Abstract
Chemical ligation of oligonucleotides enables assembly of long DNA constructs essential for genome engineering, DNA nanotechnology, and molecular diagnostics, but current methods often require external activators and suffer from reactive intermediate instability. Here we show a reagent-free DNA autoligation strategy based on intramolecular cross-activation between 3′-phosphorothioate (PS) and 5′-dinitrobenzenesulfonamide (DNBSA) termini on a splint DNA, yielding a P3′ → N5′ phosphoramidate linkage under near-physiological conditions. Ligation proceeds with over 80% yield at 37 °C and pH 8 without external reagents. The DNBSA group exhibits exceptional aqueous stability, and in situ formation of reactive intermediates contributes to high efficiency. This strategy expands the current toolkit for assembling DNA constructs and may facilitate future biotechnological and therapeutic studies.
Similar content being viewed by others
Introduction
Oligonucleotide (ON) ligation to concatenate two ONs has been applied in various biotechnologies1,2,3. Generally, chemical ON ligation requires a third ON (splint ON) that is complementary to the ONs to be ligated. Two ligating ONs with reactive functional groups are set appropriately close to each other on the splint ON, thereby increasing the local concentration of the reactive substrates and accelerating the ligation reaction. Chemical ON ligation is useful in the following two aspects: the ON ligation products themselves are useful materials in biochemical and medical applications, such as genome-oriented DNA synthesis4,5,6 and structural DNA nanotechnology7,8,9,10,11. Furthermore, the ligation reaction triggers appropriate detection signals or codes structural information that are used for biotechnological applications, such as nucleic acid detection12,13,14,15,16,17,18,19 and DNA-encoded libraries20,21,22. In comparison to enzymatic ligation reactions23,24,25, chemical ligation reactions offer higher tolerance to diverse modified structures that lead to higher functionality of conjugated products, easier induction of signal diversity conjugated to the ligation reaction, and cost merits that are important in large-scale synthesis.
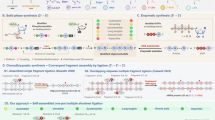
The reported chemical ligation reactions can be broadly classified into the following two types. In one method, the functional groups of the ON fragments are ligated to each other by adding external chemical reagents. Typical examples include the activation of the terminal phosphate group of an ON with EDCI, cyanogen bromide, imidazole derivatives, and a condensation reaction with the nucleophilic terminus of the other ON (Fig. 1A)9,26,27,28,29. Additionally, copper-mediated click chemistry4,30,31,32, which gives a more unnatural linkage structure, is also widely used (Fig. 1B). The second type of chemical ligation reaction does not require the addition of external reagents. These methods are especially advantageous in contexts where introducing additional reagents is impractical. Applications in RNA imaging, detection, and pharmaceuticals have been widely reported. Examples include copper-free click chemistry with distorted alkynes33,34, tetrazines35,36,37, photo-mediated cycloaddition reaction38,39,40, and nucleophilic substitution reactions with leaving group/PS pairs6,12,16,41,42,43.
On the other hand, we have developed a novel method to convert PS groups into electrophilic functional groups using 1-fluoro-2,4-dinitrobenzene (DNFB) for chemical ligation reactions. (Fig. 1C)43 This method chemoselectively activated the PS group in the ON to form electrophilic phosphorothioester (EPT) groups with dinitrobenzene, and the resulting EPT ONs could be purified by HPLC. By inducing electrophilic substitution reactions between EPT oligomers and amino oligomers on ON splints, DNA and RNA strands with phosphoramidate linkages can be prepared. However, the isolated EPT ONs lost their activity owing to gradual hydrolysis in an aqueous solution.
Crich et al. reported the peptide ligation reaction of a dinitrobenzene sulfonamide-protected peptide fragment with a thioacid44. In this reaction, the dinitrosulfonyl group is transferred from a peptide with a dinitrosulfonamide-protected peptide to a thioacid-terminated peptide, resulting in the formation of an activated thioacetate ester and amino terminus peptide. Then the activated thioacetate ester reacts with the amino terminus of the counter peptide fragment. We hypothesized that the use of dinitrobenzene sulfonamide (DNBSA) and PS ONs would similarly allow the formation of EPT ONs on the ON splint (Fig. 1D). Compared to EPT groups, ONs with DNBSA would be stable in an aqueous solution, and the PS-derived reactive species for the ligation reaction would be formed only in situ, and they would promptly react with the resulting free amino group of the counter ON in close proximity to afford the ligated ON, thereby avoiding hydrolysis of the active species and enabling efficient ligation reactions. Here, DNA with a dinitrobenzenesulfonamide (DNBSA) group at the 5’-terminus and DNA with a phosphorothioate (PS) group at the 3’-terminus react on the splint DNA strand to give linkage products with P3’ → N5’ phosphoramidate bonds28,43,45,46. Because the reaction proceeds under near-physiological conditions without auxiliary reagents, it could provide a means for stepwise synthesis of long DNA strands within cellular settings. Additionally, since non-natural DNA with a phosphoramidate linkage structure is similar to the phosphodiester backbone of natural DNA, it is expected to function as a transcriptional template for mRNA synthesis28,46 and produce proteins with high efficiency.
Results and discussion
Initially, a thymidine-based phosphoramidite with a DNBSA group at the 5’ position was synthesized (Scheme 1) for the preparation of 5’-DNBSA DNA. Thymidine 1 was converted to the corresponding tosylate 2 in 51% yield. Sodium azide was then reacted with 2 to prepare 5’ -azide form 3 in 62% yield. Subsequent palladium-catalyzed hydrogenation afforded amino derivative 4, which was then reacted with 2,4-dinitrobenzenesulfonyl chloride (DNBSCl) without isolation to afford 5’-sulfonamide derivative 5 in 77% yield over two steps. Finally, the desired phosphoramidite 6 was obtained in 52% yield by the phosphitylation of the 3’-hydroxyl group under acidic conditions. Based on this synthetic scheme, the desired phosphoramidite 6 was obtained in a total yield of 13%. It was confirmed that compound 6 was stable for at least one month when stored at −30 °C under Ar atmosphere based on 31P NMR measurement (Fig. S1). To establish suitable deprotection conditions for 5′-DNBSA DNA, several deprotection conditions were tested using poly(T) model DNA substrate (Fig. 2). In most cases, undesired removal of the DNSBA group proceeded to afford 5’ - amino DNA (b) as a major byproduct. The optimal conditions were found to be incubation in a 28% aqueous ammonia solution at room temperature for 2 h. It was also demonstrated that this deprotection condition is applicable to DNA with a mixed base sequence of ACGT by using DNA phosphoramidites with ultramild protecting groups (Fig. S8 and S17). To evaluate the designed ligation reaction with the DNBSA and PS groups, 5’-DNBSA DNA (14 mer), 3’ -PS DNA (11 mer) with fluorescein at the 5’ terminus, and the corresponding splint DNA (21 mer) were designed as shown in Fig. 3A. Each DNA sample was prepared with an automated oligonucleotide synthesizer on the solid phase, deprotected, released under appropriate conditions, and purified by reverse-phase HPLC (Table S1). To examine the efficiency of the ligation reaction of the synthesized DNAs, ligation reactions were performed in the presence of the splint DNA strand at pH 6–8. The samples were separated from the reaction solution at regular time intervals, and the reaction mixture was analyzed using 15% denaturing polyacrylamide electrophoresis (dPAGE). The gel was irradiated with UV light to detect the starting material (fluorescein-labelled 3’-PS DNA) and the ligation product (Fig. 3B). When analyzing the ligation reaction by electrophoresis, ligation reaction efficiency can be calculated by comparing the fluorescence signal intensity of the 25 mer of the ligation reaction product and 14 mer of the starting material. The efficiency of the ligation reaction clearly showed dependencies on the reaction time (1–12 h) and pH (6–8), as shown in Fig. 3C. Among the initial investigated conditions, the highest conversion (46%) was observed for the reaction at pH 8 and 12 h.
A Sequence and structure of the DNA fragments and the splint DNA. The reaction was performed with 8 μM of 3’-PS DNA, 5’-DNBSA DNA and splint DNA at different pH 6 to 8 for 1 to 12 hr at 37 °C. B dPAGE analysis of the crude mixture of the ligation reaction, C Ligation yield of the reaction at each time point and pH.
To further improve the efficiency of the ligation reaction, the concentration of DNA oligomers and the reaction time were changed, and the annealing treatment was tested (Fig. 4A) over a broader reaction time range (up to 48 h). We examined whether ligation reaction efficiency could be improved under these conditions. Similarly, from the results of gel analysis, the efficiency of the ligation reaction was calculated by comparing the fluorescence intensity of fluorescein-labelled 3’-PS DNA and the ligation product (Fig. S29). The ligation reaction proceeded more efficiently when the concentration of each oligomer was changed from 8 to 20 µM and annealing was performed. The yield also increased when the ligation reaction time was increased, with a maximum yield of 84% at a reaction time of 48 h. These results suggest that higher concentrations of DNA and annealing treatment promoted the formation of ternary complexes with DNBSA, PS-DNA, and splint DNA. A time course analysis of the ligation efficiency was also performed over 12 h (Fig. S2), which showed that the ligation efficiency reached 55% after 1 h. Concerning the ligation reaction, there would be two possible mechanisms, concerted or stepwise reaction (Fig. 4B). In a concerted mechanism, the deprotected amino group through dinitrophenyl group transfer would directly attack the activated phosphorothioate species thus generated. Considering that the reaction exhibited pH dependence, the actual reaction mechanism is presumed to be closer to a stepwise type. Additionally, the strong pH dependency around pH 7 to 8 may reflect the pKaH+ of the 5’-amino nucleoside, which was 7.847. To verify the reaction mechanism more directly, an aliquot of the reaction mixture was taken 15 min after initiation and analyzed by LC-MS (Fig. S24). The thioester species, an intermediate in the stepwise reaction mechanism, was detected, suggesting that the stepwise mechanism would be dominant. Furthermore, in the absence of splint DNA, the ligation did not proceed even at a high DNA concentration (100 μM) (Fig. S6), suggesting that this ligation reaction can be used not only for simple two-fragment ligations but also for sequential ligation reactions. In addition, ligation reactions were performed using 67 nt Long 3′-PS DNA and 46 nt Long 5′-DNBSA DNA (Figs. S4 and S5). A dPAGE analysis of the crude reaction mixture revealed a ligation efficiency of 63% after 48 h. This demonstrates that the new ligation reaction is also applicable to longer DNA sequences, albeit with a slightly lower yield. To explore the compatibility of the auto-ligation with abundant intracellular nucleophiles, reactions were also performed in the presence of 0–10 mM reduced glutathione (GSH) at 37 °C for 48 h (Fig. S3). The ligation yield declined as GSH concentration increased, yet approximately 25% conversion was still observed at 10 mM GSH, corresponding to the upper range of reported cytosolic concentrations48. We further examined the reaction in crude HeLa S3 cytosolic extract49 (Fig. S7). Although the ligation reaction proceeded, its rate was slower than under the test-tube conditions, and significant oligonucleotide degradation was observed after 4 h. These observations underscore that additional optimization will be required before this reaction can be translated to intracellular applications.
A Chemical ligation yield under modified reaction conditions; (a) 20 μM of 3’-PS DNA, 5’-DNBSA DNA and splint DNA without annealing, (b) 20 μM of 3’-PS DNA, 5’-DNBSA DNA and 40 μM of splint DNA without annealing, (c) 20 μM of 3’-PS DNA, 5’-DNBSA DNA and splint DNA with annealing before the reaction (90 °C, 3 min), B Possible reaction mechanism; (a) concerted mechanism, (b) stepwise mechanism.
Conclusion
In summary, we have established a new DNA chemical autoligation based on intramolecular activation of the 3’-phosphorothioate groups, which was mediated by the 5’-sulfonamide group of the counter DNA. 5’-sulfonamide DNA was effectively prepared by choosing mild deprotection conditions after phosphoramidite-based ON synthesis without decomposition of the functional group. The ligation reaction between 3’-phosphorothioate DNA and 5’-sulfonamide DNA proceeded smoothly and nearly quantitatively at pH 8, suggesting that the designed cross-activation of the phosphorothioate group actually proceeded. The investigation of its application in various biotechnologies and pharmaceutics is ongoing in our group.
Methods
Organic synthesis and characterization
See Supplementary Information and Supplementary Fig. S1.
Oligonucleotide synthesis and characterization
See Supplementary Information, Supplementary Table S1, Supplementary Figs. S8–S14, S18–S23 and S27, S28.
Typical procedure for chemical ligation
The DNA samples (3´-PS DNA, 5´-DNBSA DNA, and splint DNA at specified concentrations) were dissolved in phosphate buffer (20 mM, pH = 6, 7, or 8) containing MgCl2 (10 mM) and NaCl (50 mM), and the solution was incubated for the designated time at 25 °C or 37 °C. If applicable, an annealing process (heating at 90 °C for 3 min, followed by gradual cooling to room temperature) was performed before starting the incubation. Ligation efficiency was evaluated from the fluorescence intensity of FAM in gel analysis.
Characterization of chemical ligation reaction
See Supplementary Figs. S2–S7, S15–S16, S17, S24, S25 and S29–S33.
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information.
References
Li, X. & Liu, D. R. DNA-templated organic synthesis: nature’s strategy for controlling chemical reactivity applied to synthetic molecules. Angew. Chem. Int. Ed. 43, 4848–4870 (2004).
Percivalle, C., Bartolo, J.-F. & Ladame, S. Oligonucleotide-templated chemical reactions: pushing the boundaries of a nature-inspired process. Org. Biomol. Chem. 11, 16–26 (2013).
Gorska, K. & Winssinger, N. Reactions templated by nucleic acids: more ways to translate oligonucleotide-based instructions into emerging function. Angew. Chem. Int. Ed. 52, 6820–6843 (2013).
Kukwikila, M., Gale, N., El-Sagheer, A. H., Brown, T. & Tavassoli, A. Assembly of a biocompatible triazole-linked gene by one-pot click-DNA ligation. Nat. Chem. 9, 1089–1098 (2017).
Manuguerra, I. et al. Gene assembly via one-pot chemical ligation of DNA promoted by DNA nanostructures. Chem. Commun. 54, 4529–4532 (2018).
Yamaoka, K. et al. Completely chemically synthesized long DNA can be transcribed in human cells. ChemBioChem 22, 3273–3276 (2021).
Onizuka, K., Nagatsugi, F., Ito, Y. & Abe, H. Automatic Pseudorotaxane formation targeting on nucleic acids using a pair of reactive oligodeoxynucleotides. J. Am. Chem. Soc. 136, 7201–7204 (2014).
Onizuka, K. et al. Pseudorotaxane formation via the slippage process with chemically cyclized oligonucleotides. Nucleic Acids Res. 45, 5036–5047 (2017).
Weizenmann, N. et al. Chemical ligation of an entire DNA origami nanostructure. Nanoscale 13, 17556–17565 (2021).
Lyu, F. et al. Topological capture of mRNA for silencing gene expression. Chem. Commun. 59, 11564–11567 (2023).
Krishnamurthy, K., Rajendran, A., Nakata, E. & Morii, T. Near quantitative ligation results in resistance of DNA Origami against nuclease and cell Lysate. Small Methods 8, 2300999 (2024).
Xu, Y., Karalkar, N. B. & Kool, E. T. Nonenzymatic autoligation in direct three-color detection of RNA and DNA point mutations. Nat. Biotechnol. 19, 148–152 (2001).
Sando, S. & Kool, E. T. Imaging of RNA in bacteria with self-ligating quenched probes. J. Am. Chem. Soc. 124, 9686–9687 (2002).
Silverman, A. P. & Kool, E. T. Quenched autoligation probes allow discrimination of live bacterial species by single nucleotide differences in rRNA. Nucleic Acids Res. 33, 4978–4986 (2005).
Silverman, A. P. & Kool, E. T. Detecting RNA and DNA with templated chemical reactions. Chem. Rev. 106, 3775–3789 (2006).
Abe, H. et al. Rapid DNA chemical ligation for amplification of RNA and DNA Signal. Bioconjug. Chem. 19, 327–333 (2008).
Kleinbaum, D. J. & Kool, E. T. Sandwich probes: two simultaneous reactions for templated nucleic acid detection. Chem. Commun. 46, 8154–8156 (2010).
Harcourt, E. M. & Kool, E. T. Amplified microRNA detection by templated chemistry. Nucleic Acids Res. 40, e65–e65 (2012).
Shibata, A., Abe, H., Ito, Y. Oligonucleotide-templated reactions for sensing nucleic acids. Molecules. 17, 2446–2463 (2012).
Keefe, A. D., Clark, M. A., Hupp, C. D., Litovchick, A. & Zhang, Y. Chemical ligation methods for the tagging of DNA-encoded chemical libraries. Curr. Opin. Chem. Biol. 26, 80–88 (2015).
Litovchick, A. et al. Encoded library synthesis using chemical ligation and the discovery of sEH inhibitors from a 334-Million Member Library. Sci. Rep. 5, 10916 (2015).
Litovchick, A. & Keefe, A. D. Chemical ligation of oligonucleotide tags to support encoded chemical library synthesis. Methods Mol. Biol. 2541, 25–32 (2022).
Cao, W. Recent developments in ligase-mediated amplification and detection. Trends Biotechnol. 22, 38–44 (2004).
Conze, T. et al. Analysis of genes, transcripts, and proteins via DNA ligation. Annu. Rev. Anal. Chem. 2, 215–239 (2009).
Paredes, E., Evans, M. & Das, S. R. RNA labeling, conjugation and ligation. Methods 54, 251–259 (2011).
Obianyor, C., Newnam, G., Clifton, B. E., Grover, M. A. & Hud, N. V. Towards efficient nonenzymatic DNA ligation: comparing key parameters for maximizing ligation rates and yields with carbodiimide activation*. ChemBioChem 21, 3359–3370 (2020).
Shabarova, Z. A. et al. Chemical ligation of DNA: the first non-enzymatic assembly of a biologically active gene. Nucleic Acids Res. 19, 4247–4251 (1991).
El-Sagheer, A. H. & Brown, T. Single tube gene synthesis by phosphoramidate chemical ligation. Chem. Commun. 53, 10700–10702 (2017).
Nakamoto, K. et al. Chemically synthesized circular RNAs with phosphoramidate linkages enable rolling circle translation. Chem. Commun. 56, 6217–6220 (2020).
El-Sagheer, A. H. & Brown, T. Click nucleic acid ligation: applications in biology and nanotechnology. Acc. Chem. Res. 45, 1258–1267 (2012).
Fantoni, N. Z., El-Sagheer, A. H. & Brown, T. A Hitchhiker’s Guide to click-chemistry with nucleic acids. Chem. Rev. 121, 7122–7154 (2021).
Kollaschinski, M. et al. Efficient DNA click reaction replaces enzymatic ligation. Bioconjug. Chem. 31, 507–512 (2020).
Heuer-Jungemann, A., Kirkwood, R., El-Sagheer, A. H., Brown, T. & Kanaras, A. G. Copper-free click chemistry as an emerging tool for the programmed ligation of DNA-functionalised gold nanoparticles. Nanoscale 5, 7209–7212 (2013).
Shelbourne, M., Chen, X., Brown, T. & El-Sagheer, A. H. Fast copper-free click DNA ligation by the ring-strain promoted alkyne-azide cycloaddition reaction. Chem. Commun. 47, 6257–6259 (2011).
Geng, P., List, E., Rönicke, F., Wagenknecht, H.-A., Two-factor fluorogenicity of tetrazine-modified Cyanine-Styryl dyes for bioorthogonal labelling of DNA. Chem. – A Eur. J. 29, e202203156 (2022).
Šečkutė, J., Yang, J. & Devaraj, N. K. Rapid oligonucleotide-templated fluorogenic tetrazine ligations. Nucleic Acids Res. 41, e148–e148 (2013).
Wu, H., Cisneros, B. T., Cole, C. M. & Devaraj, N. K. Bioorthogonal Tetrazine-mediated transfer reactions facilitate reaction turnover in nucleic acid-templated detection of MicroRNA. J. Am. Chem. Soc. 136, 17942–17945 (2014).
Fujimoto, K. et al. Site-specific transition of cytosine to uracil via reversible DNA photoligation. Chem. Commun. 11, 3223–3225 (2006).
Ihara, T., Fujii, T., Mukae, M., Kitamura, Y. & Jyo, A. Photochemical ligation of DNA conjugates through Anthracene Cyclodimer formation and its fidelity to the template sequences. J. Am. Chem. Soc. 126, 8880–8881 (2004).
Ogino, M., Taya, Y. & Fujimoto, K. Detection of methylcytosine by DNA photoligation via hydrophobic interaction of the alkyl group. Org. Biomol. Chem. 7, 3163–3167 (2009).
Maruyama, H. et al. An intracellular buildup reaction of active siRNA species from short RNA fragments. Chem. Commun. 50, 1284–1287 (2014).
Xu, Y. & Kool, E. T. High sequence fidelity in a non-enzymatic DNA autoligation reaction. Nucleic Acids Res. 27, 875–881 (1999).
Maruyama, H. et al. Chemical ligation of oligonucleotides using an electrophilic phosphorothioester. Nucleic Acids Res. 45, 7042–7048 (2017).
Crich, D. & Sharma, I. Triblock peptide and peptide thioester synthesis with reactivity-differentiated sulfonamides and peptidyl thioacids. Angew. Chem. Int. Ed. 48, 7591–7594 (2009).
Chen, J., Baker, Y. R., Brown, A., El-Sagheer, A. H. & Brown, T. Enzyme-free synthesis of cyclic single-stranded DNA constructs containing a single triazole, amide or phosphoramidate backbone linkage and their use as templates for rolling circle amplification and nanoflower formation. Chem. Sci. 9, 8110–8120 (2018).
Nguyen, H., Abramov, M., Eremeeva, E. & Herdewijn, P. In Vivo Expression of Genetic Information from Phosphoramidate–DNA. ChemBioChem 21, 272–278 (2020).
Chen, M. S., Ward, D. C. & Prusoff, W. H. 5-Iodo-5’-amino-2’,5’-dideoxyuridine-5’-N’-triphosphate. Synthesis, chemical properties, and effect on Escherichia coli thymidine kinase activity. J. Biol. Chem. 251, 4839–4842 (1976).
Lu, S. C. Glutathione synthesis. Biochim. et. Biophys. Acta (BBA) - Gen. Subj. 1830, 3143–3153 (2013).
Kobayashi, T., Machida, K., Imataka, H. Human cell extract-derived cell-free systems for virus synthesis. In Cell-Free Protein Synthesis: Methods and Protocols, Alexandrov, K.; Johnston, W. A., Eds. Humana Press: Totowa, NJ; pp 149-156 (2014).
Acknowledgements
This work was supported by the Japan Society for the Promotion of Science (JSPS) [Grant-in-Aid for Scientific Research (B) 22H02219 and International Leading Research JP22K21346 to Y.K.], the Japan Science and Technology Agency (JST) [CREST JPMJCR18S1 and JPMJCR23N1 to H.A.), Japan Agency for Medical Research and Development (AMED) [JP22am0401008, JP22gm0010008, JP22fk0310506, JP23bm1223009, JP22fk0310506, JP243fa827006, JP23ab0123456, JP243fa827032, JP23fk0310506 to H.A., JP23fk0210133 to Y.K.], JST SPRING [Grant Number JPMJSP2125]. H. Y. would like to take this opportunity to thank Iwadare Scholarship Foundation and the THERS Make New Standards Program for the Next Generation Researchers.
Author information
Authors and Affiliations
Contributions
H.Y.: Investigation, Methodology, Data Collection, Data Analysis, Writing – Revised Manuscript Preparation, K.K.: Investigation, Methodology, Data Collection, Data Analysis, Writing – Original Draft Preparation, K.Y.: Investigation, Methodology, Data Collection, Data Analysis, R.O.: Investigation, Methodology, Data Collection, Data Analysis, F.T.: Investigation, Methodology, N.A.: Investigation, Methodology, Y.K. Project Administration, Funding Acquisition, Writing – Original Draft Preparation, Writing – Review & Editing, H.A. Supervision, Project Administration, Funding Acquisition, Writing – Review & Editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks Nicolas Winssinger and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yokoe, H., Kokubo, K., Yamaoka, K. et al. Chemical autoligation with phosphorothioate- and sulfonamide-terminated DNA via intramolecular cross-activation. Commun Chem 8, 232 (2025). https://doi.org/10.1038/s42004-025-01631-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42004-025-01631-x