Abstract
Mineral and rock additions to the environment have been proposed as a pathway to remove atmospheric CO2. This process occurs when hydrated minerals or rocks increase alkalinity, promoting the formation of bicarbonate. In this study, we evaluate the potential of commonly used hydrated rock and mineral powders to enhance alkalinity and react with both atmospheric and concentrated CO2. Silicate minerals and rocks exhibit minimal reactivity with atmospheric CO2 and provide moderate alkalinity enhancement. Volcanic rocks like basalt were shown to release CO2. Ground cement and Mg(OH)2, refined from CO2-free ultramafic rock, significantly increase alkalinity and mineralize both atmospheric and concentrated CO2. However, the effectiveness of cement waste is limit by its variable CaO content and potential heavy metal contributions. Overall, Mg(OH)2, derived from silicates, offers a promising pathway for the removal and storage of CO2.

Similar content being viewed by others
Introduction
A wide variety of rocks and minerals have been proposed to provide benefits for CO2 removal when introduced into soil systems using Enhanced Rock Weathering (ERW)1. The benefits and/or pathways of CO2 reduction via near-surface application of rocks and minerals may be direct2 (i.e., reacting with CO2) or indirect (i.e., enhancing biochemical/biogeochemical carbon binding pathways or as a ‘downstream’ alkalinity enhancement for bicarbonate formation3). As soil systems are diverse and chemically complex, characterizing how one reactant provides direct or indirect benefits requires years of field studies and advancement in monitoring methods.
Measurement and determination of CO2 removal and/or capture using ERW is ongoing4. Enhanced rock weathering requires that rock/mineral soil additions promote bicarbonate formation, typically by an increase in alkalinity. Ultimately, these soil bicarbonates will have ‘flow on’ contributions to rivers and ultimately to the oceans5. Due to ERW involving a multitude of factors, no standardized methodology, excluding modeling, has been accepted to directly verify the claims of ERW6. One of the first challenges of ERW is to assess how rocks/minerals may react when added to a soil environment.
In this study, we focus on the most common rocks and minerals used in ERW and their ability to react with atmospheric and concentrated CO2. We recognize that soil systems are more complex than the experiments presented here; however, this study provides a foundational baseline for material reactivity and interactions with atmospheric and concentrated CO2. Additionally, we employed methods to examine these interactions that others can utilize. Furthermore, we examine applications of each material for CO2 removal and alkalinity enhancement and their potential impact on global climate change.
Results and discussion
Direct air capture and high-concentration mineralization of CO2
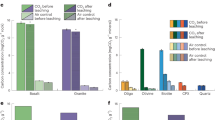
Carbon dioxide removal, reactivity and mineralization abilities for basalt, basalt/ultramafic rock mix, olivine, serpentine, ground cement, and Mg(OH)2 [See Methods] under atmospheric and high concentrations of CO2 are presented in Fig. 1. These rocks and minerals are being considered and/or used for a variety of enhanced rock weathering CO2 removal projects7,8,9,10. Mg(OH)2 was synthesized from olivine using methods similar to Scott et. al11. In Fig. 1a, CO2 concentrations over time are shown in a Direct Air Capture (DAC) closed-loop unit with a packed bed made of each sorbent [See Methods]. The average initial atmospheric CO2 concentration (520 ppm) is representative of the CO2 concentration initially present in the closed DAC loop. Please note that all rocks/minerals without the presence of water were not able to sequester or react with CO2 from our experiments and water by itself, in the time frame of these experiments, did not take in any measurable atmospheric CO2.
a CO2 present in the DAC closed loop versus time for different hydrated materials as shown. The dashed line represents the atmospheric CO2 present in the loop when closed and the average external atmospheric CO2 concentration. Accounting for the accuracy of the CO2 meter and the external atmospheric CO2 concentrations provides the basis for the gray box. b XRD patterns for each of the carbonated products after reactions with high concentration CO2 are presented. Peaks highlighted in light blue are hydromagnesite. Peaks highlighted in light gray are calcite.
In Fig. 1a, rocks/minerals are categorized into three groups based on their interactions with CO2: sorbents that were minimally reactive with CO2 (i.e., CO2 unreactive), sorbents that strongly reacted with CO2 (i.e., CO2 remover), and sorbents that released CO2 into the closed loop (i.e., CO2 contributor). Basalt was a CO2 contributor. Olivine and serpentine were generally CO2 unreactive for 1.5 h; however, CO2 did decrease by ~20-40 ppm suggesting that water may have had enough time to release some component from the rock that could react with CO2. Cement waste and Mg(OH)2 were CO2 removers. Both reached the same concentration of CO2 quickly (i.e., the lower detection limit on the detector) indicating all CO2 was removed.
Figure 1b shows XRD analysis results for the samples subjected to high CO2 concentrations in slurry tests [see Methods]. The purpose of this CO2-sorbent treatment was to provide adequate CO2 to identify potential reactivity to CO2 as well as secondary mineral formation (i.e., carbonate formation). Before and after CO2 treatment, no carbonates were present in serpentine. Similarly, the basalt/ultramafic rock mix, olivine, and basalts showed no carbonates before or after the CO2 treatment. The composition of the cement waste changed significantly; 7% calcite to 18% calcite after reacting with CO2. Magnesium hydroxide was 100% converted to a carbonate (i.e., hydromagnesite) following CO2 treatment.
In the DAC loop, basalts and the basalt-ultramafic rock mixture increased CO2 levels. Basalts are extrusive igneous (i.e., volcanic) rocks that entrain gases such as CO2 and water upon eruption. During the cooling/crystallization of basaltic lavas, CO2 is trapped (e.g., bubbles/vesicles) in the rock’s matrix12,13. Basalts naturally release CO2 upon weathering14. When basalt is crushed and allowed to react with water, this entrained CO2 is released, leading to an observed increase of CO2 levels in the DAC closed-loop unit. Concentrations of ~1 wt. % CO2 in basalt are not uncommon15 and could be higher depending on the amount of CO2 trapped during crystallization/solidification. Additionally, basalts may contain carbonates, especially those that solidified and/or interacted with seawater. To these points, even though basalt is a silicate rock, it is not a carbon or CO2-free rock.
Basalts are being proposed and used as a soil additive or as a cement feedstock to reduce global atmospheric CO216,17. Ground/powdered basalts release CO2, as shown in Fig. 1a prior and following the addition of water. This mechanism as well as carbonates present have been systemically overlooked in current carbon accounting practices. The long-term impact of weathering reactions on basalt’s CO2 release requires further investigation, particularly regarding soil dynamics and land use implications. When reacted with concentrated CO2 (Fig. 1b), basalts did not form any detectable carbonate, demonstrating low CO2 reactivity.
Olivine was obtained from an intrusive igneous rock in which CO2 was not involved during its formation. Serpentine, derived from the metamorphism of an olivine-rich rock, is typically CO2-free; however, serpentine-rich rocks may have carbonates present related to the introduction of CO2-rich fluids. Olivine and serpentine exhibited minimal reactivity with CO2, resulting in CO2 decreases of 3% and 1%, respectively, in the DAC closed-loop experiments (Fig. 1a). Furthermore, when exposed to concentrated CO2, no carbonate was detected for olivine or serpentine samples (Fig. 1b). The observed CO2 decrease of 15 ppm after 5 h in the DAC loop, along with the absence of carbonate in the presence of concentrated CO2, suggests that surface reactions, such as adsorption may be occurring. (i.e., CO2 is not mineralized but chemically bound as a surface complex). The implication is that surface complexation will provide a less durable means to store CO2 compared to mineralization and/or lead to surface passivation where mineral cations such as Mg2+ and Ca2+ may decrease in availability for alkalinity enhancement and CO2 interactions. It is important to note that these materials, as well as basalt, are silicates, which typically exhibit slower chemical reactivity with water compared to salts and some oxides/hydroxides13. This is simply a matter of the type and proportion of ionic versus covalent bonds that are present; minerals with a higher proportion of covalent bonds will take longer to dissolve and supply Mg2+ and Ca2+. Beneficial effects with regards to the removal of CO2 from the atmosphere would occur on the order of years to hundreds of years, consistent with silicate weathering rates18.
Cement waste and Mg(OH)2 sourced from ultramafic rock were able to react with all CO2 in the DAC loop. The cement waste contains unreacted CaO (Fig. 1b; determined by XRD) providing a chemical pathway to react with CO2 forming a carbonate. Additionally, cementation may occur with CaO in the presence of reactive silica to form calcium-silicate-hydrate (CSH). Mg(OH)2, with its higher purity, has the capacity to capture more CO2 (per mass) than cement. The CO2 reacting with Mg(OH)2 formed a hydrous carbonate (hydromagnesite). Both materials reacted with CO2, in contrast to the adsorption seen in serpentine and olivine, and they appear to be effective sorbents for quickly capturing CO2 from the atmosphere or providing timely alkalinity enhancements. To mineralize 1 tonne of CO2, ~1.3 tonnes of Mg(OH)2 is required. Cement waste initially had 7% calcite pre-CO2 treatment and 18% calcite post-CO2 treatment. To mineralize 1 tonne of CO2, ~20 tonnes of cement waste would be required.
Applications of sorbents for CO2 removal
Understanding the functions of sorbents extends beyond their CO2 removal capabilities to encompass broader environmental impacts, such as their effect on pH. As sorbents are considered for applications in various environments, including a wide variety of soils in different climate regions, pH measurements serve as crucial indicators of their potential efficacy and compatibility, especially in terms of alkalinity enhancement (i.e., a major consideration/variable for ERW). Figure 2 provides pH measurements for each sample in a slurry (i.e., soil pH), shedding light on their alkalinity and acidity characteristics. Ground cement had the highest pH of 12.6; Mg(OH)2 had the second highest pH of 10.3. Olivine-containing samples were able to increase the pH to ~9. The serpentine and basalt resulted in slightly basic pH values. Increases in alkalinity using olivine or serpentine (pH 8.9 and 7.9, respectively) may provide some benefits to acidic soils by increasing carbonate species such as bicarbonate and increasing pH, but would be detrimental in alkaline soils with high pH values (>8).
Increasing alkalinity and enhancing bicarbonate formation in soil have been proposed to be major pathways for the removal of atmospheric CO25. Both the cement waste and Mg(OH)2 were able to increase alkalinity with carbonate ions (CO32-) being the dominant carbon species at pH values greater than 9. MgO, which can be converted from Mg(OH)2 via heating, has been used as a soil additive for decades and has an equilibrium pH of 10.3. The equilibrium pH of Mg(OH)2 is 10.5 agreeing with the measured pH value of 10.3 ± 0.3 from the Mg(OH)2 used in this study. The equilibrium pH of CaO is 12.5 and 9 for Ca(OH)2. The pH of the cement waste from this study was 12.6 ± 0.3 supporting that CaO, not Ca(OH)2, was the major active phase in the cement waste. It should be noted that CaO increased the pH more than any of the other materials evaluated in this study. Lime has been a soil additive for decades; however, using CaO and other components in cement such as reactive silica as a soil additive requires further investigation. Cement has been widely used to immobilize waste with heavy metal contamination, but its effectiveness remains debated, typically limited to acidic waste sites with high metal contamination levels19. Soils used for agriculture, however, differ from waste sites and tend to have a closer-to-neutral pH values.
For alkalinity enhancements to provide a benefit with regards to ERW, several factors must be considered. Soils must be acidic with a pH value less than 5.8 and cannot be alkaline. Approximately 3,950 million ha of arable land have some degree of soil acidity20 with not all these soils having pHs less than 5.8. This means that there is a limitation to the type/amount of soils in which ERW would work. To promote alkalinity enhancement pathways and ultimately CO2 removal, the pH of the soil needs to increased from 5.8 to 6.2 (i.e., taking advantage of carbonic acid dissociation to bicarbonate). The enhancement additive would also have to overcome soil buffering. This means that the quantity/mass of basalt to adjust soil pH would be much greater compared to olivine, Mg(OH)2, and cement waste (see Fig. 2) provided other factors such as reactive surface area were equal. For example, the percent of basalt, Mg(OH)2, and cement waste mixed into soil to raise it from 5.8 to 6.2 (without considering buffering) would be 19%, 8.5%, and 6%, respectively. If a soil had a pH ~4, an estimated soil mix of >50% basalt would be required to raise the pH to 6.2. As basalt contains and releases CO2 as shown in Fig. 1a, there may be no benefit to adding basalt for CO2 removal; potentially providing the opposite outcome of being an overall CO2 emitter. For basalt to be considered for ERW or as feedstock for other purposes, this fundamental point needs to be addressed. The benefit of adding Mg(OH)2 or lime to soils is that less material is needed to provide pH adjustments.
Adding products to soil is not limited to their benefits, but how they may adversely affect the systems, such as the contribution of heavy metals like Cd, As, Co, Cr, and Pb. Heavy metals can be elevated in cement due to the source materials and processing21,22. When cement is mixed with water some of these heavy metals dissolve/leach out into the water, in particular Cr, Cu, and Pb23,24,25. While some current research suggests that leached heavy metal concentrations from cement are within acceptable limits for soil contamination26, it is important to note the lack of studies on fully ground and dissolved rock compared to cement block testing. Additionally, olivine commonly contains high levels of Ni and Co which could be detrimental if released into the environment27. Note that Mg(OH)2 in this study was engineered from an olivine-rich feedstock in which metals of concern such as Ni and Co were removed. While it may be advantageous to add cement waste or olivine to soils for CO2 capture and alkalinity enhancements, these materials create potential risks with regards to the introduction of heavy metals.
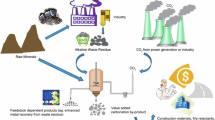
Rocks and minerals would require different methodologies to capture or offset CO2 from the atmosphere and industrial processes. Figure 3 provides a simplified and scaled up visual representation of the applications of each material for CO2 removal and capture for the following bulleted points.
-
Carbon emissions associated with current cement production emits ~0.5 tonnes of CO2 for every tonne of cement produced28. The majority of these emissions are due to the calcination of CaO. Thus, emissions could be decreased by capturing CO2 from the process (point source capture), capturing CO2 from the atmosphere (offsets via DAC), or simply reducing the amount of CaO used. This serves as a baseline for evaluating the potential impact of alternative sorbents on CO2 emissions within the cement industry, but it could be extended to any industry with CO2 emissions.
-
Basalts and ultramafic rocks are being used to offset the emissions via ERW applications including soil additions16,17. Basalts would contribute carbon, whereas, using ultramafic rocks would not contribute or react with CO2 on a short-term basis.
-
Cement waste is used similarly to enhanced weathering and spread on the soil. While some CO2 is captured, it is constrained by available CaO, and it cannot capture more CO2 than what was originally emitted during the calcination process.
-
Alternatively, substituting a portion of cement with alternative low-carbon supplementary cementitious materials would achieve an equivalent decrease in CO2 via abatement as the emissions are never generated. Thus, instead of focusing solely on using cement waste for carbon dioxide capture and storage (CDR), reducing cement consumption by using low-carbon SCMs and alternatives could offer a more sustainable approach to climate change mitigation.
-
Magnesium hydroxide sourced from a silicate that contains no CO2 offers a comprehensive solution. Overall, magnesium hydroxide can capture CO2 emissions without being a source of CO2 as well as providing alkalinity enhancement for ERW pathways.
-
All materials in this study could provide an alkalinity enhancement; however, CaO and Mg(OH)2 are well suited to do so in terms of their equilibrium pH.
We acknowledge the wide geochemical variation present in the rock/mineral types presented here as well as the short timescales of these investigations. For others exploring enhanced rock weathering in terms of their feedstock, they should consider the release and reactivity of CO2 in their rock/mineral feedstock as part of the overall carbon lifecycle analysis. Simple bulk methods to do so are presented in Supplementary Information: Materials and Methods. Additionally, future studies could expand to investigate different environmental conditions, such as water content, and react the materials over long-term timescales.
The potential and benefit of Mg(OH)2
Mg(OH)2 sourced form ultramafic rock (i.e., created without producing/releasing CO2) was able to quickly and directly mineralize atmospheric CO2 and provide alkalinity enhancements to soils. One of the main questions is whether this material is scalable to make fundamental changes to Earth’s atmosphere (i.e., slow down or possibly reverse climate change); especially, in context to the energy needed to extract, process, and supply this material. First, we discuss scalability in terms of the feedstock required and how it could lower CO2 by directly mineralizing the gas to form a durable carbonate.
Conversion of ultramafic feedstock to CO2 mineralized is provided below:
-
1 tonne of ultramafic rock produces 0.5 tonne of Mg(OH)2
-
1 tonne of Mg(OH)2 mineralizes 0.75 tonne of CO2 if into nesquehonite or 0.6 tonne of CO2 if into hydromagnesite.
-
2.7 tonnes of ultramafic rock are needed to mineralize 1 tonne of CO2
To demonstrate the scalability potential we provide two scenarios below.
Scenario 1: To remove 1,000 tonnes of CO2, 2650 tonnes of ultramafic rock would need to be processed. A simple quarry operation such as those in Invercargill, New Zealand, could provide that amount of material in less than a day.
Scenario 2: To reduce Earth’s atmospheric CO2 (416 ppm) to preindustrial levels (280 ppm) would require a change of 136 ppm (i.e., removing 1,095 billion tonnes of CO2), therefore, 2.9 × 103 billions of tonnes of ultramafic rock would need to be processed to supply the required Mg(OH)2 to ‘reset’ Earth’s atmosphere to preindustrial levels. One of the largest deposits of accessible ultramafic rocks is located in Oman. The size of the Semail Ophiolite in Oman is estimated to be 2.3×105 billion tonnes11. Only 1.2% of the Oman deposit would be required to correct for worldwide climate change. The carbonate (i.e., hydromagnesite) produced would have innumerable applications in construction, agriculture, manufacturing, etc. or it could be used to remediate the landscape in which the ultramafic feedstock was extracted by replacing a rock with a rock. Deposits of ultramafic rocks are distributed worldwide and are often located near to industrial centers allowing for an international effort, not the responsibility of one deposit or country.
Constraints for Mg(OH)2 production are ultimately related to energy and water. A cursory assessment of the energy used per tonne of atmospheric CO2 removed using data from Fig. 1a was calculated for each material. Please note that factors such as bicarbonate formation or potential reactions that may occur over longer periods (months to years) than those presented in Fig. 1a were not considered. Energy required for (a) grinding each material (0.02 MWh per tonne of material ground), (b) calcination for producing CaO (1.6 MWh per tonne), and c) processing ultramafic rocks for Mg(OH)2 (1.5-3 MWh per tonne based on the process described by Scott et al.11) was considered. No energy calculation was performed for basalt due to producing CO2. The energy required per one tonne of CO2 removal is olivine/serpentine 1 × 1010 MWh, cement waste 32 MWh, and Mg(OH)2 1.5-3 MWh.
Despite olivine and serpentine only requiring energy for grinding, low CO2 uptake resulted in these minerals demonstrating the highest energy expenditure for CO2 removal. Mg(OH)2 required more energy than olivine and serpentine to produce; however, the overall amount of energy needed to remove CO2 was the least due to its effectiveness at removing CO2. In addition to energy, there are limitations with regard to water. Enhanced rock weathering assumes water-rich soils (such as those in tropical climates) over longer time periods (years to decades), exceeding the time frame of these experiments. Although Mg(OH)2 needs more water related to its processing than mining rock/minerals, its short-term effectiveness means that it will need comparatively less water than longer-term enhanced rock weathering. Overall, Mg(OH)2 demonstrates promising results for balancing effectiveness/efficiency in relation to the energy and water demand needed to produce it.
Conclusions
We have shown in laboratory experiments the capabilities of different rocks and minerals, which are currently proposed for enhanced removal of CO2. Adding engineered products to the environment requires that the product be effective, safe, and demonstrate proven capabilities to reduce and/or store atmospheric CO2. Based on the evidence provided here, one product is a source of CO2 (basalt) and others (basalt, serpentine and olivine) are constrained to silicate dissolution rates. Cement waste shows potential in CO2 reduction; however, its overall impact is limited due to the emissions generated during cement production. From this study, Mg(OH)2 sourced from ultramafic rocks was the only heavy metal-free product to decrease CO2 from the air, mineralize CO2, and increase alkalinity. Magnesium oxides have been used as soil additives for decades29; however, most of this material is sourced from the breakdown of Mg carbonates, which releases CO2. Until now, there has been no overall carbon benefit for adding this product to soil systems. With Mg(OH)2 being sourced from CO2-free silicates, there is a net benefit in terms of overall carbon offsets and reductions as well as an alkalinity enhancement. However, this material is constrained by the energy required for processing. Overall, future enhanced rock weathering (ERW) research should consider the release of gases entrained in rocks/minerals as part of the overall carbon lifecycle analysis. We suggest using simplified methods, such as those followed in this study, to allow for verifiable CO2 balance, as well as identifying the optimum parameters for faster mineralization and reactivity.
Methods
Direct air capture closed-loop design
Supplementary Fig. 1 provides a schematic and photo of the experimental setup for the Direct Air Capture (DAC) closed-loop system. A peristaltic pump cycles ‘trapped’ air across a CO2 meter inside the loop and across a packed bed of mineral/rock powders. Outside of the closed loop is another CO2 meter measuring the external atmospheric CO2 levels.
Packed bed material (i.e., rock/mineral powders) in the DAC loop is prepared by combining 25 g of sorbent mixed with deionized water. Moisture is necessary to aid CO2 reactions (i.e., no measurable reaction with mineral/rock/CO2 occurred without water) and creates a porous tacky packed bed. Sorbent was placed in the packed-bed column between steel mesh sheets to hold the bed in place, and the lid to the column was put on (i.e., closing the loop). The peristaltic pump was started, allowing the cycling air to pass across the packed bed. The peristaltic pump moves the air at a rate of 60 ml/min; ~15 minutes for the air to cycle through the loop one time. The test was run for a total of 5 h. During the 5-h test for each sample, internal and external temperatures varied between 19.9-22°C. Relative humidity was between 30-40% externally and 30-80% internally. Internal humidity values increased with time, consistent with air circulating through a partially saturated packed bed.
CO2 concentrations were measured in ppm using an S8 Aranet4 CO2 meter. This CO2 meter uses a high-performing non-dispersive (NDIR) sensor with an accuracy of ±70 ppm. The concentration of CO2 (ppm), relative humidity (%), and temperature (°C) were recorded every minute. CO2 concentration in the room (external) varied throughout the test due to the presence of people and HVAC operation with external CO2 values averaging 520 ppm. CO2 and relative humidity measurements in the DAC loop were only affected by the packed bed of sorbent.
Rocks, minerals, and cement waste
Six separate rocks/minerals/cement material were trialed in the packed bed as the sorbent: magnesium hydroxide Mg(OH)2, serpentine (Mg2Si2O5(OH)4), olivine ((Mg0.9Fe0.1)2SiO4), basalt (minerals present include plagioclase, olivine, pyroxene, and opaques), cement/mortar waste, and an ultramafic/basalt mix. Magnesium hydroxide was synthesized from ultramafic rocks that released no CO2 during its production. Cement waste was processed from mortar testing cubes made with standard Portland cement. The remaining rocks were all New Zealand sourced with locations specified in Supplementary Table 1. All the samples were finely ground in a puck mill for 1 minute. Material properties (BET surface area, DFT pore volume, average pore diameter) for each sample and mass and water for each packed bed are provided in Supplementary Table 1. Whole rock/mineral chemistry performed by ALS Chemex for all samples is provided in SupplementaryTables 2 and 3.
High-concentration CO2 slurry test
Each sorbent was reacted with concentrated CO2 to identify potential carbonates that may form. A slurry was made with 700 g of filtered H2O and 20 g of product. The solution was put into a plastic bottle and attached to a SodaStreamTM carbonation unit. The unit remains closed and under pressure until the end of the experiment. The liquid ratio was in excess of the saturation limit for each sample, and the total amount of CO2 added was greater than the CO2 stoichiometrically required for the sequestration. For each trial, a single sample was sealed into a bottle, which was secured with a CO2 injection nozzle. Approximately 2.5 g of CO2 was injected into the solution every 10 minutes for 1 h. The total amount of CO2 injected was greater than the amount required stoichiometrically to fully carbonate the sample. This was determined by assuming that all the magnesium and calcium in the sample would convert into carbonate, forming magnesium or calcium carbonate according to Eqs. (1) and (2):
Water is essential for the carbonation reaction. CO2 dissolves into the water to produce carbonic acid, which then reacts with the hydroxide to form carbonate and water. The SodaStream unit is pressurized at 55 psi. At complete CO2 saturation, the solution could dissolve 4.46 g CO2 at this pressure. We added less than the saturation limit for CO2 in water to ensure the CO2 could be dissolved and used. As the material carbonates, water is created and can be used to produce more carbonic acid.
At the end of the test, the solution was placed into an oven at 105°C and dried for over 48 h. The sample was then analyzed with a Rigaku model Smartlab 3 kV X-ray diffractometer (XRD). Samples were ground into a fine powder with a mortar and pestle and then pressed into a 10-disc auto-sample-changer stage. Samples were run from 5 to 65 2-theta at intervals of 0.02. The voltage and current were set to 40 kV and 30 mA, respectively.
pH/Alkalinity
Rock/mineral pH values were measured to provide an indication of how each sample would affect alkalinity and/or potentially react with CO2. pH values were measured by using a 2:1 ratio of water to solids by mass using a Thermo-Scientific Orion-Star A211 pH probe.
References
Holzer, I. O., Nocco, M. A. & Houlton, B. Z. Direct evidence for atmospheric carbon dioxide removal via enhanced weathering in cropland soil. Environ. Res Commun. 5, 101004 (2023).
McQueen, N., Kelemen, P., Dipple, G., Renforth, P. & Wilcox, J. Ambient weathering of magnesium oxide for CO2 removal from air. Nat. Commun. 11, 3299 (2020).
Li, C., Frolking, S. & Harriss, R. Modeling carbon biogeochemistry in agricultural soils. Glob. Biogeochem. Cycles 8, 237–254 (1994).
Buckingham, F. L., Henderson, G. M., Holdship, P. & Renforth, P. Soil core study indicates limited CO2 removal by enhanced weathering in dry croplands in the UK. Appl. Geochem. 147, 105482 (2022).
Renforth, P. The negative emission potential of alkaline materials. Nat. Commun. 10, 1401 (2019).
Fisher, J. Can ‘enhanced rock weathering’ help combat climate change? BBC Sci. Environ. (2023).
Skov, K. et al. Initial agronomic benefits of enhanced weathering using basalt: a study of spring oat in a temperate climate. PLoS ONE 19, e0295031 (2024).
McDermott, F., Bryson, M. & van Acken, D. An investigation of crushed returned concrete (CRC) as a soil amendment for atmospheric CO2 removal. In: EGU General Assembly 2022-4824 (Vienna, 2022).
Beerling, D. J., Kantzas, E. P. & Lomas, M. R. Potential for large-scale CO2 removal via enhanced rock weathering with croplands. Nature 583, 242–248 (2020).
Wolf, A., Marklein, A. & Chang, E. Methodology for CO2 Removal by Enhanced Mineralization on Cropland. (Eion Corp Whitepaper, 2023).
Scott, A. et al. Transformation of abundant magnesium silicate minerals for enhanced CO2 sequestration. Commun. Earth. Environ. 2, 25 (2021).
Evans, M. J., Derry, L. A. & France-Lanord, C. Degassing of metamorphic carbon dioxide from the Nepal Himalaya. Geochem. Geophys. Geosyst. 9, https://doi.org/10.1029/2007GC001796 (2008).
Zondervan, J. R. et al. Rock organic carbon oxidation CO2 release offsets silicate weathering sink. Nature 623, 329–333 (2023).
Bottinga, Y. & Javoy, M. MORB degassing: evolution of CO2. Earth Planet. Sci. Lett. 95, 215–225 (1989).
Lowenstern, J. B. Carbon dioxide in magmas and implications for hydrothermal systems. Min. Depos. 36, 490–502 (2001).
Kantola, I. B. et al. Improved net carbon budgets in the US Midwest through direct measured impacts of enhanced weathering. Glob. Chang. Biol. 29, 7012–7028 (2023).
Larkin, C. S. et al. Quantification of CO2 removal in a large-scale enhanced weathering field trial on an oil palm plantation in Sabah, Malaysia. Front. Clim. 4 (2022).
Berner, R. A., Lasaga, A. C. & Garrels, R. M. The carbonate-silicate geochemical cycle and its effect on atmospheric carbon dioxide over the past 100 million years. Am. J. Sci. 283 (1983).
Schlögl, S., Diendorfer, P., Baldermann, A. & Vollprecht, D. Use of industrial residues for heavy metals immobilization in contaminated site remediation: a brief review. Int. J. Environ. Sci. Technol. 20, 2313–2326 (2023).
Bian, M., Zhou, M., Sun, D. & Li, C. Molecular approaches unravel the mechanism of acid soil tolerance in plants. Crop J. 1, 91–104 (2013).
Chang, S. W. et al. Analysis of heavy metal contents in gray and white MTA and 2 kinds of Portland cement: a preliminary study. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endodontol. 109, 642–646 (2010).
Achternbosch, M. et al Heavy metals in cement and concrete resulting from the co-incineration of wastes in cement kilns with regard to the legitimacy of waste utilisation. Karlsruhe: Forschungszentrum Karlsruhe GmbH (2003).
Estokova, A., Oravec, J. & Kovalcikova, M. Environmental impact assessment of the concrete composites in terms of the selected toxic metals leaching. Chem. Eng. Trans. 43, 1915–1920 (2015).
Ogunkunle, C. O. & Fatoba, P. O. Contamination and spatial distribution of heavy metals in topsoil surrounding a mega cement factory. Atmos. Pollut. Res. 5, 270–282 (2014).
Akpambang, V. O. E., Ebuzeme, G. C. & Akinola, J. O. Heavy metal contamination of topsoil around a cement factory—a case study of Obajana Cement Plc. Environ. Pollutants Bioavailability 34, 12–20 (2022).
Natural Resources Conservation Service, U. Hazardous Ranking Score (HRS) Reference Package—REFERENCE #62 (2016).
Kierczak, J., Pietranik, A. & Pędziwiatr, A. Ultramafic geoecosystems as a natural source of Ni, Cr, and Co to the environment: a review. Sci. Total Environ. 755, https://doi.org/10.1016/j.scitotenv.2020.142620 (2021).
Andrew, R. M. Global CO2 emissions from cement production. Earth Syst. Sci. Data 10, 195–217 (2018).
Yan, B. & Hou, Y. Effect of soil magnesium on plants: a review. IOP Conf. Ser.: Earth Environ. Sci. 170, 022168 (2018).
Acknowledgements
The authors acknowledge support from Mark Chadderton, Katherine Izumi, Anna Reid, Georgia Sigglekow, and the Breakthrough Energy Fellows Program.
Author information
Authors and Affiliations
Contributions
M.D. and C.O. contributed equally to the research, development, and writing presented in this article.
Corresponding author
Ethics declarations
Competing interests
M.D. and C.O. are employed at a for-profit company (Aspiring Materials) that manufactures Mg hydroxide for industrial alkalinity applications and CO2 mineralization. C.O. has a US patent for Mg hydroxide (Title: Soda magcite composition, methods of manufacture and use in carbon dioxide (CO2) sequestration; Authors: Scott, A. and Oze, C.; Patent No. US 11,890,572 B1). All authors declare no competing interests regarding the content of this paper.
Peer review
Peer review information
Communications Chemistry thanks Fakhreza Abdul, Ranjith Pathegama, and Long Jiang for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Danczyk, M., Oze, C. Suitability of rocks, minerals, and cement waste for CO2 removal via enhanced rock weathering. Commun Chem 7, 272 (2024). https://doi.org/10.1038/s42004-024-01361-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42004-024-01361-6