Abstract
Zwitterionic polysaccharides (ZPSs) present on the surface of a common gut commensal Bacteroides fragilis are endowed with unique immunological properties as they can directly bind to T-cells in the absence of protein conjugation. ZPSs are therefore considered to be potential antigens for the development of totally carbohydrate-based vaccines. Herein, we disclose the first total synthesis of a highly branched phosphorylated zwitterionic capsular polysaccharide repeating unit of Bacteroides fragilis. The hexasaccharide repeating unit bearing six different monosaccharides comprises three 1,2-cis-glycosidic linkages, a challenging 1,2-trans linkage in D-QuipNAc-β-(1→4)-D-Gal motif, and a 2-aminoethyl phosphonate appendage. The synthesis of target ZPS was accomplished utilizing an expeditious, highly stereoselective and convergent (1 + 2 + 2 + 1) one-pot glycosylation strategy. The striking features include efficient synthesis of rare deoxy amino sugars D- and L-quinovosamine, stereoselective installation of three 1,2-cis glycosidic linkages, glycosylation of D-quinovosamine donor with a sterically crowded, poorly reactive 4-OH galactose moiety, as well as late stage phosphorylation.

Similar content being viewed by others
Introduction
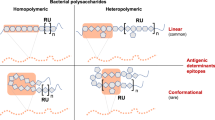
Bacterial cell-surface glycoconjugates often contain a variety of rare deoxy amino sugars which are not present on the host cells1,2. This structural difference between the host and bacterial cells can be utilized for the development of glycoconjugate vaccines3,4,5. In comparison to the human glycome, which is made up of only ten monosaccharides, bacterial glycome comprises hundreds of structurally unique monosaccharides and saccharide modifications6,7,8. The bacterial cell-surface glycoconjugates being the first point of contact with the host cells during the infection process, such bacteria-specific glycans are looked upon as potential vaccine candidates. However, carbohydrates on their own are known to be poorly immunogenic in nature as they elicit T-cell independent immune response, which is not sufficient to provide protection to high risk groups such as infants and children below 2 years of age3. This problem is overcome by conjugating them with carrier proteins to elicit T-cell dependent response that imparts sufficient levels of immunological memory for efficacious protection9,10. The flip side of this is, since the carrier proteins are self-immunogenic in nature, they can also promote the growth of peptide-specific antibodies, which can reduce immunity to the specified carbohydrate antigens11. Nevertheless, this does not hold true for a unique class of polysaccharides, isolated from the capsules of commensal bacteria, known as Zwitterionic polysaccharides (ZPSs) which can directly activate a major histocompatibility complex class II (MHCII)-mediated T-cell-dependent immune response without protein conjugation12,13,14. These ZPSs are densely decorated with positive (amine) and negative charges (phosphate or carboxylate) on adjacent monosaccharides in their structural framework. ZPSs possess unique immunological properties and can be employed as a cargo to reach out to T cells. In essence, ZPS is a carbohydrate that behaves like a protein when it comes to T-cell recognition. Indeed, a ZPS has been successfully used in place of a carrier protein to construct a totally carbohydrate antigen candidate for anticancer vaccine15,16. There has been a growing interest in isolating, characterizing and synthesizing ZPSs from pathogens. The most important ZPSs have been isolated from a common gut commensal Bacteroides fragilis (Fig. 1).
Bacterial polysaccharide PS A1, PS A2 and PS B contain rare deoxy amino sugars and display zwitterionic character due to the presence of carboxylic acids or phosphates and amino groups. PS A1 repeating unit has been synthesized by several groups. This study reports first total synthesis of PS B phosphorylated hexasaccharide unit.
Bacteroides are the most abundant opportunistic anaerobic pathogens which account for over 25% of the bacterial species colonizing the human colon17. While Bacteroides species are essential to the human metabolic system, they can also cause serious, sometimes fatal diseases like sepsis and abdominal abscesses, especially in immunocompromised patients. Among Bacteroides species, Bacteroides fragilis is the most virulent pathogen due to its virulence factors such as hemagglutinin, adhesions, polysaccharide capsule and fimbria. Although the numbers of B. fragilis isolates are 10- to 100-fold less than those of other intestinal Bacteroides species, it is most frequently isolated from clinical species in many infectious processes, including intraabdominal abscesses. Abscesses are formed specifically as a host response to B. fragilis infection which comes with significant morbidity and mortality. Therefore, this Gram-negative bacterium has been studied extensively in recent years. Studies have shown that it was associated with various human diseases such as inflammatory bowel disease (IBD), colorectal cancer, gynaecological infections, gangrenous appendicitis, brain abscesses, meningitis, endocarditis, pericarditis, and bacteraemia. The bacterium is also involved in diarrheal, bone and joint infections particularly in young children under 5 years of age14,18. B. fragilis is resistant to most antibiotics including cefoxitin, clindamycin, metronidazole, carbapenems and fluoroquinolones. Prophylaxis strategies are therefore required to tackle this pathogen.
Seminal studies by Kasper, Tzianabos and co-workers have established that ZPSs from B. fragilis are capable of eliciting T-cell proliferation and modulating the course of abscess formation12,13,14. This may serve as protection against a wide variety of abscess inducing microorganisms, including B. fragilis, Streptococcus pneumoniae, Staphylococcus aureus, and other synergistic microbes19,20,21,22,23. Importantly, it has been found that removing the zwitterionic charge from ZPS renders it non-immunogenic, highlighting the significance of the zwitterionic character for immune activation12,13,14. The unique immunogenic properties of ZPSs makes them an attractive synthetic target.
Over the past few years, tremendous effort has been put in by the synthetic chemists to synthesize such zwitterionic oligosaccharides due to their appealing biological activity, complex structures and zwitterionic nature5,24. Synthetic ZPSs are consistent, homogeneous, well-defined molecules with a single length and predefined substitution patterns, unlike naturally derived polysaccharides15,16,22,25. This makes them perfectly suited for structural studies aimed at gaining a deeper insight into their mode of action and interaction with binding partners on molecular level.
Amongst all the isolated ZPSs, PS A1 from Bacteroides fragilis is the most well studied ZPS repeating unit (RU). Van der Marel and co-workers were successful in the assembly of the protected tetrasaccharide, albeit in a low yield26. Seeberger identified the correct order of glycosylation and accomplished the first total synthesis of PS A127. Subsequently, Andreana28, and Kulkarni29 groups have accomplished the total synthesis of ZPS PS A1 tetrasaccharide RU to study its immune stimulatory properties. Very recently, Codée and co-workers synthesized a trimer repeat unit of PS A1 oligosaccharide for detailed structural studies30.
In comparison, no synthetic studies have been reported for PS B and PS A2 (Fig. 1). PS B isolated from B. fragilis of NCTC 934331 is a novel phosphorylated O-polysaccharide comprising of rare deoxy amino sugars viz. 2-acetamido-2,6-dideoxy-D-glucopyronanose (D-QuipNAc) and 2-acetamido-2,6-dideoxy-L-glucopyronanose (L-QuipNAc). In addition to this, PS B also contains 2-aminoethyl phosphonate moiety (AEP) which is believed to play a crucial role in its potent bioactivity as well as stability against enzymatic degradation32. The structure of hexasaccharide repeating unit of the O-polysaccharide was established as →3)-β-D-QuipNAc-(1→4)-[α-L-Fucp-(1→2)-β-D-GalpA(1→3)[[2-AEP-4]]-β-D-GlcpNAc-(1→3)]-α-D-Galp-(1→4)-α-L-QuipNAc-(1→. It should be noted that the positive charge on amino group of 2-AEP and the negative charge on phosphonate or carboxylate group imparts the zwitterionic character. Currently, there are no licensed vaccines available against B. fragilis, although there are many studies in this direction which have evaluated a range of antigens including outer-membrane proteins, O-polysaccharides, and exopolysaccharides. The rare sugars (both L and D) containing hexasaccharide RU is an attractive synthetic target for vaccine development. Over past decade, our laboratory has developed efficient and highly regioselective protocols for the synthesis of orthogonally protected rare deoxy amino sugar building blocks via one-pot SN2 displacements of D and L-hexopyranosidic 2,4-bis-triflates which enabled us to assemble a varity of complex bacterial glycans including the RU of PS A129,33,34,35,36,37. Herein, we report the first total synthesis of the structurally complex phosphorylated branched hexasaccharide RU of PS B via an efficient and stereoselective one-pot assembly.
Results and discussion
The synthesis of phosphorylated hexasaccharide from Bacteroides fragilis poses numerous challenges (Scheme 1). The hexasaccharide RU has a complex structure including six different monosaccharides with highly branched, sterically congested D-galactose and D-glucosamine moieties. It should be noted that low reactivity and high steric hindrance often cause problems in glycosylation with C4-OH of the D-galactopyranose bearing a sugar substituent at its C3-OH position and vice versa38,39,40,41. Similarly C4-OH group of D-glucosamine is as such highly unreactive42. Moreover, the D-galacturonate donors are well known to be significantly less reactive than their non-oxidized D-galactose counterparts in glycosylation reactions24. The rare deoxy amino sugars D and L-quinovosamine (QuipNAc) are difficult to obtain because these sugars are commercially not available and need multiple steps to synthesize. The installation of three 1,2-cis linkages particularly the one in L-QuipNAc is challenging41,43,44, and demands fine tuning of the reaction conditions to attain α selectivity in glycosylations. In addition to this, the incorporation of β-D-QuipNAc in sterically congested position, presence of multiple functional groups (three acetamido, one amino, carboxylate and phosphonate), and late stage phosphorylation makes the total synthesis a formidable task.
Retrosynthetic analysis of target molecule 1 is outlined in Scheme 1. A convergent one-pot synthetic strategy and protecting group normalization was designed with the aim of efficiently synthesizing the complex hexasaccharide. Specifically, benzyl (Bn), 2-naphthylmethyl (NAP) and benzyl carbamate (Cbz) are utilized as permanent protecting groups that could be deprotected under mild hydrogenolysis conditions with contemporaneous transformation of the NH-trichloroacetyl (NHTCA) group into the corresponding acetamido (NHAc). The paramethoxyphenyl (PMP) group at the reducing end and NAP group at the non-reducing end are installed strategically at the connection points of the repeating unit. Their orthogonal deprotection would enable chain elongation of oligosaccharide45. Target molecule 1 can be potentially obtained from phosphorylated hexasaccharide 2 through hydrogenolysis, which could be accessed by regioselective O4 reductive benzylidene ring opening of fully protected hexasaccharide 3, followed by phosphorylation. The construction of hexasaccharide 3 was envisioned via a convergent (1 + 2 + 2 + 1) orthogonal glycosylation in a one-pot manner. For a high-yielding one-pot glycosylation, glycosyl donors and acceptors must be energetically well-differentiated, which allows for selective donor activation and subsequent coupling with the acceptor. In order to maintain orthogonality and optimize the electronic properties of the glycosyl donors and acceptors, a judicious choice of protecting groups is required46. Accordingly, the fucose donor 447 was protected with arming benzyl (Bn) protecting group which can be chemoselectively activated and coupled with 2′-OH disaccharide thioglycoside acceptor 5. Acceptor 5 can be obtained by glycosylation of D-galacturonate donor 8 and 3-OH D-glucosamine acceptor 947 followed by deacylation. Disaccharide 3,4-diol acceptor 6 can be constructed by the union of orthogonally protected D-galactose donor 10 with 4-OH L-quinovosamine acceptor 11 followed by acetonide removal. Stereoselective 1,2-cis coupling of L-quinovosamine donor 12 with 4-methoxy phenol (PMPOH) followed by desilylation and conversion of N3 to NHTCA would afford 11. The α-selectivity can be achieved by placing a nonparticipating group such as N3 in the donor at C2 position and employing participating solvent Et2O48. It was envisioned that the conversion of N3 to NHTCA at this stage would enable final one-step global deprotection under hydrogenolytic conditions. The trichloroacetamido (TCA) group was chosen to mask amino groups in compounds 9 and 7 to enable the stereoselective installation of β-glycosidic bond. The 3-OH and 4-OH groups of D-galactopyranose 10 were capped with a transient acetonide protecting group that can be easily removed prior to glycosylation of C3′-OH of disaccharide 6 with the corresponding trisaccharide donor (formed by coupling of 4 and 5) and finally coupling of C4′-OH of the so formed pentasaccharide with D-quinovosamine donor 7. Quinovosamine derivatives 12 and 7 in turn can be easily accessed from rhamnosyl triols 13 and 14 via C2 inversion of triflates, respectively. With this retrosynthetic plan, we embarked on the total synthesis of target molecule 1.
The synthesis of appropriately protected building blocks is shown in Scheme 2. The reducing end L-quinovosamine donor 12 was prepared via C2 inversion of L-rhamnose derivative 13, along the lines of our reported protocol (Scheme 2a)44. The easily accessible L- rhamnose triol 1349 was first converted into the corresponding stannylene ketal and further treated with benzyl bromide (BnBr) and tetrabutylammonium bromide (TBAB) at 85 °C to afford 3-OBn derivative 15 as a single regioisomer in 82% yield over two steps. Selective silylation of 4-OH of 2,4-diol 15 using 1.5 equiv of tert-butyldimethylsilyl chloride (TBSCl) and imidazole at 85 °C furnished 2-OH rhamnose derivative 16 in 84% yield. The C-2 OH was subjected to triflation using triflic anhydride (Tf2O), pyridine in CH2Cl2 and the so formed C2-O-triflate was subsequently displaced by sodium azide (NaN3) to give L-quinovosamine donor 12 in 86% yield over two steps.
The synthesis of D-galactose donors 19, 22 and D-galacturonate donor 8 was initiated with known precursors 18 and 2047, as shown in Scheme 2b. Reaction of 18 with 2,2-dimethoxypropane (2,2-DMP) in the presence of p-toluenesulphonic acid (p-TSA) at RT for 2 h followed by refluxing with MeOH:H2O (10:1) at 70 °C for 4 h afforded 3,4-acetonide protected compound (74%), which was then subjected to benzylation using NaH and BnBr to give the fully protected thioglycoside donor 19 in 92% yield. For the synthesis of the galactose donor 22, the easily accessible 2,3-diol 20 was converted to the corresponding stannylene ketal by treating it with Bu2SnO in toluene at 110 °C and further reacting with BnBr and TBAB at 85 °C to furnish the corresponding 3-OBn derivative (74% yield over two steps), which upon borane mediated reductive ring opening at O6 gave 6-OH derivative 21 in 85% yield. Treatment of compound 21 with benzoyl chloride (BzCl) and pyridine in CH2Cl2 provided fully functionalized thioglycoside donor 22 (85%). In parallel, the D-galacturonate donor 8 was obtained upon selective TEMPO oxidation of 2,6-diol 21 followed by esterification using BnBr and NaHCO3 and subsequent capping of C2-OH with a chloroacetyl group in a very good overall yield.
Next, we moved to the synthesis of key building block D-quinovosamine 7, starting from β-D-rhamnose triol 1434, as shown in Scheme 2c. Accordingly, triol 14 was treated with 2,2-DMP in the presence of a catalytic amount of CSA to afford the corresponding 2,3-isopropylidene derivative and the remaining free 4-OH was benzylated using NaH and BnBr in DMF at 0 °C to give a fully protected derivative of D-rhamnose36. Subsequently, rhamnose derivative was again treated with p-TSA in MeOH: H2O (1:1) at room temperature to obtain 2,3-diol 23 in 78% yield, over three steps. Stannylene mediated 2-naphthylmethylation on C-3 OH of compound 23 gave 3-ONAP derivative of D-rhamnnoside 24. The remaining free hydroxyl group was treated with Tf2O and pyridine in CH2Cl2 at 0 °C and the so formed triflate group was displaced by azide using NaN3 in DMF, to smoothly furnish D-quinovosamine derivative 25, in 85% yield over two steps. For obtaining requisite β-selectivity in the ensuing (5 + 1) glycosylation via neighbouring group participation, azido group of compound 25 was converted to amine, which was subsequently capped with TCA group using trichloacetyl chloride in THF to afford orthogonally protected D-quinovosamine donor 7. It should be noted that there is no significant reactivity difference between SPh and STol glycosides when it comes to glycosylation. STol is preferred over SPh due to less offensive odor and crystalline solid compounds it forms. In case of 7, we opted for SPh due to the ready availability of triol 1434.
Synthesis of L-quinovosamine acceptor 11
After having all the desired building blocks in our hand, we went ahead towards the glycosylation of L-quinovosamine donor 12 with PMPOH. Achieving α-selectivity in L-quinovosamine is a challenging task41,43,44. So, we screened several conditions to optimize the selectivity by varying leaving groups and temperature, as shown in Table 1. In order get α-selectivity, diethylether (Et2O) is commonly used as a participating solvent48. Glycosylation of thioglycoside 12 under NIS/TMSOTf activation conditions in a dichloromethane/diethyl ether solvent mixture (1:3) with acceptor PMPOH at −30 °C afforded the desired product 26 in good yield but with moderate selectivity (α:β = 4:1) (Table 1, entry 1). By lowering the temperature to −60 °C (entry 2), we carried out glycosylation under identical conditions; in this case, though the yield of the reaction was greatly improved (98%), there was no significant enhancement in selectivity. Thioglycoside 12 being a stable and flexible donor could be readily converted into the corresponding Schmidt donor 12a (entries 3 and 4). A remarkable improvement in selectivity (α:β = 20:1) and yield (91% over two steps) was achieved by performing glycosylation reaction using imidate donor 12a with PMPOH, TMSOTf activation conditions in a dichloromethane/diethyl ether solvent mixture (1:3) at −78 °C (Table 1, entry 4). A doublet at 5.48 ppm with coupling constant J = 3.2 Hz for H-1 and 13C peak at 98.1 ppm for C-1 clearly confirmed that the newly formed linkage is 1,2-cis (α) (See Supplementary Information and Supplementary Data 1). The successful installation of α-linkage in this case can be attributed to the presence of Bn and TBS groups which results into electronic arming of the L-quinovosamine donor that in turn allows us to conduct the reaction at a low temperature of −78 °C using diethyl ether as a participating solvent. Subsequently, TBS group in 26 was cleaved in the presence of tetrabutylammonium fluoride (TBAF) in THF and the azido group was reduced to amine using zinc in acetic acid. The so formed amine was capped with TCA group using TCACl and THF to cleanly afford L-quinovosamine acceptor 11, in 78% yield over three steps.
Synthesis of disaccharide acceptor 6
Next, we moved on to the synthesis of right-hand side disaccharide acceptor diol 6 (Scheme 3). The β-thiogalactoside donor 10 was activated by NIS and TMSOTf promoter in CH2Cl2:Et2O (1:3) as the participating solvent and glycosylated with L-quinovosamine acceptor 11 at −60 °C to cleanly afford the desired α-linked disaccharide 27, as a sole isomer in 82% yield {1H NMR, δ 5.34 (d, J = 3.5 Hz, 1H, H-1), 5.24 (d, J = 3.0 Hz, 1H, H-1′)} (See Supplementary Information and Supplementary Data 1). Acidic hydrolysis of isopropylidine acetal in 27 in the presence of p-TSA furnished desired disaccharide diol 6 in 81% yield.
Attempted synthesis of disaccharide 28
For the synthesis of disaccharide acceptor 5, we attempted a coupling of thioglycoside donor 8 derived trichloroacetimidate 8a and N-phenyl trifluoroacetimidate 8a′ with C3-OH D-glucosamine acceptor 9 (Table 2). For this purpose, we screened a few conditions as shown in Table 2. Initially, we tried glycosylation of trichloroacetimidate donor 8a with acceptor 9 using TMSOTf or TfOH as a promoter at −30 °C as well as 0 °C. Unfortunately, we were not able to get our desired product. In each case, donor got decomposed with time and acceptor was recovered as such (Table 2, entries 1 and 2). Changing from imidate donor 8a to a more stable N-phenyltrifluoroacetimidate donor 8a′, under identical condition led to same result (Table 2, entry 2). Alternatively, the coupling of thioglycoside 8 with acceptor 9 under the activation of Ph2SO, Tf2O, TTBP at −60 °C50 did not give the desired product as well. In this reaction, orthoester 29 (34%) was obtained (Table 2, entry 3) and the remaining unreacted acceptor was recovered. Attempted rearrangement of orthoester 29 using TfOH did not lead to desired disaccharide 28. The electron withdrawing chloroacetyl group at C2 position and the ester group of galacturonate donor may account for the low reactivity in glycosylation. Therefore, we changed the strategy to a post-glycosylation oxidation and esterification as shown in Scheme 4.
Regio- and stereoselective assembly of pentasaccharide 34
Accordingly, we converted thiogalactoside 22 into trichlroacetamidate 30 in two steps via hydrolysis of thiogalactoside by using NBS, and subsequent treatment of the so formed hemiacetal with trichloroacetonitrile and DBU. Glycosylation of trichloroacetamidate donor 30 and acceptor 9 with TMSOTf as the promotor at 0 °C furnished β-linked disaccharide 31 in 65% yield, along with imidate rearranged side product. To enhance the yield of the reaction, we performed glycosylation reaction at −40 °C using TMSOTf and gradually warming up to room temperature. Gratifyingly, the reaction led to exclusive formation of 31 in 87% yield. After successfully synthesizing disaccharide 31 on gram scale, both the benzoate groups were cleaved using NaOMe in MeOH:CH2Cl2 under reflux to yield diol 32 in excellent yield. Subsequently, the primary hydroxyl group of compound 32 was regioselectively oxidized to its corresponding carboxylic acid using TEMPO/BAIB and concomitant esterification employing BnBr and NaHCO3 in DMF furnished C2′-OH disaccharide acceptor 5 in 85% over two steps.
The assembly of pentasaccharide was examined next. A highly chemoselective glycosylation reaction of known L-fucose donor 447 with disaccharide acceptor 5 at −60 °C in the presence of NIS (1.2 equiv) and cat. TMSOTf (0.2 equiv) promoter in CH2Cl2: Et2O (1:1) as a participating solvent afforded the desired α-linked trisaccharide 33 as a sole isomer with excellent yield. The newly formed 1,2- cis glycosidic linkage was confirmed by 1H NMR δ 5.38 (d, J = 3.6 Hz, 1H, H-1′′), 13C NMR (δ 98.6) and 2D NMR (1H-1H COSY, HSQC) (See Supplementary Information and Supplementary Data 1).
With the desired building blocks 33 and 6 in hand, the stage was set for the regioselective (3 + 2) glycosylation. Accordingly, regioselective O3 glycosylation of 3,4-diol acceptor 6 with trisaccharide donor 33 in the presence of NIS and TMSOTf promoter in CH2Cl2 at −60 °C cleanly furnished β-linked pentasaccharide acceptor 34 with good yield. The β-stereochemistry of the newly formed glycosidic linkage was confirmed by measuring 1JC–H coupling constant of the anomeric carbon of D-glucosamine unit (13C{1H} δ 100.1 (1JC–H = 161.2 Hz) ppm). The regioselectivity was confirmed by acetylation of C4′-OH in 34 using acetic anhydride, triethylamine and DMAP to obtain 34a in 87% yield. The downfield shift of a doublet at δ 5.13 ppm, J = 3.2 Hz in 1H NMR spectrum clearly confirmed the presence of acetate group at C-4′ position in 34a (See Supplementary Information and Supplementary Data 1).
Synthesis of hexasaccharide 3
After successfully synthesizing pentasaccharide acceptor 34 and D-quinovosamine donor 7, a challenging stereoselective β-(1,4) glycosylation was undertaken. It should be noted that stereoselective glycosylation of poorly nucleophilic C4-OH of the 1,3-disubstituated galactose moiety is very difficult38,39,40,41. For the synthesis of hexasaccharide, we scrutinized several conditions such as variation of leaving groups on donor, promoter and stoichiometry of donor (Table 3). First, we tried coupling of thioglycoside donor 7 with poorly nucleophilic 4′-OH pentasaccharide acceptor 34 in the presence of NIS and TMSOTf promoter in CH2Cl2 at −60 °C to afford β-linked hexasaccharide 3 in a low yield of 25% and rest of the acceptor remained unreacted while donor was hydrolysed (Table 3, entry 1). Replacing TMSOTf with a mild promoter AgOTf under identical conditions did not lead to product (Table 3, entry 2). To improve the yield of the reaction, we performed the glycosylation reaction by using a reactive trichloroacetimidate donor and the corresponding stable N-phenyltrifluoroacetimidate donor under the similar conditions, which led to moderate improvement in the yield (Table 3, entries 3 and 4). The modest yield of the glycosylation reaction can be attributed to steric hinderance to form highly branched (1,3,4-tri-substituent) galactopyranose containing hexasaccharide. Due to this, a significant portion of donor was hydrolysed. So, it was envisioned that increasing the donor concentration would probably increase the yield of reaction. Accordingly, we increased the equivalents of donor and performed the coupling with 2 equivalents of imidate donor with pentasaccharide acceptor 34. To our delight, the yield of the reaction was improved to 64% under this condition and the remaining acceptor was recovered (Table 3, entry 5). Encouraged by this result, we further increased the equivalent of quinovosamine donor (4 equivalent) and performed coupling with acceptor 34 in the presence of TMSOTf promoter in CH2Cl2 at −60 °C. The reaction showed a steady progress and after an hour ~20% product was visible on TLC along with intense spots of donor and acceptor. After 2 h, about 30% of donor was still prevalent in the reaction, along with small amount of acceptor (TLC analysis). Both donor and acceptors were consumed after 3 h and spots of product and hemiacetal resulting from hydrolysis of excess donor were seen on TLC. Gratifyingly, this time, we were able to greatly increase the yield of 3 to 94% (Table 3, entry 6). The selectivity is attributed to the well-known neighbouring group participation of the C-2 amide group of NHTCA. The β-stereochemistry of the newly formed glycosidic linkage was confirmed by 1JC–H coupling constant of the anomeric carbon of D-quinovosamine unit (13C{1H} δ 99.6 (1JC–H = 162.3 Hz) ppm) (See Supplementary Information and Supplementary Data 1).
One-pot (1 + 2 + 2 + 1) assembly of hexasaccharide 3
With the optimized conditions for the synthesis of hexasaccharide in hand, we next investigated the crucial one-pot synthesis of hexasaccharide 3 as shown in Scheme 5. Accordingly, a chemoselective glycosylation of a highly reactive per-O-benzylated thiotolyl fucose donor 8 and 2′-OH disaccharide acceptor 9 using NIS/TMSOTf as the promoter employing CH2Cl2:Et2O as a solvent mixture at −60 °C was conducted as before to cleanly afford the corresponding trisaccharide intermediate 33 in 3 h. Further, addition of glycosyl acceptor 6 in the same pot in the presence of NIS/TMSOTf promoter led to the formation of the corresponding 4′-OH pentasaccharide acceptor 34. After 2.5 h, donor 7a (4 eq) and TMSOTf (0.8 eq) were added in the same pot at −60 °C to furnish hexasaccharide 3 in 68% yield over three steps in a one-pot manner (7.5 h total time), after a single column chromatographic purification. Compound 3 can serve as a suitable right hand terminal hexasaccharide unit for chain elongation. The corresponding chain elongation repeating unit 3′ bearing a C2-azido group was also synthesized via a one-pot glycosylation using disaccharide 6′ under optimized conditions in 67% overall yield (see Supplementary Information Scheme S1 for the synthesis of 6′).
Phosphorylation and global deprotection
With the successful synthesis of hexasaccharide 3, we went ahead to complete the synthesis of hexasaccharide 1 as delineated in Scheme 6. Using Et3SiH and TfOH51 at −78 °C, a regioselective O4 reductive benzylidene ring opening of fully functionalized hexasaccharide 3, led to the desired 4-OH nucleophile 36 in 75% yield. Next, we attempted phosphorylation of compound 36 with phosphonic acid 3552 in the presence of DCC53. However, no phosphorylated compound was detected in TLC or in mass spectrum and the hexasaccharide 36 remained as such. Although different types of bases such as 1H-terazole, pyridine, Et3N or DIPEA along with DCC54 were tried for coupling of 4-OH 36 with phosphonate linker 35, no phosphorylated compound was obtained. The poor nucleophilicity of 4-OH D-glucosamine in the sterically crowded hexasaccharide 36 was considered to be the main factor behind the failure of the coupling42. Therefore, we anticipated that phosphorylation on such kind of structurally complex moiety would be a difficult task and this would require a highly electrophilic phosphorus (V) coupling partners. Seeberger and co-workers have shown a method to install phosphonate onto hindered nucleophiles using bis(chloro)-(2-azidoethyl) phosphonate55 as an electrophile and 1H-tetrazole activator. Accordingly, upon reaction with an alcohol nucleophile, rapid esterification leads to the formation of the C–P bond. At this stage, addition of water leads to the target motif. Gratifyingly, phosphorylation of 4-OH hexasaccharide 3 with freshly prepared bis(chloro)-(2-azidoethyl) phosphonate 37 in the presence of 0.45 M tetrazole in CH3CN and DIPEA, followed by addition of water in the same pot resulted in the formation of the corresponding phosphorylated hexasaccharide which was confirmed from 31P phosphorus NMR and HRMS. Finally, hydrogenolysis of all the Bn, NAP groups and conversion of NHTCA to NHAc was performed using H2, Pd(OH)2/C in EtOH at room temperature followed by purification through Sephadex G25 column to furnish the phosphorylated hexasaccharide repeating unit 1 of PS B from Bacteroides fragilis in 61% yield over three steps.
Conclusion
In conclusion, we have achieved the first total synthesis of phosphorylated zwitterionic hexasaccharide repeating unit of PS B from Bacteroides fragilis via a longest linear sequence of 21 steps in 5.5% overall yield. The convergent synthesis involves efficient synthesis of orthogonally functionalized rare deoxy amino sugar D/L-quinovosamine building blocks and its further elaboration into target molecule via one pot assembly of hexasaccharide moiety, its phosphorylation and a one-step global deprotection, in excellent overall yield. En route, we discovered suitable conditions for 1,2-cis selective glycosylation of L-quinovosamine donor, and installation of a 1,2-trans linked D-quinovosamine at sterically congested position while glycosylating a poorly nucleophilic acceptor. The protected hexasaccharide RU 3′ with placement of PMP and NAP groups at the reducing and non-reducing ends, respectively, and an azido group at C2, is well-poised for chain elongation to access higher oligomers of PS B. The synthesized ZPS RU 1 is now ready for immunological studies directed toward development of carbohydrate-based vaccine.
Methods
Compound 15
L-Rhamnose triol 1449 (7.0 g, 25.893 mmol) was dissolved in dry toluene (80 mL), and to this stirred solution, Bu2SnO (7.73 g, 31.072 mmol) was added and reaction mixture was kept at 110 °C for 6 h. After completion of the reaction, the solvent was removed under reduced pressure and the crude compound was kept under vacuum for 2 h.
Above crude compound was dissolved in dry toluene (60 mL), and TBAB (12.52 g, 38.839 mmol) followed by BnBr (4.62 mL, 38.839 mmol) were added and the reaction mixture was heated at 85 °C for 6 h. After completion of the reaction (confirmed by TLC), the reaction was diluted with EtOAc and organic layer was washed with brine solution twice. The separated organic layer was dried over anhydrous Na2SO4, concentrated, and purified by column chromatography on silica gel (30% ethyl acetate: petroleum ether) to obtain 15 as an amorphous white solid (7.63 g, 82%).
Compound 16
To a stirred solution of 15 (3.5 g, 9.709 mmol) in DMF (65 mL) was added TBSCl (2.19 g, 14.564 mmol) and imidazole (1.65 g, 24.274 mmol) sequentially. Then the reaction mixture was kept for stirring at 85 °C for 3 h. After complete conversion of starting material into product (as judge by TLC), reaction mixture was diluted with EtOAc and washed with brine. The separated organic layer was dried over Na2SO4 and concentrated in vacuo. The crude product was purified by column chromatography on silica gel (10% ethyl acetate:petroleum ether) to obtain 16 (3.87 g, 84%) as a white foam.
Compound 12
Trifluoromethanesulfonic anhydride (1.40 mL, 8.626 mmol) and pyridine (1.4 mL, 17.252 mmol) were added sequentially at 0 °C to a stirred solution of 16 (2.73 g, 5.751 mmol) in CH2Cl2 (55 mL). Then the reaction mixture was gradually warmed to rt over 1 h. After complete consumption of the starting material, the solvent was directly removed under reduced pressure to get crude triflated compound, which was kept under high vacuum for 30 min and used for the next step without purification.
The crude product which was obtained after the removal of solvents was dissolved in DMF (17 mL), and to this, NaN3 (3.74 g, 57.506 mmol) was added at rt and the reaction mixture was stirred at the same temperature for 12 h. After complete consumption of starting material, it was diluted with EtOAc and washed with brine solution. The separated organic layer was dried over Na2SO4 and concentrated in vacuo. The crude product was purified by column chromatography on silica gel (10% ethyl acetate:petroleum ether) to obtain 12 (2.47 g, 86% over two steps) as a pale yellowish viscous liquid.
Compound 19
2, 2-Dimethoxypropane (26.6 mL) and p-toluenesulfonic acid (0.21 g, 1.223 mmol) were added to a solution of compound 1847 (3.50 g, 12.223 mmol). After complete consumption of starting material, the reaction mixture was quenched with Et3N and solvents were removed under reduced pressure. The resulting crude compound was dissolved in MeOH: H2O (10:1, 120 mL) and then refluxed at 70 °C. After 4 h, solvent was removed in vacuo and chromatographed on silica gel (40% ethyl acetate and petroleum ether) to afford 3, 4-isopropylidine compound as a brown viscous liquid (2.95 g, 74% over two steps).
The above obtained compound (2.95 g, 9.037 mmol) was dissolved in DMF (30 mL) and then benzyl bromide (4.3 mL, 36.148 mmol) and sodium hydride (0.87 g, 36.148 mmol) were added sequentially at 0 °C. After complete consumption of starting material (as judge by TLC), reaction mixture was diluted with EtOAc and then washed with brine. The separated organic layer was dried over Na2SO4 and concentrated in vacuo. The obtained crude product was purified by column chromatography (10% ethyl acetate:petroleum ether) to obtain 19 as a white foam (4.2 g, 92%).
Compound 21
To a stirred solution of 2047 (2.04 g, 5.447 mmol) in toluene (40 mL) was added Bu2SnO (1.62 g, 6.537 mmol). Then the reaction mixture was kept for stirring at 110 °C for 6 h. After complete consumption of the starting material (as judged by TLC), solvent was removed under reduced pressure and the crude product was kept for drying under high vacuum. The crude product was dissolved in toluene (20 mL), and TBAB (2.63 g, 8.171 mmol) and BnBr (0.97 mL, 8.171 mmol) were added. Then the reaction mixture was allowed to stir at 60 °C for 6 h. After complete conversion of starting material, reaction mixture was diluted with EtOAc and washed with water. Separated organic layer was dried over Na2SO4, concentrated, and purified by column chromatography (30% ethyl acetate: pet ether) to give 3-OBn galactose derivative as a white solid (1.87 g, 74%).
To a clear solution of 3-OBn galactose derivative (0.94 g, 1.70 mmol) in CH2Cl2 (6 mL) were added 1 M BH3 in THF (5.5 mL) and trifloromethane sulfonate (TMSOTf, 0.09 mL, 0.51 mmol) at 0 °C. After 4 h, the reaction mixture was quenched with methanol, concentrated in vacuo, and chromatographed on silica gel (30% ethyl acetate:petroleum ether) to obtain the desired product 21 as a white solid (0.78 g, 85%).
Compound 22
Anhydrous pyridine (1.7 mL, 21.3 mmol) and benzoyl chloride (1.7 mL, 21.3 mmol) were sequentially added dropwise at 0 °C to the clear solution of compound 21 (0.667 g, 1.65 mmol) in dry CH2Cl2 (23.5 mL) and the solution was stirred at same temperature for 3 h. After completion of the reaction (confirmed by TLC), reaction mixture was washed with 1 M HCl, aq. NaHCO3 and aq. brine solution. Separated organic layer was dried over anhydrous Na2SO4, filtered, concentrated and. chromatographed on silica gel (20% ethyl acetate:petroleum ether) to obtain the desired product 22 as a white solid (0.946 g, 85%).
Compound 8
To a stirred solution of galactose diol 21 (103 mg, 0.221 mmol) in CH2Cl2/H2O (1.8 mL, 3:1) were added TEMPO (7 mg, 0.044 mmol) and BAIB (177 mg, 0.552 mmol) at 0 °C and the reaction was stirred for 2 h at room temperature. The reaction mixture was diluted with EtOAc and washed with aq. Na2S2O3 solution. The separated organic layer was dried over anhydrous Na2SO4, filtered, and concentrated. It was then kept under high vacuum for 1 h and used for the next step without purification.
The crude product was dissolved in DMF (1.2 mL) and treated with BnBr (0.05 mL, 0.442 mmol) and NaHCO3 (111 mg, 1.325 mmol) at rt overnight. After complete consumption of starting material, the mixture was diluted with EtOAc and washed with brine. The separated organic layer was dried over anhydrous Na2SO4, concentrated, and purified by silica gel chromatography using 30% ethyl acetate:petroleum ether as an eluent to afford galactose ester derivative as a colourless viscous liquid (96 mg, 76%).
C-2 OH galactose derivative (96 mg, 0.168 mmol) was dissolved in CH2Cl2 (2 mL) and to this stirring solution, pyridine (0.081 mL, 1.008 mmol) and chloroacetylchloride (27 µL, 0.336 mmol) were added sequentially at 0 °C and allowed to stir for 6 h at room temperature. After complete consumption of starting material (as judged by TLC), reaction mixture was diluted with CH2Cl2 and then washed with 1 M HCl, NaHCO3 and brine. The separated organic layer was dried over Na2SO4 and concentrated in vacuo. The crude product was purified by column chromatography (20% ethyl acetate: petroleum ether) to obtain 8 as a white foam (93 mg, 85%).
Compound 23
Known D-rhamnnose triol 1434 (1.3 g, 5.072 mmol) was dissolved in 2, 2-dimethoxypropane (13.3 mL) and camphorsulfonic acid (0.117 g, 0.507 mmol) was added to it at room temperature. After complete consumption of starting material, the reaction mixture was quenched with Et3N and solvents were removed under reduced pressure to obtain 2,3-diacetonide D-rhamnose derivative.
The above obtained compound was dissolved in DMF (20 mL) and then benzyl bromide (0.91 mL, 7.608 mmol) and sodium hydride (0.365 g, 15.216 mmol) were added sequentially at 0 °C. After complete consumption of starting material (as judged by TLC), reaction mixture was diluted with EtOAc and then washed with brine. The separated organic layer was dried over Na2SO4 and concentrated in vacuo. The obtained crude product was used for the next step without purification.
The above obtained crude compound was dissolved in MeOH:CH2Cl2 (1:1, 20 mL) and then p-toluene sulfonic acid (0.436 g, 2.536 mmol) was added at rt. After 10 h, reaction mixture was quenched with Et3N and solvent was removed in vacuo. The obtained crude product was chromatographed on silica gel (40% ethyl acetate: petroleum ether) to afford diol compound 23 as a viscous liquid (1.37 g, 78% over three steps).
Compound 24
D-Rhamnose diol 23 (1.3 g, 3.752 mmol) was dissolved in dry toluene (30 mL), and to this stirred solution, Bu2SnO (1.12 g, 4.503 mmol) was added and reaction mixture was kept at 110 °C for 6 h. After completion of the reaction, solvent was removed under reduced pressure, the crude compound was kept under vacuum for 2 h.
Above crude compound was dissolved in dry toluene (20 mL), and TBAB (1.9 g, 5.852 mmol) followed by NAPBr (0.99 g, 4.503 mmol) were added and the reaction mixture was heated at 60 °C for 6 h. After completion of the reaction (confirmed by TLC), the reaction was diluted with EtOAc and organic layer was washed with brine solution twice, dried over anhydrous Na2SO4. The separated organic layer was concentrated, and purified by column chromatography on silica gel (20% ethyl acetate: petroleum ether) to obtain 24 as a white solid (1.42 g, 78%).
Compound 25
Tf2O (0.67 mL, 3.98 mmol) and dry pyridine (0.64 mL, 7.95 mmol) were added dropwise to the stirring solution of compound 24 (1.29 g, 2.65 mmol) in dry CH2Cl2 (17.5 mL) at 0 °C and allowed to stir at same temperature for 1 h. After completion of the reaction, reaction mixture was diluted with CH2Cl2 (20 mL), and washed with 1 M HCl and aq. NaHCO3 solution. Separated organic layer was dried over anhydrous Na2SO4, concentrated and dried under vacuum to give corresponding triflate derivative quantitatively.
NaN3 (2.6 g, 39.75 mmol) was added to the solution of above obtained compound in dry DMF (15 mL) and kept for stirring at rt for 12 h. After completion of reaction (indicated by TLC), reaction mixture was diluted with EtOAc (20 mL) and washed with brine solution. Separated organic layer was dried over anhydrous Na2SO4, concentrated and purified by silica gel column chromatography (15% ethyl acetate: petroleum ether) to afford compound 25 (1.15 g, 85%) as white solid.
Compound 7
To a solution of 25 (800 mg, 1.56 mmol) in THF (10.0 mL), Zn dust (1.5 g) and acetic acid (10 mL) were added and the mixture was stirred at rt for 3 h. After completion of reaction (judged by TLC), the mixture was filtered through Celite and the filtrate was concentrated and kept under vacuum. The obtained crude material was dissolved in THF (10 mL) with activated molecular sieves (500 mg) and trichloroacetyl chloride (0.8 mL, 3.12 mmol) was added drop wise at 0 oC. The mixture was stirred for 45 minutes until complete consumption of the starting material and then neutralized with triethyl amine. The mixture was concentrated and purified by column chromatography (10% ethylacetate: petroleum ether) to obtain 7 (835 mg, 85% over two steps) as white solid.
Compound 26
Compound 12 (1.23 g, 2.532 mmol) was dissolved in THF: H2O (33 mL: 11 mL). To this stirred solution N-bromosuccinamide (1.35 g, 7.597 mmol) was added to it at rt. After complete consumption of starting material, the reaction mixture was diluted with EtOAc and washed with Na2S2O3. The separated organic layers were dried over Na2SO4, concentrated on rotor and chromatographed on silica gel (20% ethyl acetate and petroleum ether) to afford desired hemiacetal as a viscous liquid (0.878 g, 88%).
To the stirring solution of hemiacetal (878 mg, 2.231 mmol) in CH2Cl2 (15 mL), activated K2CO3 (1.32 g) and trichloroacetonitrile (1.12 mL, 11.154 mmol) were added to it at rtand the reaction was stired overnight. After completion of the reaction, the solid residue was filtered off through Celite bed and the filtrate was concentrated under reduced pressure to obtain Schimdt donor 12a as a viscous liquid (quantitative yield). This donor was used for glycosylation reaction without purification. Both donor 12a (1.2 g, 2.2307 mmol) and acceptor para methoxy phenol (332 mg, 2.676 mmol) were mixed and azeotroped with toluene under nitrogen and kept under high vacuum for 30 min.
Freshly vacuum-dried MS 3 Å (1.5 g) were added to a clear solution of donor 12a and acceptor in CH2Cl2: Et2O (1:3, 5 mL: 15 mL) and the resultant turbid solution was stirred at room temperature for 30 min. After that reaction mixture was cooled to −78 °C and then, TMSOTf (81 μL, 0.446 mmol) was added dropwise. After stirring the reaction mixture at the same temperature for 2.5 h, the reaction mixture was filtered through a Celite pad and concentrated. The crude residue was purified by column chromatography on silica gel (10% ethyl acetate:petroleum ether) to give 26 as a viscous liquid (1.05 g, 91%).
Synthesis of compound 11
To the clear solution of compound 26 (1.1 g, 2.201 mmol) in THF (20 mL), tetra butyl ammonium fluoride (2 mL) was added dropwise at room temperature. After completion of the reaction, solvent was removed under reduced pressure and it was used for next reaction without purification.
The obtained crude product was dissolved in THF (20 mL) and acetic acid (10 mL). To this stirring solution, activated zinc (2.4 g) was added at room temperature and allowed to stir for 3 h at the same temperature. After complete consumption of starting material, solid residue was filtered off through filter paper. The collected filtrate was concentrated on rotor and azetroped with toluene. The obtained crude product was kept under high vacuum for 1 h and used for the next step.
The vacuum dried compound was dissolved in THF (20 mL) and to this clear solution, trichloroacetyl chloride (0.50 mL, 4.403 mmol) was added dropwise at 0 °C. After complete consumption of starting material (as judge by TLC), solvent was removed under reduced pressure and chromatographed on silica gel (20% ethyl acetate:petroleum ether) to obtain the desired compound 11 as a sticky liquid (866 mg, 78% over three steps).
Compound 27
Freshly vacuum-dried MS 3 Å (1.6 g) were added to a clear solution of 10 (903 mg, 1.783 mmol) and 11 (720 mg, 1.426 mmol) in CH2Cl2: Et2O (1:3, 5 mL: 15 mL) and the resultant turbid solution was stirred at room temperature for 30 min. After that reaction mixture was cooled to −60 °C and then, NIS (802 mg, 3.565 mmol) and TMSOTf (64 μL, 0.356 mmol) were added sequentially at −60 °C. After stirring the reaction mixture at the same temperature for 4 h, the reaction mixture was filtered through a Celite pad and washed with CH2Cl2. Filtrate was washed twice with aq. Na2S2O3, and the separated organic layer was dried over Na2SO4, filtered, and concentrated. The crude residue was purified by column chromatography on silica gel (20% ethyl acetate: petroleum ether) to give 27 as oily liquid (1.04 g, 82%).
Compound 6
To the clear solution of compound 27 (376 mg, 0.424 mmol) in CH2Cl2 (3 mL) and MeOH (9 mL), p-toluene sulfonic acid (36 mg, 0.21 mmol) was added at room temperature and allowed to stir overnight. After completion of the reaction, reaction mixture was quenched by adding few drops of triethylamine, the solvents were removed under reduced pressure and chromatographed on silica gel (40% ethyl acetate:petroleum ether) to afford 6 as a foam (290 mg, 81%).
Compound 29
Tf2O (37 µL, 0.218 mmol) was added to a cold solution of 8 (0.101 g, 0.156 mmol), Ph2SO (88 mg, 0.437 mmol) and TTBP (116 mg, 0.468 mmol) in CH2Cl2 (4 mL) at −60 °C. After 10 min, to this cold solution, acceptor 9 (97 mg, 0.187 mmol) in CH2Cl2 (2 mL) was added dropwise. After 1 h, reaction mixture was diluted with CH2Cl2 and filtered through celite. The filtrate was washed with aq. NaHCO3 and the separated organic layer was dried over Na2SO4, concentrated and purified by column chromatography (20% ethyl acetate:pet ether) to give 29 as a viscous liquid (55 mg, 34%).
Compound 31
Compound 22 (874 mg, 1.080 mmol) was dissolved in THF: H2O (12 mL: 4 mL). To this stirred solution, N-bromosuccinamide (577 mg, 3.241 mmol) was added to it at rt. After complete consumption of starting material, the reaction mixture was diluted with EtOAc and washed with Na2S2O3. The separated organic layers were dried over anhydrous Na2SO4, concentrated on rotor and chromatographed on silica gel (20% ethyl acetate:petroleum ether) to afford desired hemiacetal (668 mg, 88%).
To the stirring solution of hemiacetal (220 mg, 0.313 mmol) in CH2Cl2 (5 mL), activated K2CO3 (330 mg) and trichloroacetonitrile (0.16 mL, 1.564 mmol) were added to it at rtand the reaction was stirred overnight. After completion of the reaction, the solid residue was filtered off through Celite bed and concentrated under reduced pressure to obtain imidate donor 30 as a viscous liquid (quantitative yield).
Freshly vacuum-dried MS 3 Å (1.5 g) were added to a clear solution of Schmidt donor 30 (0.844 g, 1.1837 mmol) and acceptor 1130 (0.552 g, 1.0653 mmol) in CH2Cl2 (10 mL) and the resultant turbid solution was stirred at room temperature for 30 min. After that, reaction mixture was cooled to −40 °C, TMSOTf (43 μL, 0.2367 mmol) was added dropwise to it and reaction temperature was gradually increased to room temperature. After stirring for 3 h, the reaction mixture was filtered through a Celite pad and concentrated. The crude residue was purified by column chromatography on silica gel (20% ethyl acetate:petroleum ether) to give 31 as a viscous liquid (0.905 g, 87%).
Compound 32
To a clear solution of compound 31 (857 mg, 0.8761 mmol) in MeOH: CH2Cl2 (20 mL: 10 mL), sodium methoxide (189 mg, 3.5044 mmol) was added at rt and allowed to stir under reflux for overnight. After completion of the reaction, reaction was quenched with amberlyte (H+) resin, filtered through filter paper, concentred in vacuo and chromatographed on silica gel (30% ethyl acetate:petroleum ether) to give 32 as viscous liquid (649 mg, 86%).
Compound 5
To a stirred solution of disaccharide diol 32 (0.122 g, 0.142 mmol) in CH2Cl2/H2O (2.4 mL, 3:1) were added TEMPO (5 mg, 0.028 mmol) and BAIB (114 mg, 0.3540 mmol) at 0 °C and to the reaction was stirred for 3 h at room temperature. The reaction was diluted with EtOAc and washed with aq. Na2S2O3 solution. The separated organic layer was dried over anhydrous Na2SO4, filtered, and concentrated. It was then kept under high vacuum for 1 h and used for the next step without purification.
The crude product was dissolved in DMF (2 mL) and treated with BnBr (0.04 mL, 0.283 mmol) and NaHCO3 (48 mg, 0.566 mmol) at rt overnight. After complete consumption of starting material, the mixture was diluted with EtOAc and washed with brine. The separated organic layer was dried over anhydrous Na2SO4, concentrated, and purified by silica gel chromatography using 30% ethyl acetate:petroleum ether as an eluent to afford 5 as a colourless viscous liquid (115 mg, 85%).
Compound 33
Freshly vacuum-dried MS 3 Å (1.0 g) were added to a clear solution of thio glycoside donor 430 (446 mg, 0.825 mmol) and acceptor 5 (557 mg, 0.577 mmol) in CH2Cl2: Et2O (1:3, 5 mL: 15 mL) and the resultant turbid solution was stirred at room temperature for 30 min. After that reaction mixture was cooled to −60 °C and then, NIS (204 mg, 0.907 mmol) and TMSOTf (30 μL, 0.1649 mmol) were added sequentially at −60 °C. After stirring the reaction mixture at the same temperature for 1 h, the reaction mixture was filtered through a Celite pad and washed with CH2Cl2. Filtrate was washed twice with aq. Na2S2O3, and the separated organic layer was dried over Na2SO4, filtered, and concentrated. The crude residue was purified by column chromatography on silica gel (25% ethyl acetate: petroleum ether) to give 33 as a white foam (697 mg, 88%).
Compound 34
A solution of trisaccharide donor 33 (289 mg, 0.209 mmol), acceptor 6 (194 mg, 0.230 mmol) and molecular sieves 3 Å MS (550 mg) in CH2Cl2 (10 mL) were stirred at room temperature for 30 min. The reaction mixture was cooled to −60 °C and then NIS (94 mg, 0.418 mmol) and TMSOTf (7 μL, 0.042 mmol) were added sequentially. After 1 h, the reaction mixture was quenched with Et3N and diluted with CH2Cl2. The solids were filtered off and the resulting residue was washed with aq. Na2S2O3 solution. The separated organic layer was dried over anhydrous Na2SO4, filtered, concentrated and purified by column chromatography over silica gel (30% ethyl acetate:petroleum ether) to afford 34 as a white foam (345 mg, 79%).
Compound 34a
Triethylamine (50 µL, 0.03 mmol), acetic anhydride (80 µL, 0.07 mmol), DMAP (2 mg, 0.016 mmol) were added sequentially to a clear solution of compound 34 (37 mg, 0.017 mmol) in CH2Cl2 (1 mL) at 0 °C, and the resulting solution was allowed to stir under nitrogen for 1 h. After complete consumption of starting material (monitored by TLC), solvent was directly removed under reduced pressure. The residue was purified by column chromatography on silica gel using 20% ethyl acetate:petroleum ether to yield compound 34a as a viscous liquid (33 mg, 87%).
Compound 3
Activated molecular sieves 3 Å (250 mg) was added to the vacuum dried azeotropic mixture of imidate donor 7a (148 mg, 0.217 mmol) and pentasaccharide acceptor 34 (114 mg, 0.054 mmol) and dissolved in dry CH2Cl2 (4 mL) at room temperature. After 30 min, reaction mixture was cooled to 60 °C and then TMSOTf (8 μL, 0.043 mmol) was added dropwise. After complete consumption of acceptor (as judged by TLC), the reaction mixture was quenched with Et3N and diluted with CH2Cl2. The solids were filtered off and the resulting residue was washed twice with aq. Na2S2O3 solution. The separated organic layer was dried over anhydrous Na2SO4, filtered, and concentrated. The crude residue was purified by column chromatography on silica gel (20% ethyl acetate:petroleum ether) to afford 3 as a white foam (134 mg, 94%).
Procedure of one-pot synthesis of hexasaccharide 3
An azeotroped mixture of donor 4 (58 mg, 0.107 mmol), acceptor 5 (82 mg, 0.085 mmol) and 3 Å MS (0.2 g) were dissolved in CH2Cl2 Et2O (1:1, 4 mL) and the reaction was stirred at room temperature for 30 mins and shifted to −60 °C. After 10 mins, NIS (29 mg, 0.128 mmol) and TMSOTf (4 μL, 21.4 μmol) were added sequentially at −60 °C. After complete consumption of starting materials (as judged by TLC), to this reaction, disaccharide acceptor 6 (72 mg, 0.0858 mmol) in CH2Cl2 (1 mL), followed by NIS (48 mg, 0.2144 mmol) and TMSOTf (4 μL, 21.4 μmol) were added at −60 °C. After 2.5 h, another donor 7a (292 mg, 0.428 mmol) in CH2Cl2 (2 mL) and TMSOTf (15 μL, 85.8 μmol) were added sequentially at −60 °C to the stirred reaction mixture. After completion of the reaction (confirmed by TLC), reaction mixture was quenched with NEt3, filtered through Celite and the obtained organic layer was washed with aq. Na2S2O3 solution. Collected organic layer was dried over anhydrous Na2SO4, concentrated and purified by column chromatography on silica gel (10–15% ethyl acetate:toluene) to afford 3 (154 mg, 68% over three steps) as a white foam.
In a similar way, compound 3′ was synthesized using diol 6′ in place of 6, under identical conditions.
Synthesis of compound 36
A turbid solution of hexasaccharide 3 (90 mg, 0.034 mmol and molecular sieves 3 Å MS (150 mg) in CH2Cl2 (1.5 mL) were stirred at room temperature for 30 min. The reaction mixture was cooled to −78 °C and then Et3SiH (19 μL, 0.119 mmol), triflic acid (9 μL, 0.009 mmol) were added sequentially at −78 °C. After complete consumption of starting material (as judge by TLC), reaction mixture was quenched with triethylamine (0.5 mL) and MeOH (1 mL). The solid residue was filtered off through Celite bed. The collected filtrate was concentrated under reduced pressure and purified by column chromatography over silica gel (30% ethyl acetate:petroleum ether) to afford 36 (67 mg, 75%).
Compound 1
The freshly prepared crude bis(chloro)(2-azidoethyl)phosphonate 3755 (9 mg, 0.045 mmol) was dissolved in toluene (1 mL) and then was added dropwise to a solution of hexasaccharide acceptor 3 (20 mg, 0.0076 mmol), DIPEA (0.026 mL, 0.152 mmol) and 1H-tetrazole (0.048 mL, 0.022 mmol, 0.45 M solution in CH3CN) in toluene (1 mL) at 0 °C. After 12 h at room temperature, 1 drop of water was added to reaction mixture and it was stirred for additional 1 h at room temperature. After completion of the reaction (as judged by TLC and 31P NMR), the reaction was quenched with water and diluted with EtOAc. The organic phase was washed with aq. NaHCO3 and brine, dried over anhydrous Na2SO4, filtered and concentrated in vacuo. The crude product was passed through the short column to give phosphodiester compound 2 and used for the next step.
Above residue was dissolved in EtOH (2 mL) and AcOH (one drop). To this solution Pd(OH)2/C (40 mg, 20 wt% Pd) was added and the reaction was stirred under an atmosphere of hydrogen for 72 h at room temperature. After complete consumption of starting material (as judged by HRMS), reaction mixture was filtered through a Celite pad with methanol/H2O as eluents. The obtained filtrate was concentrated under reduced pressure and purified on Sephadex G-25 gel using H2O as eluent to give 1 as white foam (6.6 mg) in 68% yield over three steps.
Data availability
The data underlying this study are available in the published article and its Supplementary Information. It contains additional schemes, detailed experimental procedures and spectral characterization data of all new compounds (PDF). A separate file “Supplementary Data 1” containing copies of 1D and 2D NMR spectra as well as mass spectra is also provided (PDF).
References
Herget, S. et al. Statistical analysis of the Bacterial Carbohydrate Structure Data Base (BCSDB): characteristics and diversity of bacterial carbohydrates in comparison with mammalian glycans. BMC Struct. Biol. 8, 35 (2008).
Dube, D. H., Champasa, K. & Wang, B. Chemical tools to discover and target bacterial glycoproteins. Chem. Commun. 47, 87–101 (2011).
Del Bino, L. et al. Synthetic glycans to improve current glycoconjugate vaccines and fight antimicrobial resistance. Chem. Rev. 122, 15672–15716 (2022).
Seeberger, P. H. & Werz, D. B. Synthesis and medical applications of oligosaccharides. Nature 446, 1046–1051 (2007).
Behera, A. & Kulkarni, S. S. Chemical synthesis of rare, deoxy-amino sugars containing bacterial glycoconjugates as potential vaccine candidates. Molecules 23, 1997 (2018).
Imperiali, B. Bacterial carbohydrate diversity—a Brave New World. Curr. Opin. Chem. Biol. 53, 1–8 (2019).
Werz, D. B. et al. Exploring the structural diversity of mammalian carbohydrates (“glycospace”) by statistical databank analysis. ACS Chem. Biol. 2, 685–691 (2007).
Adibekian, A. et al. Comparative bioinformatics analysis of the mammalian and bacterial glycomes. Chem. Sci. 2, 337–344 (2011).
Berti, F. & Adamo, R. Recent mechanistic insights on glycoconjugate vaccines and future perspectives. ACS Chem. Biol. 8, 1653–1663 (2013).
Daniels, C. C., Rogers, P. D. & Shelton, C. M. A review of pneumococcal vaccines: current polysaccharide vaccine recommendations and future protein antigens. J. Pediatr. Pharmacol. Ther. 21, 27–35 (2016).
Herzenberg, L. A., Tokuhisa, T. & Herzenberg, L. A. Carrier-priming leads to hapten-specific suppression. Nature 285, 664–667 (1980).
Tzianabos, A. O., Onderdonk, A. B., Rosner, B., Cisneros, R. L. & Kasper, D. L. Structural features of polysaccharides that induce intra-abdominal abscesses. Science 262, 416–419 (1993).
Cobb, B. A., Wang, Q., Tzianabos, A. O. & Kasper, D. L. Polysaccharide processing and presentation by the MHCII pathway. Cell 117, 677–687 (2004).
Avci, F. Y. & Kasper, D. L. How bacterial carbohydrates influence the adaptive immune system. Annu. Rev. Immunol. 28, 107–110 (2010).
Nishat, S. & Andreana, P. R. Entirely carbohydrate-based vaccines: an emerging field for specific and selective immune responses. Vaccines 4, 19 (2016).
De Silva, R. A., Wang, Q., Chidley, T., Appulage, D. K. & Andreana, P. R. Immunological response from an entirely carbohydrate antigen: design of synthetic vaccines based on Tn–PS A1 conjugates. J. Am. Chem. Soc. 131, 9622–9623 (2009).
Wexler, H. M. Bacteroides: the good, the bad, and the nitty-gritty. Clin. Microbiol. Rev. 20, 593–621 (2007).
San Joaquin, V. H., Griffis, J. C., Lee, C. & Sears, C. L. Association of Bacteroides fragilis with childhood diarrhea. Scand. J. Infect. Dis. 27, 211–215 (1995).
Brubaker, J. O., Li, Q., Tzianabos, A. O., Kasper, D. L. & Finberg, R. W. Mitogenic activity of purified capsular polysaccharide A from Bacteroides fragilis: differential stimulatory effect on mouse and rat lymphocytes in vitro. J. Immunol. 162, 2235–2242 (1999).
Tzianabos, A. O., Onderdonk, A. B., Zaleznik, D. F., Smith, R. S. & Kasper, D. L. Structural characteristics of polysaccharides that induce protection against intra-abdominal abscess formation. Infect. Immun. 62, 4881–4886 (1994).
Tzianabos, A. O., Kasper, D. L., Cisneros, R. L., Smith, R. S. & Onderdonk, A. B. Polysaccharide-mediated protection against abscess formation in experimental intra-abdominal sepsis. J. Clin. Investig. 96, 2727–2731 (1995).
Kalka-Moll, W. M. et al. Effect of molecular size on the ability of zwitterionic polysaccharides to stimulate cellular immunity. J. Immunol. 164, 719–724 (2000).
Tzianabos, A. O. et al. T cells activated by zwitterionic molecules prevent abscesses induced by pathogenic bacteria. J. Biol. Chem. 275, 6733–6740 (2000).
Zhang, Q., Overkleeft, H. S., van der Marel, G. A. & Codée, J. D. C. Synthetic zwitterionic polysaccharides. Curr. Opin. Chem. Biol. 40, 95–101 (2017).
Shi, M., Kleski, K. A., Trabbic, K. R., Bourgault, J.-P. & Andreana, P. R. Sialyl-Tn polysaccharide A1 as an entirely carbohydrate immunogen: synthesis and immunological evaluation. J. Am. Chem. Soc. 138, 14264–14272 (2016).
van den Bos, L. J. et al. A synthetic study towards the PSA1 tetrasaccharide repeating unit. Tetrahedron Lett. 48, 2697–2700 (2007).
Pragani, R. & Seeberger, P. H. Total synthesis of the Bacteroides fragilis zwitterionic polysaccharide A1 repeating unit. J. Am. Chem. Soc. 133, 102–107 (2011).
Eradi, P., Ghosh, S. & Andreana, P. R. Total synthesis of zwitterionic tetrasaccharide repeating unit from Bacteroides fragilis ATCC 25285/NCTC 9343 capsular polysaccharide PS A1 with alternating charges on adjacent monosaccharides. Org. Lett. 20, 4526–4530 (2018).
Pathan, E. K., Ghosh, B., Podilapu, A. R. & Kulkarni, S. S. Total synthesis of the repeating unit of Bacteroides fragilis zwitterionic polysaccharide A1. J. Org. Chem. 86, 6090–6099 (2021).
Wang, Z. et al. Total synthesis and structural studies of zwitterionic Bacteroides fragilis polysaccharide A1 fragments. J. Am. Chem. Soc. 145, 14052–14063 (2023).
Baumann, H., Tzianabos, A. O., Brisson, J. R., Kasper, D. L. & Jennings, H. Structural elucidation of two capsular polysaccharides from one strain of Bacteroides fragilis using high-resolution NMR spectroscopy.J. Biochemistry 31, 4081–4089 (1992).
Horsman, G. P. & Zechel, D. L. Phosphonate biochemistry.Chem. Rev. 117, 5704–5783 (2017).
Sanapala, S. R. & Kulkarni, S. S. Expedient route to access rare deoxy amino L-sugar building blocks for the assembly of bacterial glycoconjugates. J. Am. Chem. Soc. 138, 4938–4947 (2016).
Emmadi, M. & Kulkarni, S. S. Recent advances in synthesis of bacterial rare sugar building blocks and their applications. Nat. Prod. Rep. 11, 870–879 (2014).
Behera, A., Rai, D. & Kulkarni, S. S. Total syntheses of conjugation-ready trisaccharide repeating units of Pseudomonas aeruginosa O11 and Staphylococcus aureus type 5 capsular polysaccharide for vaccine development. J. Am. Chem. Soc. 142, 456–457 (2020).
Paul, A. et al. Total synthesis of a structurally complex tetrasaccharide repeating unit of Vibrio cholerae O43. Org. Lett. 25, 6413–6418 (2023).
Rai, D. & Kulkarni, S. S. Total synthesis of conjugation-ready tetrasaccharide repeating units of a multidrug-resistant pathogen Acinetobacter baumannii strain 34 and O5. Org. Lett. 25, 8332–8337 (2023).
Mondal, P. K., Liao, G., Mondal, M. A. & Guo, Z. Chemical synthesis of the repeating unit of type Ia group B Streptococcus capsular polysaccharide. Org. Lett. 17, 1102–1105 (2015).
Gao, J. & Guo, Z. Chemical synthesis of the repeating unit of type V group B Streptococcus Capsular polysaccharide. Org. Lett. 18, 5552–5555 (2016).
Bandara, M. D., Stine, K. J. & Demchenko, A. V. Chemical synthesis of human milk oligosaccharides: lacto-N-hexose Galβ1→3GlcNAcβ1→3 [Galβ1→4GlcNAcβ1→6] Galβ1→4Glc. J. Org. Chem. 84, 16192–16198 (2019).
Zhang, H. et al. Total synthesis of the tetrasaccharide haptens of Vibrio vulnificus MO6-24 and BO62316 and immunological evaluation of their protein conjugates. JACS Au 2, 97–108 (2022).
Crich, D. & Dudkin, V. Why are the hydroxy groups of partially protected N-acetylglucosamine derivatives such poor glycosyl acceptors, and what can be done about it? a comparative study of the reactivity of N-acetyl-, N-phthalimido-, and 2-azido-2-deoxy-glucosamine derivatives in glycosylation. 2-picolinyl ethers as reactivity-enhancing replacements for benzyl ethers. J. Am. Chem. Soc. 123, 6819–6825 (2001).
Paul, A. & Kulkarni, S. S. Total synthesis of the repeating units of Proteus penneri 26 and Proteus vulgaris TG155 via a common disaccharide. Org. Lett. 25, 4400–4405 (2023).
Ghosh, A. & Kulkarni, S. S. Total synthesis of a linear tetrasaccharide repeating unit of Vibrio vulnificus MO6-24. Org. Lett. 25, 7242–7246 (2023).
Romeo, J. R., McDermott, L. & Bennett, C. S. Reagent controlled α-selective dehydrative glycosylation of 2,6-dideoxy sugars: construction of the arugomycin tetrasaccharide. Org. Lett. 22, 3649–3654 (2020).
Kulkarni, S. S. et al. One-pot protection, glycosylation, and protection–glycosylation strategies of carbohydrates. Chem. Rev. 118, 8025–8104 (2018).
Wang, Z., Zhou, L., El-Boubbou, K., Ye, X.-S. & Huang, X. Multi-component one-pot synthesis of the tumor-associated carbohydrate antigen globo-h based on preactivation of thioglycosyl donors. J. Org. Chem. 72, 6409–6420 (2007).
Kafle, A., Liu, J. & Cui, L. Controlling the stereoselectivity of glycosylation via solvent effects. Can. J. Chem. 94, 894–901 (2016).
Wang, P. et al. Chemical synthesis and immunological evaluation of pertussis like pentasaccharide bearing multiple rare sugars as a potential anti-pertussis vaccine. Angew. Chem. Int. Ed. 59, 6451–6458 (2020).
Vohra, Y., Buskas, T. & Boons, G. J. Rapid assembly of oligosaccharides: a highly convergent strategy for the assembly of a glycosylated amino acid derived from PSGL-1. J. Org. Chem. 74, 6064–6071 (2009).
Sakagami, M. & Hamana, H. A selective ring opening reaction of 4,6-O-benzylidene acetals in carbohydrates using trialkylsilane derivatives. Tetrahedron Lett. 41, 5547–5551 (2000).
Yamauchi, K., Ohtsuki, S. & Kinoshita, M. Synthesis of peptide analogs containing (2-aminoethyl)phosphonic acid (ciliatine). J. Org. Chem. 49, 1158–1163 (1984).
Galler, D. J. & Parker, K. A. Five easy pieces. the total synthesis of phosphoiodyn A (and placotylene A). Org. Lett. 17, 5544–5546 (2015).
Tanaka, F., Kinoshita, K., Tanimura, R. & Fujii, I. Relaxing substrate specificity in antibody-catalyzed reactions: enantioselective hydrolysis of N-Cbz-amino acid esters. J. Am. Chem. Soc. 118, 2332–2339 (1996).
Lee, B. Y., Seeberger, P. H. & Varon Silva, D. Synthesis of glycosylphosphatidylinositol (GPI)-anchor glycolipids bearing unsaturated lipids. Chem. Commun. 52, 1586–1589 (2016).
Acknowledgements
S.S.K. thanks the Science and Education Research Board (Grant SPR/2021/000328), and KP thanks UGC, New Delhi for fellowships. The authors thank Dr. Bhaswati Ghosh for her assistance during the initial stages of the project.
Author information
Authors and Affiliations
Contributions
S.S.K. conceptualized the research. K.P. performed the experiments. S.S.K and K.P. prepared the manuscript and the SI. S.S.K. supervised the project and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interest.
Peer review
Peer review information
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Puri, K., Kulkarni, S.S. Total synthesis of a structurally complex zwitterionic hexasaccharide repeating unit of polysaccharide B from Bacteroides fragilis via one-pot glycosylation. Commun Chem 7, 204 (2024). https://doi.org/10.1038/s42004-024-01296-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42004-024-01296-y