Abstract
Homologation is an important organic transformation which extends the carbon chain of a parent molecule, and many procedures have been established. However, although carbonylation reactions are now well developed as valuable methods for the synthesis of carbonyl-containing compounds, studies of carbonylative homologation are limited. Here we report a nickel-catalysed carbonylative homologation of aryl iodides. With molybdenum hexacarbonyl as the solid carbon monoxide source and silane as the deoxygenation reagent, benzylic units can be effectively produced. Various (hetero)arenes can be successfully benzylated and give the corresponding products in moderate to excellent yields.
Similar content being viewed by others
Introduction
Homologation is an important organic transformation for extending the carbon chain of parent molecules, which increases their complexity and also offers the possibility for further transformations. The importance of homologation reactions has been recognized by several named reactions as well, such as Kiliani-Fischer synthesis, Arndt-Eistert reaction, Kowalski ester homologation, Seyferth-Gilbert homologation, and more1,2,3,4,5,6.
On the other hand, transition metal-catalyzed carbonylation reactions have experienced tremendous progress since the 1940s7,8,9,10,11,12,13,14. Nowadays, carbonylation reactions have emerged as one of the most valuable methods for the synthesis of carbonyl-containing compounds, and many carbonylative procedures have even been industrialized. In particular, the aminohomologation of alkenes has been successfully explored, yielding a domino process consisting of three steps: (1) transition metal-catalyzed hydroformylation of alkenes into the corresponding aldehydes; (2) the aldehyde condensation with amine to give imine/enamine; and (3) transition metal-catalyzed hydrogenation of the imine/enamine intermediate to give the final aliphatic amines15. Conversely, carbonylative homologation of aryl halides is still challenging and rarely reported. In the latter case, benzylic units can be produced and used for the benzylation of various substrates.
Concerning the catalysts studied in carbonylation chemistry, expensive palladium and rhodium catalysts together with even more costly phosphine ligands are frequently studied. Nickel, as an abundant and non-expensive metal, has been seldom studied in carbonylative transformation16,17,18,19,20. One of the main reasons for this situation is the formation of Ni(CO)4, which is a highly toxic liquid (boiling point = 43 °C). Additionally, Ni(CO)4 has limited reactivity in oxidative addition with substrates, due to the full coordination of CO on the metal center. Based on our previous experience in developing new CO-gas-free carbonylation reactions21,22,23,24,25,26,27, we assume the usage of Mo(CO)6 as a solid CO source could be an ideal option for exploring nickel catalyst in carbonylation chemistry 28,29.
Here we describe a nickel-catalyzed carbonylative homologation of aryl iodides with Mo(CO)6 as the solid carbon monoxide source and silane as the deoxygenation reagent. Various (hetero)arenes can be successfully benzylated and can produce the corresponding products in moderate to excellent yields.
Results
Optimization
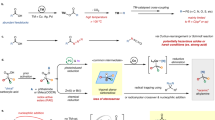
In order to study this hypothesis and also the known importance of indole derivatives30,31,32, we choose iodobenzene 1a and N-methyl indole 2a as the model substrates to establish the catalytic system (Fig. 1). To our delight, when the model substrates were treated with Mo(CO)6 under the catalysis of Ni(OTf)2 and 4,4′-di-tert-butyl-2,2′-bipyridine (dtbbpy) in toluene without external reductant, the desired methylene product 3-benzyl-1-methyl-1H-indole 3aa was obtained successfully, albeit in a low yield (Table 1, entry 1). Diaryl ketone 4aa was detected as the major product. Polar solvents such as DMF (dimethylformamide) and MeCN (acetonitrile) were not suitable for this reaction and no carbonylation products could be obtained (for details see Supplementary Table 1). Then we tested a series of reductants in this reaction. Zinc powder and formic acid were inefficient in this catalytic system and no desired product was obtained (Table 1, entry 2; for details see Supplementary Table 2). Silanes turned out to be a suitable hydrogen source, and 38% yield of 3aa was obtained when Ph2SiH2 was used as the reductant (Table 1, entry 7). Increasing the amount of iodobenzene 1a improved the total conversion and resulted in higher yield of 65% (Table 1, entry 8). Other catalyst precursors such as NiCl2, NiI2, and Ni(acac)2 were all effective in catalyzing this reaction and produced 3aa in comparable yields (for details see Supplementary Table 3). A slightly higher yield of 67% was obtained when NiBr2 was used as the catalyst (Table 1, entry 9). Subsequently, the ligand effect was investigated with a series of nitrogen- and phosphine-ligands. To our delight, when 2,2′-bipyridine (bpy) was used as the ligand, the desired product 3aa was obtained in a high yield of 88% (Table 1, entry 10). Other ligands including 1,10-phenanthroline and PPh3 were also effective for this transformation, but with lower yields (Table 1, entries 11 and 12, for details see Supplementary Table 4). Screening of the amount of CO and reductant revealed that 0.5 equivalent of Mo(CO)6 and 2 equivalents of Ph2SiH2 were the optimal concentrations (see Supplementary Tables 5, 7, 8). Further screening of non-silane-based hydrogen sources such as isopropanol and ammonia borane failed to improve the yields (Table 1, entries 13 and 14, for details see Supplementary Table 6). Considering that Ni(OTf)2 leads to complete conversion of the substrate, we tested it again under our new system and found that Ni(OTf)2/bpy catalyst system provides better yield. The desired product 3aa was isolated in an excellent yield of 91% (Table 1, entry 15). Notably, benzaldehyde could be detected as well during the optimization process.
Model system used for reaction optimization. Conditions used for optimization of the catalyst, ligand, and deoxygenation reagent can be found in Table 1
Substrate scope
With the optimized reaction conditions in hand (Table 1, entry 15), we examined the substrate scope of this reaction with respect to aryl iodides, and these results are summarized in Fig. 2. Both electron-donating (3aa-3la) and electron-withdrawing substituents (3ma-3pa) on iodobenzene were well-tolerated and produced the methylene products in moderate to excellent yields. The electronic property of the substituent played an important role in the reaction yields. Generally, substrates with electron-donating groups gave higher yields than those with electron-withdrawing groups. Steric effect on aryl iodides also affected the reaction yields. For example, ortho-methyl iodobenzene gave a lower yield than meta- and para-methyl iodobenzene (3ba vs 3ca, 3da). Fluoro- and chloro-substituents were compatible in this reaction and produced the desired products in 58–86% yields (3ma, 3na, and 3oa). In addition to iodobenzene, β- and α-iodo-naphthalene were also subjected to the optimized reaction conditions, and the corresponding products were conveniently obtained in 61% and 59% yields, respectively (3qa and 3ra). Moreover, heteroaryl iodides such as 3-iodothiophene were tolerated as well, and the corresponding product 3sa was delivered in 63% yield. However, no desired transformation could be detected when bromobenzene was applied as the substrate.
Then, we turned our attention to test the generality of the indole coupling partner of the arylmethylation reaction. As highlighted in Fig. 3, firstly, we examined a series of different N-substituted indoles. N-alkyl, N-benzyl, and N-phenyl-indoles were all suitable substrates and produced the corresponding products in good yields (3ib–3af). Interestingly, when non-protected indole was applied in this reaction, the C3-arylmethylated product 3ag was obtained in 27% yield. A N-benzoyl-indoline was observed as a by-product (Fig. 4). However, when electron-withdrawing groups (such as Boc, Piv and Ts) were attached to the nitrogen of indole, no desired products were obtained. This might have resulted from the decreased nucleophilicity of the indoles due to the strong electron-withdrawing effect. Subsequently, a range of substitutes N-methylindoles were subjected to the optimized conditions. All reactions proceeded smoothly and provided the corresponding products in moderate to excellent yields (3ah–3al). Interestingly, the steric effect on indole did not affect the yield. For example, 2-methylindole substrate reacted smoothly and afforded the desired product 3am in 87% yield. It should be noted that C3-alkyl substituted indole was also tolerated in this reaction, where the arylmethylation took place at the C2-position of the indole. For example, 1,3-dimethyl-1H-indole 2n reacted with different substituted iodobenzenes and provided the corresponding C2-benzylated products in acceptable yields (3an, 3fn, and 3mn).
Besides indoles, other (hetero)arenes are proper substrates for this transformation as well (Fig. 5). Five-membered heterocycles, including thiophene, furan, pyrrole, benzofuran, and benzothiophene, are all suitable reaction partners, and moderate to good yields of the corresponding products can be obtained (Fig. 5, 3ie–3il). Ferrocene can be selectively benzylated as well; the corresponding product 3im was isolated in 42% yield. Simple arenes were tested under standard conditions; it was found that moderate yields of the desired products can be obtained (Fig. 5, 3in–3ir). Interestingly, in the case of 1,3-dimethoxybenzene, 16% of double benzylated product (3ip′) can be obtained as well.
Control experiments
To examine the reaction pathway of this nickel-catalyzed arylmethylation reaction, a series of control experiments were conducted. First, when benzylic alcohol 5 was treated with N-methyl indole 2a under the standard condition, no methylated product 3aa could be observed (Fig. 6a). However, when benzaldehyde 6 was used in this reaction, the desired product 3aa was obtained in 32% yield (Fig. 6b). During the optimization of the reaction conditions, a diaryl ketone 4aa was detected as a by-product. So we examined the direct transformation of the ketone to the methylene group. When 4aa was subjected to the standard condition, the desired product 3aa was obtained in 20% yield (Fig. 6c). Besides, 3aa was obtained in 50% yield when alcohol 7 was treated under the standard condition (Fig. 6d). These observations indicate that benzaldehyde 6, diaryl ketone 4, and diaryl methanol 7 can serve as intermediates in this catalytic transformation. In addition, no desired product 3aa was obtained when diaryl ketone 4aa was treated under standard condition without nickel catalyst (Fig. 6e). Moreover, decreased yield was obtained from alcohol 7 in the absence of nickel catalyst (Fig. 6f). These results indicate that nickel catalyst plays a crucial role in the deoxygenation step.
Control experiments. The experiments support the intermediacy of a benzylic carbonyl, which undergoes two-step reduction, and the necessity of the nickel catalyst for reduction. a Benzyl alcohol does not react under standard conditions. b Benzaldehyde undergoes homologation under standard conditions. c Diaryl ketone, observed as a reaction by-product, is reduced under standard conditions. d Diaryl alcohol is reduced under standard conditions. Nickel is required for the reduction of e diaryl ketone and f diaryl alcohol
Discussion
Based on these observations and previous literatures, a plausible mechanism is proposed and shown in Fig. 7. Initially, the oxidative addition of aryl iodide to the in situ-generated Ni(O) forms an aryl nickel complex 8, which was converted to the acyl nickel intermediate 9 after coordination and insertion of CO. Then, acyl-nickel complex reacted with indole to produce the ketone 4; the catalytic active Ni(O) was regenerated in this step. Ketone 4 was reduced by silane to give the alcohol or its silyl ether 7. It should be mentioned that the formation of 7 via the intermediacy of aldehyde 6 cannot be excluded. Subsequently, hydrogenation of 7 by silane provided the final methylene product 3. It is also important to state that molybdenum hexacarbonyl is not only a solid carbon monoxide source but also participates in the deoxygenation process as a catalyst.
Proposed mechanism. Based on control experiments, (Fig. 6) a plausible mechanism involving oxidative addition to in situ generated Ni(O) followed by insertion of CO reaction to form a ketone or aldehyde, and finally Ni-mediated hydrogenation by silane
In summary, we have developed an interesting nickel-catalyzed carbonylative homologation of aryl iodides. With Mo(CO)6 as the solid CO source and silane as the deoxygenation reagent, benzylic units can be effectively produced. Various (hetero)arenes can be successfully benzylated and can produce the corresponding products in moderate to excellent yields.
Methods
Synthesis and characterization
See Supplementary Methods for general information about chemicals and analytical methods, synthetic procedures and characterization for substrates and products. For 1H and 13C NMR data see Supplementary Figures 1-46.
Optimization
See Supplementary Table 1 (Optimization of solvent), Supplementary Table 2 (Optimization of additives), Supplementary Table 3 (Optimization of catalysts), Supplementary Table 4 (Optimization of ligands), Supplementary Table 5 (Optimization of CO source), Supplementary Table 6 (Optimization of reductants), Supplementary Table 7 (Optimization of the amount of Mo(CO)6), and Supplementary Table 8 (Optimization of the amount of Ph2SiH2).
General procedure
Ni(OTf)2 (8.9 mg, 5.0 mol%), bpy (3.9 mg, 5 mol%), and Mo(CO)6 (132.0 mg, 1.0 equiv.) were transferred into a 15-mL tube filled with nitrogen. N-methyl indole (63 μL, 0.5 mmol), iodobenzene (143 μL, 1.5 mmol) and toluene (2 mL) were added to the reaction tube. Then the tube was sealed and the mixture was stirred at 120 °C for 24 h. After cooling to room temperature, the reaction tube was adding sodium hydroxide methanol solution (0.2 mol/L, 10 mL) and stirred for 1 h. The reaction was quenched with H2O and diluted with EtOAc. The phases were separated and the aqueous phase was washed with EtOAc (30 mL). Combined organic phases was dried over Na2SO4, filtered, and concentrated under vacuum. The crude product was purified by column chromatography on silica gel to afford the corresponding product.
Data availability
The data sets generated and analyzed during the current study are included in the Supplementary Information file and also available from the corresponding authors on request.
References
Kowalski, C. J., Haque, M. S. & Fields, K. W. Ester homologation via.alpha.-bromo.alpha.-keto dianion rearrangement. J. Am. Chem. Soc. 107, 1429–1430 (1985).
Huggett, C., Arnold, R. T. & Taylor, T. I. The mechanism of the Arndt-Eistert reaction. J. Am. Chem. Soc. 64, 3043–3044 (1942).
Katritzky, A. R., Zhang, S., Hussein, A. H. M., Fang, Y. & Steel, P. J. One-carbon homologation of carboxylic acids via BtCH2TMS: a safe alternative to the Arndt-Eistert reaction. J. Org. Chem. 66, 5606–5612 (2001).
Roth, G., Liepold, B., Müller, S. & Bestmann, H. J. Further improvements of the synthesis of alkynes from aldehydes. Synthesis 59–62 (2004).
Sui, X., Ding, L. & Gu, Z. The palladium/norbornene-catalyzed ortho-silylmethylation reaction: a practical protocol for ortho-functionalized one-carbon homologation of aryl iodides. Chem. Commun. 52, 13999–14002 (2016).
Ding, L., Sui, X. & Gu, Z. Enantioselective synthesis of biaryl atropisomers via Pd/norbornene-catalyzed three-component cross-couplings. ACS Catal. 8, 5630–5635 (2018).
Wu, X.-F., Neumann, H. & Beller, M. Palladium-catalyzed carbonylative coupling reactions between Ar–X and carbon nucleophiles. Chem. Soc. Rev. 40, 4986–5009 (2011).
Gabriele, B., Mancuso, R. & Salerno, G. Oxidative carbonylation as a powerful tool for the direct synthesis of carbonylated heterocycles. Eur. J. Org. Chem. 6825–6839 (2012).
Peng, J.-B., Qi, X. & Wu, X.-F. Recent achievements in carbonylation reactions: a personal account. Synlett 28, 175–194 (2017).
Wu, X.-F. Palladium-catalyzed carbonylative transformation of aryl chlorides and aryl tosylates. RSC Adv. 6, 83831–83837 (2016).
Peng, J.-B., Qi, X. & Wu, X.-F. Visible light-induced carbonylation reactions with organic dyes as the photosensitizers. ChemSusChem 9, 2279–2283 (2016).
Peng, J.-B. & Wu, X.-F. Ligand- and solvent-controlled regio- and chemodivergent carbonylative reactions. Angew. Chem., Int. Ed. 57, 1152–1160 (2018).
Peng, J.-B., Wu, F.-P. & Wu, X.-F. First-row transition-metal-catalyzed carbonylative transformations of carbon electrophiles. Chem. Rev. 2018, https://doi.org/10.1021/acs.chemrev.8b00068.
Bai, Y., Davis, D. C. & Dai, M. Natural product synthesis via palladium-catalyzed carbonylation. J. Org. Chem. 82, 2319–2328 (2017).
Kalck, P. & Urrutigoïty, M. Tandem hydroaminomethylation reaction to synthesize amines from alkenes. Chem. Rev. 118, 3833–3861 (2018).
Andersen, T. L., Donslund, A. S., Neumann, K. T. & Skrydstrup, T. Carbonylative coupling of alkyl zinc reagents with benzyl bromides catalyzed by a nickel/NN2 pincer ligand complex. Angew. Chem., Int. Ed. 57, 800–804 (2018).
Peng, J.-B. et al. Nickel-catalyzed molybdenum-promoted carbonylative synthesis of benzophenones. J. Org. Chem. 83, 6788–6792 (2018).
Peng, J.-B. et al. Nickel-catalyzed carbonylative synthesis of functionalized alkyl iodides. iScience 8, 175–182 (2018).
Cassar, L. & Foá, M. Nickel-catalyzed carbonylation of aromatic halides at atmospheric pressure of carbon monoxide. J. Organo. Chem. 51, 381–393 (1973).
Yu, H., Gao, B., Hu, B. & Huang, H. Charge-transfer complex promoted C-N bond activation for Ni-catalyzed carbonylation. Org. Lett. 19, 3520–3523 (2017).
Peng, J.-B., Wu, F.-P., Li, C.-L., Qi, X. & Wu, X.-F. A convenient and efficient palladium-catalyzed carbonylative sonogashira transformation with formic acid as the CO source. Eur. J. Org. Chem. 1434-1437 (2017).
Wu, F.-P., Peng, J.-B., Meng, L.-S., Qi, X. & Wu, X.-F. Palladium-catalyzed ligand-controlled selective synthesis of aldehydes and acids from aryl halides and formic acid. ChemCatChem 9, 3121–3124 (2017).
Wu, F.-P., Peng, J.-B., Qi, X. & Wu, X.-F. Palladium-catalyzed carbonylative transformation of organic halides with formic acid as the coupling partner and CO source: synthesis of carboxylic acids. J. Org. Chem. 82, 9710–9714 (2017).
Wu, F.-P., Peng, J.-B., Fu, L.-Y., Qi, X. & Wu, X.-F. Direct palladium-catalyzed carbonylative transformation of allylic alcohols and related derivatives. Org. Lett. 19, 5474–5477 (2017).
Wu, F.-P., Peng, J.-B., Qi, X. & Wu, X.-F. Palladium-catalyzed carbonylative sonogashira coupling of aryl diazonium salts with formic acid as the CO source: the effect of 1,3-butadiene. Catal. Sci. Technol. 7, 4924–4928 (2017).
Wu, F.-P., Peng, J.-B., Qi, X. & Wu, X.-F. Palladium-catalyzed carbonylative homocoupling of aryl iodides for the synthesis of symmetrical diaryl ketones with formic acid. ChemCatChem 10, 173–177 (2018).
Peng, J.-B. et al. Direct synthesis of benzylic amines by palladium-catalyzed carbonylative aminohomologation of aryl halides. Commun. Chem. 1, 29 (2018).
Odell, L. R., Russo, F. & Larhed, M. Molybdenum hexacarbonyl-mediated CO gas-free carbonylative reactions. Synlett 23, 685–698 (2012).
Åkerbladh, L., Odell, L. R. & Larhed, M. Palladium-catalyzed molybdenum hexacarbonyl-mediated gas-free carbonylative reactions. Synlett https://doi.org/10.1055/s-0037-1610294 (2018).
Kawasaki, T. & Higuchi, K. Simple indole alkaloids and those with a nonrearranged monoterpenoid unit. Nat. Prod. Rep. 22, 761–793 (2005).
O’Connor, S. E. & Maresh, J. J. Chemistry and biology of monoterpene indole alkaloid biosynthesis. Nat. Prod. Rep. 23, 532–547 (2006).
Kochanowska-Karamyan, A. J. & Hamann, M. T. Marine indole alkaloids: potential new drug leads for the control of depression and anxiety. Chem. Rev. 110, 4489–4497 (2010).
Acknowledgements
The authors thank the financial supports from NSFC (21472174, 21772177) and Zhejiang Natural Science Fund for Distinguished Young Scholars (LR16B020002). The publication of this article was funded by the Open Access Fund of the Leibniz Association.
Author information
Authors and Affiliations
Contributions
X.-F.W. and J.-B.P. conceived and supervised the project. F.-P.W. performed experiments and prepared the supporting information. X.Q. and J.Y. participated in the discussions. X.-F.W. and J.-B.P. wrote and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Peng, JB., Wu, FP., Qi, X. et al. Nickel-catalysed carbonylative homologation of aryl iodides. Commun Chem 1, 87 (2018). https://doi.org/10.1038/s42004-018-0091-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42004-018-0091-2