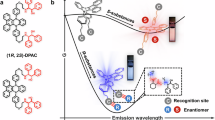
Abstract
Chirality plays a pivotal role in the properties of biologically active molecules, with enantiomers exhibiting divergent pharmacological and toxicological profiles. Enantioselective recognition is thus crucial in drug development, asymmetric synthesis, and environmental monitoring. Luminescence sensing has emerged as a powerful strategy for enantioselective recognition due to its fast response and visual readout capabilities. Covalent-organic frameworks (COFs) offer a promising platform for such applications by combining structural robustness, modular functionality, and inherent porosity. However, achieving both high enantioselectivity and quantitative sensing within a single system remains highly challenging. Herein, we present a cation-induced strategy for enantioselective sensing using a terbium-loaded chiral COF, Tb@CD-COF. Through a facile cation exchange of piperazine cations of CD-COF with Tb3+ ions, we revealed a synergistic integration of cation-enhanced luminescence and chiral cavity-based enantioselective recognition mechanism. Tb@CD-COF demonstrates visually discernible colorimetric responses and quantitative enantiomer discrimination, offering a robust and efficient platform for advanced enantioselective sensing applications.
Similar content being viewed by others
Introduction
Chirality is a widespread phenomenon in nature and plays a vital role in many chemical and biological systems1,2. Many molecules, particularly those involved in biological processes, are inherently chiral. These molecules often exhibit distinct pharmacological activities, metabolic behaviors, and toxicity. Hence, the selective recognition of chiral molecules—enantioselective recognition—is critically important across diverse fields, including drug development, asymmetric synthesis, and environmental monitoring3,4,5. However, constructing sensing systems that can differentiate enantiomers with both high sensitivity and efficiency remains a long-standing challenge in supramolecular and analytical chemistry because of the very similar structures and properties of the enantiomers.
Among various sensing strategies, luminescence-based sensing has garnered significant attention due to its high sensitivity, non-invasive property, and potential for real-time monitoring6,7,8,9,10, which has been applied in the sensing towards ions, volatile organic compounds, and persistent organic pollutants. Integrated chiral recognition motifs, luminescent sensors enable enantioselective detection with quantifiable and often visually distinguishable outputs, offering an efficient platform for both qualitative and quantitative chiral analysis11,12,13,14,15,16,17. However, the development of efficient luminescent chiral sensors faces several critical challenges. The synthesis of chiral units often relies on expensive or enantiomerically pure starting materials, significantly increasing the overall cost and limiting large-scale applications. Additionally, the construction of stable and well-defined chiral architectures with integrated luminescent properties remains synthetically demanding, requiring precise control over stereochemistry and framework assembly. Even when successful, many reported systems exhibit only subtle or ambiguous signal changes upon enantiomer binding, making accurate detection and quantification difficult, especially under practical sensing conditions. These limitations underscore the need for more accessible, robust, and responsive chiral luminescent materials for real-world applications.
Covalent-organic frameworks (COFs), a class of crystalline porous polymers assembled via covalent bonds, offer several intrinsic advantages for sensing applications, including high thermal and chemical stability, ordered porosity, and designable modularity18,19,20. Compared to other luminescent sensing materials such as small-molecule sensors, metal-organic frameworks (MOFs), and coordination polymers, COFs provide structural stability and enable precise spatial arrangement of functional groups without metal nodes. Notably, the incorporation of chiral centers into COF backbones leads to chiral COFs21,22,23,24, which integrate enantioselective recognition with luminescent output within a robust and tunable scaffold.
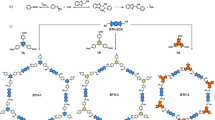
To date, achieving both quantitative recognition and strong enantioselectivity within a single COF-based sensor remains rare. Herein, we report a cation-induced enhancement strategy for enantioselective sensing based on a chiral luminescent COF, Tb@CD-COF (CD = cyclodextrin), prepared via a facile cation exchange of piperazine (PPZ) cations in CD-COF25 with Tb3+ ions (Fig. 1). Cation-induced strategy for enhanced certain properties has been applied in advanced porous materials due to its facile, designable, and costless characteristics26. The synergistic effect of cation-enhanced luminescence and framework-embedded chiral cavities results in markedly improved sensitivity, distinct colorimetric changes, and quantitative discrimination of enantiomers, offering a powerful platform for high-performance enantioselective sensing. The combination of Tb3+ ions with the analytes weakened the interactions between Tb3+ ions and COF framework, and hence recovered the luminescence of the original COF, while the enantioselectivity comes from the differences in the adsorption of enantiomers by the chiral framework.
Results
Basic characterization
γ-CD is a cyclic oligosaccharide composed of eight α-D-glucopyranose units linked by α-1,4-glycosidic bonds. It adopts a toroidal structure featuring a hydrophobic inner cavity and a hydrophilic outer surface. This unique architecture enables γ-CD to form host–guest inclusion complexes with a broad range of molecules27,28. Herein, an anionic COF, denoted as CD-COF (Fig. 1), was synthesized via the condensation reaction between γ-CD and trimethyl borate25. CD-COF is selected for this study because of its costless and facile synthesis, ionic framework structures, stable and permanent pore channels, and appropriate energy transfer ability with desired luminescence performance. Unlike the high-energy microwave-assisted synthesis reported in the literature25, a solvothermal method using an autoclave was employed. The phase purity of the as-synthesized CD-COF was confirmed by powder X-ray diffraction (PXRD) (Supplementary Fig. S1). The activated CD-COF showed a type-I behavior of the N2 adsorption isotherm at 77 K (Supplementary Fig. S2). The Brunauer–Emmett–Teller (BET) surface area was calculated to be 504 m2 g−1, which is consistent with the reported value of 494 m2 g−125. PXRD analysis of CD-COF after N2 adsorption was also performed (Supplementary Fig. S3), showing an improved signal-to-noise ratio (Supplementary Fig. S4), which may be due to the removal of disordered guest solvents.
Tb3+ ions were introduced into CD-COF to serve as the luminescence center to give Tb@CD-COF. The content of Tb3+ relies on the concentration of TbCl3, soaking time, and temperature. A dual-center luminescence sensor with visible dual emission peaks is established by adopting this procedure. The successful incorporation of Tb3+ was confirmed by inductively coupled plasma (ICP) analysis. PXRD patterns further verified that the crystallinity and phase purity of the framework were retained after ion exchange (Supplementary Fig. S5). Infrared (IR) spectra revealed similar functional groups before and after Tb3+ incorporation, indicating that the chemical structure remained unchanged (Supplementary Fig. S6). Additionally, thermogravimetric analysis (TGA) showed similar weight loss profiles (Supplementary Fig. S7). Solid-state circular dichroism spectra of the frameworks with and without Tb3+ ions, both exhibited Cotton effects consistent with that of pure γ-CD, confirming the preservation of chiral characteristics (Supplementary Fig. S8). Moreover, from the liquid 1H nuclear magnetic resonance (NMR) spectra of the diggested COFs, the decrease in the characteristic peak area of PPZ in the channel after Tb3+ introduction further supports the partial replacement of PPZ exchanged by Tb3+ ions (Supplementary Fig. S9).
Luminescent sensing
The solid-state luminescence spectra of CD-COF and Tb@CD-COF were measured. Excited at 252 and 338 nm, CD-COF possesses similar emission spectra with the emission peak at 402 nm (Fig. 2a). Excited at 296 nm, Tb@CD-COF possesses comparable emission intensities at 402 and 542 nm (Fig. 2b), while Tb@CD-COF exhibits lower emission intensities compared with CD-COF, indicating inefficient energy transfer in Tb@CD-COF (Fig. 2c). The time-dependent emission intensities of Tb@CD-COF were stable (Supplementary Figs. S10–S12), demonstrating that Tb@CD-COF is suitable for sensing application.
a Excitation and emission spectra of CD-COF. b Excitation and emission spectra of Tb@CD-COF. c Emission spectra of CD-COF and Tb@CD-COF excited at 296 nm. d Response time of Tb@CD-COF. e Anti-interference tests of Tb@CD-COF towards scan slit. f Anti-interference tests of Tb@CD-COF towards excitation wavelength.
To investigate the enantioselective sensing capabilities of Tb@CD-COF, several chiral molecules including 1,2-propanediol, 2-amino-1-propanol, and 2-amino-1-butanol, which are important industrial raw materials and pharmaceutical intermediates29,30,31,32 were selected as target analytes. Upon gradual additions of these analytes, the emission intensities of the COF framework increased, while the characteristic emission of Tb3+ ions significantly decreased (Supplementary Figs. S13–S18) and stabilized within the first 30 s (Fig. 2d), demonstrating a rapid and dynamic luminescence response. Furthermore, the anti-interference property of Tb@CD-COF was evaluated under variations in scan slit width and excitation wavelength. The results showed excellent stability and reproducibility under these conditions (Fig. 2e, f), highlighting the robustness of the sensing platform, which often originates from modulations in the energy transfer process, thereby further improving the reliability and reproducibility of the sensing results. All the above results confirm Tb@CD-COF an effective and instant enantioselective sensing material.
To evaluate the sensing capacities, the linear Benesi-Hildebrand (B-H) equation is used to describe the enhancement-type sensing of the framework: I0/(I − I0) = KBH/[C] + b, wherein KBH is the association constant, C is the concentration of the added analytes, b is a constant33,34. With the additions of these analytes, the intensities of Tb@CD-COF at 402 nm can be well fitted by the B-H equations (Fig. 3a–c), and the KBH values are given in Fig. 3d. The differences of the KBH values for Tb@CD-COF towards these enantiomers are 1.25–1.45 times, which confirms its great enantioselective sensing performance. The limits of detection were 8.9/11.8 μM (R-/S-1,2-propanediol), 7.5/10.7 μM (R-/S-2-amino-1-propanol), and 8.6/10.8 μM (R-/S-2-amino-1-butanol), respectively, according to the 3σ IUPAC criteria35. Furthermore, the relationships between the concentrations of the enantiomers and the ratio-metric luminescence intensity changes can be well described by the exponential equations (Supplementary Figs. S19–S21), which induces the better anti-interference quantitative concentration analysis.
a Emission intensity changes of Tb@CD-COF towards 1,2-propanediol at 402 nm. b Emission intensity changes of Tb@CD-COF towards 2-amino-1-propanol at 402 nm. c Emission intensity changes of Tb@CD-COF towards 2-amino-1-butanol at 402 nm. d KBH values and the enantioselective ratios of Tb@CD-COF towards the enantiomers.
Sensing mechanism
As for the sensing mechanism, a series of characterizations and analyses were carried out. Firstly, the PXRD patterns reveal no structural distortion of the framework after the sensing experiments (Supplementary Fig. S22), indicating that the sensing function can be attributed to the COF structure but not the structural collapse15. There are limited overlaps between the ultraviolet-visible (UV-vis) absorption spectra of the analytes and the UV-vis absorption and the emission spectra of Tb@CD-COF (Supplementary Figs. S23 and S24), indicating the absence of the competitive absorption and Förster resonance energy transfer (FRET) process15. As determined by density functional theory (DFT) calculations, the lowest unoccupied molecular orbital (LUMO) energy level of γ-CD is lower than the analytes (Supplementary Fig. S25), suggesting that the photoinduced electron transfer (PET) mechanism is absent15.
Solid-state UV-vis spectrum of γ-CD and the phosphorescence spectrum at 77 K of isostructural Gd@CD-COF were tested (Supplementary Figs. S26 and S27). The singlet-state energy level of this COF is calculated as 4.95 × 104 cm−1, while the triplet-state energy level is 2.17 × 104 cm−1. The energy gap is 2.78 × 104 cm−1, which is larger than 5000 cm−1, indicating that the intersystem crossing process in Tb@CD-COF is effective (Supplementary Fig. S28)36. The 5D4 energy level of Tb3+ ion is 2.04 × 104 cm-1, so the calculated energy difference between the lowest triplet-state of γ-CD and the 5D4 energy level of Tb3+ ion is 1.3 × 103 cm-1, which is lower than 1.5 × 103 cm−1, indicating the inefficient “antenna effect”37. This explains the much lower intensities of Tb@CD-COF compared with CD-COF (Fig. 2c) and provides chances to the luminescence recovery. Furthermore, Tb3+ ions were added to a N,N-dimethylformamide (DMF) solution of γ-CD to evaluate the luminescence ability. As the concentration of Tb3+ ions increased, a corresponding enhancement in the emission intensity was observed (Supplementary Fig. S29), indicating that the luminescence of Tb3+ in this system is concentration-dependent and arises from their coordination with γ-CD. Comparative studies were also conducted using Tb3+ ions in the presence of γ-CD and the analytes under the same concentration, respectively. The Tb3+–γ-CD complex exhibited significantly higher emission intensity based on Tb3+ compared to the combinations of Tb3+ with the analytes (Supplementary Fig. S30), indicating a more effective energy transfer process between γ-CD and Tb3+ compared with the analytes.
In summary, the inefficient “antenna effect” of γ-CD induces the decreased emission of Tb@CD-COF compared with CD-COF. With the additions of the analytes, the decrease of the luminescence of Tb3+ at 542 nm is due to the weaker luminescence of Tb3+-analytes compared with Tb3+–γ-CD, while the enhanced luminescence of CD-COF framework is due to the competitive binding of the analytes with Tb3+ ion (Fig. 4). Compared with primary framework sensing materials constructed by post-synthetic modification methods using luminescent frameworks and modified chiral centers14,26, this work uses the cheap and natural chiral molecule to construct chiral framework and introduced luminescence center, which gives rise to the more stable chiral centers and enhanced-type luminescence response. Meanwhile, the enantioselective sensing performance may be caused by their different combination capacities of the enantiomers with Tb3+ ions within the COF framework (Fig. 4).
The luminescence lifetimes also confirm the combination mechanism of Tb3+ and analytes. With the additions of the analytes, the luminescence lifetimes possess no obvious changes (Supplementary Figs. S31–S42 and Table S1), indicating the static mechanism when the non-luminescent ground-state complex is formed between the donor and acceptor38,39. The quantum yield results further confirm this mechanism. With the additions of the analytes (Supplementary Figs. S43–S48), the total quantum yields increased obviously (Supplementary Figs. S49–S51 and Table S2), confirming the decreased energy-transfer-caused energy loss. Besides, the different increasement of the quantum yields towards enantiomers also confirm the enantioselective capacities (Supplementary Figs. S49–S51 and Table S2).
Discussion
In summary, the development of Tb@CD-COF as a luminescent enantioselective sensor highlights the potential of integrating chiral recognition motifs within a single COF scaffold. The observed improvements in sensitivity and enantiomeric discrimination are attributed to the synergistic interplay between the framework’s spatially confined chiral cavities and the luminescence amplification imparted by Tb3+ ions. Three pairs of small-molecule enantiomers with coordination capabilities have been recognized effectively and selectively, which can be employed for monitoring the enantiomeric purity and the progress of stereoselective reactions. This strategy not only enhances emission intensity and signal contrast but also provides a pathway for fine-tuning host–guest interactions at a molecular level. Compared to conventional sensing materials, Tb@CD-COF exhibits several key advantages: (i) structural rigidity and ordered porosity for stable selective enantiomer access, (ii) modular design enabling facile functionalization and post-synthetic modification, and (iii) enhanced and ratio-metric luminescent responses for intuitive readout and quantitative analysis. These features collectively advance the current state-of-the-art in enantioselective sensing, particularly in the systems that demand both robustness and real-time feedback.
Nonetheless, several challenges and opportunities still remain. For instance, improving water stability and compatibility with biologically relevant conditions will be essential for practical development in pharmaceutical and clinical settings. Furthermore, expanding the range of detectable chiral analytes will require tailored design of chiral ligands and coordination environments. Incorporation of stimuli-responsive or dynamic features could further enhance the selectivity and adaptability. Looking ahead, the tunability and modularity of COFs position them as versatile scaffolds not only for chiral sensing but also for applications in asymmetric catalysis, optoelectronics, and molecular separation. The cation-induced enhancement strategy demonstrated here opens new avenues for designing multifunctional COF-based platforms that combine recognition, analysis, and response within a single system. Future work will focus on exploring other lanthanide ions, diverse chiral building blocks, and hybrid sensing modalities to broaden the utility of this approach across interdisciplinary applications.
Methods
Materials
All reagents were commercially available and used without further purification. Liquid 1H NMR spectra were recorded on a Bruker AV 400 MHz NMR spectrometer. PXRD measurements were performed using a Rigaku SmartLab SE X-ray diffractometer with Cu-Kα radiation. TGA data were obtained under nitrogen atmosphere on a Mettler Toledo TGA 2 thermogravimetric analyzer from 40 °C to 800 °C. Circular dichroism spectra were measured on a JASCO J-715 circular dichroism spectrometer. Elemental analyses for C, H, and N were carried out using a Vario EL cube elemental analyzer. ICP analysis was conducted using a Thermo IRIS Advantage instrument. Luminescence spectra, luminescence lifetimes and quantum yields were recorded on an Edinburgh FS5 fluorescence spectrophotometer equipped with Xenon lamp, pulsed flash lamps and an integrating sphere. UV-vis absorption spectra were measured by a Shimadzu UV-2600 spectrometer. Gas adsorptions were performed at a Micromeritics 2020 gas adsorption apparatus. IR spectra were tested by a Bruker Tensor 27 spectrometer. PXRD refinement was done by Jana40.
Synthesis of CD-COF
CD-COF was synthesized following a reported method25 with slight modifications. A solution of γ-CD (65 mg, 0.05 mmol), B(OMe)3 (28 µL, 0.25 mmol), PPZ (22 mg, 0.25 mmol), super dry 1,3,5-trimethylbenzene (TMB, 3.5 mL) and super dry DMF (4 mL) was added in a 23 mL Teflon-sealed autoclave in a glove bag. The autoclave was heated in an oven at 120 °C for 4 days. The obtained white powder was collected by centrifugation, purified by repeated washing with DMF. Elemental analysis (%) calculated for C69.7H122.6N7O45B2 ({(H2PPZ)[(γ-CD)(BO4)2]}·0.3TMB·5DMF): C 46.26, H 6.86, N 5.47; found: C 46.30, H 6.63, N 5.48. For N2 isotherm, CD-COF was soaked and exchanged by CH2Cl2 and hexane for three times, and activated at 120 °C under vacuum for 12 h.
Synthesis of Tb@CD-COF
50 mg CD-COF was soaked in 5 mL 10 mM TbCl3 DMF solution for 24 h to give Tb@CD-COF. The product was collected by filtering and washed with fresh DMF until the eluate exhibited no obvious luminescence signal. The content of Tb based on ICP is ~1%.
Synthesis of Gd@CD-COF
50 mg CD-COF was soaked in 5 mL 10 mM GdCl3 DMF solution for 24 h to give Gd@CD-COF. The product was collected by filtering and washed with fresh DMF until the eluate exhibited no obvious luminescence signal. The content of Gd based on ICP is ~1%.
Data availability
Data supporting findings from this work are available within this Article and the Supplementary Information. All other relevant data supporting findings are available from the corresponding author on request.
References
Zhu, H. & Yakobson, B. I. Creating chirality in the nearly two dimensions. Nat. Mater. 23, 316–322 (2024).
Jones, R. R. et al. Chirality conferral enables the observation of hyper-Raman optical activity. Nat. Photon. 18, 982–989 (2024).
Yang, X.-L. et al. Chiral MOF-derived wearable logic sensor for intuitive discrimination of physiologically active enantiomer. Adv. Mater. 35, 2304046 (2023).
Kim, M. et al. Bio-templated chiral zeolitic imidazolate framework for enantioselective chemoresistive sensing. Angew. Chem. Int. Ed. 62, e202305646 (2023).
Han, Z. et al. Tailoring chirality and porosity in metal-organic frameworks for efficient enantioselective recognition. Chem 9, 2561–2572 (2023).
Gong, W., Chen, Z., Dong, J., Liu, Y. & Cui, Y. Chiral metal-organic frameworks. Chem. Rev. 122, 9078–9144 (2022).
Han, Z., Wang, K., Zhou, H.-C., Cheng, P. & Shi, W. Preparation and quantitative analysis of multicenter luminescence materials for sensing function. Nat. Protoc. 18, 1621–1640 (2023).
Chen, Z. et al. Rapid extraction of trace benzene by a crown-ether-based metal-organic framework. Natl Sci. Rev. 11, nwae342 (2024).
Lustig, W. P. et al. Metal-organic frameworks: functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 46, 3242–3285 (2017).
Chen, Z. et al. Efficient recognition and removal of persistent organic pollutants by a bifunctional molecular material. J. Am. Chem. Soc. 145, 260–267 (2023).
Wanderley, M. M., Wang, C., Wu, C. D. & Lin, W. B. A chiral porous metal-organic framework for highly sensitive and enantioselective fluorescence sensing of amino alcohols. J. Am. Chem. Soc. 134, 9050–9053 (2012).
Xuan, W., Zhang, M., Liu, Y., Chen, Z. & Cui, Y. A chiral quadruple-stranded helicate cage for enantioselective recognition and separation. J. Am. Chem. Soc. 134, 6904–6907 (2012).
Li, P. et al. A homochiral microporous hydrogen-bonded organic framework for highly enantioselective separation of secondary alcohols. J. Am. Chem. Soc. 136, 547–549 (2014).
Han, Z. et al. Chiral linker installation in a metal-organic framework for enantioselective luminescent sensing. J. Am. Chem. Soc. 146, 15446–15452 (2024).
Zhao, Y., Zeng, H., Zhu, X.-W., Lu, W. & Li, D. Metal-organic frameworks as photoluminescent biosensing platforms: mechanisms and applications. Chem. Soc. Rev. 50, 4484–4513 (2021).
Zhao, Y.-W., Wang, Y. & Zhang, X.-M. Homochiral MOF as circular dichroism sensor for enantioselective recognition on nature and chirality of unmodified amino acids. ACS Appl. Mater. Interfaces 9, 20991–20999 (2017).
Thoonen, S. et al. Enhancing enantioselectivity in chiral metal organic framework fluorescent sensors. Inorg. Chem. Front. 11, 3877–3888 (2024).
Dincă, M. & Long, J. R. Introduction: porous framework chemistry. Chem. Rev. 120, 8037–8038 (2020).
Cote, A. P. et al. Porous, crystalline, covalent organic frameworks. Science 310, 1166–1170 (2005).
Ding, S. Y. & Wang, W. Covalent organic frameworks (COFs): from design to applications. Chem. Soc. Rev. 42, 548–568 (2013).
Qian, H. L., Yang, C. X. & Yan, X. P. Bottom-up synthesis of chiral covalent organic frameworks and their bound capillaries for chiral separation. Nat. Commun. 7, 12104 (2016).
Han, X. et al. Chiral induction in covalent organic frameworks. Nat. Commun. 9, 1294 (2018).
Xu, H.-S., Ding, S.-Y., An, W.-K., Wu, H. & Wang, W. Constructing crystalline covalent organic frameworks from chiral building blocks. J. Am. Chem. Soc. 138, 11489–11492 (2016).
Han, X. et al. Chiral covalent organic frameworks: design, synthesis and property. Chem. Soc. Rev. 49, 6248–6272 (2020).
Zhang, Y. et al. Three-dimensional anionic cyclodextrin-based covalent organic frameworks. Angew. Chem. Int. Ed. 56, 16313–16317 (2017).
Han, Z. et al. Cation-induced chirality in a bifunctional metal-organic framework for quantitative enantioselective recognition. Nat. Commun. 10, 5117 (2019).
Tian, H. et al. Supramolecular spectral/visual detection of urinary polyamines through synergetic/competitive complexation with γ-CD and CB[7]. Chem. Commun. 57, 1806–1809 (2021).
Fan, S.-T. et al. Ultrahigh carbon dioxide-selective composite membrane containing a γ-CD-MOF layer. ACS Appl. Mater. Interfaces 13, 13034–13043 (2021).
Tao, Y. et al. A comprehensive review on microbial production of 1,2-propanediol: micro-organisms, metabolic pathways, and metabolic engineering. Biotechnol. Biofuels 14, 216 (2021).
Główka, M. & Krawczyk, T. New trends and perspectives in production of 1,2-propanediol. ACS Sustain. Chem. Eng. 11, 7274–7287 (2023).
Ishikawa, W. & Sato, S. Synthesis of amino acids in intense laser-irradiated primary amine solutions. Phys. Chem. Chem. Phys. 27, 3504–3509 (2025).
Lv, Y. et al. Conversion of S-2-amino-1-butanol-l-tartrate to S-2-amino-1-butanol by using bipolar membrane electrodialysis for post-treatment of direct enantioseparation. ACS Sustain. Chem. Eng. 9, 3542–3551 (2021).
Arunkumar, E., Ajayaghosh, A. & Daub, J. Selective calcium ion sensing with a bichromophoric squaraine foldamer. J. Am. Chem. Soc. 127, 3156–3164 (2005).
Chu, Q., Medvetz, D. A. & Pang, Y. A polymeric colorimetric sensor with excited-state intramolecular proton transfer for anionic species. Chem. Mater. 19, 6421–6429 (2007).
Committee, A. M. Recommendations for the definition, estimation and use of the detection limit. Analyst 112, 199–204 (1987).
Steemers, F. J., Verboom, W., Reinhoudt, D. N., vander Tol, E. B. & Verhoeven, J. W. New sensitizer-modified calix [4] arenes enabling near-UV excitation of complexed luminescent lanthanide ions. J. Am. Chem. Soc. 117, 9408–9414 (1995).
Binnemans, K. Lanthanide-based luminescent hybrid materials. Chem. Rev. 109, 4283–4374 (2009).
Wu, S. ; et al. Rapid detection of the biomarkers for carcinoid tumors by a water stable luminescent lanthanide metal-organic framework sensor. Adv. Funct. Mater. 28, 1707169 (2018).
Wang, M. ; et al. Confinement of p-xylene in the pores of a bilanthanide metal-organic framework for highly selective recognition. Angew. Chem. Int. Ed. 63, e202318722 (2024).
Petríček, V., Dušek, M. & Palatinus, L. Crystallographic computing system JANA2006: General features. Z. Kristallogr. 229, 345–352 (2014).
Acknowledgements
This work was supported by the National Key Research and Development Program of China (Grant No. 2024YFE0211600) and the National Natural Science Foundation of China (Grant Nos. 22471130, 22261132509, 22121005, and 22435002).
Author information
Authors and Affiliations
Contributions
Z.H. did the main experiment, collected and interpreted the data, and wrote the paper. T.S. and P.C. modified the paper. W.S. designed the experiments and modified the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Han, Z., Sun, T., Cheng, P. et al. Cation-induced enhanced enantioselective recognition by a chiral covalent-organic framework. Commun Chem 8, 206 (2025). https://doi.org/10.1038/s42004-025-01605-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42004-025-01605-z