Abstract
Visual perception appears largely stable in time. However, psychophysical studies have revealed that low frequency (0.5 – 7 Hz) oscillatory dynamics can modulate perception and have been linked to various cognitive states and functions. Neither the contribution of waves around 5 Hz (theta or alpha-like) to cortical activity nor their impact during aberrant brain states have been resolved at high spatiotemporal scales. Here, using cortex-wide population voltage imaging in awake mice, we found that bouts of 5-Hz oscillations in the visual cortex are accompanied by similar oscillations in the retrosplenial cortex, occurring both spontaneously and evoked by visual stimulation. Injection of psychotropic 5-HT2AR agonist induced a significant increase in spontaneous 5-Hz oscillations, and also increased the power, occurrence probability and temporal persistence of visually evoked 5-Hz oscillations. This modulation of 5-Hz oscillations in both cortical areas indicates a strengthening of top-down control of perception, supporting an underlying mechanism of perceptual filling and visual hallucinations.

Similar content being viewed by others
Introduction
Oscillatory activity of cortical neuronal populations at various frequencies have been shown to be associated with brain state-dependent cognition and perception1,2,3,4. The involvement of low frequency oscillations in visual processing has been particularly well studied. Cortical theta (4–7 Hz) and alpha-like (3–5 Hz) oscillations are hypothesized to reflect rhythmic sampling5, modulations of attention6 and memory consolidation during periodic replay7,8,9. In mice, alpha-like oscillations strongly correlate with activity in the visual thalamus9. More recently, it has been suggested that familiarity of visual stimuli can frequently trigger and modulate theta oscillations in the primary visual cortex (V1) of mice10,11,12,13,14.
Neuromodulators regulate the dynamics of cortical interactions15 and thereby shape the brain-wide rhythms of activity16,17,18,19,20. For instance, serotonin (5-HT) suppresses theta oscillations in the hippocampus21,22 and modulates the strength of evoked activity in the visual cortex23,24,25,26,27, while hippocampal oscillations have also been shown to actively modulate neuronal activity within V128. Elevated 5-HT signaling can induce deviations from normal visual perception18. A well-known case is the acute effects of serotonergic psychedelics, which can lead to profound changes in perception, cognition, mood29,30, and motor-related behaviors such as hyperactivity, stereotypy24, and head-twitch response in mice31,32,33. In humans, psychedelic actions are often accompanied by visual hallucinations. Visual hallucinations can also appear as a symptom of certain brain disorders34, such as during episodes of psychosis, where visual hallucinations are accompanied by reduced activity in V1 (external inputs) and perception driven more strongly by internal mechanisms35. However, how serotonergic psychedelics act on cortical circuit dynamics is largely unknown.
Currently, 5-HT-dependent changes in rhythmic brain activities36,37,38,39,40,41 have mainly been derived from observations using electrical measurements of brain activity (e.g. using electroencephalography or local field potential recordings). However, such methods are limited by spatial resolution and/or coverage and capture activity blind to cell type. To overcome these limitations, here we used optical imaging of genetically encoded voltage indicators (GEVIs) in pyramidal neurons, which enables direct monitoring of cortex-wide voltage activity selectively from pyramidal cells in awake head-fixed mice42,43, using a cortex-wide preparation and a mesoscopic imaging approach that achieves high spatial coverage. In contrast to calcium imaging approaches, mesoscopic voltage imaging readily resolves subthreshold, suprathreshold and hyperpolarizing population activities.
Capitalizing on this powerful approach, we first observed episodes of 5-Hz oscillatory activity occurring in V1 both spontaneously and evoked by visual simulation. Through the high spatial coverage of our approach, we detected similar and co-occurring oscillatory activity in the retrosplenial cortex (RSC), indicating a coupling between these two regions. Then, we administered a serotonergic psychedelic to investigate how 5-HT receptor activation modulates the 5-Hz oscillatory activity in the cortex. We observed that properties of spontaneous and sensory evoked oscillatory episodes are facilitated by an injection of a 5-HT2AR agonist (either 2,5-Dimethoxy-4-iodoamphetamine (DOI) or TCB2). Collectively, the present study advances our concepts of how serotonergic psychedelics alter perception and consciousness.
Results
5-Hz oscillations occur in primary visual cortex (V1) pyramidal neurons both spontaneously and evoked by a visual stimulus
We performed widefield optical voltage imaging in awake mice. To facilitate stable optical recordings, the mice were head-fixed but otherwise allowed to run freely on a treadmill (Fig. 1a). All mice were first habituated to the setup prior to optical imaging but were not exposed to any specific stimuli or task training.
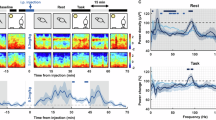
a Experimental configuration for FRET optical imaging in head-fixed awake mice (see “Methods” section). b Widefield image of cortical vasculature in a transcranial cortex-wide window preparation, with cortical regions of the right hemisphere (white outline) estimated using the Allen Mouse Brain Atlas77 (Supplementary Fig. 1). Green outline indicates primary visual cortex (V1). c Examples of single trial V1 population voltage activity (spatially averaged across V1 marked green in b). Episodes of 5-Hz oscillations occurring both spontaneously (yellow highlight) and evoked (yellow highlight with black outline) by a visual stimulus (moving grating at 100% contrast, grey vertical bar marks onset and duration). d Time-resolved 5-Hz power in V1 population voltage activity in individual trials across a single imaging session (same session as that shown in c). Visual stimulus presented at time zero (vertical line) for a duration of 0.2 s.
We measured ongoing and evoked changes in membrane potential across both cortical hemispheres using FRET optical imaging (Fig. 1a, b) of layer II/III or II/III/V pyramidal neurons that express genetically encoded voltage indicators42,43. We interleaved fluorescence measurements with light reflectance measurements at 200 Hz, to obtain voltage and hemodynamic signals quasi-simultaneously. The hemodynamic measurements were subsequently used for offline correction for hemodynamic components of the fluorescence signals (see “Methods” section).
We observed spontaneously occurring bouts of 5-Hz oscillations in V1 (Fig. 1c, highlighted in yellow) during normal waking state while mice were exposed to an isoluminant blank screen. These bouts occurred at a median rate of 0.025 oscillatory episodes per second (mean = 0.032 Hz ± 0.046 standard deviation), consistent with the findings of Einstein et al., 201710. In addition, we also binocularly presented a short-lasting (200 ms duration) moving grating visual stimulus, and detected 5-Hz oscillations that occurred time-locked to the visual stimulus presentation. Trial-by-trial time-resolved 5-Hz power (Fig. 1d) shows the occurrence of both spontaneously occurring and evoked 5-Hz bouts across imaging trials.
5-HT2AR agonist treatment increases the occurrence of spontaneous 5-Hz oscillations in V1, but not their power or duration
Next, to modulate cognitive function, we systemically applied a 5-HT2AR agonist (DOI or TCB2) half-way through an experimental session, generating a set of trials before and after injection (Fig. 2a).
a Experimental timeline for trials before and after 5-HT2A receptor agonist injection. b 5-Hz time resolved spectral analysis of V1 membrane voltage for a single session after injection of DOI. The visual stimulus is presented at time zero (vertical black line). Vertical black line on the colour bar indicates the threshold for 5-Hz power counted as 5-Hz bout (see “Methods” section). c Quantification of spontaneous 5-Hz oscillations occurring in trials before (blue, n = 80) and after (orange, n = 87) injection, based on a subset of experiments with longer recordings prior to visual stimulation (20-s instead of 2 or 3-s in all other experiments) to capture extended periods of spontaneous activity (***p-value = 2.93e-05, Welch’s t-test. N = 3 mice, data points representing each trial while symbol shape indicates individual mouse experiments; Boxplot follows Tukey’s method: median (middle line), 25th, 75th percentile (box), ± 2.7 sigma (whiskers), outliers are plotted as ‘o’).
We first examined the effect of 5-HT2AR agonist treatment on the spontaneously occurring 5-Hz oscillations. Time-resolved spectral analysis of V1 population voltage activity indicated the presence of both spontaneous and evoked 5-Hz oscillations in imaging sessions after agonist treatment (Fig. 2b; in this example using DOI). Compared to trials before injection, 5-HT2AR activation caused a significant increase in the rate of spontaneous 5-Hz oscillations (p < 0.001, Welch’s t test, N = 78 (before), 87 (after) trials from 3 mice, Fig. 2c), with a median rate of 0.05 episodes/s. We observed no statistically significant increase in the power and duration (p-value = 0.885 and 0.654, respectively, Welch’s t test, N = 55 (before) and 152 (after) oscillations) of these spontaneously occurring episodes of 5-Hz oscillations.
5-HT2AR agonist treatment increases the power and duration of visually evoked 5-Hz oscillations in V1
Next, we characterised the dynamics of the visually evoked oscillations. We therefore first separated all trials into two categories - those with evoked oscillations vs those without (Fig. 3ai, ii show mean traces of trials for each category, respectively; Fig. 3b depicts the corresponding wavelet analysis for trials before injection and with oscillations). Compared to the spontaneously occurring oscillations, visually evoked oscillations appeared similar in shape and power (p-value = 0.885, Welch’s t test), and showed a statistically non-significant tendency of longer duration (median duration for spontaneous bouts: 540 ms and evoked bouts: 700 ms; p-value = 0.0579, Welch’s t test). The across-trial average of evoked oscillations (Fig. 3ai) shows that each cycle of oscillations involved a phase of strong depolarization followed by hyperpolarization of the entire V1 pyramidal cell population. Figure 3aii depicts the mean of trials that showed no evoked bouts and only a single depolarisation-inhibition primary sensory responses were detected.
a Trial averaged V1 population membrane voltage activity (shading represents SEM) before (i-ii) and after (iii-iv) treatment of the 5-HT2AR agonist (TCB2 in this example). b Time-resolved spectral analysis of the evoked oscillations shown in a-i. Time zero indicates onset of visual stimulus. c Same as b after drug treatment (a-iii), showing an increase in both duration and amplitude of 5-Hz oscillations. d Duration of oscillations before and after treatment comparing the mean across all experiments (indicated inside, *p-value = 0.0144, paired t test) and individual trials (indicated outside, ***p-value = 1.35e-06, Welch’s t-test, nbefore = 109 and nafter = 189). Data based upon N = 7 experiments in 5 mice, symbol shape indicates individual mouse experiments. e Same as d, for mean oscillatory power across all experiments (indicated inside, *p-value = 0.016, paired t test) and individual trials (indicated outside, ***p-value = 5.91e-04, Welch’s t-test).
After injection of the 5-HT2AR agonist, we observed an increase in the duration and power of the evoked 5-Hz oscillations in V1 (Fig. 3aiii and Fig. 3c). These effects were consistent across all 7 experiments (Fig. 3d and Fig. 3e) and effective for either agonist used. TCB2 showed a significant increase in the power of the evoked oscillations (Supplementary Fig. 2a, **p-value = 1.09e-03, Welch’s t-test) and a non-significant tendency to increase in duration (Supplementary Fig. 2b, p-value = 0.0796, Welch’s t-test). The changes were not explained by other factors such as trial length or the number of imaging sessions carried out in the same mouse (Supplementary Fig. 3). A comparison between spontaneous and evoked oscillations after injection revealed a significant increase in the duration of the visually evoked 5-Hz oscillations (median duration for spontaneous bouts: 480 ms and evoked bouts: 1070 ms; p-value = 1.26e-9, Welch’s t test).
Evoked 5-Hz oscillations co-occur in V1 and the retrosplenial cortex (RSC)
Our widefield imaging approach covers the entire dorsal brain surface (Fig. 1b), so we next investigated whether visually evoked oscillations were present in other cortical regions. We only detected episodes of 5-Hz oscillations in the retrosplenial cortex (Fig. 4a, outlined in pink; RSC), which co-occurred with oscillations in V1 (Fig. 4b and Supplementary video 1; Supplementary Fig. 4). We additionally examined several other cortical areas including another primary sensory modality (barrel field) and motor areas (primary and secondary motor areas), and we did not detect any notable occurrence of 5-Hz oscillations (Supplementary Fig. 4c, d). The correlation of V1 and RSC 5-Hz oscillations suggests a strong interactive function between these cortical areas13. In support of such specific relationship, we observed correlation in the duration of the oscillations between these two regions, which persisted after 5-HT2AR agonist application (Fig. 4c–e).
a A series of single trial cortex-wide voltage maps of visual stimulus-evoked oscillation of population membrane voltage in both V1 and RSC after TCB2 injection (Supplementary video 1 as an additional example for data shown in Fig. 3a, b). b Changes in membrane voltage averaged across V1 and RSC (regions marked in a) following visual stimulus presentation (black bar). c Quantification of the lag time (calculated by cross correlation) between signals (based on a subset with high power) in V1 and RSC across single trials before (blue, n = 28) and after injection (orange, n = 59). Cross-correlation plots are shown in Supplementary Fig. 5b–d. d Linear regression and correlation between the duration of evoked 5-Hz oscillations in V1 and RSC, both before (left; R2 = 0.95 and **p-value = 0.00189, n = 64) and after injection of 5-HT2A agonist (right; R2 = 0.695 and **p-value = 0.00217, n = 158). e Same as d for oscillation power before (left; R2 = 0.71 and n.s, p-value = 0.718) and after injection of 5-HT2A agonist (right, R2 = 0.692 and ***p-value = 6.28e-04). (All R-values were calculated using bisquare weights and p-values depict the Pearson’s correlation.).
Notably, the oscillatory power between the two regions correlated only after injection of a 5-HT2AR agonist (Fig. 4e). This indicates that the 5-HT2AR agonist strengthened the interactions between V1 and RSC. To explore whether this interaction was direct or whether it resulted from a common source, we quantified the lag between the two signals. The cross-correlation of co-occurring V1 and RSC oscillations revealed a consistent mean lag for both before and after injection of the agonist, of -18.6 ms and -17.7 ms respectively (Fig. 4c; a similar result was found using FFT analysis to calculate the phase difference, Supplementary Fig. 5).
5-HT2AR agonist treatment increased the occurrence probability of 5-Hz oscillations
We found that 5-HT2AR activation enhanced the occurrence probability of both spontaneous and stimulus-evoked 5-Hz oscillations in V1 and RSC in a similar manner (Fig. 5a, b). As in V1, we also see a significant increase in the rate of spontaneous oscillations occurring in the RSC after agonist treatment (Fig. 5b; before: mean = 0.085 Hz +/- 0.093, after: mean = 0.132 Hz +/- 0.086). TCB2 produced a more pronounced increase in probability for evoked oscillations compared to DOI (Supplementary Fig. 2c).
a Wavelet analysis detected evoked 5-Hz oscillations in V1 (**p-value = 0.002, paired T test) and RSC (**p-value = 0.00275, paired T test) in an increased percentage of trials after 5-HT2AR agonist treatment (N = 7 experiments in 5 mice). b Increase in frequency of spontaneous 5-Hz oscillations in RSC by 5-HT2A agonist. (***p-value = 1.26e-04, Welch’s t-test. N = 3 mice, individual data points represent each trial, data point shape represents the individual mouse. nbefore = 75 and nafter = 84). c Distribution of spontaneous (left) and evoked (right) oscillations occurring only in V1, RSC or co-occurring in both regions (see Methods).
Further, we found that evoked 5-Hz oscillations predominately either co-occurred in both regions or in V1 alone, but rarely in RSC alone (Fig. 5c). This observation is maintained after agonist treatment. The rare occasion of an evoked oscillation only in RSC was also combined with power and duration on the border of detection (Supplementary Fig. 6a, b), suggesting spurious RSC-only positives and further lending weight to the interpretation of a V1-driven effect upon visual stimulus.
Overall, the increase of visually evoked oscillations in V1 and RSC suggests an enhanced recruitment of underlying processes such as memory retrieval44, stimulus interpretation, and prediction.
Discussion
Cortical oscillations can modulate perception and have been linked to various cognitive states and functions1,2,3,4. While psychedelics are also generally known to alter perception and states of consciousness, whether these serotonergic hallucinogens modulate oscillatory activities in the cortex remains largely unknown. Here, we measured neuronal population voltage signals of pyramidal neurons across the entire mouse dorsal cortex at high spatiotemporal resolutions and coverage using epifluorescence widefield imaging in awake head-fixed mice. We detected episodes of 5-Hz oscillations in the primary visual cortex (V1), occurring both spontaneously and evoked by presenting a visual stimulus. We observed experimental evidence for how serotonergic psychedelics modulate oscillatory events, revealing a possible correlate of the altered brain-state. Expanding beyond V1, we also observed 5-Hz oscillatory activities in the retrosplenial cortex (RSC). We further obtained experimental measures of how inter-areal cortical activities between V1 and RSC are altered by psychedelics, possibly through increasing interregional connectivity strengths.
Our focus on oscillations in the theta range was driven by its implicated role in many cognitive functions, including cortical information processing45, working memory8, memory encoding and retrieval10,11,12,13. The existence of 5-Hz oscillations in V1 has previously been reported and their occurrence has been strongly correlated to the degree of familiarity to a given visual stimulus10,11,12. Specifically, others have reported that V1 5-Hz oscillations displayed an increase in duration, power, and probability of occurrence when mice perceived a visual stimulus repeatedly presented over consecutive days11,12. Our study does not provide additional evidence for 5-Hz oscillations in V1 relating to perceptual familiarity, as we do not have a measure of familiarity beyond the trial number. Also, our data did not show an increased occurrence of 5-Hz oscillations with increasing trial number.
Our experimental paradigm involved the passive viewing of a visual stimulus rather than behavioral task conditions where a visual stimulus may have an attached valence that could influence its processing. Hence, the observed oscillations could likewise reflect attentional disengagement or distraction from repetitive stimuli46,47. While we cannot rule out this possibility, under the assumption that an animal is likely to be more attentive and engaging at the beginning of an imaging session than at the end, we did not observe any changes of 5-Hz oscillations across trials of a given imaging session. Therefore, making this possibility unlikely, nevertheless, future studies may empirically test this possibility.
Psychedelics are primarily known to influence visual processing through activation of 5-HT2A receptors29,48. We observed 5-HT2A receptor agonists (DOI and TCB-2) facilitate significant modulation on bouts of 5-Hz oscillations in V1 and the RSC. First, we considered these observations in the context of psychedelics’ capacity to alter the brain state and the reported possibility of pupil dilation induced by the acute effect of psychedelic administration49. There is accumulating evidence which supports that cortical state changes are intricately interwoven with interoceptive signals50,51 and visual responses52. Moreover, recent studies suggest theta-range rhythms may emerge from sensorimotor sampling frequency53,54. However, though psychedelics may induce pupil dilation49, recent studies in humans suggest that N,N-dimethyltryptamine (DMT, a 5-HT2AR serotonergic psychedelic55 similar to those used in this study) can modulate V1 population receptive field sizes devoid of motor-related signals from eye or head movements56. In fact, in mice, previous observations by others have shown a lack of changes in both pupil diameter and in general behavioural state following administration of DOI24 (as used in the current study). In line with this, we did not observe any correlation in our data between properties of the 5-Hz oscillation and trial number after drug injection. This led us to conclude that the observed psychedelic-induced changes on 5-Hz oscillations were not due to changes in pupillometry or in the animals’ behavioural state. Subsequently, considering the effects induced by the systemic injection itself, we found that properties of V1 5-Hz oscillations were altered by both types of 5-HT2A agonists used, although significantly modulated more by TCB2 than by DOI (Supplementary Fig. 2). This drug dependency makes a pure stress-induced artifact originating from the injection procedure unlikely. Taken together, this led us to consider the alterations in cortical 5-Hz oscillations as a physiological effect of the psychedelic agents.
Capitalizing on the spatial coverage of our approach, we expanded our investigation to beyond the visual cortex and across both cortical hemispheres. We found that visually-evoked oscillations in V1 frequently co-occurred with similar bouts in the RSC, a major hub connecting hippocampus with the cerebral cortex. Bouts of 5-Hz oscillations occur in both V1 and in the RSC with a delay of ~18 ms. Given a distance of 1.5–3.2 mm (Supplementary Fig. 1e) between V1 and RSC, this translates to a propagation speed of 0.083–0. 12 m/s, a value in the range of traveling waves in the cortices of the mouse (0.08–0.15 ms-157,58), cat (0.1–0.3 ms-159,60,61), monkey (0.25–1.35 ms-162,63) and human (0.3–0.5 ms-164). Also, the range of calculated propagation speed aligns with those found for unmyelinated (long-range) axonal conduction speeds within superficial cortical layers65, which suggests that V1 occasionally triggers oscillations in RSC upon visual input. This is further supported by the fact that visually-evoked oscillations in the RSC were rarely found alone.
We observed no notable co-occurrence of 5-Hz oscillations between V1 and other cortical regions, other than the RSC, including cortical areas for other sensory modalities (e.g., the barrel field cortex) or motor areas (primary and secondary motor cortices). The spatially restricted existence of these 5-Hz oscillations strongly suggests their specific involvement in the processing of visual information, extending importance to the RSC which is increasingly implicated in integrating internal representations with visual input66.
Oscillatory activity around 5 Hz has been observed in subcortical structures, including the thalamus and hippocampus, which in some instances appear to co-occur with oscillations in V1. Seminal work of McCormick and colleagues have tied V1 oscillatory activity in the 3–5 Hz range to the thalamocortical loop, specifically, when animals were engaged in active sensing associated with arousal and movements9. More recently, however, under passive viewing conditions paired with extracellular recordings have shown that V1 oscillations do not originate in the thalamus13. That is, inactivation of the hippocampus did not affect V1 oscillations, indicating that these oscillations emerge independent of each other13 and contingent on behavioural context. The independent emergence of these oscillations may contribute to the seeming discrepancy between the facilitatory effects on cortical 5-Hz oscillations by serotonergic psychedelics (i.e., 5-HT2A agonists) seen here vs the depression in the power of similar oscillations in the hippocampus induced by serotonin21,22 acting on different 5-HTR simultaneously13.
While the cellular and circuit mechanisms of cortical 5-Hz oscillations remain to be elucidated, 5-Hz oscillations could in principle emerge from a strengthening of local circuitries within V1 (see ref. 5 for a recent review), in particular, when exposed to a specific familiar stimulus13. Interestingly, familiarity-evoked theta oscillations were longer and less stimulus specific in SERT knockout (KO) mice14. On a mechanistic level, activation of the 5-HT2A receptor by specific agonists affects pyramidal cell dendritic excitability and interneuron interactions and could for example modulate slow oscillations via somatostatin-expressing neurons36.
We found a strong facilitatory effect of 5-HT2A agonists on spontaneous and visually-evoked 5-Hz oscillations within both V1 and RSC. Following changes to the cortical state through systemic activation of such a single receptor type, the detected enhancement in V1–RSC interaction may be interpreted as a “cascade-effect” of bouts in V1 on bouts in the RSC67,68. This supports the concept that brain states under the influence of hallucinogenic agents increase internally driven and/or decrease externally driven activity, resulting in visual perceptions being under increased top-down control69,70,71 where they are overly guided by internally stored or acutely generated associations.
Materials and methods
Key Resources
Animals
We have complied with all relevant ethical regulations for animal use. All procedures were conducted in accordance with the guidelines of with the European Union Community Council guidelines and approved by the German Animal Care and Use Committee under the Deutsches Tierschutzgesetz (Az.: 84-02.04.2019.A477, 84-02.04.2019.A483) and the NIH guidelines. The experiments were carried out on 5 (4 male, 1 female) mice, aged 4-5 months, using strains Ai87XAi93 (N = 2) and CamK-tTA;chiVSFP (N = 3), expressing voltage-indicators in layer II/III or in layer II/III plus layer V pyramidal neurons, respectively. After preparatory surgery of the cortical window, mice were housed in standard vivarium conditions (temperature maintained at 20–22 °C, 30–70% humidity) and kept in 12 h light/dark cycle with food and water ad libitum.
Surgical procedure
Surgery was carried out as previously described23,74. In brief, animals were anesthetized with isoflurane (3% induction and 1.5–2% for maintenance via a nose mask) and received a subcutaneous bolus of isotonic 0.9% NaCl solution mixed with Buprenorphine (0.1 µg/g bodyweight) and Atropine (0.05 µg/g bodyweight). A heating pad (set to 37 °C) was placed below the animal during surgery to maintain body temperature. Before sagittal incision along the midline, 2% Lidocaine was applied to provide additional local anaesthesia. The skull was slowly thinned using a microdrill until the surface blood vessels became clearly visible.
The thinned skull was then covered with transparent dental cement and a glass cover for protection and to reduce light scattering. Finally, a custom head holder was attached to the skull in order to provide a clear and easily accessible imaging window for chronic experiments. Mice were allowed to recover for at least one week before beginning habituation to the imaging setup.
Voltage imaging
Simultaneous recording of Fluorescence at 515–569 nm and >594 nm as well as reflectance recording at 515–569 nm and 620–660 nm was performed using interlaced imaging with different light sources switching every 5 ms. The fluorescence signals (FRET donor and acceptor) carry a combination of the voltage and hemodynamic signals while the reflectance signals carry only the hemodynamic signals (blood volume and blood oxygenation). The fluorescence signals were then modelled as a linear combination of the reflectance (hemodynamic) signals and simple regressors. Using this model, it was possible to remove the hemodynamic component from the fluorescence signal, extracting the voltage signal.
Visual stimulus
A monitor (60 Hz, mean luminance 55 cd/m2, LG 24BK55WV-B) was placed 30 cm away from the stimulated eye, covering ~56 × 70 degrees of the visual field. A semipermeable zero power contact lens was used to prevent the eyes from drying out. The visual stimuli consisted of a moving grating with a 90 degree orientation, 100% contrast and a duration of either 0.2 or 0.5 s. This was followed by a uniform grey screen (blank condition) presented for 15 s during the interstimulus intervals. Each experiment consisted of 30–110 trials without and then with treatment of the 5-HT agonist.
5-HT2AR agonist treatment
TCB-2 (Tocris Bioscience, 10 mg/kg bodyweight in saline) or DOI (Sigma, 10 mg/kg bodyweight in saline) was administered subcutaneously24 and after waiting ~20 minutes (time till effect) recordings continued with the same experimental protocol as before. The dosage was chosen to meet literature standards of intraperitoneal injections24,31,32,68,75,76. As mice were head fixed, injections were performed without removing the animal from the setting to stabilise the transition between conditions.
Data analysis
Voltage imaging data was preprocessed and functionally aligned to the Allen Mouse Brain Atlas projected upon the imaging plane (Fig. 1b, Supplementary Fig. 1), as previously described73.
All data analysis was performed in MATLAB (R2024b Update 2), unless stated otherwise all functions refer to MATLAB built-in functions. For the analysis of the membrane potential (Vm), 5-Hz oscillations were detected by wavelet analysis (using the cwt function). For each mouse the right hemisphere was analyzed. The 5-Hz oscillations were identified by those traces which had a 5-Hz magnitude (calculated using the mean of the 4.8–5.2 Hz band) 4 standard deviations above the baseline (pre-stimulus) for a duration of at least 0.2 s (one 5-Hz oscillation). Detection was also verified by manual detection (using a custom Python 3.12.7 script GUI) and Fast Fourier transform (using the fft function. Evoked oscillations were classified as co-occurring if oscillations time-locked to the visual stimulus were identified in both V1 and RSC, further verified by calculating the time lag between the signals. The time lag was calculated by cross correlation (using the xcorr function) and then compared to FFT lag time. For FFT, the signals were transformed into the frequency domain (using the fft function) and then calculated the phase (using the angle function) of the closest bin to 5 Hz. The subsequent phase difference between the signals was then transformed into time lag by dividing the angular frequency (\(\omega =\,2\pi f=2\pi 5\)).
Spontaneous oscillations were identified using the same thresholding method as evoked (without the time-locked restriction), creating a binarized vector of on-going activity and subsequently testing co-occurrence if V1 and RSC oscillations overlapped by at least one image frame.
Statistics and reproducibility
Statistical analysis was performed using MATLAB (R2024b Update 2), unless stated otherwise all functions refer to MATLAB built-in functions. All statistical analysis was performed using a two-sided independent T-test (using the ttest2 function). Boxplots used follow Tukey’s method: median (middle line), 25th, 75th percentile (box), ± 2.7 sigma (whiskers), outliers are plotted as an outlined ‘o’. For comparison of uneven sample sizes, we used Welch’s T-test (using the ttest2 function with “Vartype” settings to “unequal”). While experiment-wise means before and after injection of the agonist were compared using a paired T-test (using the ttest function). We calculated the linear regression (using the fit function with settings “poly1” and “bisquare” robustness) between V1 and RSC, while p-values represented the Pearson correlation (using the corr function).
The standard identification of statistical significance was used: *p < 0.05, **p < 0.01, ***p < 0.001.
Data availability
The data supporting the findings of this study and custom MATLAB code used for analysis are available within the main text, the Supplementary Information file, the Supplementary Data, or from the corresponding authors upon request.
References
Lozano-Soldevilla, D. & VanRullen, R. The Hidden Spatial Dimension of Alpha: 10-Hz Perceptual Echoes Propagate as Periodic Traveling Waves in the Human Brain. Cell Rep 26, 374–380.e4 (2019).
Luo, C., Brüers, S., Berry, I., VanRullen, R. & Reddy, L. Tentative fMRI signatures of perceptual echoes in early visual cortex. NeuroImage 237, 118053 (2021).
Schwenk, J. C. B., VanRullen, R. & Bremmer, F. Dynamics of Visual Perceptual Echoes Following Short-Term Visual Deprivation. Cereb. Cortex Commun. 1, tgaa012 (2020).
McCormick, D. A., McGinley, M. J. & Salkoff, D. B. Brain state dependent activity in the cortex and thalamus. Curr. Opin. Neurobiol. 31, 133–140 (2015).
Kienitz, R., Schmid, M. C. & Dugué, L. Rhythmic sampling revisited: Experimental paradigms and neural mechanisms. Eur. J. Neurosci. 55, 3010–3024 (2022).
Fiebelkorn, I. C., Pinsk, M. A. & Kastner, S. The mediodorsal pulvinar coordinates the macaque fronto-parietal network during rhythmic spatial attention. Nat. Commun. 10, 215 (2019).
Sederberg, P. B., Kahana, M. J., Howard, M. W., Donner, E. J. & Madsen, J. R. Theta and Gamma Oscillations during Encoding Predict Subsequent Recall. J. Neurosci. 23, 10809–10814 (2003).
Fuentemilla, L., Penny, W. D., Cashdollar, N., Bunzeck, N. & Düzel, E. Theta-Coupled Periodic Replay in Working Memory. Curr. Biol. 20, 606–612 (2010).
Nestvogel, D. B. & McCormick, D. A. Visual thalamocortical mechanisms of waking state-dependent activity and alpha oscillations. Neuron 110, 120–138.e4 (2022).
Einstein, M. C., Polack, P.-O., Tran, D. T. & Golshani, P. Visually Evoked 3–5 Hz Membrane Potential Oscillations Reduce the Responsiveness of Visual Cortex Neurons in Awake Behaving Mice. J. Neurosci. 37, 5084–5098 (2017).
Gao, M., Lim, S. & Chubykin, A. A. Visual Familiarity Induced 5-Hz Oscillations and Improved Orientation and Direction Selectivities in V1. J. Neurosci. 41, 2656–2667 (2021).
Kissinger, S. T., Pak, A., Tang, Y., Masmanidis, S. C. & Chubykin, A. A. Oscillatory Encoding of Visual Stimulus Familiarity. J. Neurosci. 38, 6223–6240 (2018).
Zimmerman, M. P. et al. Origin of visual experience-dependent theta oscillations. Curr. Biol. 35, 87–99.e6 (2025).
Pak, A. & Chubykin, A. A. Cortical Tuning is Impaired After Perceptual Experience in Primary Visual Cortex of Serotonin Transporter-Deficient Mice. Cereb. Cortex Commun. 1, tgaa066 (2020).
Pollak Dorocic, I. et al. A Whole-Brain Atlas of Inputs to Serotonergic Neurons of the Dorsal and Median Raphe Nuclei. Neuron 83, 663–678 (2014).
Atasoy, S. et al. Connectome-harmonic decomposition of human brain activity reveals dynamical repertoire re-organization under LSD. Sci. Rep. 7, 17661 (2017).
Deco, G. et al. Whole-Brain Multimodal Neuroimaging Model Using Serotonin Receptor Maps Explains Non-linear Functional Effects of LSD. Curr. Biol. 28, 3065–3074.e6 (2018).
Jancke, D., Herlitze, S., Kringelbach, M. L. & Deco, G. Bridging the gap between single receptor type activity and whole-brain dynamics. FEBS J 289, 2067–2084 (2022).
Kringelbach, M. L. et al. Dynamic coupling of whole-brain neuronal and neurotransmitter systems. Proc. Natl. Acad. Sci. 117, 9566–9576 (2020).
Kringelbach, M. L. & Deco, G. Brain States and Transitions: Insights from Computational Neuroscience. Cell Rep 32, 108128 (2020).
Ly, S., Pishdari, B., Lok, L. L., Hajos, M. & Kocsis, B. Activation of 5-HT6 Receptors Modulates Sleep–Wake Activity and Hippocampal Theta Oscillation. ACS Chem. Neurosci. 4, 191–199 (2013).
Sörman, E., Wang, D., Hajos, M. & Kocsis, B. Control of hippocampal theta rhythm by serotonin: Role of 5-HT2c receptors. Neuropharmacology 61, 489–494 (2011).
Azimi, Z. et al. Separable gain control of ongoing and evoked activity in the visual cortex by serotonergic input. eLife 9, e53552 (2020).
Michaiel, A. M., Parker, P. R. L. & Niell, C. M. A Hallucinogenic Serotonin-2A Receptor Agonist Reduces Visual Response Gain and Alters Temporal Dynamics in Mouse V1. Cell Rep 26, 3475–3483.e4 (2019).
Seillier, L. et al. Serotonin Decreases the Gain of Visual Responses in Awake Macaque V1. J. Neurosci. 37, 11390–11405 (2017).
Watakabe, A. et al. Enriched Expression of Serotonin 1B and 2A Receptor Genes in Macaque Visual Cortex and their Bidirectional Modulatory Effects on Neuronal Responses. Cereb. Cortex 19, 1915–1928 (2009).
Waterhouse, B. D., Ausim Azizi, S., Burne, R. A. & Woodward, D. J. Modulation of rat cortical area 17 neuronal responses to moving visual stimuli during norepinephrine and serotonin microiontophoresis. Brain Res 514, 276–292 (1990).
Fournier, J. et al. Mouse Visual Cortex Is Modulated by Distance Traveled and by Theta Oscillations. Curr. Biol. 30, 3811–3817.e6 (2020).
Kwan, A. C., Olson, D. E., Preller, K. H. & Roth, B. L. The neural basis of psychedelic action. Nat. Neurosci. 25, 1407–1419 (2022).
Sharp, T. & Ippolito, A. Neuropsychopharmacology of hallucinogenic and non-hallucinogenic 5-HT2A receptor agonists. Br. J. Pharmacol. https://doi.org/10.1111/bph.70050 (2025).
González-Maeso, J. et al. Hallucinogens Recruit Specific Cortical 5-HT2A Receptor-Mediated Signaling Pathways to Affect Behavior. Neuron 53, 439–452 (2007).
Garcia, E. E., Smith, R. L. & Sanders-Bush, E. Role of Gq protein in behavioral effects of the hallucinogenic drug 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane. Neuropharmacology 52, 1671–1677 (2007).
López-Giménez, J. F. & González-Maeso, J. Hallucinogens and Serotonin 5-HT2A Receptor-Mediated Signaling Pathways. in Behavioral Neurobiology of Psychedelic Drugs (eds. Halberstadt, A. L., Vollenweider, F. X. & Nichols, D. E.) 45–73 (Springer, Berlin, Heidelberg, https://doi.org/10.1007/7854_2017_4782018).
Bernasconi, F. et al. Theta oscillations and minor hallucinations in Parkinson’s disease reveal decrease in frontal lobe functions and later cognitive decline. Nat. Ment. Health 1, 477–488 (2023).
van Ommen, M. M., van Laar, T., Renken, R., Cornelissen, F. W. & Bruggeman, R. Visual Hallucinations in Psychosis: The Curious Absence of the Primary Visual Cortex. Schizophr. Bull. 49, S68–S81 (2023).
de Filippo, R. et al. Somatostatin interneurons activated by 5-HT2A receptor suppress slow oscillations in medial entorhinal cortex. eLife 10, e66960 (2021).
Grandjean, J. et al. A brain-wide functional map of the serotonergic responses to acute stress and fluoxetine. Nat. Commun. 10, 350 (2019).
Roth, G. I., Walton, P. L. & Yamamoto, W. S. Area postrema: Abrupt EEG synchronization following close intra-arterial perfusion with serotonin. Brain Res 23, 223–233 (1970).
Salomon, R. M. & Cowan, R. L. Oscillatory serotonin function in depression. Synapse 67, 801–820 (2013).
Vanderwolf, C. H. The electrocorticogram in relation to physiology and behavior: a new analysis. Electroencephalogr. Clin. Neurophysiol. 82, 165–175 (1992).
Vanderwolf, C. H. & Baker, G. B. Evidence that serotonin mediates non-cholinergic neocortical low voltage fast activity, non-cholinergic hippocampal rhythmical slow activity and contributes to intelligent behavior. Brain Res 374, 342–356 (1986).
Akemann, W. et al. Imaging neural circuit dynamics with a voltage-sensitive fluorescent protein. J. Neurophysiol. 108, 2323–2337 (2012).
Knöpfel, T. & Song, C. Optical voltage imaging in neurons: moving from technology development to practical tool. Nat. Rev. Neurosci. 20, 719–727 (2019).
Ortega, B. A., Weisstaub, N. V. & Katche, C. Retrosplenial cortex 5-HT2A receptors critically contribute to recognition memory processing. Front. Cell. Neurosci. 19, 1711777 (2025).
Kienitz, R. et al. Theta, but Not Gamma Oscillations in Area V4 Depend on Input from Primary Visual Cortex. Curr. Biol. 31, 635–642.e3 (2021).
Spyropoulos, G., Bosman, C. A. & Fries, P. A theta rhythm in macaque visual cortex and its attentional modulation. Proc. Natl. Acad. Sci. 115, E5614–E5623 (2018).
Han, H.-B., Lee, K. E. & Choi, J. H. Functional Dissociation of θ Oscillations in the Frontal and Visual Cortices and Their Long-Range Network during Sustained Attention. eNeuro 6, 6 (2019).
Schmitz, G. P. et al. Psychedelic compounds directly excite 5-HT2A layer V medial prefrontal cortex neurons through 5-HT2A Gq activation. Transl. Psychiatry 15, 381 (2025).
Al-Imam, A. Could Hallucinogens Induce Permanent Pupillary Changes in (Ab)users? A Case Report from New Zealand. Case Rep. Neurol. Med. 2017, 2503762 (2017).
Bagur, S. et al. Breathing-driven prefrontal oscillations regulate maintenance of conditioned-fear evoked freezing independently of initiation. Nat. Commun. 12, 2605 (2021).
Karalis, N. & Sirota, A. Breathing coordinates cortico-hippocampal dynamics in mice during offline states. Nat. Commun. 13, 467 (2022).
Leupin, V. & Britz, J. Interoceptive signals shape the earliest markers and neural pathway to awareness at the visual threshold. Proc. Natl. Acad. Sci. 121, e2311953121 (2024).
Joshi, A. et al. Dynamic synchronization between hippocampal representations and stepping. Nature 617, 125–131 (2023).
Forli, A., Fan, W., Qi, K. K. & Yartsev, M. M. Replay and representation dynamics in the hippocampus of freely flying bats. Nature 1–7 https://doi.org/10.1038/s41586-025-09341-z.(2025).
Alamia, A., Timmermann, C., Nutt, D. J., VanRullen, R. & Carhart-Harris, R. L. DMT alters cortical travelling waves. eLife 9, e59784 (2020).
Pais, M. L. et al. Rapid effects of tryptamine psychedelics on perceptual distortions and early visual cortical population receptive fields. NeuroImage 297, 120718 (2024).
Fehérvári, T. D., Okazaki, Y., Sawai, H. & Yagi, T. In Vivo Voltage-Sensitive Dye Study of Lateral Spreading of Cortical Activity in Mouse Primary Visual Cortex Induced by a Current Impulse. PLOS ONE 10, e0133853 (2015).
Liang, Y. et al. Complexity of cortical wave patterns of the wake mouse cortex. Nat. Commun. 14, 1434 (2023).
Bringuier, V., Chavane, F., Glaeser, L. & Frégnac, Y. Horizontal propagation of visual activity in the synaptic integration field of area 17 neurons. Science 283, 695–699 (1999).
Jancke, D., Chavane, F., Naaman, S. & Grinvald, A. Imaging cortical correlates of illusion in early visual cortex. Nature 428, 423–426 (2004).
Benucci, A., Frazor, R. A. & Carandini, M. Standing Waves and Traveling Waves Distinguish Two Circuits in Visual Cortex. Neuron 55, 103–117 (2007).
Muller, L., Reynaud, A., Chavane, F. & Destexhe, A. The stimulus-evoked population response in visual cortex of awake monkey is a propagating wave. Nat. Commun. 5, 3675 (2014).
Grinvald, A., Lieke, E. E., Frostig, R. D. & Hildesheim, R. Cortical point-spread function and long-range lateral interactions revealed by real-time optical imaging of macaque monkey primary visual cortex. J. Neurosci. 14, 2545–2568 (1994).
Fakche, C. & Dugué, L. Perceptual Cycles Travel Across Retinotopic Space. J. Cogn. Neurosci. 36, 200–216 (2024).
Girard, P., Hupé, J. M. & Bullier, J. Feedforward and feedback connections between areas V1 and V2 of the monkey have similar rapid conduction velocities. J. Neurophysiol. 85, 1328–1331 (2001).
Alexander, A. S., Place, R., Starrett, M. J., Chrastil, E. R. & Nitz, D. A. Rethinking retrosplenial cortex: Perspectives and predictions. Neuron 111, 150–175 (2023).
Spens, E. & Burgess, N. A generative model of memory construction and consolidation. Nat. Hum. Behav. 8, 526–543 (2024).
Freo, U., Holloway, H. W., Kalogeras, K., Rapoport, S. I. & Soncrant, T. T. Adrenalectomy or metyrapone-pretreatment abolishes cerebral metabolic responses to the serotonin agonist 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) in the hippocampus. Brain Res 586, 256–264 (1992).
Carhart-Harris, R. L. & Friston, K. J. REBUS and the Anarchic Brain: Toward a Unified Model of the Brain Action of Psychedelicss. Pharmacol. Rev. 71, 316–344 (2019).
Deco, G. et al. Awakening: Predicting external stimulation to force transitions between different brain states. Proc. Natl. Acad. Sci. 116, 18088–18097 (2019).
Carhart-Harris, R. L. et al. The entropic brain: a theory of conscious states informed by neuroimaging research with psychedelic drugs. Front. Hum. Neurosci. 8, 20 (2014).
Mishina, Y., Mutoh, H., Song, C. & Knöpfel, T. Exploration of genetically encoded voltage indicators based on a chimeric voltage sensing domain. Front. Mol. Neurosci. 7, 78 (2014).
Song, C., Piscopo, D. M., Niell, C. M. & Knöpfel, T. Cortical signatures of wakeful somatosensory processing. Sci. Rep. 8, 11977 (2018).
Barzan, R. et al. Gain control of sensory input across polysynaptic circuitries in mouse visual cortex by a single G protein-coupled receptor type (5-HT2A). Nat. Commun. 15, 8078 (2024).
Eickelbeck, D. et al. CaMello-XR enables visualization and optogenetic control of Gq/11 signals and receptor trafficking in GPCR-specific domains. Commun. Biol. 2, 1–16 (2019).
Aulakh, C. S., Hill, J. L., Yoney, H. T. & Murphy, D. L. Evidence for involvement of 5-HT1C and 5-HT2 receptors in the food intake suppressant effects of 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI). Psychopharmacology (Berl.) 109, 444–448 (1992).
Wang, Q. et al. The Allen Mouse Brain Common Coordinate Framework: A 3D Reference Atlas. Cell 181, 936–953.e20 (2020).
Acknowledgements
We thank Stefan Dobers and Henning Knoop and the RUB mechanical shop for technical support. This work was supported by Deutsche Forschungsgemeinschaft (DFG) grants: Project ID 122679504 - SFB 874, D.J.; JA 945/5-1, D.J.; “MoNN&Di”, Project number 492434978 - GRK 2862/1, Subproject 10, D.J.; BMBF, ERA-Net Neuron “Horizon 2020”, 01EW2104B, D.J.; US National Institutes of Health BRAIN Initiative Grant (5U01NS099573), T.K.; Lee Kuan Yew Postdoctoral Fellowship administered by Nanyang Technological University Singapore (022506-00001), C.S.; Open Fund Young Individual Research Grant (MOH-001720) administered by the Singapore Ministry of Health’s National Medical Research Council, C.S.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
These authors contributed equally: Zohre Azimi, Robert Staadt and Callum M. White. These authors jointly supervised this work: Dirk Jancke and Thomas Knöpfel. Conceptualization: T.K. and D.J. Data curation: C.M.W. and D.J. Formal analysis: C.M.W. and D.J. Data acquisition: Z.A. and R.S. Data visualization: C.M.W. and D.J. Software: C.M.W., T.K. and R.S. Supervision: T.K. and D.J. Funding acquisition and resources: C.S. T.K. and D.J. Writing—original draft: C.M.W., C.S., T.K. and D.J.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Hio-Been Han and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Jasmine Pan. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
White, C.M., Azimi, Z., Staadt, R. et al. Psychedelic 5-HT2A agonist increases spontaneous and evoked 5-Hz oscillations in visual and retrosplenial cortex. Commun Biol 9, 216 (2026). https://doi.org/10.1038/s42003-025-09492-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-09492-9