integrated-gut-liver-on-a-chip”, which utilizes separate connecting perfusable bioreactors with hepatic and gut cell lines to enable a MASLD phenotype72, and the hLiMT and patient-derived 3D MASH spheroids, using static multicellular hepatic spheroids combined with physiologically relevant metabolic perturbations for therapeutic intervention73,74. As such, the choice of physiologically reflective defined values of insulin, FFA, and glucose in our model, combined with the choice of microfluidic bioreactor, allows for reproducible molecular measurements and provides a baseline for assays across all in vitro models.
We realize that while elevation of insulin alone was enough to induce aspects of the insulin-resistant phenotype, increasing glucose or FFAs alone, or in combination, did markedly induce metabolic changes in the hepatocellular phenotype (Fig. 4), and as such, are more reflective of a MASLD condition4. By investigating combinations of these three metabolic agonists in physiologically normal and diseased levels, we uncover immunologically reactive hepatocytes that produce increased amounts of bile acids and have higher intracellular triglyceride content (Fig. 4). Further, the Condition 2 transcriptomic signatures share strong similarities across other models and human datasets (Fig. 5C)50, and are representative of an early MASLD to MASH model (Fig. 5E, F); hampered response to insulin, with significant metabolic reprogramming, but likely not as immunologically reactive as late-stage75,76. These data further posit this model as one that reflects multiple aspects of MASLD, but warrant multiple follow-up studies investigating the complex regulatory effects of metabolic stress in driving aberrant signaling patterns and immunological shifts.
To more accurately recapitulate the full spectrum of MASLD progression, particularly the transition to MASH, future iterations may benefit from longer hepatic incubation times in the Condition 2 media, the addition of TGF-β, or the inclusion of NPCs to elicit a more physiologically relevant MASLD to MASH profile77. Indeed, it is possible that such an inclusion would also modify the hepatocellular response to certain drugs15,78. This is observed similarly in another perfusable MPS model, whereby hepatocytes cultured with stellate cells, kupffer cells, and endothelial cells have a mechanistically different response to resmetirom in comparison with hepatocytes cultured alone, possibly coinciding with the noticeable decrease in steatosis, but paradoxical increase in inflammatory cues seen in our model77. Our data adds to other pre-clinical data that report counterintuitive findings associated with thyroid receptor β agonism79,80, and, given that only 30% of patients benefit from treatment with resmetirom, they underscore the substantial need for the development of new therapeutic agents25,81. At the same time, our model only assayed the effect of a single concentration of the drug, 2 µM, selected to approximate human Cmax at 100 mg dosing over a 48-h period25. Future studies should examine a dose-response of resmetirom to map response ranges and compare against in vivo Cmax fluctuations and EC50 values. Several mechanistic questions are additionally raised by our data and warrant deeper investigation. These include the role of additional thyroid hormone transporter complexes, OATP1B3 & MCT8, in regulating bioavailability of resmetirom, the contribution of MT activity/redox imbalance to chemokine induction (CXCL1 and IL8), sexual dimorphism in resmetirom response, and the regulatory influence of NPCs on hepatocellular reactivity.
There are additional notable limitations of our in vitro model of human MASLD. First, the MPS platform lacks in situ imaging capabilities, limiting the interpretation of certain morphological metrics in real-time. Second, studying hepatocytes alone limits interpretability and certain comparisons with animal models; however, it provides for a more focused analysis and platform for dissecting hepatocyte-specific molecular drivers of metabolic disease, as previously mentioned, and as we have showcased in Fig. 7 with a therapeutic intervention. Co-culture of hepatocytes with islets is an appealing solution to better mimic the normal physiologic insulin delivery82, which exhibits a pulsatility lost in T2D83, but scaling the cell masses and medium volumes to obtain physiological variation is a significant burden84. Further, we chose to perform transcriptomic analyses on hepatocytes cultured in the C1 or C2 condition (+/− Insulin treatment) at a single 15-day endpoint to capture gene- and pathway-level perturbations that aligned with the functional impairment (altered insulin uptake, glucose output, and chemokine expression patterns) observed at a similar timepoint. Nonetheless, we believe additional probes into the temporal transcriptomic landscape (day 3, 7, and 9) in the C1 and C2 conditions would lead to valuable insights that are missed in our MPS model of MASLD.
In conclusion, our MPS model using primary human hepatocytes exposed to pathophysiological concentrations of insulin and nutrients to mimic the hyperinsulinemia, hyperglycemia, and increased FFAs seen in healthy and metabolic syndrome recapitulates vital aspects of insulin resistance and MASLD. This model of MASLD offers a means to better understand the molecular mechanisms involved in insulin resistance, metabolic reprogramming, and hepatocyte reactivity and provides a baseline for additional therapeutic targets and avenues.
Methods
Liver MPS assembly and operation during culture
Two nearly identical (Liverchip™ (re-useable) and the disposable PhysioMimix™ (both from CNBio Innovations, Cambridge, UK)) versions of a polycarbonate micro-machined platform supporting 12 continuously perfused liver MPS-channels were used, according to already established methods18,20. The fluidic wells were primed by adding 1.6 mL priming media (1% BSA, 1% PenStrep in PBS) to each well continuously at 1 µL/s for 24 h at 37 °C. Priming media was aspirated to the level of the retaining ring immediately ahead of seeding. Following the seeding phase (see below), flow was maintained at 1 µL/s. Recirculating media was replaced every 48 hours by aspirating old media, adding 400 µL new media for a 3-minute downward flow rinse step, followed by a full 1.6 mL media replacement.
Cells and media
The study was performed with primary human hepatocytes from a 50 year old male Caucasian donor with a BMI of 29.9 (BioIVT; lot #SMC). Select experiments were repeated with cells from a 63 year old male donor (BioIVT, lot #AQL) and 62 year old female donor (Thermo, lot #HU8406). Human cells purchased from BioIVT and ThermoFischer are collected with the informed consent of the donor. These donors were screened and chosen for their ability to effectively remove insulin from media and produce albumin at consistent levels over the course of 10 days (data not shown). Cells were thawed in Cryopreserved Hepatocyte Recovery Media (ThermoFisher), spun down at 100 × g for 5 minutes, and resuspended in seeding medium; William’s E with 2 mM GlutaMax, 15 mM HEPES, 5% FBS, 1% P/S, 100 nM hydrocortisone, and glucose and insulin supplemented at levels corresponding to the proper experimental group. Typically, cells were seeded by adding 600,000 cells in 300 µL seeding medium to each well, allowing the cells to settle into the scaffold, and then adding an additional 1.3 mL of seeding media (for total reactor volume of 1.6 mL). Experiments optimizing insulin uptake/removal from media as a function of cell concentration were performed in triplicates using primary human hepatocytes (lot HU2098, ThermoFisher): 100,000; 200,000; 400,000; 600,000. Flow was initiated in a downward 1 µL/s mode for 8 hours to facilitate cell attachment to the scaffold and then reversed to an upward 1 µL/s flow for the remainder of the experiment. At 24 h post-seeding, cells were switched to maintenance media; William’s E plus 6.25 µg/mL transferrin, 6.25 ng/mL selenium, 0.125% fatty acid-free BSA (18.8 µM), 2 mM GlutaMax, 15 mM HEPES, 100 nM hydrocortisone, 0.5% PenStep, and FFA, glucose, and insulin, with concentrations adjusted to mimic various healthy and disease-inducing states18,20. Media states were designated as follows: “Condition 1” (200 pM insulin, 5.5 mM glucose, 20 µM FFA), “Condition 1 + G (200 pm insulin, 11 mM glucose, 20 µM FFA), “Condition 1 + FFA” (200 pM insulin, 5.5 mM glucose, 100 µM FFA), “Condition 1 + G + FFA” (200 pM insulin, 11 mM glucose, 100 µM FFA), and “Condition 2” (800 pM insulin, 11 mM glucose, 100 µM FFA). Baseline glucose of 5.5 mM corresponds to healthy fasting blood glucose levels and the elevated level of 11 mM corresponds to fasting blood glucose levels of diabetic patients85. FFAs were supplemented at a baseline of 20 µM of linoleic acid (normal maintenance condition for primary human hepatocytes) or 100 µM (with a physiologic ratio of 25% poly-unsaturated, 30% saturated, 45% mono-unsaturated e.g., 27.5 μM linoleic acid (18:2), 33 μM palmitic acid (16:0), and 49.5 μM oleic acid (18:1)) based on human plasma lipid composition86. Physionormic (200 pM) and high physiological (800 pM) insulin concentrations stem from well-established collected normal, T2D, and MASLD human sera values87.
Insulin uptake/removal from media
Insulin concentrations were measured by ELISA (Abcam, ab200011) in 50 µl media samples collected at 2, 4, 8, 24, and 48 h after full media replacement. The insulin uptake/removal from media percentage was calculated by dividing insulin concentrations in collected samples by the initial concentration and multiplying by 100.
Cytokine, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) expression
Media was collected in male and female hepatocytes after 48 h on days 3, 9, 14, and 18, and assayed by ELISA (Abcam, IL-8; ab214030, CCL2; ab179886, CXCL1; ab190805, CXCL10; ab316903, ALT; ab234578, AST; ab263881) and bioluminescence (Promega, LDH; J2380) according to the manufacturer instructions.
Hepatic glucose production
Cells were first transitioned to a basal state by exchanging the medium to a formulation containing no glucose and no insulin and circulating for an hour. Media was then replaced with glucose-free media + 20 mM lactate and 1 mM pyruvate + insulin (0, 0.01, 0.1, 1, 10, or 100 nM as separate conditions). Cells were maintained in “glucose output” media for 24 h followed by media collection for glucose concentration determination using the Amplex Red kit (Invitrogen, cat. no. A22189) or transcriptomics (see below).
Cell harvest for molecular assays
Endpoint analysis of cellular molecular properties (RT-qPCR, transcriptome sequencing, phospho-AKT, intracellular triglycerides, and metabolomics) was accomplished by lysing cells from the scaffolds at the end of each experiment. A unique lysis protocol was required for each type of assay. In cases where multiple analytes were measured in a single experiment, scaffolds were divided evenly between each assay to ensure at least n = 2 technical replicates per assay.
RT-qPCR
Scaffolds were rinsed in chilled DPBS and snap-frozen in liquid nitrogen. RNA was isolated using 1 mL Trizol per scaffold. Scaffolds were sonicated for 30 s before adding 200 µL chloroform and spun down at 18,000 × g for 20 minutes. Supernatant was transferred and mixed with 500 µL IPA for 10 minutes and spun at 18,000 × g for 15 minutes. Pellets were rinsed twice with 75% EtOH and eluted into RNAse-free water. RNA purity and concentration were determined using a Nanodrop One (Thermo-Fischer Scientific). Select samples with low or high 260 / 280 values were discarded. RNA was converted to cDNA using High-Capacity RNA-to-cDNA kit (Life Technologies, cat. no. 4387406) and qPCR was performed using TaqMan Fast Advanced Master Mix (cat. no. 4444556) with probes for PCK1 (Hs00159918_m1), G6PC (Hs00609178_m1), DIO1 (Hs00174944), CPT1A (HS00912671), SLCO1B1 (Hs00272374_m1), PPARGC1A (Hs00173304), SOD2 (Hs00167309), and internal reference 18S (Hs03928990_g1). Data were analyzed for fold change using the delta-Ct method88 and normalized to the 0 nM insulin group previously incubated in Condition 1 media for PCK1 and G6PC analyses.
pAKT
Phosphorylation of AKT (AKT Serine/Threonine Kinase 1) was measured by starving cells for 30 minutes in insulin-free media and stimulating with insulin in basal media at doses ranging from 0 to 100 nM for 30 minutes. pAKT was measured using Milliplex MAP kit for total and phospho-AKT 1/2 (Millipore, cat. no. 48-618MAG) according to manufacturer instructions. After 30 minutes of insulin stimulation, cells were transferred immediately to kit lysis buffer for 30 minutes at 4 °C with 1:100 protease inhibitor (Millipore, cat no. 535140), spun down at 3000 × g for 5 minutes, and collected for downstream quantification. Equivalent amounts of protein were determined and loaded in each assay using normalized BCA quantified lysate concentrations. For each biological replicate, the phospho/total AKT ratio was plotted and fit using a 3-parameter logarithmic curve in GraphPad Prism (version 8).
Intracellular triglycerides
Intracellular triglycerides were quantified using the Zen-Bio TG kit (Zen-Bio, cat. no. TG-1-NC) according to manufacturer instructions. Cells were lysed in 150 µL lysis buffer for 30 minutes at 4 °C and frozen at −80 °C. Upon thawing, 150 µL of wash buffer and 40 µL reagent B were added to scaffolds and incubated at 37 °C for 2 h. Lysates were diluted 1:2 in assay wash buffer followed by reagent A for detection of glycerol at 540 nm using a standard plate reader. Triglyceride content was normalized to total protein levels obtained through Bicinchoninic Acid assay (Thermo; 23235).
Analysis of bile acids
MPS were disassembled and scaffolds with cells were extracted, rinsed once with 0.9% NaCl, and immediately placed in tubes with 1 mL ice-cold LCMS-grade isopropanol (Thermo Fisher) containing 100 nM d4-cholic acid (CDN isotopes) as an internal standard. Samples were vortexed for 10 minutes at 4 °C and cleared by centrifugation at 8000 × g for 10 minutes at 4 °C. 500 μL of supernatant was transferred to a new tube and evaporated under a stream of nitrogen. Dried extracts were resuspended in 50 µL acetonitrile/water (50/50 volume/volume) and 5 µL were injected into an ACUITY BEH C18 column (Waters, 3 × 100 mm × 1.7 μm). The HPLC system was a Dionex UltiMate 3000 HPLC coupled to a QExactive mass spectrometer equipped with an Ion Max source and a HESI II probe (Thermo Fisher Scientific, San Jose, CA). External mass calibration was performed using the standard calibration mixture every 7 days. Mobile Phase A was 0.1% formic acid in water and Mobile Phase B was 0.1% formic acid in acetonitrile. The column oven was set at 40 °C and the autosampler was kept at 4 °C. The flow rate was 0.6 mL min−1 and the gradient was as follows: 0–1.5 min: hold 2% B; 1.5–2.0 min: linear gradient 2–35% B; 2.0–15.0 min: linear gradient 35–75% B; 15.0–15.1 min: linear gradient 75–100% B; 15.1–17.0 min: hold at 100% B; 17.0–17.1 min: linear gradient 100–2% B; 17.1–19.0 min: hold at 2% B. Data were acquired in full-scan, negative mode, with a scan range of m/z = 200–600. The resolution was 70,000, with an AGC target of 1 × 106, and IT at 80 ms. Relative quantitation of bile acids were performed using TraceFinder™ 4.1 (Thermo Fisher Scientific) using a 5 ppm mass tolerance and referencing an in-house library of chemical standards. Raw peak areas were normalized to d4-cholic acid.
Transcriptome analyses
Total RNA from hepatocyte scaffolds were extracted as previously detailed (see “RT-qPCR”) after 15 days in culture in either Condition 1 media, Condition 2 media, or 14 days Condition 1 media + 24 h insulin treatment, or 14 days Condition 2 media + 24 h insulin treatment. RNA purity and concentration were determined using a Nanodrop One (Thermo-Fischer Scientific). The preparation of RNA library and whole transcriptome sequencing was performed by EuroFins. The method for differentially expressed gene (DEG) determination was edgeR using the open-source R/BioConductor software89. EnrichR and clusterProfiler (Gene Ontology: Biological Process) were used to detect pathway-level differences among sample sets40,90. The effects of separate RNA isolation dates were treated as covariates and batch-corrected using the ComBat normalization method91. Comparison of variance in transcriptome data between Condition 1 and 2 hepatocytes, an MPS model of MASH48, and human MASLD & MASH data was achieved using Latent in vitro to in vivo Translation47, where all scripts utilized for this approach are open source (https://github.com/NickMeim/FattyLiverModeling).
Multi-photon label-free imaging
Following 15 days in culture, hepatocytes were mechanically dissociated from the LiverChip™ scaffold, and imaged using label-free multiphoton microscopy36,37. The laser scanning system comprised excitation from 1100 nm, 200 fs laser pulses (Light Conversion, Cronus-3P), a 1.4 numerical aperture objective (Zeiss, Plan-Apochromat 40×/1.4 Oil DIC), and emission measurements via photomultiplier tubes (PMTs; Hamamatsu, H16201). The emission was split using dichroic mirrors (Chroma, T412lpxt, T505lpxr), and the PMTs were fitted with bandpass filters to detect the following wavelength ranges: 365–375 nm, 417–477 nm, 580–638 nm (Chroma, ZET365/20×; Edmund Optics, 84-095; Semrock, FF01-609/57). The first detection band was designed to include the third harmonic generation signal, which forms at refractive index boundaries, and which hence revealed the lipid droplets inside the cells92. The second detection band was designed to capture the 3-photon-autofluorescence signal from NAD(P)H, and similarly, the third detection band was designed to capture the 2-photon autofluorescence signal from FAD93. Quantification of scanned images was performed using automated scripts written in Qupath that segmented cells and performed positive pixel analysis94,95. Briefly, normalization of the image signal in Qupath was performed against the average internal cell merged pixel intensity in each acquired image to segment cells. Quantification of FAD, NAD(P)H, and lipids was performed following cell segmentation by calculating the total amount of positive pixels per channel in each cell, dividing by total cell area, and multiplying by 100.
Statistics and reproducibility
All data shown are mean ± SD. All statistical comparisons were performed using one-way or two-way ANOVA in GraphPad Prism version 8, or as indicated. If the interaction term was not significant, p values were reported for the column factor (typically experimental condition, # p < 0.05, ## p < 0.01, ### p < 0.001) and row factor (either dose-response or time course, $ p < 0.05, $$ p < 0.01, $$$ p < 0.001). If the interaction term was significant, post-hoc multiple comparison analyses were run using either Tukey test (3 or more groups) or Sidak test (2 groups). For dose-response curves with 3 or more groups, Tukey post-hoc test compared the main effect between experimental conditions. For time courses or dose-response curves with only 2 experimental groups, a post-hoc test compared the experimental condition effect within each time point or dose. For all post-hoc tests, p values were reported for the post-hoc test only (* p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001). For insulin dose-response, a nonlinear regression 3 parameter fit ([Agonist] vs. dose-response) was performed to determine IC50 values. For metabolomics data, the discovery of significant metabolites was performed using multiple t-tests in GraphPad Prism of normalized expression values corrected for multiple comparisons using an FDR threshold of 10%.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
RNA-Seq data were deposited into the Gene Expression Omnibus database under accession number GSE313774 and are available at the following URL: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE313774. The experimental data, including Supplementary Data 1 and other source material, that support the findings of this study are available in Figshare with the identifier https://doi.org/10.6084/m9.figshare.30888380.
References
Targher, G., Corey, K. E., Byrne, C. D. & Roden, M. The complex link between NAFLD and type 2 diabetes mellitus—mechanisms and treatments. Nat. Rev. Gastroenterol. Hepatol. 18, 599–612 (2021).
Wang, S. & Friedman, S. L. Found in translation—Fibrosis in metabolic dysfunction-associated steatohepatitis (MASH). Sci. Transl. Med. 15, eadi0759 (2023).
Roden, M. & Shulman, G. I. The integrative biology of type 2 diabetes. Nature 576, 51–60 (2019).
Petersen, M. C. & Shulman, G. I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 98, 2133–2223 (2018).
Green, C. J. et al. Studying non-alcoholic fatty liver disease: the ins and outs of in vivo, ex vivo and in vitro human models. Horm. Mol. Biol. Clin. Investig. https://doi.org/10.1515/hmbci-2018-0038 (2018).
Saydmohammed, M. et al. Quantifying the progression of non-alcoholic fatty liver disease in human biomimetic liver microphysiology systems with fluorescent protein biosensors. Exp. Biol. Med. 246, 2420–2441 (2021).
Sakurai, Y., Kubota, N., Yamauchi, T. & Kadowaki, T. Role of insulin resistance in MAFLD. Int. J. Mol. Sci. 22, 4156 (2021).
Najjar, S. M. & Perdomo, G. Hepatic insulin clearance: mechanism and physiology. Physiology 34, 198–215 (2019).
Ter Horst, K. W. et al. Hepatic insulin resistance is not pathway selective in humans with nonalcoholic fatty liver disease. Diabetes Care 44, 489–498 (2021).
Bell, C. C. et al. Characterization of primary human hepatocyte spheroids as a model system for drug-induced liver injury, liver function and disease. Sci. Rep. 6, 25187 (2016).
Aizenshtadt, A. et al. Pump-Less, recirculating organ-on-chip (rOoC) platform to model the metabolic crosstalk between islets and liver. Adv. Healthc. Mater. 13, 2303785 (2024).
Rigual, M. d. M. et al. Macrophages harness hepatocyte glutamate to boost liver regeneration. Nature 641, 1005–1016 (2025).
Wang, J., Wu, X., Zhao, J., Ren, H. & Zhao, Y. Developing liver microphysiological systems for biomedical applications. Adv. Healthc. Mater. 13, 2302217 (2024).
Messelmani, T. et al. Liver organ-on-chip models for toxicity studies and risk assessment. Lab Chip 22, 2423–2450 (2022).
Kostrzewski, T. et al. A microphysiological system for studying nonalcoholic steatohepatitis. Hepatol. Commun. 4, 77–91 (2020).
Reaven, G. M. Role of insulin resistance in human disease. Diabetes 37, 1595–1607 (1988).
Sarkar, U. et al. Metabolite profiling and pharmacokinetic evaluation of hydrocortisone in a perfused three-dimensional human liver bioreactor. Drug Metab. Dispos. 43, 1091–1099 (2015).
Tsamandouras, N. et al. Quantitative assessment of population variability in hepatic drug metabolism using a perfused three-dimensional human liver microphysiological system. J. Pharmacol. Exp. Ther. 360, 95–105 (2017).
Vacca, M. et al. Bone morphogenetic protein 8B promotes the progression of non-alcoholic steatohepatitis. Nat. Metab. 2, 514–531 (2020).
Long, T. J. et al. Modeling therapeutic antibody-small molecule drug-drug interactions using a three-dimensional perfusable human liver coculture platform. Drug Metab. Dispos. 44, 1940–1948 (2016).
Clark, A. et al. A liver microphysiological system of tumor cell dormancy and inflammatory responsiveness is affected by scaffold properties. Lab Chip. 17, 156–168 (2017).
Vaupel, P. & Multhoff, G. Blood supply and oxygenation status of the liver: from physiology to malignancy. In Oxygen Transport to Tissue XLIII. 263–267 (Springer International Publishing, Cham, 2022).
Domansky, K. et al. Perfused multiwell plate for 3D liver tissue engineering. Lab Chip 10, 51–58 (2010).
Rubiano, A. et al. Characterizing the reproducibility in using a liver microphysiological system for assaying drug toxicity, metabolism and accumulation. Clin. Transl. Sci. https://doi.org/10.1111/cts.12969 (2020).
Harrison, S. A. et al. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. N. Engl. J. Med. 390, 497–509 (2024).
Song, S. H. et al. Direct measurement of pulsatile insulin secretion from the portal vein in human subjects. J. Clin. Endocrinol. Metab. 85, 4491–4499 (2000).
Porksen, N. et al. Human insulin release processes measured by intraportal sampling. Am. J. Physiol. Endocrinol. Metab. 282, E695–E702 (2002).
Kiselyov, V. V., Versteyhe, S., Gauguin, L. & De Meyts, P. Harmonic oscillator model of the insulin and IGF1 receptors’ allosteric binding and activation. Mol. Syst. Biol. 5, 243 (2009).
Hori, S. S., Kurland, I. J. & DiStefano, J. J. 3rd Role of endosomal trafficking dynamics on the regulation of hepatic insulin receptor activity: models for Fao cells. Ann. Biomed. Eng. 34, 879–892 (2006).
Matsumoto, M., Pocai, A., Rossetti, L., DePinho, R. A. & Accili, D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metabolism 6, 208–216 (2007).
Li, T. et al. Glucose and insulin induction of bile acid synthesis: mechanisms and implication in diabetes and obesity. J. Biol. Chem. 287, 1861–1873 (2012).
Anstee, Q. M., Reeves, H. L., Kotsiliti, E., Govaere, O. & Heikenwalder, M. From NASH to HCC: current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 16, 411–428 (2019).
Mittendorfer, B. Sexual dimorphism in human lipid metabolism. J. Nutr. 135, 681–686 (2005).
Jamalinia, M., Lonardo, A. & Weiskirchen, R. Sex and gender differences in liver fibrosis: pathomechanisms and clinical outcomes. Fibrosis 2, 10006 (2024).
Kimura, M. et al. En masse organoid phenotyping informs metabolic-associated genetic susceptibility to NASH. Cell 185, 4216–4232. e16 (2022).
You, S. et al. Intravital imaging by simultaneous label-free autofluorescence-multiharmonic microscopy. Nat. Commun. 9, 2125 (2018).
Liu, K. et al. Deep and dynamic metabolic and structural imaging in living tissues. Sci. Adv. 10, eadp2438 (2024).
Stéphenne, X., Najimi, M. & Sokal, E. M. Hepatocyte cryopreservation: is it time to change the strategy? World J. Gastroenterol. 16, 1 (2010).
Khetani, S. R. et al. Microengineered liver tissues for drug testing. J. Lab. Autom. 20, 216–250 (2015).
Chen, E. Y. et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 14, 1–14 (2013).
Su, A. I. et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc. Natl. Acad. Sci. USA 101, 6062–6067 (2004).
Kanehisa, M. & Goto, S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30 (2000).
Ashburner, M. et al. Gene ontology: tool for the unification of biology. Nat. Genet. 25, 25–29 (2000).
Grissa, D., Junge, A., Oprea, T. I. & Jensen, L. J. Diseases 2.0: a weekly updated database of disease–gene associations from text mining and data integration. Database. https://doi.org/10.1093/database/baac019 (2022).
Piñero, J. et al. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 48, D845–D855 (2019).
Keenan, A. B. et al. ChEA3: transcription factor enrichment analysis by orthogonal omics integration. Nucleic Acids Res. 47, W212–W224 (2019).
Cadavid, J. L., Meimetis, N., Griffith, L. G. & Lauffenburger, D. A. Systems biology framework for rational design of operational conditions for <em>in vitro / in vivo</em> translation of microphysiological systems. Preprint at bioRxiv https://doi.org/10.1101/2025.01.17.633624 (2025).
Kostrzewski, T. et al. Modelling human liver fibrosis in the context of non-alcoholic steatohepatitis using a microphysiological system. Commun. Biol. 4, 1080 (2021).
Hoang, S. A. et al. Gene expression predicts histological severity and reveals distinct molecular profiles of nonalcoholic fatty liver disease. Sci. Rep. 9, 12541 (2019).
Feaver, R. E. et al. Development of an in vitro human liver system for interrogating nonalcoholic steatohepatitis. JCI Insight 1, e90954 (2016).
Cariou, B. et al. Fasting plasma chenodeoxycholic acid and cholic acid concentrations are inversely correlated with insulin sensitivity in adults. Nutr. Metab. 8, 1–6 (2011).
Arab, J. P., Karpen, S. J., Dawson, P. A., Arrese, M. & Trauner, M. Bile acids and nonalcoholic fatty liver disease: molecular insights and therapeutic perspectives. Hepatology 65, 350–362 (2017).
Schott, C. et al. GAS6 and AXL promote insulin resistance by rewiring insulin signaling and increasing insulin receptor trafficking to endosomes. Diabetes 73, 1648–1661 (2024).
Tian, T. et al. Dietary quality and relationships with metabolic dysfunction-associated fatty liver disease (MAFLD) among United States adults, results from NHANES 2017–2018. Nutrients 14, 4505 (2022).
Beygi, M., Ahi, S., Zolghadri, S. & Stanek, A. Management of metabolic-associated fatty liver disease/metabolic dysfunction-associated steatotic liver disease: from medication therapy to nutritional interventions. Nutrients 16, 2220 (2024).
Marino, L., Kim, A., Ni, B. & Celi, F. S. Thyroid hormone action and liver disease, a complex interplay. Hepatology 81, 651–669 (2025).
Li, J., Ayada, I. & Pan, Q. Resmetirom directly inhibits lipid accumulation in human liver-derived organoids. J. Hepatol. 83, e86–e87 (2025).
Kannt, A. et al. Activation of thyroid hormone receptor-β improved disease activity and metabolism independent of body weight in a mouse model of non-alcoholic steatohepatitis and fibrosis. Br. J. Pharmacol. 178, 2412–2423 (2021).
Wang, X., Wang, L., Geng, L., Tanaka, N. & Ye, B. Resmetirom ameliorates NASH-model mice by suppressing STAT3 and NF-κB signaling pathways in an RGS5-dependent manner. Int. J. Mol. Sci. 24, 5843 (2023).
Polyzos, S. A. & Targher, G. Hepatic thyroid hormone receptor-β signalling: Mechanisms and recent advancements in the treatment of metabolic dysfunction-associated steatohepatitis. Diabetes Obes. Metab. 27, 1635–1647 (2025).
Rius-Pérez, S., Torres-Cuevas, I., Millán, I., Ortega, ÁL. & Pérez, S. PGC-1α, inflammation, and oxidative stress: an integrative view in metabolism. Oxid. Med. Cell. Longev. 2020, 1452696 (2020).
Zou, X. et al. Manganese superoxide dismutase (SOD2): is there a center in the universe of mitochondrial redox signaling? J. Bioenerg. Biomembr. 49, 325–333 (2017).
Caro, J. F. et al. Studies on the mechanism of insulin resistance in the liver from humans with noninsulin-dependent diabetes. Insulin action and binding in isolated hepatocytes, insulin receptor structure, and kinase activity. J. Clin. Invest. 78, 249–258 (1986).
Koh, H. E., Cao, C. & Mittendorfer, B. Insulin clearance in obesity and type 2 Diabetes. Int. J. Mol. Sci. https://doi.org/10.3390/ijms23020596 (2022).
Duriez, M. et al. A 3D human liver model of nonalcoholic steatohepatitis. J. Clin. Transl. Hepatol. 8, 359–370 (2020).
Kozyra, M. et al. Human hepatic 3D spheroids as a model for steatosis and insulin resistance. Sci. Rep. 8, 14297 (2018).
Cordero-Herrera, I. et al. AMP-activated protein kinase activation and NADPH oxidase inhibition by inorganic nitrate and nitrite prevent liver steatosis. Proc. Natl. Acad. Sci. USA 116, 217–226 (2019).
Davidson, M. D., Pickrell, J. & Khetani, S. R. Physiologically inspired culture medium prolongs the lifetime and insulin sensitivity of human hepatocytes in micropatterned co-cultures. Toxicology 449, 152662 (2021).
Sarkar, U. et al. Integrated assessment of diclofenac biotransformation, pharmacokinetics, and omics-based toxicity in a three-dimensional human liver-immunocompetent coculture system. Drug Metab. Dispos. 45, 855–866 (2017).
Jang, K. J. et al. Reproducing human and cross-species drug toxicities using a Liver-Chip. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aax5516 (2019).
Otumala, A. E. et al. Opportunities and considerations for studying liver disease with microphysiological systems on a chip. Lab Chip 23, 2877–2898 (2023).
Yang, J. et al. Integrated-gut-liver-on-a-chip platform as an in vitro human model of non-alcoholic fatty liver disease. Commun. Biol. 6, 310 (2023).
Ströbel, S. et al. A 3D primary human cell-based in vitro model of non-alcoholic steatohepatitis for efficacy testing of clinical drug candidates. Sci Rep. 11, 22765 (2021). Nov 23.
Youhanna, S. et al. Chemogenomic screening in a patient-derived 3D fatty liver disease model reveals the CHRM1-TRPM8 axis as a novel module for targeted intervention. Adv. Sci. 12, 2407572 (2025).
Gorden, D. L. et al. Biomarkers of NAFLD progression: a lipidomics approach to an epidemic. J. Lipid Res. 56, 722–736 (2015).
McPherson, S. et al. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J. Hepatol. 62, 1148–1155 (2015).
Liu, H. et al. Dissecting acute drug-induced hepatotoxicity and therapeutic responses of steatotic liver disease using primary mouse liver and blood cells in a liver-on-a-chip model. Adv. Sci. 11, 2403516 (2024).
Lauschke, V. M., Hendriks, D. F., Bell, C. C., Andersson, T. B. & Ingelman-Sundberg, M. Novel 3D culture systems for studies of human liver function and assessments of the hepatotoxicity of drugs and drug candidates. Chem. Res. Toxicol. 29, 1936–1955 (2016).
Lopez-Alcantara, N., Oelkrug, R., Sentis, S. C., Kirchner, H. & Mittag, J. Lack of thyroid hormone receptor beta is not detrimental for non-alcoholic steatohepatitis progression. Iscience 26, 108064 (2023).
Xia, M. et al. Comparison of wild-type and high-risk PNPLA3 variants in a human biomimetic liver microphysiology system for metabolic dysfunction-associated steatotic liver disease precision therapy. Front. Cell Dev. Biol. 12, 1423936 (2024).
Ezhilarasan, D. Thyromimetics and MASLD: unveiling the novel molecules beyond resmetirom. J. Gastroenterol. Hepatol. 40, 367–378 (2025).
Satin, L. S., Butler, P. C., Ha, J. & Sherman, A. S. Pulsatile insulin secretion, impaired glucose tolerance and type 2 diabetes. Mol. Aspects Med. 42, 61–77 (2015).
Laurenti, M. C. et al. Diabetes-associated genetic variation in TCF7L2 alters pulsatile insulin secretion in humans. JCI Insight. https://doi.org/10.1172/jci.insight.136136 (2020).
Bauer, S. et al. Functional coupling of human pancreatic islets and liver spheroids on-a-chip: Towards a novel human ex vivo type 2 diabetes model. Sci Rep. 7, 14620 (2017).
Inzucchi, S. E. et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 38, 140–149 (2015).
Hodson, L., Skeaff, C. M. & Fielding, B. A. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog. Lipid Res. 47, 348–380 (2008).
Groop, L., Widen, E. & Ferrannini, E. Insulin resistance and insulin deficiency in the pathogenesis of type 2 (non-insulin-dependent) diabetes mellitus: errors of metabolism or of methods? Diabetologia 36, 1326–1331 (1993).
Pfaffl, M. W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 29, e45–e45 (2001).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Wu, T. et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innovation 2, 100141 (2021).
Zhang, Y., Parmigiani, G. & Johnson, W. E. ComBat-seq: batch effect adjustment for RNA-seq count data. NAR Genom. Bioinform. 2, lqaa078 (2020).
Weigelin, B., Bakker, G.-J. & Friedl, P. Third harmonic generation microscopy of cells and tissue organization. J. Cell Sci. 129, 245–255 (2016).
Georgakoudi, I. & Quinn, K. P. Label-free optical metabolic imaging in cells and tissues. Ann. Rev. Biomed. Eng. 25, 413–443 (2023).
Hellen, D. J. & Karpen, S. J. LiverQuant: an improved method for quantitative analysis of liver pathology. Bio-Protoc. 13, e477 (2023).
Bankhead, P. et al. QuPath: open source software for digital pathology image analysis. Sci. Rep. 7, 1–7 (2017).
Acknowledgements
Dominick J. Hellen received funding from the NIH (5T32ES007020-50). Erin Tevonian received funding from the NIH (Douglas A. Lauffenburger; R01-DK108056) and a National Science Foundation Graduate Research Fellowship (1745302). This work was funded by NovoNordisk via a sponsored research agreement with the Massachusetts Institute of Technology. The authors thank Jose L. Cadavid, Rachelle P. Braun, Kairav K. Maniar, Saul J. Karpen, and Douglas A. Lauffenburger for their constructive discussions and methodological insights.
Author information
Authors and Affiliations
Contributions
Conceptualization: D.J.H., J.U., & L.G.G.; Data Collection: D.J.H., J.U., E.T., P.S., P.R., F.P., A.M.W., R.O.C., C.A.L., J.J., & D.D.; Data Analysis: D.J.H., J.U., & N.M.; Original Draft: D.J.H.; Data Interpretation & Draft Editing: D.J.H., J.U., E.T., S.Y., D.D., & L.G.G. All authors contributed to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare the following competing interests: L.G.G. is an inventor on patents licensed to CN BioInnovations. J.J. and D.D. are employed/shareholders of NovoNordisk A/S. All other authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Stephen Fowler and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Dr Katharine Irvine and Dr Ophelia Bu. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hellen, D.J., Ungerleider, J., Tevonian, E. et al. A microphysiological model of human MASLD reveals paradoxical response to resmetirom. Commun Biol 9, 148 (2026). https://doi.org/10.1038/s42003-025-09484-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-09484-9