Abstract
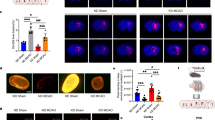
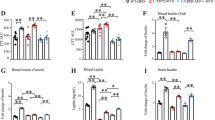
Cognitive impairment is a known complication of metabolic dysfunction-associated steatotic liver disease (MASLD), and β-hydroxybutyrate (BHB), a ketone body providing alternative brain energy under metabolic stress, may exert neuroprotective effects. This study explored BHB’s role in MASLD-related cognitive impairment and its underlying mechanisms using a 20-week high-fat diet (HFD)-induced MASLD mouse model with cognitive dysfunction, comparing 3-hydroxy-3-methylglutaryl-CoA synthase 2 (Hmgcs2) knockout (KO), wild-type (WT), and exogenous BHB-supplemented mice. Key outcomes included hippocampal pathology, neuroinflammation, insulin resistance, amyloid-β (Aβ) deposition, tau phosphorylation, glucose/lipid homeostasis, and cognitive function. Results showed Hmgcs2 KO mice exhibited worse metabolic dysregulation (elevated triglycerides, cholesterol, hepatic lipid accumulation, impaired glucose tolerance, increased insulin, reduced BHB), cognitive decline (confirmed by Y-maze and novel object recognition tests), hippocampal p-Tau/Aβ aggregation, neuroinflammation (elevated iNOS, COX-2, IL-1β), and impaired IRS/PI3K/AKT/GSK3β signaling, whereas exogenous BHB supplementation alleviated these phenotypes. Collectively, reduced Hmgcs2 expression and BHB levels critically contribute to MASLD-induced cognitive impairment via cerebral insulin signaling disruption and neuroinflammation, highlighting BHB’s therapeutic potential.
Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information. Uncropped and unedited blot images are shown in Supplementary Figs. 5–11. Source data for the main figures are provided in Supplementary Data File 1. Additional data are available from the corresponding author upon request.
Code availability
This paper does not report any new generated original code.
References
Le, M. H. et al. Forecasted 2040 global prevalence of nonalcoholic fatty liver disease using hierarchical Bayesian approach. Clin. Mol. Hepatol. 28, 841–850 (2022).
Wong, V. W., Ekstedt, M., Wong, G. L. & Hagstrom, H. Changing epidemiology, global trends and implications for outcomes of NAFLD. J. Hepatol. 79, 842–852 (2023).
Targher, G., Tilg, H. & Byrne, C. D. Non-alcoholic fatty liver disease: a multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol. Hepatol. 6, 578–588 (2021).
Colognesi, M., Gabbia, D. & De Martin, S. Depression and cognitive impairment-extrahepatic manifestations of NAFLD and NASH. Biomedicines 8, E229 (2020).
Yu, Q. et al. Association between metabolic dysfunction-associated fatty liver disease and cognitive impairment. J. Clin. Transl. Hepatol. 10, 1034–1041 (2022).
Cushman, M. et al. Nonalcoholic fatty liver disease and cognitive impairment: a prospective cohort study. PLoS One 18, e0282633 (2023).
Doward, L. C. et al. Development of a patient-reported outcome measure for non-alcoholic steatohepatitis (NASH-CHECK): results of a qualitative study. Patient 14, 533–543 (2021).
Kennedy-Martin, T., Bae, J. P., Paczkowski, R. & Freeman, E. Health-related quality of life burden of nonalcoholic steatohepatitis: a robust pragmatic literature review. J. Patient Rep. Outcomes 2, 28 (2017).
Dede, A. J., Wixted, J. T., Hopkins, R. O. & Squire, L. R. Hippocampal damage impairs recognition memory broadly, affecting both parameters in two prominent models of memory. Proc. Natl. Acad. Sci. USA 110, 6577–6582 (2013).
Gold, S. M. et al. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia 50, 711–719 (2007).
Cheon, S. Y. & Song, J. Novel insights into non-alcoholic fatty liver disease and dementia: insulin resistance, hyperammonemia, gut dysbiosis, vascular impairment, and inflammation. Cell Biosci. 12, 99 (2022).
Kaya, E. & Yılmaz, Y. Association of metabolic dysfunction-associated fatty liver disease with cognitive impairment and all-cause dementia: a comprehensive review. Turk. J. Gastroenterol. 35, 76–82 (2024).
Kim, J. H. et al. Protective effects of krill oil on high fat diet-induced cognitive impairment by regulation of oxidative stress. Free Radic. Res. 55, 799–809 (2021).
Xu, J. et al. Melatonin alleviates cognition impairment by antagonizing brain insulin resistance in aged rats fed a high-fat diet. J. Pineal Res. 67, e12584 (2019).
Xu, J. et al. Docosahexaenoic acid enhances hippocampal insulin sensitivity to promote cognitive function of aged rats on a high-fat diet. J. Adv. Res. 45, 31–42 (2023).
Newman, J. C. & Verdin, E. Beta-hydroxybutyrate: a signaling metabolite. Annu. Rev. Nutr. 37, 51–76 (2017).
Croci, I. et al. Whole-body substrate metabolism is associated with disease severity in patients with non-alcoholic fatty liver disease. Gut 62, 1625–1633 (2013).
Nasser, S. et al. Ketogenic diet administration to mice after a high-fat-diet regimen promotes weight loss, glycemic normalization and induces adaptations of ketogenic pathways in liver and kidney. Mol. Metab. 65, 101578 (2022).
Ohashi, T. et al. Conophylline inhibits high fat diet-induced non-alcoholic fatty liver disease in mice. PLoS One 14, e0210068 (2019).
Asif, S. et al. Hmgcs2-mediated ketogenesis modulates high-fat diet-induced hepatosteatosis. Mol. Metab. 61, 101494 (2022).
Yan, A. et al. Beta-hydroxybutyrate upregulates FGF21 expression through inhibition of histone deacetylases in hepatocytes. Open Life Sci. 17, 856–864 (2022).
Hu, L. T. et al. HMGCS2-induced autophagic degradation of tau involves ketone body and ANKRD24. J. Alzheimers Dis. 91, 407–426 (2023).
Wu, Y. et al. BHBA treatment improves cognitive function by targeting pleiotropic mechanisms in transgenic mouse model of Alzheimer’s disease. FASEB J. 34, 1412–1429 (2020).
Iwanaga, T. & Kishimoto, A. Cellular distributions of monocarboxylate transporters: a review. Biomed. Res. 36, 279–301 (2015).
Al Haj Ahmad, R. M., Ababneh, N. A. & Al-Domi, H. A. Brain insulin resistance as a mechanistic mediator links peripheral metabolic disorders with declining cognition. Diab. Metab. Syndr. 16, 102468 (2022).
Zheng, M. et al. Research progress on the association of insulin resistance with type 2 diabetes mellitus and Alzheimer’s disease. Metab. Brain Dis. 40, 35 (2024).
Gao, Z. et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 58, 1509–1517 (2009).
Bae, H. R. et al. Beta-hydroxybutyrate suppresses inflammasome formation by ameliorating endoplasmic reticulum stress via AMPK activation. Oncotarget 7, 66444–66454 (2016).
Duda, P. et al. Targeting GSK3 signaling as a potential therapy of neurodegenerative diseases and aging. Expert Opin. Ther. Targets 22, 833–848 (2018).
Zhang, L. et al. Colorimetric and surface-enhanced Raman scattering dual-mode magnetic immunosensor for ultrasensitive detection of blood phosphorylated tau in Alzheimer’s disease. Biosens. Bioelectron. 222, 114935 (2023).
Tang, Z. et al. Treponema denticola Induces Alzheimer-like tau hyperphosphorylation by activating hippocampal neuroinflammation in mice. J. Dent. Res 101, 992–1001 (2022).
Chen, X. Q. et al. γ-Secretase modulator BPN15606 reduced Aβ42 and Aβ40 and countered alzheimer-related pathologies in a mouse model of down syndrome. Ann. Neurol. 96, 390–404 (2024).
De Strooper, B. & Karran, E. New precision medicine avenues to the prevention of Alzheimer’s disease from insights into the structure and function of γ-secretases. Embo J. 43, 887–903 (2024).
Chen, S. Y., Koch, M., Chávez-Gutiérrez, L. & Zacharias, M. How modulator binding at the amyloid β-γ-secretase interface enhances substrate binding and attenuates membrane distortion. J. Med Chem. 66, 16772–16782 (2023).
Kjærgaard, K. et al. Cognitive dysfunction in early experimental metabolic dysfunction-associated steatotic liver disease is associated with systemic inflammation and neuroinflammation. JHEP Rep. 6, 100992 (2024).
Zuo, W. et al. CA3 pyramidal neuron activation promotes cognitive resilience to inflammation-induced cognitive inflexibility. CNS Neurosci. Ther. 31, e70271 (2025).
Liu, L. & Chan, C. The role of inflammasome in Alzheimer’s disease. Ageing Res. Rev. 15, 6–15 (2014).
Bomfim, T. R. et al. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease- associated Aβ oligomers. J. Clin. Invest. 122, 1339–1353 (2012).
Gage, M. C. & Thippeswamy, T. Inhibitors of Src family kinases, inducible nitric oxide synthase, and NADPH oxidase as potential CNS drug targets for neurological diseases. CNS Drugs 35, 1–20 (2021).
Picca, A. et al. Age-associated glia remodeling and mitochondrial dysfunction in neurodegeneration: antioxidant supplementation as a possible intervention. Nutrients 14, 2406 (2022).
Kajitani, N. et al. Prefrontal cortex infusion of beta-hydroxybutyrate, an endogenous NLRP3 inflammasome inhibitor, produces antidepressant-like effects in a rodent model of depression. Neuropsychopharmacol. Rep. 40, 157–165 (2020).
Bai, Y. P. et al. β-Hydroxybutyrate suppresses M1 macrophage polarization through β-hydroxybutyrylation of the STAT1 protein. Cell Death Dis. 15, 874 (2024).
Cotter, D. G. et al. Ketogenesis prevents diet-induced fatty liver injury and hyperglycemia. J. Clin. Invest. 124, 5175–5190 (2014).
d’Avignon, D. A. et al. Hepatic ketogenic insufficiency reprograms hepatic glycogen metabolism and the lipidome. JCI Insight 3, e99762 (2018).
Bragoszewski, P., Habior, A., Walewska-Zielecka, B. & Ostrowski, J. Expression of genes encoding mitochondrial proteins can distinguish nonalcoholic steatosis from steatohepatitis. Acta Biochim. Pol. 54, 341–348 (2007).
Yang, X. et al. Beta-hydroxybutyrate alleviates learning and memory impairment through the SIRT1 pathway in D-galactose-injured mice. Front. Pharmacol. 12, 751028 (2021).
Hu, E. et al. Beta-hydroxybutyrate promotes the expression of bdnf in hippocampal neurons under adequate glucose supply. Neuroscience 386, 315–325 (2018).
Youm, Y. H. et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 21, 263–269 (2015).
Wang, R. et al. β-Hydroxybutyrate alleviates brain aging through the MTA1 pathway in D-galactose injured mice. Eur. J. Pharmacol. 983, 176959 (2024).
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant number 82474318), the Jiangsu Administration of Traditional Chinese Medicine (grant number QN202411), the special fund for training outstanding young doctors in the second session of Jiangsu Province Hospital of Chinese Medicine (grant number 2024QB002), the project funded by Jiangsu Province Hospital of Chinese Medicine (grant number KKZX01), and the Hefei Municipal Health Science and Technology Project (grant number Hwk2025zd017).
Author information
Authors and Affiliations
Contributions
This study was conceptualized and designed by L.N., Q.X., and X.Z. L.N. performed the majority of the experiments, acquired and analyzed the data, and drafted the manuscript. J.S. and W.X. contributed some immunofluorescence and immunohistochemistry experiments, and analyzed some data. X.Y., G.W., and Y.W. assisted in the performance of animal experiments and reproduction of HMGCS2 KO mice. T.J., Y.C., and H.C. contributed to the performance of H&E stainings and immunoblotting. Q.X. revised the manuscript and provided critical feedback. X.Z. supervised the study, had full access to the data, and is responsible for the integrity of the data and accuracy of the analysis. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Norifumi Kawada and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Ibrahim Javed and Benjamin Bessieres.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nie, L., Sun, J., Xu, W. et al. Hepatic HMGCS2-derived β-hydroxybutyrate attenuates hippocampal insulin resistance and neuroinflammation to promote MASLD-induced cognitive function. Commun Biol (2026). https://doi.org/10.1038/s42003-026-09513-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-026-09513-1